Abstract
OBJECTIVES
We have previously demonstrated beneficial cardiac protection with hypothermic polarizing cardioplegia compared to a hyperkalemic depolarizing cardioplegia. In this study, a porcine model of cardiopulmonary bypass was used to compare the protective effects of normothermic blood-based polarizing and depolarizing cardioplegia during cardiac arrest.
METHODS
Thirteen pigs were randomized to receive either normothermic polarizing (n = 8) or depolarizing (n = 5) blood-based cardioplegia. After initiation of cardiopulmonary bypass, normothermic arrest (34°C, 60 min) was followed by 60 min of on-pump and 90 min of off-pump reperfusion. Primary outcome was myocardial injury measured as arterial myocardial creatine kinase concentration. Secondary outcome was haemodynamic function and the energy state of the hearts.
RESULTS
During reperfusion, release of myocardial creatine kinase was comparable between groups (P = 0.36). In addition, most haemodynamic parameters showed comparable results between groups, but stroke volume (P = 0.03) was significantly lower in the polarizing group. Adenosine triphosphate levels were significantly (18.41 ± 3.86 vs 22.97 ± 2.73 nmol/mg; P = 0.03) lower in polarizing hearts, and the requirement for noradrenaline administration (P = 0.002) and temporary pacing (6 vs 0; P = 0.02) during reperfusion were significantly higher in polarizing hearts.
CONCLUSIONS
Under normothermic conditions, polarizing blood cardioplegia was associated with similar myocardial injury to depolarizing blood cardioplegia. Reduced haemodynamic and metabolic outcome and a higher need for temporary pacing with polarized arrest may be associated with the blood-based dilution of this solution.
Keywords: Cardioplegia, Polarized arrest, Depolarized arrest, Normothermia, Ischaemia–reperfusion, Cardioprotection
Today, patients undergoing cardiac surgery are older and have more co-morbidities than 30 years ago [1].
INTRODUCTION
Today, patients undergoing cardiac surgery are older and have more co-morbidities than 30 years ago [1]. Thus, the need for optimized cardioplegic protection has become more important than ever before [2]. Although the ideal form of cardioplegia remains controversial and can be adjusted to the individual patient’s demand or surgeon’s personal experience, depolarizing cardioplegic solutions, containing relatively high levels of potassium, have been the gold standard for myocardial protection during cardiac arrest for decades. Various options of cardioplegic solutions are available to cardiac surgeons: crystalloid versus blood mixed, depolarizing versus polarizing and warm versus cold solutions. One of the most widely used cardioplegic solutions [3] is the St Thomas’ Hospital cardioplegia inducing a depolarized arrest. This depolarized arrest, however, potentially leads to intracellular accumulation of sodium and calcium, contraction and, consequently, cell death [4]. Existing literature about the ideal cardioplegic formulation is still controversial and with the advent of new polarizing cardioplegic solutions, complexity of the scientific discussion further increases. Using a recently developed polarized arrest solution, comprising esmolol, adenosine and magnesium in a cardiopulmonary bypass (CPB) model in pigs, we recently demonstrated decreased cardiac enzyme release and improved haemodynamic recovery following cold polarizing versus depolarizing blood cardioplegia [5, 6]. The reported benefits of blood-mixed solutions are reduced ischaemic injury, decreased metabolism during arrest, better functional recovery [7] and, consequently, improved myocardial protection [8]. Tepid temperature (32–34°C) is beneficial in case of ventricular hypertrophy [9], associated with reduced postoperative enzyme release (cardiac troponin, CK-MB) and improved cardiac index [10], but with less endothelial protection [11], whereas cold administration (4°C) has shown improved clinical outcome in patients with diffuse coronary artery disease [12].
