Abstract
Introduction
Co-use of tobacco and marijuana is common, and research suggests that marijuana use may be a barrier to smoking cessation. Research to date has not evaluated how marijuana use affects e-cigarette switching behaviors and related outcomes in a harm reduction trial.
Aims and Methods
This secondary analysis includes African American (48%) and Latinx (52%) adult smokers randomized to the e-cigarette group (N = 114) of a harm reduction clinical trial from 2018 to 2019. Participants were provided JUUL e-cigarettes and encouraged to make an exclusive switch for 6 weeks. Our primary outcome was cigarettes smoked per week. Secondary health outcomes were e-cigarette substitution (calculated by measuring e-cigarette pod use), expired carbon monoxide (CO), and respiratory symptoms. Marijuana products were recorded at three timepoints and coded for combustion.
Results
Marijuana use during the study (n = 52, 46%) was not associated with week 6 cigarettes smoked or e-cigarette substitution, and combustible marijuana use was not associated with week 6 respiratory symptoms (ps > .05). After controlling for cigarettes smoked at week 6, combustible marijuana use was significantly associated with a 4.4 ppm increase in CO compared with no use of marijuana (p = .001).
Conclusions
Marijuana use was not a barrier to switching to e-cigarettes in this 6-week trial. Marijuana use contributed to elevated CO, reflecting greater exposure to toxic combustion products, beyond the effects of cigarette smoking. Marijuana co-use may increase risk of adverse health outcomes and may be a confounding factor when using CO as an endpoint to bioverify exclusive e-cigarette use.
Implications
This is the first known study to examine the effects of marijuana use on smokers switching to e-cigarettes. Marijuana use was not a barrier to cigarette reduction in a 6-week randomized clinical trial. Marijuana use uniquely contributed to higher carbon monoxide among cigarette smokers, indicating greater exposure to toxic combustion products, which could increase risk of adverse health outcomes. Furthermore, combustible marijuana use may be a confounding factor when CO is used as an endpoint to bioverify exclusive e-cigarette use.
Introduction
Electronic cigarettes (e-cigarettes) are an emerging harm reduction strategy, replacing smoking tobacco and the associated deleterious health effects with the less harmful effects of “vaping.” 1–3 Cigarette smoking delivers nicotine through inhaling the smoke from combusted tobacco, whereas vaping delivers nicotine through inhaling an aerosol produced from heating e-liquid. Given the lack of combustion, e-cigarette use is associated with reduced carbon monoxide (CO),4–6 a marker for gas-phase exposure to combustibles, and fewer self-reported respiratory symptoms for exclusive users compared with dual users.7 For current smokers, use of e-cigarettes satisfies urges to smoke4 and withdrawal symptoms8 while reducing harms associated with combustion. However, some smokers engage in additional behaviors involving combustion that may diminish the harm reduction potential of switching to e-cigarettes.
Marijuana may be used through multiple routes including ingestion or vaporization, but is typically smoked, and is marked by the psychoactive properties of tetrahydrocannabinol.9 Co-use of combusted (ie, smoked) marijuana is frequent among cigarette smokers and shares similar health concerns from combustion such as exposure to CO, tar, and many harmful compounds also found in tobacco smoke.10–12 Marijuana use among smokers ranges from 18% to 40%13–16 varying by study, population, and survey questions used. Combustion of plant-based material exposes marijuana and tobacco users alike to CO, which impedes oxygen delivery in the body by competing with oxygen for binding to hemoglobin.17 Smoking blunts, containing both marijuana and tobacco, results in higher CO than smoking marijuana in the form of joints,18 while noncombustibles (ie, vaporization) do not increase CO.19 Smoking a joint increases blood carboxyhemoglobin saturation four times that of a single filtered tobacco cigarette, indicative of greater CO exposure.20 Furthermore, marijuana use is associated with morning cough, sputum production, and wheeze among cigarette smokers, even after adjusting for current and cumulative (pack-years) tobacco smoking.21 Given the effects of smoking marijuana, it is plausible that marijuana use will diminish the expected reduction in CO and improvement of respiratory symptoms that would occur when switching from cigarettes to e-cigarettes.
Shared sensory and behavioral associations between marijuana and cigarette smoking raise the concern that marijuana co-use may impede switching from combustible cigarettes to e-cigarettes. Observational studies show marijuana use is associated with continued tobacco use,13,22 and marijuana use has been a barrier to cigarette reduction in previous intervention studies.23–25 Marijuana use was associated with reduced likelihood of smoking abstinence in a 12-week study administering varenicline,23 in a 12-month study using online support groups and counseling,24 and a 26-week study using nicotine replacement therapy.25 In an intervention using nicotine replacement therapy plus behavioral support for 8 weeks, smoking abstinence rates did not vary between marijuana and nonmarijuana users.26 To date, no study has investigated how marijuana use affects smokers switching to e-cigarettes and related health effects in a clinical trial.
This study tests the contribution of marijuana use on cigarettes smoked per week at week 6, the primary outcome point of a harm reduction trial, hypothesizing more cigarettes smoked for marijuana users. Secondary aims test the contribution of marijuana use on e-cigarette substitution, CO levels, and respiratory symptoms at week 6. It was hypothesized that marijuana use would contribute to lower e-cigarette substitution, higher CO levels, and increased respiratory symptoms.
