Abstract
Purpose
Autism spectrum disorder is a highly complex neurological and psychosocial disorder characterized by social dysfunction, severe reduction in speech, and a single stereotyped behavior. The treatment methods are currently limited, and children with autism generally suffer from constipation and sleep disorders. It is urgent to find an alternative psychotropic drug, given the drug dependence and adverse reactions that may occur with long-term medication.
Patients and Methods
This retrospective study included 49 children with autism at the first affiliated Hospital of Guangdong Pharmaceutical University, who received washed fecal microbiota transplantation (WMT) treatment between June 2019 and July 2021 and compared the sleep disorder scores between the constipation group, control group and blank group.
Results
Second WMT could significantly improve the sleep disorder scores in the constipation group (p=0.026) and the decrease in sleep disturbance scale for children (SDSC) score was synchronized with the increase in Bristol stool form scale (BSFS) score. However, there was no significant difference between patients without constipation (p=0.54), and the behavior of autism improved in both groups.
Conclusion
WMT could relieve constipation and improve sleep disorders in children with autism, with no deterioration in stool morphology and sleep disorders in other children. Moreover, there were no obvious serious adverse clinical events after WMT.
Keywords: SDSC, ABC, CARS, BSFS, microbiota-gut-brain axis
Introduction
Autism is a mental illness characterized by the repetition of stereotyped behavior, severe lack of speech, and social communication disorders. Patients can harm themselves and attack others in serious cases.1,2 According to 2014 data, an average of one in 59 people in the United States had autism.3 By 2020, 2.21% of Americans were found to have autism, as per the Centers for Disease Control and Prevention (CDC) findings. According to a conservative estimate by China Autism Rehabilitation Association, the number of people with autism under the age of 13 years in China has reached more than two million. The head computerized tomography (CT), magnetic resonance imaging (MRI), X-ray and routine examination of blood, urine and feces of children with autism are similar to normal children, which makes diagnosis at an early stage very difficult until the typical noncommunicative and unresponsive symptoms of autism emerge. There is no specific drug or intervention4 available to cure autism worldwide due to its unclear pathogenesis, and the application of antipsychotics causes several side effects and adverse reactions.5
Autism spectrum disorder (ASD) is related to the genetic variation and flora imbalance of intestinal microbiota.6 Children with autism often suffer from gastrointestinal (GI) disorders, such as constipation and diarrhea, especially constipation.7 Many children with autism are picky eaters, which worsens the symptoms of constipation.8 These GI symptoms increase the physical burden and suffering of children with autism. In addition, it is difficult for children with autism to express their mental and physical pain, and their long-term emotions are not vented in a timely manner because of the social disorders of autism itself, which triggers extreme behaviors such as head banging, biting and assaulting others.2 Several studies reported9,10 that dysbiosis in the gut microbiota of children with autism spectrum disorders, which may be a determining factor on child development through the microbiota-gut-brain axis. For instance, children with ASD showed a higher abundance of Roseburia and Candida genera, and lower abundance of Dialister, Bilophila, Veillonella, Streptococcus, Coprococcus and Prevotella genera. Hom et al11 found that anxiety and loneliness are associated with sleep disturbances and the deterioration of sleep leads to extreme daytime behavior in children with autism.12 Sleep disorders in children with autism are more pronounced compared with normal children.13 Some children with autism accompanied by serious sleep disorders and intense aggressive behavior need to take sleeping pills or sedatives.14 Parents of Chinese children with autism are reluctant to give them long-term medication since these psychotropic drugs cause drug dependence, digestive symptoms such as nausea, vomiting, and central nervous system inhibitions such as hallucinations, delusions, and so on.
Washed fecal microbiota transplantation (WMT) is the extract of beneficial intestinal flora from healthy people and transplanted into patients with intestinal flora disorders to stabilize intestinal flora and treat intestinal and intestinal-related diseases.15 WMT has been widely used in intestinal diseases, such as Crohn's, ulcerative colitis, irritable bowel syndrome, functional constipation and diarrhea in recent years.16,17 Nowadays, fecal bacteria transplantation is widely researched and applied in the fields of GI digestive diseases, psychiatry and metabolic diseases.18 The view of “gut-microbiota-brain axis” is based on the close relationship between the central nervous system and GI nerves, which indicates the possible treatment of mental diseases from the GI tract.19,20 Recent studies have shown that after normal mice were transplanted with feces from children with autism, the social behavior of their offspring changed and became more autistic.21 The development of WMT has greatly reduced the adverse reactions and ensured safety.22,23
In this study, we investigated the effects of washed fecal bacteria transplantation on sleep quality, characteristics of the stools and manifestations in Chinese children with autism, thus providing basic information for the curative effect of WMT on children with autism in clinical applications.