Since data on warm polarized arrest are still scarce, the aim of this study was to evaluate warm polarized blood cardioplegia versus a conventional blood-mixed depolarizing cardioplegia. Based on the results of our previous study [6], we hypothesized that warm blood-based polarizing cardioplegia will be superior to warm blood-based depolarizing cardioplegia. The primary outcome parameter is CK-MB release during reperfusion, which is assumed to reflect myocardial damage. Secondary outcomes are haemodynamic function and energy state.
METHODS
Ethics statement
The study protocol was approved by the Animal Ethics Committee of the Medical University of Vienna and by the Austrian Ministry of Science and Technology (GZ: 66.009/0171-II/3b/2011).
Animals
In total, 14 pigs (Austrian Landrace) were included into the study and randomized to 2 groups: blood-based polarizing cardioplegia (POL, n = 8) and blood-based depolarizing cardioplegia (DEPOL, n = 5; 1 additional animal in this group was excluded due to technical difficulties prior to CPB). Animal housing is described in the Supplementary Material.
Protocol
The detailed surgical CPB protocol is given in the Supplementary Material. According to the protocol (Fig. 1), all pigs were subjected to 60 min of ischaemia, 60 min of on-pump reperfusion and another 90 min of off-pump reperfusion after decannulation and administration of protamine (300 IU/kg). Prior to sacrificing (pentobarbital 300 mg/kg i.v.), samples from the anterior wall of the left ventricle were harvested for the analysis of high-energy phosphates (HEP; Fig. 1).
Figure 1:
Experimental protocol. After baseline haemodynamic assessment, cardiopulmonary bypass was started, aorta was crossclamped and the first dose of cardioplegia was applied (polarizing blood cardioplegia, depolarizing blood cardioplegia). After 30 min of ischaemia, the second dose of cardioplegia was administered and ischaemia was maintained for additional 30 min (in total: 60 min ischaemia). After declamping the aorta and 60 min of on-pump reperfusion including weaning from cardiopulmonary bypass 90 min of off-pump reperfusion was performed. The indicated times refer to sampling points. DEPOL: depolarizing blood cardioplegia; POL: polarizing blood cardioplegia.
Cardioplegic solutions
The cardioplegic solutions used in this study were based on modifications in the St Thomas’ Hospital cardioplegic solution No. 2 (STH-2) or a cardioplegic solution containing esmolol, adenosine and magnesium [the St Thomas’ Hospital Polarizing solution (STH-Pol)]. STH-2 (Na+: 110.0 mmol/l, K+: 16.0 mmol/l, Mg2+: 16.0 mmol/l; Ca2+: 1.2 mmol/l) was provided as a 1000 ml solution by the hospital pharmacy of the General Hospital Linz, Austria, and was mixed (1:2; blood:crystalloid; total volume: 1500 ml) with 500 ml of pig blood for the composition of DEPOL. The basic composition of STH-Pol was 1000 ml of Ringer’s solution with 1.0 mmol/l esmolol (Baxter, Vienna, Austria), 0.5 mmol/l adenosine (Sigma Aldrich, St. Louis, USA) and 10.0 mmol/l magnesium gluconate (G.L. Pharma GmbH, Lannach, Austria), which was mixed with 500 ml of pig blood (1:2; blood:crystalloid; total volume: 1500 ml) to form POL immediately before administration. Hence, both cardioplegic solutions were diluted by one-third of their original concentrations to produce these blood-based solutions. After aortic cross clamping, 1000 ml of the respective cardioplegia was infused with a pressure of 60 mmHg and a temperature of 34°C via the aortic root; after 30 min of ischaemia, an additional 500 ml of the cardioplegic solutions were infused. The final molar concentrations of both cardioplegic solutions are presented in Table 1.