Methods
This is a secondary analysis from the first reported randomized clinical trial of nicotine salt pod system e-cigarettes testing effects on toxicant exposure among members of the two largest racial/ethnic groups in the United States, African American and Latinx smokers.27 The trial was registered with ClinicalTrials.gov (Identifier: NCT03511001) and specific inclusion/exclusion criteria are found in the main outcomes paper.27 In brief, participants were 21 years or older, smoked 5 cigarettes per day for at least 6 months, had expired CO greater than 5 parts per million (ppm) at baseline, and were willing to switch from smoking cigarettes to e-cigarettes for 6 weeks. This is a secondary analysis and was not preregistered and the results should be considered exploratory. This multisite study was conducted from July 2018 to May 2019 and consisted of a baseline, week 2, and week 6 visit. Participants (N = 187) were randomly assigned in a 2:1 ratio, stratified by study site (African American in Kansas City, Missouri and Latinx in San Diego, CA) to the “e-cigarette group” or “smoking as usual” control group. Legality of marijuana use also differed by site, with medicinal-only use (ie, authorized for those with a qualifying medical issue) in Missouri and recreational use (ie, authorized for those age 21 years or older) in California. Those randomized to e-cigarettes received a JUUL e-cigarette and pods in a choice of Virginia tobacco, classic menthol, cool mint, or mango flavor pods (5% nicotine) for 6 weeks. Participants were given brief education, training, and action planning for making a complete switch to e-cigarettes. At week 6, those in the e-cigarette group demonstrated significant reductions in cigarettes smoked, CO, and respiratory symptoms compared with the “smoking as usual” control group. The study was approved by the Institutional Review Board at California State University San Marcos and the University of Kansas School of Medicine and written consent obtained for each participant.
Measures
Tobacco and Marijuana Use
Tobacco and marijuana product use were measured by a 7-day Timeline Followback (TLFB) method at baseline, week 2, and week 6. TLFB is a reliable and valid source of measuring cigarettes, e-cigarettes, and marijuana use.6,28,29 TLFB has been verified with objective tobacco measures such as interactive voice response, cigarette butt counts, and biological markers of cigarette use (ie, cotinine).30 Researchers used a paper calendar to first write down participants’ schedules and memorable events for the past 7 days. Using the calendar to assist with recall, participants were asked about tobacco and marijuana use on each day and quantities recorded. Quantities for the following marijuana products were recorded: blunt (cigar or cigarillo with marijuana), joint (marijuana rolled in paper), spliff (marijuana and tobacco rolled in paper), bowl (pipe/bong with marijuana), mole (pipe/bong with marijuana and tobacco), hookah with marijuana and tobacco, dabs or vaporized marijuana, edible marijuana, other form of marijuana only, and other form of tobacco with marijuana. Marijuana use was dichotomized into any marijuana use or no marijuana use at baseline, week 2, or week 6, in addition to any or no marijuana use during the 6-week study. Combustible marijuana use was classified for use of blunt, joint, spliff, bowl, pipe/bong with marijuana and tobacco, or hookah with tobacco and marijuana.
Tobacco products were also measured by TLFB including cigarettes, little cigars, cigarillos, hand-rolled cigarettes, full-sized cigars, pipes, bidis, hookah/water pipes, and spit/snuff/smokeless tobacco. An objective measure of e-cigarette use was obtained by counting and weighing participants’ used pods at week 6. Combustible tobacco product use was classified for use of little cigars, cigarillos, hand-rolled cigarettes, full-sized cigars, pipes, bidis, or hookah/water pipes.
E-Cigarette Substitution
E-cigarette substitution was calculated using cigarettes smoked and the number of pods returned at week 6. Cigarettes smoked at week 6 were recorded as the number of cigarettes smoked in the past 7 days using TLFB. As described elsewhere, e-cigarette pods were counted and weighed at week 6 and converted into cigarette equivalents (one pod contains 0.57 g e-liquid, equivalent to about 20 cigarettes).31,32 E-cigarette substitution was calculated as a percent by dividing the total amount of e-cigarettes consumed per week by the sum of total cigarette and e-cigarette consumption.
Carbon Monoxide
CO was measured in parts per million (ppm) from an exhaled breath test using a coVita Smokerlyzer device. Expired CO has a half-life of 2–6 hours and is only sensitive to recent smoking.33 CO levels of 6 or more ppm were used as inclusion criteria to enroll in the study and recorded at baseline, week 2, and week 6.
Respiratory Symptoms
Respiratory symptoms were assessed using the American Thoracic Society Questionnaire scale.34,35 This 8-item, self-report questionnaire measures respiratory symptom frequency (eg, cough first thing in the morning, getting very tired in a short time) with responses of 0 (never), 1 (less than once per week), 2 (1–2 times per week), 3 (several times per week), and 4 (every day). Scores were summed and range from 0 to 32, with higher scores indicating increased respiratory symptoms.27,34 This scale displays psychometric validity in adolescent smokers, with Cronbach’s α = 0.80 and α = 0.86 in a matched pairs study.34 Scales in the present study display good internal consistency at baseline α = 0.86, week 2 α = 0.83, and week 6 α =0.86.
Statistical Analyses
Independent samples t tests and chi-square tests were used to compare baseline characteristics of those who reported marijuana use during the study period with those that did not report marijuana use. Four separate multiple linear regressions tested the effect of marijuana use on the following measures at week 6: cigarettes smoked, e-cigarette substitution, CO, and respiratory symptoms. Any marijuana use during the 6 weeks was used for analyses predicting cigarettes smoked and e-cigarette substitution, whereas combustible marijuana use was used for analyses predicting CO and respiratory symptoms. Due to the time sensitivity of CO, this analysis used combustible marijuana at the week 6 timepoint.
Multiple linear regressions were conducted controlling for gender, race/ethnicity or site, age, number of cigarettes smoked at baseline, and use of other combustible tobacco products. Baseline respiratory symptoms were added as a control for the model predicting week 6 respiratory symptoms. Winsorization was applied to outlying continuous variables in which z-scores were less than −3.29 or greater than 3.29.36 This procedure was applied to three TLFB cigarette values (two at baseline; one at week 6) and four CO values (two at baseline; two at week 6). Missing data were excluded using listwise deletion and all analyses were conducted using SPSS v24.0.
