Abstract
The central nervous system (CNS) was previously thought to be the only organ system lacking lymphatic vessels to remove waste products from the interstitial space. Recently, based on the results from animal experiments, the glymphatic system was hypothesized. In this hypothesis, cerebrospinal fluid (CSF) enters the periarterial spaces, enters the interstitial space of the brain parenchyma via aquaporin-4 (AQP4) channels in the astrocyte end feet, and then exits through the perivenous space, thereby clearing waste products. From the perivenous space, the interstitial fluid drains into the subarachnoid space and meningeal lymphatics of the parasagittal dura. It has been reported that the glymphatic system is particularly active during sleep. Impairment of glymphatic system function might be a cause of various neurodegenerative diseases such as Alzheimer’s disease, normal pressure hydrocephalus, glaucoma, and others. Meningeal lymphatics regulate immunity in the CNS. Many researchers have attempted to visualize the function and structure of the glymphatic system and meningeal lymphatics in vivo using MR imaging. In this review, we aim to summarize these in vivo MR imaging studies and discuss the significance, current limitations, and future directions. We also discuss the significance of the perivenous cyst formation along the superior sagittal sinus, which is recently discovered in the downstream of the glymphatic system.
Keywords: magnetic resonance imaging, gadolinium, endolymphatic hydrops, glymphatic system, sleep
Introduction
For years, it has been thought that the brain lacks lymphatic vessels like those found in other parts of the body.1 However more recently, a mechanism of brain waste clearance has been actively studied. Based on the results from animal studies, a hypothesis for the “glymphatic system” has been proposed.2,3 The movement of ventricular and subarachnoid cerebrospinal fluid (CSF) into the brain parenchyma was evaluated after infusion of fluorescent tracer into the lateral ventricle or cisterna magna. Based on in vivo two-photon microscopic imaging of small fluorescent tracers, Iliff et al. showed that CSF enters the parenchyma along the paravascular spaces that surround penetrating arteries and that brain interstitial fluid (ISF) is cleared along the paravenous drainage pathways. Animals lacking the water channel aquaporin-4 (AQP4) in astrocytes exhibit slowed CSF influx through this system and a ~70% reduction in interstitial solute clearance, suggesting that the bulk fluid flow between these anatomical influx and efflux routes is supported by astrocytic water transport.3
In this system, CSF travels through the perivascular space around the arteries to the deeper brain regions, flowing into the brain parenchyma through AQP4 channels in the astrocytic end feet.1,4 The ISF within the brain parenchyma is flushed out of the perivascular space around the veins, thereby clearing the waste products. This is a brief description of the glymphatic system or para-vascular ISF pathway, which has the role of lymphatic vessels in the brain.1,2,5 The name “glymphatic” was coined from glia plus lymphatic.
Another hypothesis, the Intramural Peri-Arterial Drainage (iPAD) pathway, has been proposed for the elimination of ISF and waste products from the brain. In the iPAD pathway, the authors hypothesized that the tracers injected in the CSF entered the cerebral cortex along the pial-glial basement membranes as there are no perivascular "spaces" around cortical arteries. Then, they exited the brain along the smooth muscle cell basement membranes.6 There is a review which suggests that the iPAD pathway is associated with the issue of gadolinium deposition in brains with a presumably intact blood brain barrier.7 However, there is no MR imaging study to visualize this hypothesized iPAD pathway in vivo. Therefore, we will mainly focus on the glymphatic system hypothesis in this review.
In this review, we will describe the glymphatic system hypothesis and recent challenges to visualize glymphatic system function using MR imaging. The words “perivascular” and “paravascular” space sometimes have different meanings. “Perivascular” space is used to describe the potential space in the vascular wall,6 and “paravascular” space is used to describe the space around and outside the vessels.3 However, both words are sometimes used interchangeably to describe the space around and outside the vessel, particularly in the field of radiology. In this review, we use both terms to describe the space around and outside the vessels.