Table 1:
Molar concentrations of cardioplegic solutions
| Components | Unit | Group |
|
|---|---|---|---|
| POL | DEPOL | ||
| (n = 8) | (n = 5) | ||
| Blood crystalloid ratio | – | 1:2 | 1:2 |
| Haematocrit | % | 10.8 ± 0.8 | 11.0 ± 0.6 |
| Esmolol | mmol/l | 0.68 | – |
| Adenosine | mmol/l | 0.33 | – |
| Magnesium | mmol/l | 6.67 | 10.7 |
| Sodium | mmol/l | 110 | 110 |
| Potassium | mmol/l | 4 | 10.7 |
| Calcium | mmol/l | 1.2 | 1.2 |
Final molar concentrations in low-dose cardioplegic solutions (POL and DEPOL). The basic composition of STH-Pol was esmolol, adenosine and magnesium gluconate mixed in 1 l of Ringer’s solution, and pig blood was mixed with the crystalloid solution immediately before administration. Haematocrit values are given as mean ± standard deviation.
DEPOL: depolarizing blood cardioplegia; POL: polarizing blood cardioplegia.
Biochemical analyses
Arterial blood samples were drawn at baseline and 1, 5, 15, 30, 60, 90, 120 and 150 min of reperfusion. During CPB, venous samples were drawn from the coronary sinus during controlled on-pump reperfusion: baseline, 1, 5, 15, 30 and 60 min of reperfusion. An immunoassay was performed for the primary outcome parameter CK-MB (Cobas immunoassay CKL, ID 0-324, Roche, Germany). The assessment of the energy status is given in the Supplementary Material.
Haemodynamic evaluation
The description of the haemodynamic variables is given in the Supplementary Material.
Statistical analysis
Graphs were drawn with GraphPad Prism (9.0, GraphPad Software, La Jolla, USA), IBM SPSS Statistics 27 (IBM Corporation, New York, USA) was used for statistical analysis and online sample size calculator (http://clincalc.com/Stats/SampleSize.aspx) was used for calculation based on a previous study in rats by Fujii and Chambers [13]. We defined a minimally relevant group difference of 25%. With a power of 80%, accepting the probability of a type I error of 5%, 5 animals per group were needed. As a safety margin, we aimed for 8 pigs/group.
Regarding the dependent variables, mixed linear models were performed. First, data were visually analysed for normal distribution at all time points and right-skewed data were log10-transformed. Normally distributed data are given as mean and standard deviation, and right-skewed data are given as geometric mean with 95% confidence interval. ‘Group’ was specified as fixed between-subjects factor with the 2 levels POL and DEPOL, and ‘time’ was specified as levels as fixed within-subjects factor. Furthermore, each animal was included as a level of a random factor. Baseline values were included as a covariate to adjust the models for pretreatment differences. Model estimation was performed using the restricted maximum likelihood method. Based on adequate covariance structures and the smallest Akaike information criterion value, a time by group interaction was tested. When a significant interaction was observed, group differences were estimated by contrasts at each time point. Otherwise, the interaction term was dropped from the model, and the main effect of ‘group’ was interpreted as group difference that applies to all time points. All reported P-values are the result of two-sided tests. P-values <0.05 were considered significant. Arterial CK-MB release was defined as primary end point; therefore, we took mean values from Fujii and Chambers [13] and aimed for a comparable reduction in the POL versus DEPOL group. All secondary outcome measures were not adjusted for multiplicity due to the exploratory nature of this study and have to be interpreted accordingly.
RESULTS
Baseline characteristics
Animals in both groups showed comparable body (POL: n = 8, 61 ± 6 kg; STH-2-B: n = 5, 64 ± 5 kg; P = 0.35) and heart weights (DEPOL: 305 ± 52 g; STH-2-B: 322 ± 19 g; P = 0.49).