Materials and Methods
Study Design and Participants
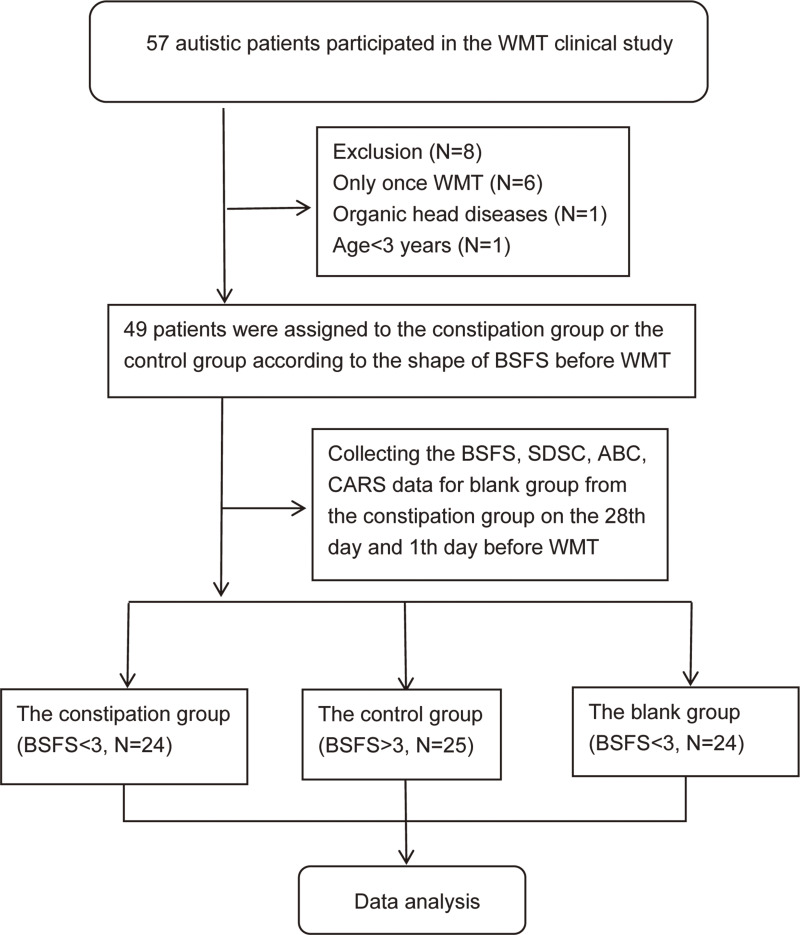
A total of 49 autistic patients aged 3–14 years were recruited from the first affiliated Hospital of Guangdong Pharmaceutical University between June 2019 and July 2021. The patients were diagnosed with autism according to the revised version of Autism Diagnostic interview (ADI-R)24 and Fifth Edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-5; APA2013)25 before WMT. Most ASD patients had moderate-to-severe GI disorders,26 and the basic conditions of the patients at the inception of the study are shown in Table 1. The patients could not get complete relief from constipation drugs, such as lactulose (667 mg:200 m Abbott Biologicals B.V.l) and Kaiserol (20 mL Baiyunshan Guangdong). The diagnosis of constipation was defined according to the Rome IV diagnostic criteria and the Bristol stool form scale (BSFS),27 and BRITOL <3 was considered to indicate constipation. All participants were divided into two groups according to whether or not they had constipation before WMT and received a course of WMT every four weeks. Twenty-four children with ASD and constipation (constipation group) and 25 children with ASD and without constipation were selected (blank control group, clinical population). Among them, 12 patients were diagnosed with mental retardation tendencies, including 5 in the constipation group and 7 in the control group. The data of the blank group were the follow-up data of the patients in the constipation group 28 days and one day before the first WMT treatment (Figure 1). All patients had used probiotics (bifidobacteria capsule, compound Lactobacillus acidophilus tablets, etc.) before and during the WMT. Among them, 1 patient had taken risperidone tablet before and after WMT due to the hallucinations and anxiety, another patient used aripiprazole for schizophrenia and has stopped one month before WMT.