Significance of the Glymphatic System
The human brain utilizes 25% of the body’s total energy and generates an estimated 7g of potentially toxic protein waste daily.8 The paravascular CSF transport system in the brain, referred to as the glymphatic system, facilitates waste removal and is most active during sleep.3 Studies have shown that the clearance of soluble Aβ increased 2-fold during slow-wave sleep when compared to wakefulness, which was associated with an increase in the ISF volume.9
This glymphatic system hypothesis received considerable attention for two main reasons. The first is that animal studies have shown that this system is more active during sleep. The importance of sleep in the maintenance of biological functions was clarified by these studies showing increased activity of the glymphatic system during sleep.1,9 The second reason is that dysfunction of the glymphatic system has been associated with the accumulation of abnormal proteins in neurodegenerative diseases such as Alzheimer’s disease, normal pressure hydrocephalus (NPH), glaucoma, and Parkinson’s disease.4,5,10–13
In addition, research on the glymphatic system has become more popular due to several other reasons. A short time after the glymphatic system was hypothesized, and lymphatic vessels within the meninges were discovered.14–16 The meningeal lymphatic vessels are located downstream of the glymphatic system. It is thought that waste products from the brain travel through the meningeal lymphatic vessels to reach the cervical lymph nodes.17 Another reason is the issue of brain accumulation of gadolinium-based contrast agents (GBCAs) used for MR imaging, which has become a hot topic of research.18–20 When this GBCA deposition in the brain was first discovered, it was not clear how the GBCA, which was thought to not cross the blood–brain barrier (BBB), entered the brain parenchyma and accumulated in the brain. A few years later, it was shown that intravenously administered GBCA can enter the CSF even in healthy subjects and can migrate into the perivascular space.21,22 It has long been known that intrathecally administered GBCA can penetrate into brain parenchyma, bypassing the BBB.23–27 Furthermore, a single intrathecal administration of a very small amount (i.e. less than 1 mL) of linearly structured GBCA resulted in deposition of gadolinium in the dentate nucleus and the globus pallidus, similar to that in patients who had received multiple regular doses of intravenously administered GBCA.28 In other words, once administered intravenously, GBCA permeates into the CSF via some unknown route. Next, the GBCA in CSF enters the brain parenchyma via the glymphatic system. While the unstable linearly structured GBCA stays in the brain for days to weeks, the GBCA is dechelated, and then the gadolinium is deposited in specific areas of the brain such as the dentate nucleus or the globus pallidus. This hypothesis is now widely accepted.29
As of September 23, 2020, a search on PubMed for the word “glymphatic” shows 508 results. However, approximately two-thirds of these articles were based on actual experiments or original data, and the rest are review articles. The concept of the glymphatic system is still a hypothesis and there are many counter arguments.30–33 Recently, the idea of “Neurofluids,” which includes the arterial, venous, CSF, and ISF systems, was proposed as a comprehensive concept for the fluid dynamics of the central nervous system (CNS). The rigid cranial cavity houses several space-competing material compartments: the brain parenchyma and the four extracellular fluids, namely arterial, venous, CSF, and ISF. Perturbing any of these fluid compartments can alter the brain dynamics, potentially increasing intracranial pressure, affecting perfusion, and hampering the clearance of metabolic waste.34–36
Debates on Glymphatic System Hypothesis
The main arguments against the glymphatic system hypothesis are as follows. Does convection really contribute to the movement of ISF in the brain, other than diffusion?37,38 If there is a convective contribution to ISF movement, the driving force is unknown and may be attributed to the pulsation of the arteries, the generation of CSF, or respiration.1,39–42 It is also debated whether the movement of ISF in the brain parenchyma is actually related to the excretion of waste products, even though the AQP4 channels only allow water to pass through.3,5,33
Despite the aforementioned arguments, many people believe in the existence of a glymphatic system or a similar waste excretion mechanism because it explains the results of many experiments and observations of the phenomena in clinical practice.30,31
Challenges to Visualizing Glymphatic System Function by MR Imaging
MRI visualization of the glymphatic system is important for elucidating the pathogenesis of many neurodegenerative diseases, determining therapeutic effects, and defining targets for treatment. Several methods have been attempted using MR imaging to visualize the glymphatic system in time and space.