Description of cardioplegic arrest
Time to asystole was comparable in both groups (POL: 336 ± 240 s; DEPOL: 300 ± 174 s; P = 0.79). Four animals showed an unstable arrest (POL: 2; DEPOL: 2). Peri-ischaemic ventricular fibrillation (VF) within the first minute of reperfusion was recorded in all groups [POL: 7 (of 8); DEPOL: 5 (of 5)], but without statistical difference. Two animals of the POL group showed VF during ischaemia; therefore, the second administration of cardioplegia was applied earlier. Six (of 8) animals in the DEPOL group required temporary pacing, while no pacing was necessary in POL (P = 0.02). There were a higher number of DC shocks required to induce sinus rhythm in POL, but this was without significance (POL: 4.7 ± 3.4; DEPOL: 2.6 ± 1.8; P = 0.34).
Myocardial damage
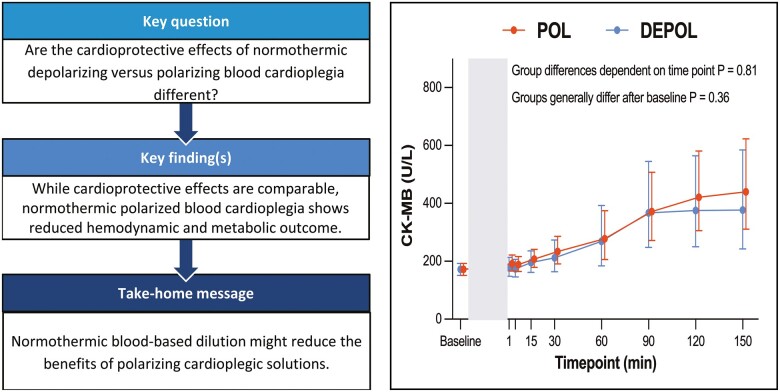
Primary outcome parameter was the analysis of arterial CK-MB as a measure of myocardial cell damage. The increase (P < 0.0001) in arterial CK-MB after ischaemia was comparable in both groups (P = 0.36; Fig. 2A). Likewise, coronary CK-MB changed over time (P = 0.002) and was also similar in both groups (P = 0.30, Supplementary Material, Fig. S1A).
Figure 2:
Effects of polarizing blood cardioplegia (POL) and depolarizing blood cardioplegia (DEPOL) applied in blood solution on the primary outcome parameter arterial myocardial creatine kinase and secondary outcome parameters. (A) There was no relevant difference between polarizing blood cardioplegia and depolarizing blood cardioplegia at all time points (P=0.36). (B) Systolic left ventricular pressure did not differ between groups (P=0.06). (C) In polarizing blood cardioplegia, there was a tendency for lower cardiac output (P=0.07). (D) The different cardioplegic solutions did not affect coronary flow (P = 0.29). (E) Polarizing blood cardioplegia resulted in lower pulmonary capillary wedge pressure but without significance (P = 0.24). (F) Stroke volume was significantly reduced in St Thomas’ Hospital Polarizing solution-B (P = 0.03). Arithmetic or geometric means (depending on whether data were log-transformed for analysis) with 95% confidence intervals estimated by a mixed linear model that adjusts for baseline differences was used for the illustrations.
Haemodynamics
Secondary outcome was parameters of haemodynamic function.
Systolic left ventricular pressure (P = 0.6, Fig. 2B), cardiac output (P = 0.07; Fig. 2C), coronary flow (P = 0.29; Fig. 2E) and wedge pressure (P = 0.24; Fig. 2F) were similar in both groups with a tendency for lower values in POL. Stroke volume was significantly reduced in POL (P = 0.03; Fig. 2G). Notably, the differences apply to the time points 90, 120 and 150 min.
In addition, the need for noradrenaline administration was significantly higher from 90 to 120 min in POL (P = 0.002; Supplementary Material, Fig. S1B). Heart rate was significantly reduced in POL at 15 and 30 min of reperfusion (P = 0.021; Supplementary Material, Fig. S1C). Systolic, mean and diastolic arterial pressure showed no differences over time in both groups (Supplementary Material, Fig. S1D–F).