A post hoc power analysis with a sample size of N = 114 was conducted using G*Power.37 Without compromising power below 0.80, an effect size of Cohen’s f2 = 0.08 was needed for one predictor using a multiple linear regression with up to seven parameters and using a two-sided p value of <.05.
Results
The present study focuses on the primary analytic sample of the e-cigarette group who completed the final week 6 visit (114/125 = 91.2%; 60 African American and 54 Latinx). The sample was 58.8% male, 52.6% African American and 47.4% Latinx, and on average 44.6 years old (Table 1). Participants smoked on average 83 cigarettes per week or a little over half a pack per day. Nearly half of the sample (n = 52, 46%) reported marijuana use at least once during the study period (baseline n = 46, 40.4%; week 2 n = 42, 38.2%; and week 6 n = 45, 39.5%). Past week marijuana days (use of any marijuana on each of 7 days) among marijuana users reveal a bimodal pattern of use. Approximately half (50%–53%) of marijuana users used on 7 out of 7 past calendar days, while another 29%–37% used on only 1 or 2 days in past 7 days (Supplementary Figure 1). A minority of marijuana users reported use on 4 or 5 days in the past 7 days.
Table 1.
Baseline Characteristics of Analytic Sample, by Study Marijuana Use; M (SD) or N (%)
| Variables at baseline | All N = 114 |
Marijuana used,g | X 2/t | p h | ||
|---|---|---|---|---|---|---|
| Nonmarijuana users (n = 62) | Marijuana users (n = 52) | |||||
| Demographics | Sitea | 4.088 | .043 | |||
| San Diego, CA | 54 (47.4) | 24 (38.7) | 30 (57.7) | |||
| Kansas City, MO | 60 (52.6) | 38 (61.3) | 22 (42.3) | |||
| Genderb | 2.094 | .148 | ||||
| Male | 67 (58.8) | 33 (53.2) | 34 (65.4) | |||
| Female | 46 (40.4) | 29 (46.8) | 17 (32.7) | |||
| Age, years | 44.6 (12.9) | 47.8 (12.1) | 40.8 (12.8) | 3.026 | .003 | |
| Marriage status | 3.209 | .073 | ||||
| Never married | 51 (44.7) | 23 (37.1) | 28 (53.8) | |||
| Otherc | 63 (55.3) | 39 (62.9) | 24 (46.2) | |||
| Federal poverty level | 0.118 | .731 | ||||
| At or below 200% | 83 (74.1) | 46 (75.4) | 37 (72.5) | |||
| Above 200% | 29 (25.9) | 15 (24.6) | 14 (27.5) | |||
| Outcome variables | Cigarettes per week smokedd | 82.6 (44.7) | 83.0 (42.5) | 82.2 (47.5) | 0.094 | .925 |
| Other combustible tobacco productsd,e | 25 (21.9) | 9 (14.5) | 16 (30.8) | 4.364 | .037 | |
| Carbon monoxide, ppm | 17.7 (9.0) | 16.8 (8.9) | 18.8 (9.2) | −1.177 | .242 | |
| Respiratory symptomsf | 11.9 (8.4) | 12.0 (8.4) | 11.7 (8.5) | 0.226 | .821 |
Boldface indicates statistical significance (p < .05).
aSite and race/ethnicity are interchangeable such that those from San Diego were Latinx and those from Kansas City were African American.
bDue to small sample size, gender excluded one participant who identified as transgender/gender nonconforming and was not included in chi-square analysis.
cMarried, divorced, separated, or widowed.
dPast 7-day Timeline Followback (TLFB).
eDichotomized into use of other combustible tobacco products or no use of other combustible tobacco products (besides cigarettes). Includes little cigars, cigarillos, hand-rolled cigarettes, full-sized cigars, pipes, bidis, and hookah/water pipes.
fMeasured with the American Thoracic Society Questionnaire, range 0–32, with higher scores indicating increased respiratory symptoms.
gDichotomized into marijuana use reported at the baseline, week 2, or week 6 visit, or no marijuana use reported.
hInferential testing for demographics based on marijuana use used t tests and chi-square tests.
Among those who used marijuana, the majority of marijuana use was combustible (1162/1411 products reported = 82% combustible use instances at baseline, 858/1106 products reported = 78% combustible use instances at week 2, and 1068/1400 products reported = 76% combustible use instances at week 6; Supplementary Figure 2). Most people who used marijuana used multiple forms, and every person who used marijuana reported a type of combustible marijuana, except for one person at week 6 who used noncombustible marijuana only. The four leading marijuana products reported were bowls (55% average across baseline, week 2, and week 6), dabs or vaporized marijuana (18% average), blunts (14% average), and joints (7% average) (Supplementary Figure 2). Bowls in this context refer to the amount of marijuana placed into the feature of a pipe or bong that is subsequently smoked.
Bowls were reported more often and on more days than any other product (Table 2). At baseline, those who used bowls (n = 24) smoked approximately 5 bowls per day on an average of 5 days per week. Those using dabs or vaporized marijuana at baseline (n = 12) reported 4 sessions per day on an average of 3 days per week. Blunt smokers at baseline (n = 24) smoked approximately 2 blunts per day on an average of 3 days per week. Joint smokers at baseline (n = 22) smoked approximately 2 joints per day on an average of 2 days per week. Data for other marijuana products and other timepoints are reported in Table 2. Weekly consumption levels of each marijuana product are provided in Supplementary Table 1.
Table 2.