Intrathecal administration of GBCA
Intrathecal administration of GBCA is contraindicated in the package insert. In cases where a large amount of GBCA was accidentally administered into the intrathecal space, gadolinium-induced encephalopathy with unconsciousness and seizure could occur. Patients with gadolinium encephalopathy sometimes have to be kept in intensive care units (ICU) for days. Deaths have also been reported in cases where large doses of GBCA were accidentally administered intrathecally.24,25,43
Alternatively, intrathecal administration of a small dose of GBCA (0.5 mL to 1 mL) can be used to detect CSF leakage or to evaluate NPH in patients as clinical research.11,17,44–46 These studies reported that patients receiving small doses of intrathecal GBCA had few side effects. Some centers have safely administered small intrathecal doses of GBCA in 100 or more patients as clinical research.47,48
A study in eight healthy human adult volunteers quantified CSF transport kinetics and brain glymphatic distribution using MR imaging following lumbar intrathecal injection of GBCA.49 After intrathecal administration, the GBCA was observed in the basal cisterns by the first imaging time point (1.8 hrs), and GBCA in the intracranial CSF spaces peaked within 1–3 hrs and started to decrease by 7 hrs. In some regions of the brain parenchyma, such as the cerebral cortex and white matter, enhancement was still increasing at the time of the final MR imaging (11 hrs). In general, the GBCA distributed in the CSF spaces, superficial to the CNS parenchyma, the ventricular systems, and then the brain parenchyma (cortical followed by subcortical). The averaged images of the intracerebral gadolinium infiltration after intrathecal GBCA administration in these healthy subjects revealed that the amount and speed of contrast transport differed by brain regions.49
Many studies using intrathecal GBCA administration have been reported in patients with NPH. There is a significant difference in the intracranial kinetics of GBCA between NPH patients and controls.23,44,45,48 However, judging from the images in the reported papers, the differences in the degree and rate of penetration into the brain parenchyma are highly individualized both in healthy individuals and in patients.44 Therefore, the use of intrathecal administration of GBCA for a specific individual diagnosis of glymphatic system function requires further research and development.
Future small-dose intrathecal administrations of GBCAs under a variety of controlled conditions may allow for the assessment of glymphatic system function in individual patients if sufficient data can be accumulated in large numbers of healthy and diseased conditions to define the normal range. However, intrathecal administration of GBCA carries certain risks.24,26 It is unlikely that this test will be widely used as a screening tool for asymptomatic patients, and would be limited to cases that required a lumbar puncture for other purposes.
Methods using diffusion weighted images
Several studies have used diffusion tensor imaging to investigate the water movement along the perivascular spaces as an assessment of glymphatic system function.50,51 In the diffusion tensor analysis along the perivascular space (DTI-ALPS) method, which is based on the assumption that the perivascular ISF movement in the white matter near lateral ventricles is dominant along the parallelly aligned medullary veins.50 The diffusivity in the X, Y, and Z directions in slices at the level of the lateral ventricular body is first calculated. Then, the ALPS-index, which is calculated from the diffusivity in each direction of the projection and association fibers’ regions, is defined to estimate the diffusivity along the perivascular space of medullary veins.50 There was a significant correlation between Mini Mental State Examination (MMSE) scores and ALPS-index in patients with Alzheimer’s disease.50 In patients with a NPH, the ALPS-index is reduced and has been shown to be different between normal subjects, patients with pseudo-NPH and patients with idiopathic NPH. The ALPS-index was reported to have a higher diagnostic performance than the Evans index.51 The DTI-ALPS does not require GBCA and uses clinically common diffusion tensor imaging data, which makes it easier to perform retrospective studies with previously acquired imaging data. However, it remains unclear whether the ALPS-index is completely unaffected by blood flow, and whether the movement of water along the perivascular space really reflects the function of the glymphatic system. Another limitation of DTI-ALPS is that the method can only evaluate a specific brain region.