High-energy phosphates
HEP analysis showed comparable preservation concerning phosphocreatine (PCr); however, there was a tendency towards lower levels of ADP in POL (4.3 ± 0.6 vs 5.2 ± 0.9 nmol/mg; P = 0.07). Adenosine triphosphate (ATP) content was significantly lower in POL (18.41 ± 3.86 vs 22.97 ± 2.73 nmol/mg; P = 0.03). The ratio of PCr/ATP and energy charge were comparable in all groups (Fig. 3).
Figure 3:
High-energy phosphates. Phosphocreatine, adenosine triphosphate, the phosphocreatine/adenosine triphosphate ratio and energy charge analysed from freeze clamped left ventricular biopsies obtained immediately after scarification at 150 min of reperfusion. Phosphocreatine showed comparable preservation within all groups (A). Adenosine triphosphate content was reduced in the left ventricle in St Thomas’ Hospital Polarizing solution-B (adenosine triphosphate: *P = 0.03; B). The ratio of phosphocreatine/adenosine triphosphate (C) and energy charge (D) showed no significant differences between groups. DEPOL: depolarizing blood cardioplegia; POL: polarizing blood cardioplegia.
DISCUSSION
Recently, we showed beneficial effects of POL compared to DEPOL under hypothermic conditions [6]. Here, we present the second study aiming to describe the effects of the novel polarizing St Thomas’ cardioplegia under normothermic conditions. As surrogate marker for myocardial damage, we used again the time course of arterial CK-MB during reperfusion. The main results of the present study are that myocardial protection with (i) DEPOL and POL did not lead to different outcomes in myocardial damage and (ii) POL led to decreased haemodynamic performance while the requirement of vasopressor support and temporary pacing was increased.
In former studies, the concept of polarized arrest has already been shown as microplegia [14], in small [13] as well as in large animal models [6, 15], and has been demonstrated to be similarly effective compared to depolarized arrest. In similar models, it has also recently been shown that polarized, blood-mixed cardioplegia reduced myocardial damage and enhanced cardiac function at 4°C [6] and 12°C [15]. The major difference of the present study to former studies was the application temperature of 34°C. This tepid application seemed to prolong time to asystole for both cardioplegic solutions (POL: 336 ± 240 s; DEPOL: 300 ± 174 s; P = 0.79), but it is likely that this was a consequence of the cardioplegic dilution of the component concentrations. Two animals of POL group showed VF during ischaemia; therefore, the second administration of cardioplegia was applied earlier. Hence, we speculate that the temperature was also a factor that led to an unstable arrest in POL-treated hearts. The lower ATP levels in POL might be caused by the fast degradation of esmolol under normothermic conditions in the polarizing solution, which then led to VF, all indicating lower energy preservation. In contrast, Aass et al. [15] described increased levels of PCr and ATP after hypothermic polarized cardiac arrest compared to depolarized arrest; in their study, the component concentrations of the arresting infusion of the polarized cardioplegia were approximately double those used in the present study.
The design of the current study to test POL in a normothermic setting was based on the concept of endothelial protection as demonstrated by Fujii and Chambers [13]. The authors performed their study in the isolated rat heart and showed that the mixture of blood with STH-Pol (1:3, haematocrit of about 10%) in an esmolol-based normothermic cardioplegia was equal to hyperkalemic arrest regarding cardioprotective efficacy. Administration was repeated every 10 min, over a global ischaemia period of 40 min. In contrast, in the present study, cardioplegia was only re-administered once after a clinically relevant interval of 30 min and the duration of global ischaemia was 60 min. It is tempting to speculate that the result of the mentioned rodent study [16] was probably due to the short re-infusion time of 10 min. With a half-life of about 9 min, the frequent re-infusions guaranteed a constant esmolol concentration throughout the entire ischaemic period. In addition, the advantage of adenosine as a component of the cardioplegic solution for preventing Ca2+ overload has already been confirmed [17]. In a warm setting, adenosine has been shown to achieve rapid arrest and a higher functional recovery [18]. However, as its half-life is below 10 s in vivo a continuous infusion was performed. Therefore, it was not surprising that in our current study, re-infusion in 2 animals of the POL had to take place earlier due to VF. One pharmacological explanation is the activity of ubiquitous esterases (from red blood cells [19]), which immediately metabolize the β-blocker esmolol at normothermia into an acid metabolite and methanol, both eliminated by the kidneys. Number of shocks to terminate VF during early reperfusion seemed to be increased in POL but without significance. In contrast, in our recent publication, we performed the same experimental protocol and dilution with blood, but under hypothermic conditions, and were able to show reliable myocardial protection with blood-mixed STH-Pol [6]. Therefore, it seems reasonable to assume that in normothermia esterase activity caused unstable arrest, subsequently reduced myocardial protection in POL-treated hearts and this normothermic protocol unmasks these negative effects.