Table 1.
Baseline Clinical Characteristics of Participants in the Constipation Group and the Control Group (N = 49)
| Group | Constipation Group | Control Group | p-value |
|---|---|---|---|
| Nation | |||
| The Han nationality | 24 | 25 | |
| Sex (M/F) | |||
| Male (percentage) | 19(76%) | 22(88%) | 0.614a |
| Female (percentage) | 5(24%) | 3(12%) | |
| Age (yr) | 5.67±3.08 | 6.72±3.94 | 0.308b |
| Other diagnosis | |||
| Mental retardation | 5 | 7 | 0.56b |
| Epilepsy | 0 | 1 | |
| Schizophrenia | 0 | 1 | |
| Abnormal behavior | |||
| Termagancy | 17 | 12 | 0.104a |
| Injure oneself and attack others | 18 | 8 | 0.003a |
| Life issues | |||
| Lack of concentration in study | 19 | 14 | 0.084a |
| Picky eaters | 21 | 12 | 0.008a |
| Other treatments | |||
| Behavior therapy | 19 | 21 | 0.66a |
Notes: Han nationality, China’s main nationality; Data are presented as Frequency (Percentage). Age is represented mean±standard deviation; aChi-square; bIndependent sample t-test.
Figure 1.
The workflow in this study.
Abbreviations: WMT, Washed fecal microbiota transplantation; BSFS, Bristol Stool Form Scale; SDSC, Sleep Dist scale for children; CARS, Child Autism Rating Scale; ABC, Autism Behavior Checklist; N, number.
Inclusion and Exclusion Criteria
The exclusion criteria were as follows: patients with severe primary cardiopulmonary, liver, brain, kidney or other serious diseases; head diseases such as head tumors or trauma; severe anemia or severe systemic infection, congenital aphasia or oroglossal muscle disease, history of thyroid disease, depression, pregnancy or lactation, inability to provide informed consent; history of abdominal or perianal surgery; history of GI surgery and digestive system diseases (tumor, inflammatory bowel disease), and history of intestinal pathogen infection; patients who were allergic to propofol; taken long-term oral laxatives; only one WMT, taken sleeping pills within one month and were not cooperating with the study protocol or had to withdraw from the study.
Donors Recruitment and Health Checkup
All donors completed a rigorous screening, including regular questionnaire surveys, fecal tests, fecal flora tests, human papilloma virus antigens, parasite egg tests, etc., to eliminate most parasites in order to prevent the transmission of infectious diseases from donors to subjects. We also ensured strict dietary requirements for donors. All fecal donors maintained a healthy and reasonable diet, with regular daily exercise, no drinking, smoking, or eating unhealthy food, such as pickled or canned food. The fecal sample from donor was collected in a 1 L sterile collection bucket (FMT Company) in the morning.
Fecal Suspension Preparation and Transplantation
Normal saline was used to prepare uniform fecal suspension to extract washed fecal bacteria. An instrument for fecal bacteria preparation (GenFMTer; FMM Medical, Nanjing, China) was used for microfiber filtration of fecal suspensions (removal of food, inflammatory substances and fungi). In addition, transendoscopic enteral tubing (TET)28 (lower digestive tract) or nasojejunal tube (middle digestive tract) (120 mL daily for six days) was injected into the patient within one hour according to the recommendation of Nanjing Consensus.29 The fecal suspensions from different donors (three donors mixed,40 mL:40 mL:40 mL) were randomly distributed to the patients according to each patient’s physical condition. Almost all patients were administered propofol anesthesia during TET and hospitalized in the gastroenterology department of Guangdong Pharmaceutical University for observation and to complete the WMT.
Data Collection
When the patients revisited the hospital for WMT, we asked them again to fill in and correct these two scales to improve the accuracy. We avoided collection of SDSC scores based on the sleep status of patients during WMT hospitalization due to the changes in sleep environment during hospitalization. Patients or their families and researchers filled out the following questionnaires every two weeks from the eighth week before WMT to the eighth week after WMT:
Sleep disturbance scale for children (SDSC)30,31 is a recognized measurement: There are 34 items about sleep difficulties, breathing disorders, arousal disorders, conversion disorders, excessive somnolence and sweating at night, to assess the extent to which various factors interfere with the quality of sleep in patients. The score for each question is 5, and the total score ranges from 34 to 170. The higher the score, the worse the sleep condition. Before scoring, we checked the patient’s medical history to exclude factors that affect sleep due to environmental changes, noise, strong light, temperature, drinking coffee or tea, depression, exams, fatigue, congenital diseases, pain, etc.