Frequency of Marijuana Product Use
| Baseline | Week 2 | Week 6 | ||||
|---|---|---|---|---|---|---|
| Days used in past 7 days | Products per day | Days used in past 7 days | Products per day | Days used in past 7 days | Products per day | |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Combustibles | ||||||
| Bowl (pipe/bong with marijuana) | 5.42 (2.43) | 5.09 (5.17) | 5.23 (2.29) | 4.89 (4.68) | 5.60 (1.98) | 6.15 (4.52) |
| n = 24 | n = 24 | n = 22 | n = 22 | n = 20 | n = 20 | |
| Mole (pipe/bong with marijuana and tobacco) | 2.00 (2.24) | 4.27 (4.06) | — | — | — | — |
| n = 5 | n = 5 | |||||
| Blunt (cigar or cigarillo with marijuana) | 3.08 (2.50) | 1.83 (1.20) | 3.23 (2.33) | 1.82 (1.25) | 3.48 (2.64) | 2.32 (1.89) |
| n = 24 | n = 24 | n = 22 | n = 22 | n = 21 | n = 21 | |
| Joint (marijuana rolled in paper) | 2.36 (2.01) | 2.19 (1.44) | 2.27 (1.79) | 3.12 (3.98) | 2.73 (2.07) | 1.45 (0.69) |
| n = 22 | n = 22 | n = 15 | n = 15 | n = 22 | n = 22 | |
| Spliff (marijuana and tobacco rolled in paper) | 3.33 (3.22) | 2.00 (1.00) | 1.00 (—) | 1.00 (—) | 1.00 (0.00) | 1.00 (—) |
| n = 3 | n = 3 | n = 1 | n = 1 | n = 2 | n = 2 | |
| Hookah with marijuana and tobacco | — | — | — | — | — | — |
| Noncombustibles | ||||||
| Dabs or vaporized marijuana (sessions) | 2.83 (2.21) | 4.12 (4.35) | 3.29 (2.64) | 3.90 (3.62) | 4.50 (2.65) | 4.36 (4.27) |
| n = 12 | n = 12 | n = 14 | n = 14 | n = 12 | n = 12 | |
| Edible marijuana | 2.33 (2.34) | 3.75 (5.12) | 2.00 (2.24) | 1.79 (1.52) | 1.43 (0.79) | 2.33 (2.69) |
| n = 6 | n = 6 | n = 7 | n = 7 | n = 7 | n = 7 | |
| Other form of marijuana only | 3.67 (3.06) | 1.67 (0.58) | — | — | 1.00 (—) | 1.00 (—) |
| n = 3 | n = 3 | n = 1 | n = 1 | |||
| Other form of tobacco with marijuana | — | — | — | — | — | — |
All marijuana products were recorded using past 7-day Timeline Followback. Means and SDs are reported. Data rows include only users of the respective product, for the respective timepoint. Products per day were calculated by taking the total units reported during the week and dividing by days used in the past 7 days.
Smokers who reported marijuana use during the study period were younger, had higher rates of other combustible tobacco product use at baseline, and were over-represented in San Diego, where recreational marijuana use is legal, compared with those with no marijuana use (Table 1). Location was interchangeable with race/ethnicity, meaning those who reported marijuana use were over-represented among Latinx versus African American participants. Smokers who reported marijuana use during the study period did not vary in baseline levels of cigarettes smoked, CO, or respiratory symptoms from those with no marijuana use.
Impact of Marijuana Use on Cigarettes Smoked
Cigarettes smoked at baseline (B = 0.236, p = .001, 95% confidence interval [CI] = 0.104, 0.369) and age (B = 0.652; p = .030, 95% CI = 0.064, 1.239) predicted cigarettes smoked at week 6, such that those who were older and smoking more at baseline were smoking more at week 6 (Table 3). Adding the predictor of marijuana use did not improve model fit: ΔF(1, 107) = 0.061, p = .805, 95% CI = −10.628, 13.654, ΔR2 = 0.000. Gender, site, and other combustible tobacco products were nonsignificant variables in the model (ps > .05).
Table 3.
Regression Analysis Results for Week 6 Outcomes
| t | p | 95% CI | Unstandardized B | Unstandardized SE | β | ΔF | df | p | ΔR2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cigarettes smokeda | ||||||||||
| Marijuana usea,b | 0.247 | .805 | −10.628, 13.654 | 1.513 | 6.124 | 0.023 | 0.061 | 1, 107 | .805 | 0.000 |
| Gender | −0.454 | .651 | −14.822, 9.296 | −2.763 | 6.083 | −0.042 | ||||
| Site | 1.563 | .121 | −3.155, 26.640 | 11.743 | 7.515 | 0.176 | ||||
| Age | 2.200 | .030 | 0.064, 1.239 | 0.652 | 0.296 | 0.251 | ||||
| Cigarettes smoked at baselinea | 3.541 | .001 | 0.104, 0.369 | 0.236 | 0.067 | 0.316 | ||||
| Other combustible tobacco productsa,c | −0.377 | .707 | −16.113, 10.966 | −2.573 | 6.830 | −0.034 | ||||
| E-cigarette substitution | ||||||||||
| Marijuana usea,b | −1.080 | .282 | −0.208, 0.061 | −0.073 | 0.068 | −0.104 | 1.167 | 1, 107 | .282 | 0.010 |
| Gender | 0.320 | .750 | −0.112, 0.155 | 0.022 | 0.068 | 0.031 | ||||
| Site | −0.337 | .737 | −0.193, 0.137 | −0.028 | 0.083 | −0.040 | ||||
| Age | −2.019 | .046 | −0.013, 0.000g | −0.007 | 0.003 | −0.243 | ||||
| Cigarettes smoked at baselinea | −1.743 | .084 | −0.003, 0.000h | −0.001 | 0.001 | −0.164 | ||||
| Other combustible tobacco productsa,c | 0.089 | .929 | −0.144. 0.157 | 0.007 | 0.076 | 0.009 | ||||
| Carbon monoxide, ppm | ||||||||||
| Marijuana use at week 6a,d | 3.460 | .001 | 1.894, 6.979 | 4.437 | 1.282 | 0.270 | 11.969 | 1, 107 | .001 | 0.066 |
| Gender | −1.058 | .293 | −3.782, 1.150 | −1.316 | 1.244 | −0.084 | ||||
| Site | −2.491 | .014 | −6.935, −0.789 | −3.862 | 1.550 | −0.241 | ||||
| Age | −1.216 | .227 | −0.198, 0.047 | −0.075 | 0.062 | −0.121 | ||||
| Cigarettes smoked at week 6a | 7.041 | <.001 | 0.095, 0.170 | 0.132 | 0.019 | 0.550 | ||||
| Other combustible tobacco productsa,c | −1.630 | .106 | −5.052, 0.492 | −2.280 | 1.398 | −0.127 | ||||
| Respiratory symptomse | ||||||||||
| Marijuana usea,f | 1.102 | .273 | −1.062, 3.717 | 1.327 | 1.205 | 0.090 | 1.214 | 1, 104 | .273 | 0.007 |
| Gender | −0.910 | .365 | −3.489, 1.292 | −1.097 | 1.205 | −0.077 | ||||
| Site | 0.505 | .615 | −2.232, 3.756 | 0.762 | 1.510 | 0.052 | ||||
| Age | 1.478 | .143 | −0.030, 0.206 | 0.088 | 0.060 | 0.154 | ||||
| Cigarettes smoked at baselinea | −2.603 | .011 | −0.063, −0.009 | −0.036 | 0.014 | −0.209 | ||||
| Other combustible tobacco productsa,c | −0.560 | .576 | −3.556, 1.989 | −0.783 | 1.398 | −0.047 | ||||
| Respiratory symptoms at baselinee | 7.074 | <.001 | 0.373, 0.664 | 0.519 | 0.073 | 0.593 |
Boldface indicates statistical significance (p < .05). CI = confidence interval.