Clinical implications of polarized arrest
Although the invention of depolarizing cardioplegic solutions made the development of cardiac surgery possible in the first place, the potential sodium and calcium overload might be harmful, especially in damaged hearts. Polarized arrest has been shown to be beneficial when administered as cold blood-based solution [6, 15]. While biomedical research will continue to search for the best administration strategy, the first-in-man study for the St Thomas Hospital Polarizing cardioplegia is currently planned. However, polarizing cardioplegic solutions (adenosin, lidocaine and Mg2+; Verona ALM) have already been proven to be safe, effective and superior to Buckberg cardioplegia in a prospective randomized clinical study [20].
Limitations
Since all animals underwent comparable housing and feeding conditions and all parameters were normalized to heart weight, where applicable, the differences in weight should not influence our main results. Although a sample size was defined, it is possible that between-group differences might be caused by a sampling error due to the low number of pigs.
This study used CK-MB to describe myocardial damage. Porcine troponins were not measured due to unavailability during the period of the experiments. In the current experiment, mixing of blood and crystalloid solution took place in the application containers of the cardioplegia immediately before administration. However, as shown above, under normothermia this seems to allow esterase interaction and consecutive degradation of esmolol. One possible option would be to mix the 2 components in the moment of administration, using a Y-connector on top of the infusion cannula, which is planned for future studies. Hence, in the POL solution in our experiments, the levels of esmolol, adenosine and magnesium were reduced as compared to a pure crystalloid application. This dilution beside degradation may account for the reduced efficacy of the POL solution under normothermic conditions.
CONCLUSION
Under normothermic conditions, polarizing cardioplegic solution was associated with similar arterial CK-MB release as compared to depolarizing solution. Reduced stroke volume and metabolic outcome as well as a higher need for temporary pacing with polarized arrest may be associated with the blood-based dilution of this solution. Therefore, to proceed with the full description of polarized arrest with STH-Pol, the consecutive evaluation of normothermic blood-based STH-Pol with increased component concentrations of the arresting infusion is required to rule out potential effects of dilution and esterase activity.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGMENTS
We thank the perfusionists of the Department of Cardiac Surgery, Universitaetsklinikum St. Poelten, Austria, Gerd Kager for HEP measurements (MUG) and Alexander Weigl, MPharm., AKH Linz, Austria, for providing St Thomas’ cardioplegia.
Funding
This study received award from King’s College London and continuous funding of the Ludwig Boltzmann Society (grant number REM 2013/16) and the Medical Scientific Fund of the Mayor of the City of Vienna Foundation (Buergermeisterfonds, project number 17056).