In addition to DTI-ALPS, there has been another attempt to use diffusion-weighted images to evaluate glymphatic function. In animals, diffusion-weighted images with multiple b-factors have been used to evaluate the effect of suppression of the AQP4 channels in astrocyte end-feet. The results of this study showed that a marker of diffusion called the S-index was significantly reduced by the suppression of AQP4 function.52 Fundamentally, to evaluate the permeability of cell membrane with and without AQP4, diffusion time is important. An in vitro study showed that multi-b and multi-diffusion-time diffusion-weighted MR imaging on AQP4-expressing and -non-expressing cells demonstrated a clear difference between the signals from the two cell types.53
Perivascular fluid movement was assessed by diffusion tensor imaging with a long echo time to attenuate the signal from the surrounding arterial blood and brain tissue. A relatively lower b-value (i.e. 107 s/mm2) was also employed to focus the fluid in the perivascular space. Using this non-invasive MRI method in healthy rats, it was shown how the CSF is driven into the brain when the blood vessels nearby expand and contract. As the vessels pulsate with each heartbeat, there was a 300% increase in the movement of fluid into the perivascular space.54
Additionally, a newly developed phase contrast MR imaging using stimulated echo preparation was employed to observe very slow flow, which has been hampered previously by diffusion weighting and phase error from gradient hardware imperfections. Flows as slow as 1 μm/s were measured and validated using controlled water flow through a pipe at 4.7T.55
Method using ultra-long echo time arterial spin labelling (ASL)
A method for non-invasive measurement of blood–CSF barrier function using tracer-free MRI to quantify rates of water delivery from arterial blood to ventricular CSF has been developed.56 This method recorded a 36% decrease in blood–CSF barrier function in aged mice, compared to a 13% decrease in parenchymal blood flow.56 However, this technique is still preliminary and analysis is limited to the CSF in the vicinity of the choroid plexus.
Observation of the movement of cerebrospinal fluid/interstitial fluid in the subarachnoid space
Capturing the movement of water in brain parenchyma is very challenging due to the need to perform very complex modeling. However, the movement of CSF in the subarachnoid space is relatively easy to observe. In animal experiments at 9.4T, diffusion tensor imaging using an ultra-long echo time and a low b-value was employed to measure the movement of water in the perivascular space of the rat brain as a part of glymphatic pathway.54 However, in this report, the analysis of water movement along the perivascular space was limited to the vicinity of the circle of Willis, and further development is awaited.
Some attempts have been made to examine the function of the glymphatic system from the movement of CSF in humans using time-resolved 3D phase contrast imaging,57 3D dynamic improved motion-sensitized driven-equilibrium steady-state free precession (3D dynamic iMSDE SSFP) imaging,58 and low-b factor diffusion weighted imaging.59 However, the association of the macroscopic movement of CSF with the microscopic movement of water by the glymphatic system within the brain parenchyma is still not well understood.