aPast 7-day Timeline Followback (TLFB).
bDichotomized into marijuana use reported at the baseline, week 2, or week 6 visit, or no marijuana use.
cOther combustible tobacco products include little cigars, cigarillos, hand-rolled cigarettes, full-sized cigars, pipes, bidis, and hookah/water pipes.
dDichotomized into combustible marijuana use at week 6 visit, or no combustible marijuana use (blunts, joints, spliffs, bowls, pipe/bong with marijuana and tobacco, and hookah with marijuana and tobacco).
eMeasured with the American Thoracic Society Questionnaire, range 0–32, with higher scores indicating increased respiratory symptoms.
fDichotomized into combustible marijuana use reported at the baseline, week 2, or week 6 visit, or no combustible marijuana use (blunts, joints, spliffs, bowls, pipe/bong with marijuana and tobacco, and hookah with marijuana and tobacco).
g−0.000119.
h0.000178.
Impact of Marijuana Use on E-Cigarette Substitution
Age predicted e-cigarette substitution at week 6, such that those who were older had lower e-cigarette substitution at week 6 (B = −0.007, p = .046, 95% CI = −0.013, 0.000; Table 3). Adding the predictor of marijuana use did not improve model fit: ΔF(1, 107) = 1.167, p = .282, 95% CI = −0.208, 0.061, ΔR2 = 0.010. Gender, site, cigarettes smoked at baseline, and other combustible tobacco products were nonsignificant variables in the model (ps > .05). A chi-square test found marijuana use was not related to the dichotomized variable of no smoking compared with any smoking/dual use (p = .135).
Impact of Combustible Marijuana Use on CO
Site (B = −3.862, p = .014, 95% CI = −6.935, −0.789) and cigarettes smoked at week 6 (B = 0.132, p < .001, 95% CI = 0.095, 0.170) predicted CO at week 6, such that those from Kansas City/African Americans and those smoking more cigarettes at week 6 had increased CO at week 6 (Table 3). Adding the predictor of combustible marijuana use at week 6 improved model fit: ΔF(1, 107) = 11.969, p = .001, 95% CI = 1.894, 6.979, ΔR2 = 0.066. Combustible marijuana use at week 6 was associated with a 4.4 ppm increase in week 6 CO compared with no use of marijuana, after controlling for cigarettes smoked at week 6. Gender, age, and other combustible tobacco products were nonsignificant variables in the model (ps > .05).
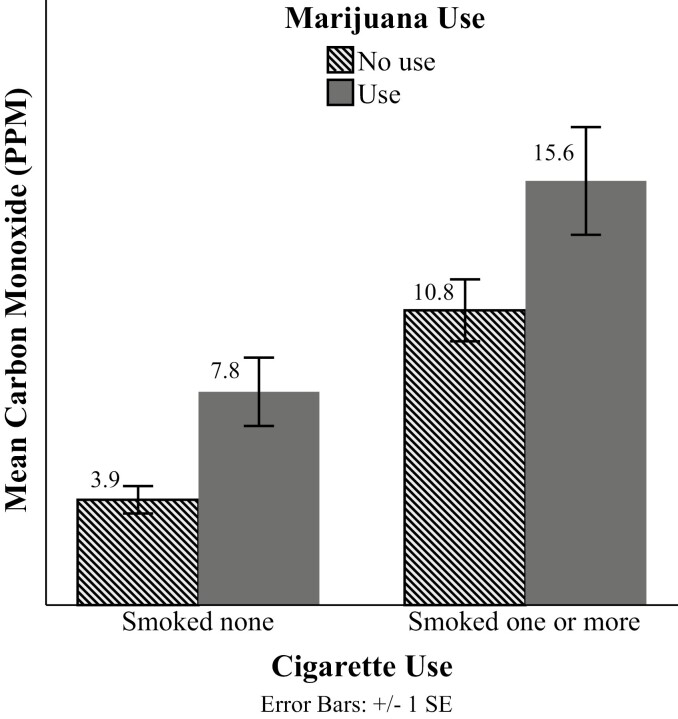
The effect of combustible marijuana use on CO at week 6 can be observed when cigarette use is dichotomized into none (0 cigarettes smoked) and any (one or more cigarettes smoked). As shown in Figure 1, combustible marijuana users have increased CO compared with nonmarijuana users for those who continued to smoke cigarettes at week 6 (15.6 vs. 10.8 ppm) and those who had exclusively switched to e-cigarettes (ie, non-cigarette smokers, 7.8 vs. 3.9 ppm). Mean CO ppm was higher for combustible marijuana users than nonusers for those reporting any cigarette use (4.8 ppm difference) and no cigarette use (3.9 ppm difference) at week 6, indicating marijuana users have higher CO regardless of cigarette smoking status at week 6.