Conflict of interest: David J. Chambers is a co-inventor of the STH-Pol. All of the other authors have nothing to disclose with regard to commercial support.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Anne-Margarethe Kramer: Data curation; Formal analysis; Investigation; Methodology; Writing—original draft. Attila Kiss: Investigation; Methodology; Resources; Writing—review & editing. Stefan Heber: Data curation; Formal analysis; Software; Validation; Visualization; Writing—review & editing. David J. Chambers: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Writing—review & editing. Seth Hallström: Formal analysis; Investigation; Methodology; Validation; Writing—review & editing. Patrick M. Pilz: Investigation; Resources. Bruno K. Podesser: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—review & editing. David Santer: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Vito Domenico Bruno, Emre Belli and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Glossary
ABBREVIATIONS
- ATP
Adenosine triphosphate
- CK-MB
Myocardial creatine kinase
- CPB
Cardiopulmonary bypass
- DEPOL
Depolarized group
- HEP
High-energy phosphates
- PCr
Phosphocreatine
- POL
Polarized group
- STH-2
St Thomas’ Hospital cardioplegic solution No. 2
- STH-Pol
St Thomas’ Hospital Polarizing solution
- VF
Ventricular fibrillation
Contributor Information
Anne-Margarethe Kramer, Ludwig Boltzmann Institute for Cardiovascular Research at the Center for Biomedical Research, Medical University of Vienna, Vienna, Austria.
Attila Kiss, Ludwig Boltzmann Institute for Cardiovascular Research at the Center for Biomedical Research, Medical University of Vienna, Vienna, Austria.
Stefan Heber, Institute of Physiology, Center for Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria.
David J Chambers, Cardiac Surgical Research, The Rayne Institute (King’s College London), Guy’s and St Thomas’ NHS Foundation Trust, St Thomas’ Hospital, London, UK.
Seth Hallström, Division of Physiological Chemistry, Otto Loewi Research Center, Medical University of Graz, Graz, Austria.
Patrick M Pilz, Ludwig Boltzmann Institute for Cardiovascular Research at the Center for Biomedical Research, Medical University of Vienna, Vienna, Austria.
Bruno K Podesser, Ludwig Boltzmann Institute for Cardiovascular Research at the Center for Biomedical Research, Medical University of Vienna, Vienna, Austria.
David Santer, Ludwig Boltzmann Institute for Cardiovascular Research at the Center for Biomedical Research, Medical University of Vienna, Vienna, Austria; Department of Cardiac Surgery, University Hospital of Basel, Basel, Switzerland.
REFERENCES
- 1. Podesser BK, Chambers DJ.. New Solutions for the Heart: An Update in Advanced Perioperative Protection. Wien/New York: Springer, 2011. [Google Scholar]
- 2. Schmidtler FW, Tischler I, Lieber M, Weingartner J, Angelis I, Wenke K. et al. Cardiac surgery for octogenarians—a suitable procedure? Twelve-year operative and post-hospital mortality in 641 patients over 80 years of age. Thorac Cardiovasc Surg 2008;56:14–9. [DOI] [PubMed] [Google Scholar]
- 3. Robinson LA, Schwarz GD, Goddard DB, Fleming WH, Galbraith TA.. Myocardial protection for acquired heart disease surgery: results of a national survey. Ann Thorac Surg 1995;59:361–72. [DOI] [PubMed] [Google Scholar]
- 4. Chambers DJ, Fallouh HB.. Cardioplegia and cardiac surgery: pharmacological arrest and cardioprotection during global ischemia and reperfusion. Pharmacol Ther 2010;127:41–52. [DOI] [PubMed] [Google Scholar]