BSFS uses the BSFS32,33 picture as a reference, including the morphological and trait characteristics of various stools,27 1 or 2 as a hard stool, 6 or 7 as a soft stool, and 3–5 as normal, which are provided to children or parents for identification.
Child Autism Rating Scale (CARS)34 consists of 15 questions, with a maximum score of 60. The higher the score, the more serious the illness is. The scores of mild to moderate and severe patients were 30–36 and >36, respectively. The scores were completed by pediatricians or qualified professionals.
Autism Behavior Checklist (ABC)35 consists of 57 items, which is a structured, validated and authoritative questionnaire widely used in ASD research to assess the severity of current ASD symptoms.36,37
Statistical Analysis
The statistical analysis was performed by IBM SPSS26 software (IBM Corporation, Somers, New York), and the graphs were made with GraphPad prism8.0.1. (GraphPad Software, San Diego, California). Cohen’s d analysis38 has been applied to calculate the magnitude of the effect size. F-test was used to test the homogeneity of variance, independent sample t-test was used for scale, and Chi-square test was used for clinical characteristics. The p-value of the second row was selected if the p-value of the first column was >0.05 and did not satisfy the variance hypothesis. The correlation strength between the variable data was reflected in the p-value with 95% confidence interval (CI).
Results
Constipation Relief During Early WMT Using BSFS
Table 1 shows that the baseline clinical characteristics of the constipation group were worse than the control group because children with autism were more susceptible to constipation (p = 0.003) and more picky eaters (p = 0.008). There was a very significant difference (p < 0.001) in the BSFS of W1 and W2 after WMT in the constipation group, as shown in Tables 2 and 3. The average BSFS score of the constipation group was higher than that of the baseline (Baseline = 1.29, W1 = 2.33, W2 = 2.92). In the control group, there was no significant difference in BSFS after W1 and W2. However, compared with the mean and standard deviation of baseline and W1, it was closer to 3 and 4.
Table 2.
The Constipation Group Results
| Mean | SD | F | p-value | T | DF | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CARS | Baseline | 35.25 | 4.36 | 1.537 | 0.221 | 1.942 | 46 | - |
| W1 | 33.15 | 3.03 | 1.942 | 41.024 | 0.059 | |||
| W2 | 32.5 | 3.1 | 1.748 | 0.193 | 2.518 | 41.550 | 0.015a | |
| ABC | Baseline | 56.21 | 16.08 | 0.192 | 0.663 | 1.080 | 46 | - |
| W1 | 50.92 | 17.81 | 1.080 | 45.531 | 0.286 | |||
| W2 | 46.54 | 16.64 | 0.005 | 0.945 | 2.046 | 45.95 | 0.046a | |
| SDSC | Baseline | 55.13 | 14.36 | 0.023 | 0.881 | 0.813 | 46 | - |
| W1 | 51.75 | 14.42 | 0.813 | 45.999 | 0.421 | |||
| W2 | 45.79 | 13.69 | 0.74 | 0.786 | 2.305 | 45.896 | 0.026a | |
| BSFS | Baseline | 1.29 | 0.46 | 4.014 | 0.051 | −6.474 | 46 | - |
| W1 | 2.33 | 0.64 | −6.474 | 42.059 | 0.000a | |||
| W2 | 2.92 | 0.93 | 2.528 | 0.118 | −7.905 | 37.256 | 0.000a | |
Notes: aIndependent sample t-test; W1, First course of WMT treatment; W2, Second course of WMT treatment.
Abbreviations: CARS, Child Autism Rating Scale; ABC, Autism Behavior Checklist; SDSC, Sleep Dist Scale for Children; BSFS, Bristol Stool Form Scale; SD, Standard deviation; F, F statistic; T, T statistic; DF, Degrees of freedom.
Table 3.