Figure 1.
Week 6 carbon monoxide (CO) by combustible marijuana use and cigarettes smoked. Combustible marijuana use refers to one or more combustible marijuana products used at the week 6 past 7-day Timeline Followback. Cigarette use refers to one or more cigarettes reported at the week 6 past 7-day Timeline Followback.
Impact of Marijuana Use on Respiratory Symptoms
Cigarettes smoked at baseline (B = −0.036, p = .011, 95% CI = −0.063, −0.009) and respiratory symptoms at baseline (B = 0.519, p < .001, 95% CI = 0.373, 0.664) predicted respiratory symptoms at week 6, such that those smoking fewer cigarettes at baseline and those with increased baseline respiratory symptoms had increased respiratory symptoms at week 6 (Table 3). Adding the predictor of combustible marijuana use did not improve model fit: ΔF(1, 104) = 1.214, p = .273, 95% CI = −1.062, 3.717, ΔR2 = 0.007. Gender, site, age, and other combustible tobacco products were nonsignificant variables in the model (ps > .05).
Discussion
In the first study to our knowledge examining the impact of marijuana use on smokers switching to e-cigarettes, marijuana use was not a barrier to cigarette reduction in a 6-week trial. Furthermore, there was no evidence that marijuana use inhibited switching to e-cigarettes. The lack of association between marijuana use and switching to e-cigarettes is encouraging, but longer term follow-up may elucidate a different pattern of results over a greater time period. Studies linking marijuana co-use to worse cigarette cessation outcomes infer that marijuana use may need to be a concurrent target of cigarette smoking cessation interventions,23–25 and results from the present e-cigarette switching study reveal marijuana users display differential health outcomes for some markers (ie, CO).
Present findings for frequency of marijuana use as well as method of use are comparable to US national studies. Past 7-day marijuana use data reveal two distinct patterns of users, those who use marijuana once or twice a week and those who use daily (50%–53% of marijuana users). A survey sample of lifetime marijuana users recruited from Twitter (mean age 43 years old) also found approximately half (46%) were daily/near daily users of flower/herbal cannabis.38 Data on marijuana product types reported are similar to a 2016–2017 survey sample reporting most common modes of cannabis consumption being bowl (ie, pipe or bong), vaporizer, blunts, and joints.39 Additionally, proportions of combustible marijuana use (82% at baseline, 78% at week 2, and 76% at week 6) are comparable to an online sample finding the majority of past-year marijuana users (1063/1270, 84%) reported marijuana smoking.9
Combustible marijuana was not associated with respiratory symptoms at week 6 once cigarette smoking and other risk factors were accounted for. Marijuana use did not compound respiratory symptoms beyond the effect of cigarette smoking and other risk factors over 6 weeks. However, it is possible that an additive effect of respiratory symptoms would emerge over a longer time or with more frequent users, as cannabis dependency (measured by the DSM-III-R) has been associated with some respiratory symptoms even after controlling for tobacco use.40
Combustible marijuana use was uniquely associated with elevated CO at week 6, beyond that explained by cigarette smoking. Marijuana use was associated with approximately four ppm more CO exposure than nonmarijuana users, after controlling for cigarette use. This was expected given that combusted marijuana is associated with increased CO exposure.19 CO is a marker of exposure to combustion toxicants, which have been associated with many diseases. CO reduces oxygen delivery from blood and can be harmful to people with preexisting cardiovascular disease or pulmonary disease.41 Higher CO levels reflect greater smoke exposure and suggest that smokers who use marijuana may be at increased risk for adverse health effects.42 Some e-cigarette switching studies include education about the benefit of reduced CO by switching from combustible to electronic cigarettes.6,27 Our study suggests that the magnitude of this benefit will not be equal for those who smoke marijuana compared with those who do not.
Almost half of our study sample reported at least one incident of marijuana use, corresponding with rates of marijuana use among cigarette smokers ranging from 27% to 46% in previous studies.13,15,43,44 Given that many smoking studies use CO as an endpoint for bioverifying tobacco use status, researchers will need to determine how to use this information. Many researchers opt to make marijuana and other drug use exclusion criteria.4,5,8,45–48 It is unknown whether excluding marijuana use was done to isolate cigarette smoking as a source of CO for bioverifying smoking reduction, or whether this exclusion was a strategy to exclude drug use. For example, dependence on chemicals other than nicotine4,8,45–48 is common exclusion criteria for e-cigarette switching studies. Interventionists should be aware that excluding cigarette smokers who use marijuana use will significantly decrease study generalizability. Researchers who wish to include combustible marijuana users in their study sample are advised to conduct a nuanced measurement of product use and account for combustible marijuana use when bioverifying tobacco use status.49
The study includes several important limitations. The study was conducted in two geographical locations in the United States and among two different racial/ethnic groups and findings may not generalize to other populations. Additionally, the product characteristics and frequency patterns of marijuana use came from a relatively small sample (n = 52) that may not generalize to other populations of marijuana users. Findings must also be taken in light of a sample of smokers willing to switch to e-cigarettes and provided e-cigarettes at no cost for 6 weeks. Self-reported marijuana use was higher at the San Diego site than the Kansas City site. Although participants were told their responses were deidentified and confidential, Kansas City participants could have felt pressure to conceal marijuana use due to the state’s medicinal-only status, compared with the San Diego site where recreational marijuana use is legal. In addition to decreased comfort of reporting due to associated legal risks, there are also practical considerations that may contribute to less use such as accessibility to purchasing and exposure to advertising. Prevalence of marijuana use has been reported highest in states with legal recreational use.9 To address reporting bias in a multisite setting, measuring a biomarker of cannabis use is recommended. It is also possible that the younger age of San Diego study participants compared with Kansas City participants contributed to differential marijuana use, as age is associated with marijuana use.12,16,26,50 Finally, study outcomes were taken at 6-week postbaseline and the long-term impact of marijuana use on cigarette reduction and related health effects is an important topic for future study. While this study captured a majority of combustible marijuana use, understanding if there are reinforcing sensory components of vaping marijuana and vaping nicotine e-liquid remains to be studied.