Investigation of CSF movement by separating the effects of cardiac pulsation and respiration has been proposed. Although the effect of cardiac pulsation has a large influence on velocity, the effect of respiration seems to have a larger effect on the movement than that of cardiac pulsation.60
With ultra-fast MR encephalography, three physiological mechanisms affecting cerebral CSF pulsations, cardiac, respiratory, and very low frequency pulsations, were detected in the human brain. The MR encephalography is based on the signal observation of the brain in the ultra-fast gradient echo images. Cardiac pulsations induce a negative MR encephalography signal change in the peri-arterial regions, which extends centrifugally and covers the brain in ≈1 Hz cycles. Respiratory ≈0.3 Hz pulsations are centripetal periodical pulses that occur dominantly in the perivenous areas. The third type of pulsation had very low frequency (0.001–0.023 Hz and 0.023–0.73 Hz) waves, which propagated in unique spatiotemporal patterns. Using this ultra-fast MR encephalography, the subjects were scanned for 10 mins, and 5822-3D brain volumes were obtained after removal of the initial T1-saturation effects. This method was 20–25 times faster than conventional functional MRI (fMRI). Ultra-fast MR encephalography could potentially facilitate new discoveries of cerebral fluid dynamics.42
Time-spatial labeling inversion pulse (Time-SLIP) technique has been utilized to evaluate the CSF flow dynamics.61–63 By employing the respiration-induced Time-SLIP method, they were able to visualize CSF movement induced by respiratory excursions. CSF moved cephalad (16.4 ± 7.7 mm) during deep inhalation and caudad (11.6 ± 3.0 mm) during deep exhalation in the prepontine cisternal area.62
Use of sleep and/or anesthesia to alter MR contrast
CSF can move along the perivascular spaces in the brain and spinal cord to distribute nutrients and clear waste.1,3 These processes are supported by AQP4 water channels, which are highly expressed in the vascular end feet of astrocytes.64 CSF influx into the brain is higher in animals during sleep than in awake conditions.9 Previous studies have shown that anesthesia emulates sleep in that fluorescent CSF tracer influx, and the rate of radiolabeled amyloid beta efflux from the brain was comparable to that in naturally sleeping animals.40,65
Some studies have attempted to distinguish the changes in the ISF space during sleep from the changes in CSF, blood flow, and ISF movement. Analysis of fMRI data acquired at high temporal resolution found a slow 20-second cycle of CSF oscillation, which was synchronous with sleep. There are also changes in blood flow in the brain parenchyma that are synchronous with the changes in the CSF.66 Other research measured the effect of sleep on the change on the apparent diffusion coefficient (ADC). Sleep, compared to wakefulness, was associated with increases in the slow-ADC in the cerebellum and the left temporal pole, and with decreases in the fast-ADC in the thalamus, insula, parahippocampus, and striatal regions. In addition, the number of sleep arousals was inversely associated with the ADC changes. The CSF volume was increased during sleep and was associated with sleep-induced changes in the ADC in the cerebellum. There were no differences in the ADC during wakefulness following sleep deprivation compared to fully rested wakefulness.67 Although they hypothesized an increased ADC with sleep, their findings uncovered both increases in the slow ADC (mostly in the cerebellum), as well as decreases in the fast ADC, which could reflect the distinct biological significance of fast-versus slow-ADC values in relation to sleep. These findings suggest a more complex sleep-related glymphatic function in the human brain.67
In animals, linearly structured GBCA was administered intravenously and gadolinium deposition in the brain parenchyma at 5-week post-administration was studied with respect to the time of administration (i.e. morning or late afternoon) and the duration of anesthesia (i.e. short or long) at the time of administration. The results showed that, depending on the time of administration and the duration of anesthesia, the amount of the gadolinium deposition in the brain was affected. Thus, the deposition of gadolinium in the brain appears to be related to sleep status at the time of administration of the GBCA, or, alternatively, the function of the glymphatic system.68
Analyses using changes in T1 values
It has been suggested that the integral result of the action of the glymphatic system could be estimated by observing the change in T1 values in the white matter perivascular space. These estimates would be dependent on the protein concentration of the ISF and would not require the use of GBCA69 (Fig. 1). Although the aforementioned diffusion-weighted images could be used to study the instantaneous movement of water, it may not be sufficient to evaluate the glymphatic system with its diurnal variability, from a single time measurement.
Fig. 1.
A 72-year-old woman with a suspicion of endolymphatic hydrops. 3D-real inversion recovery (TR 15130/TE 549/TI 2700) images of the whole brain were obtained before (a, c) and 4 hrs after (b, d) IV-GBCA for the evaluation of endolymphatic hydrops. A slice at the basal ganglia level (a, b) and a slice at the body of the lateral ventricles level are shown (c, d). The PVS in the basal ganglia shows lower to similar signal intensity compared to the brain parenchyma (a, arrows) in the pre-contrast scan. The PVS in the basal ganglia show marked enhancement at 4 hrs after IV-GBCA (b, arrows). Note that the CSF in the subarachnoid space also shows contrast enhancement (b). The PVS in the white matter shows high signal intensity in the pre-contrast scan (c, short arrows) and no apparent enhancement is seen at 4 hrs after the IV-GBCA (d, short arrows). CSF, cerebrospinal fluid; IV-GBCA, intravenous administration of a single dose of GBCA; PVS, perivascular space.
