Atopic dermatitis (AD) is a chronic, recurrent inflammatory skin condition that affects approximately 10–20% of children and 2–15% of adults in developed countries (1). In Singapore, the prevalence of AD among children and adolescents is 20.8% (2). Itchiness, the most common complaint, results in behavioural and social impairments among paediatric patients with AD, which in turn impacts on the wellbeing of caregivers, in particular family members (3, 4). A sick child can markedly affect normal family life and the mental and social wellbeing of other family members (5). Their family members, specifically parents, may experience feelings of helplessness and stress as they struggle to treat their child’s symptoms, and this can lead to feelings of guilt as they feel they are failing in their duty to care for their offspring (6). In return, family quality of life (QoL) can greatly influence patient-related outcomes. Previous studies have demonstrated that paediatric AD can significantly affect family life; however, there is limited quantitative data on the factors influencing family life and functions (6). Therefore, the aim of this study was to gain in-depth insights into the family burden caused by paediatric AD, and to explore the social and clinical factors potentially impacting family life and function.
METHODS
A cross-sectional survey was conducted during 2016 and 2017 at 2 paediatric dermatology clinics in Singapore. Families were recruited if the patient was: (i) aged 0–16 years; and (ii) fulfilled the Hanifin & Rajka criteria for AD (7).
The following information and instruments were included in the study: (i) the Dermatitis Family Impact (DFI) questionnaire, which assesses the impact of AD on family life and function (8); (ii) RAND-36, which assesses the physical and mental health of caregivers, whereby a lower score indicates poorer health or functioning (9); and (iii) Infants’ Dermatitis Quality of Life Index (IDQOL) in infants (0–3 years) and Children’s Dermatology Life Quality Index (CDLQI) in children (4–16 years) were merged into the health-related quality of life (HRQoL) score in order to evaluate AD-related QoL among paediatric patients; with this instrument, a higher score indicates a greater degree of impairment of QoL (10, 11).
Eczema severity data was extracted from electronic medical records (EMR). For patients whose severity of symptoms was not explicitly reported in the EMR, symptoms and information on affected areas were extracted from EMR and assessed by the researcher (XX) using the Eczema Area and Severity Index (EASI) method (12).
Statistical analysis was carried out using Stata software package (version 14.2) (StataCorp, College Station, TX, USA). The Wilcoxon rank-sum (or Mann–Whitney) test and the Kruskal–Wallis test were used to determine the statistical association between socio-demographic characteristics and DFI scores along with their subdomains. Subsequently, a negative binomial regression model was used to demonstrate the relationship between socio-demographic variables and DFI measures. Univariable and multivariable incidence rate ratios (IRR) were calculated and reported with 95% confidence intervals (CIs). A 2-sided p-value of < 0.05 was considered statistically significant, and the 95% CIs are presented below.
RESULTS
In total, 559 families participated in the study. The ages of paediatric patients in the families ranged from 1 month to 16 years, with a mean age of 6.6 ±4.6 years. Disease severity was mild in 56% of cases, moderate in 24%, and severe in 11%. Of the participants, 72% were Chinese, and Indian and Malay participants accounted for 16% and 6%, respectively. A great majority of caregivers (81%) were educated to at least tertiary-level educational attainment. The majority were employed (81%), and 19% were unemployed or retired.
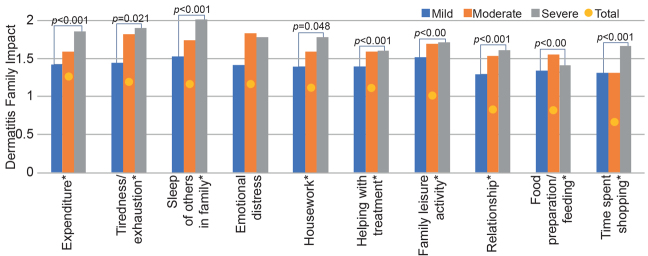
More than 94% of families reported that their family life and function were affected by their child’s AD (DFI > 0). The mean DFI score in affected families was 9.19 ±7.28. Sleep disturbance, emotional distress and tiredness/exhaustion were the subscales with the largest impacts. Significant differences among different disease severity groups was observed in terms of overall DFI score. In 9 out of 10 subdomains, there were indications that families with children with more severe AD had poorer DFI scores than families with children with mild AD (Fig. 1).
Fig. 1.
Association between mean score of reported Dermatitis Family Impact subdomains and children’s disease severity. X-axis: Dermatitis Family Impact (DFI) subdomains; y-axis: mean score of dermatitis family impact. *Significant differences in dermatitis family impact between different severity groups. ˜missing data: n = 46.