Conclusion
Marijuana use did not inhibit cigarette reduction or e-cigarette substitution when smokers were provided a fourth-generation nicotine salt pod system e-cigarette and encouraged to switch for 6 weeks. Combustible marijuana use was linked to elevated CO, reflecting exposure to smoke, and may create increased risk for a variety of health issues.42 Furthermore, combustible marijuana use may be a confounding factor when CO is used as an endpoint to bioverify exclusive e-cigarette use. This sample of smokers was comprised of nearly half marijuana co-users. A little over half of marijuana users were daily users and a large majority of products reported were combustible. Tobacco interventionists should keep in mind the proportion of enrolled smokers who could also be smoking marijuana and be aware that combustion is a general mechanism affecting health outcomes.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We are grateful for the time and effort of Tricia Snow, Brian Hernandez, Michael Arnold, Ana Leon, Jennifer Mosley-Garcia, Amanda Dean, Crystal Marez, Dalia Hipolito, Mirella Orozco, Justin Sanchez, Juan Alva, John Le, Madison Garrett, Nathan Au-Yeung, Jeremy Mills-Shimell, Shyla Everett, Alexis Osuna, Daniell Derry Garlejo, Flavia Ponce, and Laura Wells for their assistance with data collection. We are also thankful for the Neighborhood Healthcare staff and Mary Baker for supporting participant enrollment, and Dr. Nancy Caine for support with initial concept development.
Contributor Information
Myra Rice, Neuroscience Interdepartmental Graduate Program, University of California, Los Angeles, Los Angeles, CA, USA.
Nicole L Nollen, Department of Population Health and the University of Kansas Cancer Center, University of Kansas School of Medicine, Kansas City, KS, USA.
Jasjit S Ahluwalia, Department of Behavioral and Social Sciences and the Center for Alcohol and Addiction Studies, School of Public Health, Brown University, Providence, RI, USA; Department of Medicine and Brown Cancer Center, Alpert Medical School, Brown University, Providence, RI, USA.
Neal Benowitz, Program in Clinical Pharmacology, Division of Cardiology, Department of Medicine, University of California, San Francisco School of Medicine, San Francisco, CA, USA.
Anna Woodcock, Department of Psychology, California State University San Marcos, San Marcos, CA, USA.
Kim Pulvers, Department of Psychology, California State University San Marcos, San Marcos, CA, USA.
Funding
This work was supported by the National Institutes of Health (5SC3GM122628). MR, NN, and KP were supported by 5SC3GM122628. JA was supported in part by P20GM130414, a National Institutes of Health funded Center of Biomedical Research Excellence (COBRE).
Declaration of Interests
NB serves as a consultant for Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has been an expert witness in litigation against tobacco companies. MR, NLN, AW, JSA, and KP have no conflicts of interest to declare.
References
- 1. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; 2018. [Google Scholar]
- 2. Notley C, Ward E, Dawkins L, Holland R. The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduct J. 2018;15(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warner KE. How to think—not feel—about tobacco harm reduction. Nicotine Tob Res. 2018;21(10):1299–1309. [DOI] [PubMed] [Google Scholar]
- 4. D’Ruiz CD, Graff DW, Robinson E. Reductions in biomarkers of exposure, impacts on smoking urge and assessment of product use and tolerability in adult smokers following partial or complete substitution of cigarettes with electronic cigarettes. BMC Public Health. 2016;16(1):543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Eissenberg T. Effects of six weeks of electronic cigarette use on smoking rate, CO, cigarette dependence, and motivation to quit smoking: a pilot study. Addict Behav. 2018;80:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pulvers K, Emami AS, Nollen NL, et al. . Tobacco consumption and toxicant exposure of cigarette smokers using electronic cigarettes. Nicotine Tob Res. 2018;20(2):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cassidy RN, Tidey JW, Colby SM. Exclusive e-cigarette users report lower levels of respiratory symptoms relative to dual e-cigarette and cigarette users. Nicotine Tob Res. 2020;22(1):S54–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220–11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steigerwald S, Wong PO, Cohen BE, et al. . Smoking, vaping, and use of edibles and other forms of marijuana among U.S. adults. Ann Intern Med. 2018;169(12):890–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10(3):239–247. [DOI] [PubMed] [Google Scholar]
- 11. Graves BM, Johnson TJ, Nishida RT, et al. . Comprehensive characterization of mainstream marijuana and tobacco smoke. Sci Rep. 2020;10(1):7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 13. Ford DE, Vu HT, Anthony JC. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug Alcohol Depend. 2002;67(3):243–248. [DOI] [PubMed] [Google Scholar]
- 14. Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M. Assessing the overlap between tobacco and marijuana: trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addict Behav. 2015;49:26–32. [DOI] [PubMed] [Google Scholar]
- 15. Rubenstein D, Aston ER, Nollen NL, et al. . Factors associated with cannabis use among African American nondaily smokers. J Addict Med. 2020;14(5):e170–e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rogers AH, Shepherd JM, Buckner JD, et al. . Current cannabis use and smoking cessation among treatment seeking combustible smokers. Drug Alcohol Depend. 2020;209:107928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palmeri R, Gupta V.. Carboxyhemoglobin Toxicity. Treasure Island, FL: StatPearls Publishing LLC; 2020. [PubMed] [Google Scholar]