Analyses using changes in T2 values
From multi-echo images, it is possible to decompose the decaying signal into separate T2 components. By adjusting the color table to create a color map, it is possible to visualize the extracellular water distribution, as well as their associated T2 values.70 Using this methodology, investigators found that the subarachnoid CSF has long T2 values, but there were short T2 components at the brain surface, the surface of dura, and around the blood vessels in the subarachnoid space, etc. They also found that in the brain parenchyma, short T2 components (longer than intracellular component but shorter than that in the CSF) exist in the white matter and choroid plexus. These may be associated with the distribution of macromolecules (waste materials) in the brain. This non-invasive method does not utilize GBCA, and might be useful to investigate pathophysiology of iNPH. The cause of enlarged CSF spaces is thought to be related to increased osmotic pressure in the CSF, and T2 is considered to be correlated with osmotic pressure.70
Chemical exchange saturation transfer (CEST)
CEST MRI was utilized to study changes in the CEST signal intensity of the brain after deep cervical lymph node ligation in animals and was correlated with behavior. In the pilot in vitro study, they showed that the CEST effect of the lymph fluid is significantly greater than those of the blood, CSF, or distilled water. The results of the in vivo study showed that the intensity of CEST effect was significantly higher in the ipsilateral than in the contralateral hippocampus. The correlation between the signal abnormality and the behavioral score was significant. These results supported the use of CEST MRI as a tool to access the brain’s glymphatic system and to predict glymphatic system dysfunction.71
17O-labeled water
The 17O isotope is the only stable isotope of oxygen that can produce an MRI signal. Direct detection of 17O requires specific hardware. Indirect method, which utilizes the T2 shortening effect by 17O, is easier to be implemented in clinical scanners. The intravenous administration of 20% 17O-labeled water (1 mL/Kg) allowed the observation of the tracer distribution in the brain parenchyma, choroid plexus, ventricles, and subarachnoid space. Although 17O-labeled water is quite precious and expensive now, it might be a promising tracer in the future.72
Intravenous administration of GBCA
In healthy animals and humans, GBCA migrates into the CSF after intravenous administration, but the concentration of GBCA in the CSF is too low to be detected routinely using T1-weighted imaging.22,73–75 Pulse sequences for endolymphatic hydrops assessment are used to detect very low concentrations of GBCA in fluid on MR images.76,77 These pulse sequences are used for imaging of endolymphatic hydrops at 4 hrs after the intravenous administration of a single dose of GBCA. The evaluation of endolymphatic hydrops is now possible clinically not only at 3T78 but also at 1.5T.79
Recently, to further increase the sensitivity of GBCA at low concentrations in fluid, a combination of longer repetition times and increased refocusing flip angles has been used.80–82 These methods are called improved hybrid of the reversed image of the positive endolymph signal and native image of positive perilymph signal (HYDROPS) imaging and improved 3D-real IR imaging. The most recent report shows that MR imaging for the evaluation of endolymphatic hydrops with a denoising technique using artificial intelligence reconstruction increased the contrast to noise ratio by more than a 4-fold.83 This technique can also be applied for the evaluation of the glymphatic system in the future.