The Control Group Results
| Mean | SD | F | p-value | T | DF | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CARS | Baseline | 36.64 | 3.38 | 0.824 | 0.369 | 2.202 | 48 | - |
| W1 | 34.54 | 3.37 | 2.202 | 47.999 | 0.033a | |||
| W2 | 33.88 | 2.61 | 0.604 | 0.441 | 3.233 | 45.139 | 0.002a | |
| ABC | Baseline | 63.52 | 20.97 | 0.109 | 0.742 | 1.028 | 48 | - |
| W1 | 57.56 | 20.04 | 1.028 | 47.902 | 0.309 | |||
| W2 | 52.88 | 16.59 | 1.249 | 0.269 | 1.990 | 45.588 | 0.053 | |
| SDSC | Baseline | 50.04 | 8.95 | 0.004 | 0.948 | 0.557 | 48 | - |
| W1 | 48.64 | 8.81 | 0.557 | 47.989 | 0.580 | |||
| W2 | 48.48 | 9.00 | 0.006 | 0.941 | 0.615 | 47.999 | 0.542 | |
| BSFS | Baseline | 4.08 | 0.95 | 1.365 | 0.248 | 0.530 | 48 | - |
| W1 | 3.96 | 0.61 | 0.530 | 40.855 | 0.599 | |||
| W2 | 4.00 | 0.50 | 2.598 | 0.114 | −0.979 | 24.336 | 0.337 | |
Notes: aIndependent sample t-test; W1, First course of WMT treatment; W2, Second course of WMT treatment.
Abbreviations: CARS, Child Autism Rating Scale; ABC, Autism Behavior Checklist; SDSC, Sleep Dist Scale for Children; BSFS, Bristol Stool Form Scale; SD, Standard deviation; F, F statistic; T, T statistic; DF, Degrees of freedom.
Sleep Disturbance Relief in the Constipation Group Using SDSC
As shown in Table 2, the SDSC score of patients in the constipation group decreased after W2, which was significantly different (p=0.026) from that of baseline. The sleep quality of children was improved after W2. Compared with the baseline, there was no statistical difference between BSFS and SDSC after W1 and W2 in the control group. After W1 and W 2, the stool shape of the constipation group was closer to 3 points. As shown in Table 3, there was also no statistical difference between SDSC and baseline in the control group after W1 and W2. In addition, there was no statistical difference between BSFS. The decrease of SDSC score was proportional to the increase in BSFS score.
No Changes in Sleep Patterns of the Control Group
In the constipation group, the mean value of SDSC decreased after W1, but there was no statistical difference (p=0.42), which was related to the small dose of fecal bacteria. Table 3 and showed that the difference between the CARS scale of W1 and the baseline was not statistically significant in the constipation group, but W2 was statistically significant (p=0.015). However, after W1 was statistically significant (p=0.033) and W2 was significantly different (p=0.002) in the control group. The ABC of the constipation group after W2 was statistically different from that of the baseline (p=0.046). However, after W1 and W2, it did not show any difference in the control group (p>0.05). This suggests that the autistic symptoms of patients in the control group improved more significantly after the first WMT at doctor’s judgment while disagreeing with the parents of control group, but it was close to the statistical difference after W2 (p= 0.053). Table 4 shows that there was neither statistical difference in CARS or ABC nor SDSCor BSFS between PW1 and PW2 in the blank group (p>0.05).
Table 4.
The Blank Group Results
| Mean | SD | F | p-value | T | DF | p-value | ||
|---|---|---|---|---|---|---|---|---|
| CARS | PW1 | 35.29 | 3.85 | 0.271 | 0.605 | 0.035 | 46 | 0.972 |
| PW2 | 35.25 | 4.36 | 0.035 | 45.301 | 0.972 | |||
| ABC | PW1 | 56.08 | 14.22 | 0.481 | 0.491 | −0.029 | 46 | 0.977 |
| PW2 | 56.21 | 16.08 | −0.029 | 45.323 | 0.977 | |||
| SDSC | PW1 | 53.75 | 11.88 | 2.371 | 0.130 | 0.193 | 46 | 0.848 |
| PW2 | 53.00 | 14.91 | 0.193 | 43.815 | 0.848 | |||
| BSFS | PW1 | 1.29 | 0.46 | 0.000 | 1.000 | 0.000 | 46 | 1.000 |
| PW2 | 1.29 | 0.46 | 0.000 | 46.000 | 1.000 | |||
Abbreviations: CARS, Child Autism Rating Scale; ABC, Autism Behavior Checklist; SDSC, Sleep Dist Scale for Children; BSFS, Bristol Stool Form Scale; SD, Standard deviation; F, F statistic; T, T statistic; DF, Degrees of freedom; PW1, First course previous WMT; PW2, Second course previous WMT.