- 5. Kiss A, Heber S, Kramer AM, Hackl M, Skalicky S, Hallstrom S. et al. MicroRNA expression profile changes after cardiopulmonary bypass and ischemia/reperfusion-injury in a porcine model of cardioplegic arrest. Diagnostics 2020;10:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santer D, Kramer A, Kiss A, Aumayr K, Hackl M, Heber S. et al. St Thomas' Hospital polarizing blood cardioplegia improves hemodynamic recovery in a porcine model of cardiopulmonary bypass. J Thorac Cardiovasc Surg 2019;158:1543–54 e8. [DOI] [PubMed] [Google Scholar]
- 7. Fremes SE, Christakis GT, Weisel RD, Mickle DA, Madonik MM, Ivanov J. et al. A clinical trial of blood and crystalloid cardioplegia. J Thorac Cardiovasc Surg 1984;88:726–41. [PubMed] [Google Scholar]
- 8. Guru V, Omura J, Alghamdi AA, Weisel R, Fremes SE.. Is blood superior to crystalloid cardioplegia? A meta-analysis of randomized clinical trials. Circulation 2006;114:I331–8. [DOI] [PubMed] [Google Scholar]
- 9. Yau TM, Ikonomidis JS, Weisel RD, Mickle DA, Hayashida N, Ivanov J. et al. Which techniques of cardioplegia prevent ischemia? Ann Thorac Surg 1993;56:1020–8. [DOI] [PubMed] [Google Scholar]
- 10. Fan Y, Zhang AM, Xiao YB, Weng YG, Hetzer R.. Warm versus cold cardioplegia for heart surgery: a meta-analysis. Eur J Cardiothorac Surg 2010;37:912–9. [DOI] [PubMed] [Google Scholar]
- 11. Kuhn EW, Choi YH, Pyun JM, Neef K, Liakopoulos OJ, Stamm C. et al. Endothelial injury associated with cold or warm blood cardioplegia during coronary artery bypass graft surgery. Biomed Res Int 2015;2015:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trescher K, Gleiss A, Boxleitner M, Dietl W, Kassal H, Holzinger C. et al. Short-term clinical outcomes for intermittent cold versus intermittent warm blood cardioplegia in 2200 adult cardiac surgery patients. J Cardiovasc Surg 2017;58:105–12. [DOI] [PubMed] [Google Scholar]
- 13. Fujii M, Chambers DJ.. Cardioprotection with esmolol cardioplegia: efficacy as a blood-based solution. Eur J Cardiothorac Surg 2013;43:619–27. [DOI] [PubMed] [Google Scholar]
- 14. Onorati F, Santini F, Dandale R, Ucci G, Pechlivanidis K, Menon T. et al. "Polarizing" microplegia improves cardiac cycle efficiency after CABG for unstable angina. Int J Cardiol 2013;167:2739–46. [DOI] [PubMed] [Google Scholar]
- 15. Aass T, Stangeland L, Chambers DJ, Hallstrom S, Rossmann C, Podesser BK. et al. Myocardial energy metabolism and ultrastructure with polarizing and depolarizing cardioplegia in a porcine model of cardiopulmonary bypass. Eur J Cardiothorac Surg 2017;52:180–188. https://doi.org/10.1093/ejcts/ezx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bessho R, Chambers DJ.. Myocardial protection: the efficacy of an ultra-short-acting beta-blocker, esmolol, as a cardioplegic agent. J Thorac Cardiovasc Surg 2001;122:993–1003. [DOI] [PubMed] [Google Scholar]
- 17. Vinten-Johansen J, Nakanishi K, Zhao ZQ, McGee DS, Tan P.. Acadesine improves surgical myocardial protection with blood cardioplegia in ischemically injured canine hearts. Circulation 1993;88:II350–8. [PubMed] [Google Scholar]
- 18. Sloots KL, Vinten-Johansen J, Dobson GP.. Warm nondepolarizing adenosine and lidocaine cardioplegia: continuous versus intermittent delivery. J Thorac Cardiovasc Surg 2007;133:1171–8. [DOI] [PubMed] [Google Scholar]
- 19. Wiest D. A review of its therapeutic efficacy and pharmacokinetic characteristics. Clin Pharmacokinet 1995;28:190–202. [DOI] [PubMed] [Google Scholar]
- 20. Onorati F, Dobson GP, San Biagio L, Abbasciano R, Fanti D, Covajes C. et al. Superior myocardial protection using "polarizing" adenosine, lidocaine, and Mg2+ cardioplegia in humans. J Am Coll Cardiol 2016;67:1751–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.