Univariate and multivariate models were used to analyse the factors influencing DFI; the results are shown in Table SI1. Families with younger children were less affected by their child’s AD (IRR 0.97, 95% CI 0.96, 0.99, p = 0.002). Disease duration and severity also significantly affect DFI: families with children with longer disease duration exhibited poorer DFI scores compared with families with children having AD for a shorter period (IRR 1.02, 95% CI 1.00, 1.04, p = 0.019). Families with children with moderate and severe AD had poorer DFI scores than those with children with mild AD (moderate IRR 1.23, 95% CI 1.09, 1.40, p = 0.001; severe IRR 1.26, 95% CI 1.06, 1.49, p = 0.008). As for the caregivers’ QoL, both the mental and physical health of caregivers can affect DFI. Better mental and physical health among caregivers is associated with a better DFI (physical health IRR 0.99, 95% CI 0.98, 0.99, p < 0.001; mental health IRR 0.99, 95% CI 0.99, 0.99, p <0.001). A family whose child has a poorer HRQoL had a poorer DFI score (IRR 1.04, 95% CI 1.03, 1.05, p < 0.001).
DISCUSSION
Of the families included in this study 94% reported their DFI to be affected by their child’s AD, with the most commonly affected domains being sleep disturbance, emotional distress, and tiredness/exhaustion. In addition, the severity of the child’s AD was found to be significantly associated with DFI and its subdomains, indicating that severe AD causes more severe impairment of family functioning. Univariate and multivariate models showed that the patient’s age and disease severity influence DFI. This study also found that the self-reported QoL in paediatric patients and the mental and physical health of the caregivers contribute to impaired family life and function. The impact of childhood AD on DFI can be substantial. It has been reported that the QoL of families with children with AD are more impaired than that of families with psoriasis patients, and is comparable to that of families with congenital ichthyosis patients (13, 14).
Family support plays a significant role in the treatment of AD, and this study provides a unique perspective into the family burden of paediatric patients with AD. Following a review of previous works (3, 6), statistical models were used to identify the influencing factors on family life, which could generate meaningful data for informing treatment decisions in clinical practice. Our sample size is large enough to be generalized to Singapore and to other multi-ethnic countries. However, there are also a number of limitations to this study that merit attention. First, the family impact data is based on parents’ self-reporting, and might not represent all family members’ points of view. Secondly, there is no healthy control group included in this study, which precludes the calculation of comparisons with healthy families.
In conclusion, this study was conducted in order to provide a more in-depth insight into the impact of paediatric AD on families, in order to improve overall family QoL, treatment of AD should not be limited to the patients; rather, the approach should be broadened to involve family members.
ACKNOWLEDGEMENTS
This research was supported through the Skin Research Grant by the Skin Research Institute of Singapore (SRIS: SRG/15022), a tripartite partnership between the Agency for Science, Technology, and Research (A*STAR), National Healthcare Group through its National Skin Centre (NHG) and the Nanyang Technological University (NTU).
The present study was approved by the Institutional Review Board of National Healthcare Group (NHG-DSRB: 2015/01228) and Nanyang Technological University (NTU IRB: IRB-2016-10-059-01).
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Vartiainen E, Petays T, Haahtela T, Jousilahti P, Pekkanen J. Allergic diseases, skin prick test responses, and IgE levels in North Karelia, Finland, and the Republic of Karelia, Russia. J Allergy Clin Immunol 2002; 109: 643–648. [DOI] [PubMed] [Google Scholar]
- 2.Tay YK, Kong KH, Khoo L, Goh CL, Giam YC. The prevalence and descriptive epidemiology of atopic dermatitis in Singapore school children. Br J Dermatol 2002; 146: 101–106. [DOI] [PubMed] [Google Scholar]
- 3.Jang HJ, Hwang S, Ahn Y, Lim DH, Sohn M, Kim JH. Family quality of life among families of children with atopic dermatitis. Asia Pacific Allergy 2016; 6: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X, van Galen LS, Koh MJA, Bajpai R, Thng S, Yew YW, et al. Factors influencing quality of life in children with atopic dermatitis and their caregivers: a cross-sectional study. Sci Rep 2019; 9: 15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun F. Caregiving stress and coping: a thematic analysis of Chinese family caregivers of persons with dementia. Dementia 2014; 13: 803–818. [DOI] [PubMed] [Google Scholar]
- 6.Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol 2010; 27: 618–623. [DOI] [PubMed] [Google Scholar]
- 7.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis Acta Derm Venereol 1980; 92: 44–47. [Google Scholar]
- 8.Higaki Y KK, Kamo T, Ueda S, Arikawa J, Kawashima M. Measurement of the impact of atopic dermatitis on patients’ quality of life: a cross-sectional and longitudinal questionnaire study using the Japanese version of Skindex-16. J Dermatol 2004; 31: 5. [DOI] [PubMed] [Google Scholar]
- 9.Mortimer D, Segal L. Comparing the incomparable? A systematic review of competing techniques for converting descriptive measures of health status into QALY-weights. Med Decis Making 2008; 28: 66–89. [DOI] [PubMed] [Google Scholar]
- 10.Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol 2001; 144: 104–110. [DOI] [PubMed] [Google Scholar]
- 11.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 1995; 132: 942–949. [DOI] [PubMed] [Google Scholar]
- 12.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10: 11–18. [DOI] [PubMed] [Google Scholar]
- 13.Gånemo A, Wahlgren CF, Svensson A. Quality of life and clinical features in Swedish children with psoriasis. Pediatr Dermatol 2011; 28: 375–379. [DOI] [PubMed] [Google Scholar]
- 14.Gånemo A. Quality of life in Swedish children with congenital ichthyosis. Dermatol Reports 2010; 2: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]