Discussion
There are many limitations in the diagnosis and treatment of early autism worldwide.39 In recent years, the relationship between gut microbiota and ASD has drawn great public attention, and it refers that this relationship between the emotional state and the abundance of intestinal microbes was named the microbiota-intestine-brain axis. The previous study has indicated that the ASD symptoms, gastrointestinal symptoms and GM composition have been improved after the intervention of prebiotics, probiotics and WMT.40 WMT was found to relieve constipation and improve sleep disorders in children with autism. Children with autism, who did not have constipation, showed no deterioration in stool morphology and sleep disorders after WMT. The study also found that WMT had a better short-term effect on autistic behavior in the children without constipation than those with constipation. It is suggested that for children with autism with constipation who are not adequately treated with WMT, improving their GI disorders as soon as possible can improve their treatment for autistic behavior. Consequently, improvement in GI symptoms is one of the key intermediate links in the treatment of autistic behavior in some children. Although this study did not show a statistical difference before WMT, the average degree of sleep disorder in children with autism accompanied by constipation was higher than that in children with autism without constipation. One reason may be that the sample size was not sufficiently large, and another reason may be that the parents of children with autism without constipation scored their children’s symptoms more strictly than parents of children with autism with constipation because they could not gauge a reduction in mental burden (anxiety, abdominal pain, abdominal distention) after the improvement of constipation in children with autism.
After WMT improved constipation, the children with autism had better sleep quality, so we hypothesized that constipation increases the time for metabolic toxins in feces to come into contact with the inner wall of the intestine. WMT alleviates the symptoms of constipation, accelerates the excretion of feces, reduces the retention of harmful metabolic wastes in the intestine, and reduces the entry of harmful metabolic wastes into the brain through the “gut-microbiota-brain axis”, thereby improving the sleep quality of children. Most children were more tolerant to gastroenterological intervention during the treatment period, and there were no obvious serious adverse clinical events after WMT.
Altogether, WMT could help relieve constipation and improve sleep disorders in children with autism, no obvious serious adverse clinical events after WMT was found. Our study provides a new and effective treatment for sleep disorders in children with autism, and an alternative for patients who are unsuitable for treatment with psychotropic drugs or who have drug dependence.
Limitations
The sample size was small and the data may be biased. Because of medical ethics, we could not use placebo on children with autism. Some children with autism with severe constipation used lactulose or mannitol or saline enema during TET, which inevitably led to alternating constipation and diarrhea, but the mild GI discomfort could be relieved within three days. Besides, validated instruments that measure the severity of gastrointestinal symptoms such as the GSI were absent.
Acknowledgments
The authors thank Pediatrician Li Zhenyuan for evaluating CARS and clinical guidance, and all children with autism and their parents for cooperating with us in nucleic acid and data collection during COVID-19.
Funding Statement
This study was funded by the Natural Science Foundation of Guangdong Province (Project No.: 2019A1515010125), the Guangdong Provincial key disciplines Scientific Research Project of Guangdong Education Department (Project No.: 2019-GDXK-0013), and the Special Research Project of COVID-19 epidemic Prevention and Control in Colleges and Universities of Guangdong Province (Project No.: 2020KZDZX1132).