- 18. Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 2009;103(3):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newmeyer MN, Swortwood MJ, Abulseoud OA, Huestis MA. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol Depend. 2017;175:67–76. [DOI] [PubMed] [Google Scholar]
- 20. Wu T-C, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med. 1988;318(6):347–351. [DOI] [PubMed] [Google Scholar]
- 21. Hancox RJ, Shin HH, Gray AR, Poulton R, Sears MR. Effects of quitting cannabis on respiratory symptoms. Eur Respir J. 2015;46(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schauer GL, King BA, McAfee TA. Prevalence, correlates, and trends in tobacco use and cessation among current, former, and never adult marijuana users with a history of tobacco use, 2005–2014. Addict Behav. 2017;73:165–171. [DOI] [PubMed] [Google Scholar]
- 23. McClure EA, Baker NL, Hood CO, et al. . Cannabis and alcohol co-use in a smoking cessation pharmacotherapy trial for adolescents and emerging adults. Nicotine Tob Res. 2020;22(8):1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vogel EA, Rubinstein ML, Prochaska JJ, Ramo DE. Associations between marijuana use and tobacco cessation outcomes in young adults. J Subst Abuse Treat. 2018;94:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gourlay SG, Forbes A, Marriner T, Pethica D, McNei JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. BMJ. 1994;309(6958):842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Metrik J, Spillane NS, Leventhal AM, Kahler CW. Marijuana use and tobacco smoking cessation among heavy alcohol drinkers. Drug Alcohol Depend. 2011;119(3):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pulvers K, Nollen NL, Rice M, et al. . Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3(11):e2026324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hjorthøj CR, Hjorthøøj AR, Nordentoft M. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances—systematic review and meta-analysis. Addict Behav. 2012;37(3):225–233. [DOI] [PubMed] [Google Scholar]
- 29. Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–162. [DOI] [PubMed] [Google Scholar]
- 30. Shiffman S, Scholl SM. Three approaches to quantifying cigarette consumption: data from nondaily smokers. Psychol Addict Behav. 2018;32(2):249–254. [DOI] [PubMed] [Google Scholar]
- 31. Arnold MJ, Nollen NL, Mayo MS, et al. . Harm reduction associated with dual use of cigarettes and e-cigarettes in Black and Latino smokers: secondary analyses from a randomized controlled e-cigarette switching trial. Nicotine Tob Res. 2021;23(11):1972–1976. doi: 10.1093/ntr/ntab069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod [published online ahead of print March 24, 2021]. Tob Control. 2021. doi: 10.1136/tobaccocontrol-2020-056367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benowitz NL, Hukkanen J, Jacob P III. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cassidy RN, Roberts ME, Colby SM. Validation of a respiratory symptom questionnaire in adolescent smokers. Tob Regul Sci. 2015;1(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Comstock GW, Tockman MS, Helsing KJ, Hennesy KM. Standardized respiratory questionnaires comparison of the old with the new. Am Rev Respir Dis. 1979;119(1):45–53. [DOI] [PubMed] [Google Scholar]
- 36. Tabachnick BG, Fidell LS.. Using Multivariate Statistics. 6th ed. Boston, MA: Pearson; 2013. [Google Scholar]
- 37. Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. [DOI] [PubMed] [Google Scholar]
- 38. Daniulaityte R, Zatreh MY, Lamy FR, et al. . A Twitter-based survey on marijuana concentrate use. Drug Alcohol Depend. 2018;187:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar N, Puljević C, Ferris J, Winstock A, Barratt MJ. Cannabis use patterns at the dawn of US cannabis reform. J Cannabis Res. 2019;1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taylor DR, Poulton R, Moffitt TE, Ramankutty P, Sears MR. The respiratory effects of cannabis dependence in young adults. Addiction. 2000;95(11):1669–1677. [DOI] [PubMed] [Google Scholar]
- 41. Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46(1):91–111. [DOI] [PubMed] [Google Scholar]
- 42. Singh A, Saluja S, Kumar A, et al. . Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. 2018;7(1):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Montgomery L. Marijuana and tobacco use and co-use among African Americans: results from the 2013, National Survey on Drug Use and Health. Addict Behav. 2015;51:18–23. [DOI] [PubMed] [Google Scholar]
- 44. Aung AT, Pickworth WB, Moolchan ET. History of marijuana use and tobacco smoking topography in tobacco-dependent adolescents. Addict Behav. 2004;29(4):699–706. [DOI] [PubMed] [Google Scholar]
- 45. Bullen C, Howe C, Laugesen M, et al. . Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–1637. [DOI] [PubMed] [Google Scholar]
- 46. Cravo AS, Bush J, Sharma G, et al. . A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul Toxicol Pharmacol. 2016;81:S1–S14. [DOI] [PubMed] [Google Scholar]
- 47. O’Connell G, Graff DW, D’Ruiz CD. Reductions in biomarkers of exposure (BoE) to harmful or potentially harmful constituents (HPHCs) following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Toxicol Mech Methods. 2016;26(6):453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walele T, Sharma G, Savioz R, Martin C, Williams J. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part B: safety and subjective effects. Regul Toxicol Pharmacol. 2016;74:193–199. [DOI] [PubMed] [Google Scholar]
- 49. Hindocha C, McClure EA. Unknown population-level harms of cannabis and tobacco co-use: if you don’t measure it, you can’t manage it. Addiction. 2020;116(7):1622–1630. doi: 10.1111/add.15290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schulenberg J, Johnston L, O’Malley P, Bachman J, Miech R, Patrick M.. Monitoring the Future National Survey Results on Drug Use, 1975–2019: Volume II, College Students and Adults Ages 19–60. Ann Arbor, MI: The University of Michigan Institute for Social Research; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.