Ethics Approval and Informed Consent
All the procedures in the study were in accordance with the ethical standards approved by the Ethics Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University [Grant number: 2020-14]. The WMT study and donor selection study have been registered in the Chinese Clinical Trial Registry (Registration Number: ChiCTR2100044807). Written informed consent was obtained from all guardians and participants in accordance with the principle of the Helsinki Declaration.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hedley D, Uljarevi M, Wilmot M, Richdale A, Dissanayake C. Understanding depression and thoughts of self-harm in autism: a potential mechanism involving loneliness. Res Autis Spe Dis. 2018;46:1–7. doi: 10.1016/j.rasd.2017.11.003 [DOI] [Google Scholar]
- 2.Culpin I, Mars B, Pearson RM, et al. Autistic traits and suicidal thoughts, plans and self-harm in late adolescence: population-based cohort study. J Am Child Psy. 2018;57(5):313–320. doi: 10.1016/j.jaac.2018.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jon B, Lisa W, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR. Surveillance summaries: morbidity and mortality weekly report. Sur Sum CDC. 2018;67:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marraffa C, Araba B. Social communication in autism spectrum disorder not improved by theory of mind interventions. J Paediatr Child Health. 2016;52:461–463. doi: 10.1001/jpc.13178 [DOI] [PubMed] [Google Scholar]
- 5.Anagnostou E, Aman MG, Handen BL, et al. Metformin for treatment of overweight induced by atypical antipsychotic medication in young people with autism spectrum disorder: a randomized clinical trial. JAMA Psy. 2016;73:928–937. doi: 10.1001/jamapsychiatry [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Mao X, Dan Z, Pei Y, Liu X. Gene variations in autism spectrum disorder are associated with alteration of gut microbiota, metabolites and cytokines. Gut Microbes. 2021;13(1):1–16. doi: 10.1080/19490976.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang DW, Adams JB, Gregory AC, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. doi: 10.1186/s40168-016-0225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor CM, Emmett PM. Picky eating in children: causes and consequences. Pro Nutri Soc. 2018;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez-González AE, Andreo-Martínez P. The role of gut microbiota in gastrointestinal symptoms of children with ASD. Medicina. 2019;55(8):408. PMID: 31357482; PMCID: PMC6722942. doi: 10.3390/medicina55080408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreo-Martínez P, Rubio-Aparicio M, Sánchez-Meca J, Veas A, Martínez-González AE. A meta-analysis of gut microbiota in children with autism. J Autism Dev Disord. 2022;52(3):1374–1387. PMID: 33948825. doi: 10.1007/s10803-021-05002-y [DOI] [PubMed] [Google Scholar]
- 11.Andreo-Martínez P, García-Martínez N, Sánchez-Samper EP, Martínez-González AE. An approach to gut microbiota profile in children with autism spectrum disorder. Environ Microbiol Rep. 2020;12(2):115–135. PMID: 31713352. doi: 10.1111/1758-2229.12810 [DOI] [PubMed] [Google Scholar]
- 12.Hom MA, Hames JL, Bodell LP, et al. Investigating insomnia as a cross-sectional and longitudinal predictor of loneliness: findings from six samples. Psychiatry Res. 2017;253:116–128. doi: 10.1016/j.psychres.2017.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson CR, Smith T, Demand A, et al. Exploring sleep quality of young children with autism spectrum disorder and disruptive behaviors. Sleep Med. 2018;44(1):61–66. doi: 10.1016/j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soe-Agnie SE, Paap M, Vandernagel J, Nijman H, Jong C. The generalizability of the structure of substance abuse and antisocial behavioral syndromes: a systematic review. Psychiatry Res. 2017;259:412–421. doi: 10.1016/j.psychres.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Zhang T, Zhu H, et al. Evolution of fecal microbiota transplantation in methodology and ethical issues. Cur Opin Pharma. 2019;49:11–16. doi: 10.1016/j.coph.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Krajicek E, Fischer M, Allegretti JR. CRK.Nuts and bolts of fecal microbiota transplantation. Clin Gas Hepatol. 2019;17(2):345–352. doi: 10.1016/j.cgh.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 17.Sokol MH, Landman C, Seksik P, Berard L, Simon T.Using fecal microbiota transplantation to help Crohn’s patients maintain remission; 2020.
- 18.Ianiro G, Segal JP, Mullish BH, Quraishi MN, Cammarota G. Fecal microbiota transplantation in gastrointestinal and extraintestinal disorders. Future Micro. 2020;15(12):1173–1183. doi: 10.2217/fmb-2020-0061 [DOI] [PubMed] [Google Scholar]
- 19.Tait C, Sayuk GS. The brain-gut-microbiotal axis: a framework for understanding functional gi illness and their therapeutic interventions. Eur J I Med. 2021;84(7):1–9. doi: 10.1016/j.ejim.2020.12.023 [DOI] [PubMed] [Google Scholar]
- 20.Quigley E. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17(12):94. doi: 10.1007/s11910-017-0802-6 [DOI] [PubMed] [Google Scholar]
- 21.Sharon G, Cruz NJ, Kang DW, et al. Gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618. doi: 10.1016/j.cell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermudez-Martin P, Becker J, Caramello N, Fernandez SP, Davidovic L. The microbial metabolite p-cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome. 2021;9(1):157. doi: 10.1186/s40168-021-01103-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T, Lu G, Zhao Z, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Pro Cell. 2020;11(4):251–266. doi: 10.1007/s13238-019-00684-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hus V, Pickles A, Cook EH, Risi S, Lord C. Using the autism diagnostic interview–revised to increase phenotypic homogeneity in genetic studies of autism. Biol Psychiatry. 2007;61(4):438–448. PMID: 17276746. doi: 10.1016/j.biopsych.2006.08.044 [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association DSM- Task Force Arlington VA US. Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). Codas. 2013;25(2):191. doi: 10.1590/s2317-17822013000200017 [DOI] [PubMed] [Google Scholar]
- 26.Kulich KR, Madisch A, Pacini F, et al. Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: a six-country study. Health Qual Life Outcomes. 2008;6:12. PMID: 18237386; PMCID: PMC2276197. doi: 10.1186/1477-7525-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane MM, Czyzewski DI, Chumpitazi BP, Shulman RJ. Reliability and validity of a modified Bristol stool form scale for children. J Pediatr. 2011;159(3):437–441.e1. PMID: 21489557; PMCID: PMC3741451. doi: 10.1016/j.jpeds.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang T, Long C, Cui B, Buch H, Zhang F. Colonic transendoscopic tube-delivered enteral therapy (with video): a prospective study. BMC Gas. 2020;20(1):135. doi: 10.1186/s12876-020-01285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fecal Microbiota Transplantation-Stan-Dardization Study Group. Nanjing consensus on methodology of washed microbiota transplantation. Chin Med. 2020;133(19):2330–2332. doi: 10.1097/CM9.0000000000000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang MM, Qian Z, Wang J, Vaughn MG, Lee YL, Dong GH. Validation of the sleep disturbance scale for children and prevalence of parent-reported sleep disorder symptoms in Chinese children. Sleep Med. 2014;15(8):923–928. PMID: 24916093. doi: 10.1016/j.sleep.2014.03.023 [DOI] [PubMed] [Google Scholar]
- 31.Lin LZ, Xu SL, Wu QZ, et al. Exposure to second-hand smoke during early life and subsequent sleep problems in children: a population-based cross-sectional study. Environ Health. 2021;20(1):127. PMID: 34920730; PMCID: PMC8684187. doi: 10.1186/s12940-021-00793-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B, Tian H, Ye C, et al. The efficacy and safety of fecal microbiota transplantation combined with biofeedback for mixed constipation: a retrospective cohort study. Front Med. 2021;8:746990. PMID: 34746183; PMCID: PMC8564017. doi: 10.3389/fmed.2021.746990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Ou Y, Zhao L, et al. Differential effects of lactobacillus casei strain shirota on patients with constipation regarding stool consistency in China. J Neurogastroenterol Motil. 2019;25(1):148–158. PMID: 30646486; PMCID: PMC6326197. doi: 10.5056/jnm17085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randall M, Egberts KJ, Samtani A, et al. Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochrane Database Syst Rev. 2018;7(7):CD009044. PMID: 30075057; PMCID: PMC6513463. doi: 10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkmar FR, Cicchetti DV, Dykens E, Sparrow SS, Leckman JF, Cohen DJ. An evaluation of the autism behavior checklist. J Autism Dev Disord. 1988;18(1):81–97. doi: 10.1007/BF02211820 [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, Xu X, Yan W, et al. Prevalence of autism spectrum disorder in China: a nationwide multi-center population-based study among children aged 6 to 12 years. Neurosci Bull. 2020;36(9):961–971. PMID: 32607739; PMCID: PMC7475160. doi: 10.1007/s12264-020-00530-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Yang T, Chen L, et al. Serum folate status is primarily associated with neurodevelopment in children with autism spectrum disorders aged three and under-a multi-center study in China. Front Nutr. 2021;8:661223. PMID: 34055856; PMCID: PMC8155683. doi: 10.3389/fnut.2021.661223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Erlbaum Associates; 1988. [Google Scholar]
- 39.Hus Y, Segal O. Challenges surrounding the diagnosis of autism in children. Neuropsychiatr Dis Treat. 2021;17:3509–3529. doi: 10.2147/NDT.S282569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-González AE, Andreo-Martínez P. Prebiotics, probiotics and fecal microbiota transplantation in autism: a systematic review. Rev Psiquiatr Salud Ment. 2020;13(3):150–164. PMID: 32684346. doi: 10.1016/j.rpsm.2020.06.002 [DOI] [PubMed] [Google Scholar]