With the intravenous method, transfer of GBCA to the CSF can be detected by sensitive MR fingerprinting and 3D-real IR imaging.84 MR fingerprinting and 3D-real IR imaging are not sensitive enough to see the T1 shortening of brain parenchyma associated with the transfer of very small amounts of GBCA administered intravenously. 3D-real IR imaging is more sensitive than MR fingerprinting to extremely low concentrations of GBCA in fluid.85
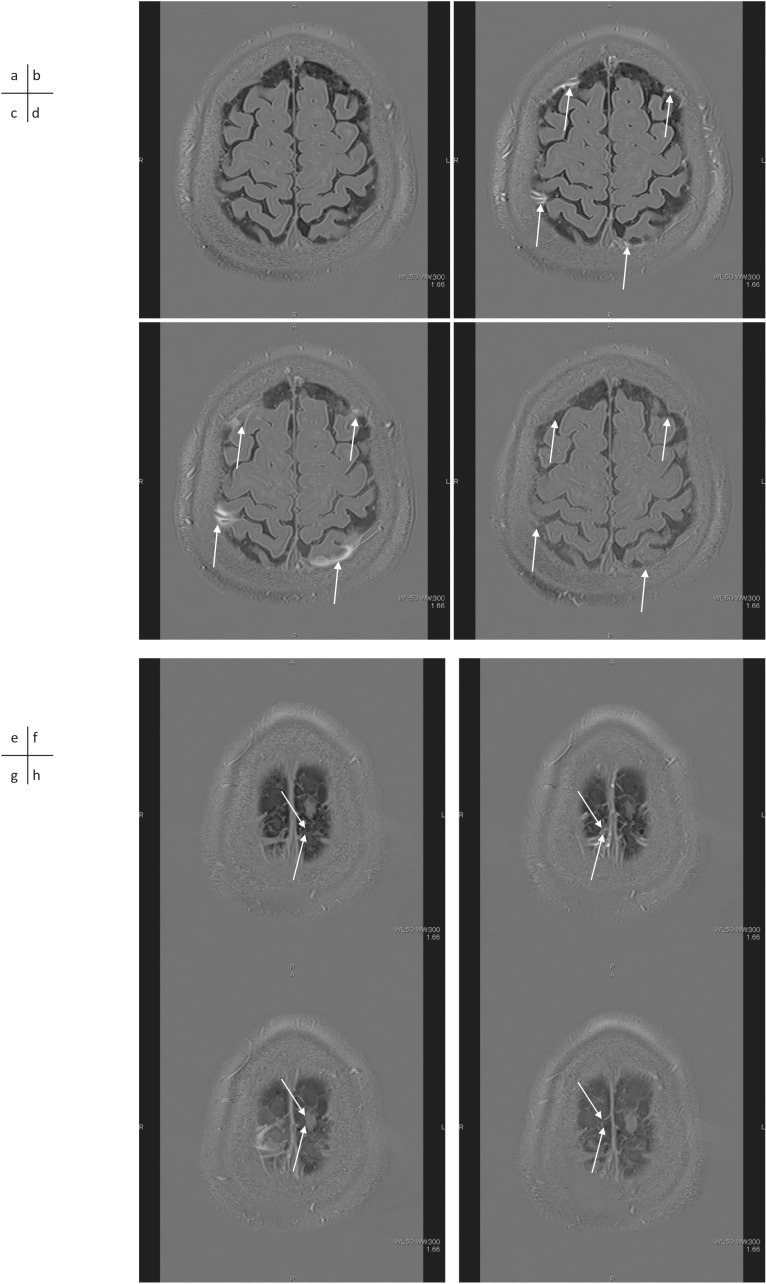
The longitudinal assessment of 3D-real IR images after the intravenous administration of GBCA can help analyze the morphological microstructure of the downstream region and understand the relationship with glymphatic system function. Furthermore, the degree of leakage of GBCA from the subpial space around the cortical veins to the surrounding subarachnoid space observed after intravenous administration of GBCA was shown to be significantly accelerated by aging86,87 (Fig. 2). In addition, continuity between the subpial space around the cortical veins and the meningeal lymphatic vessels on both sides of the superior sagittal sinus has been suggested on MR images.88 The subpial space around the cortical veins is enhanced 5–10 mins after intravenous administration of GBCA in humans. This subpial space around the cortical veins in humans seemed to be continuous from the perivenous space in the brain and corresponded to images obtained in animals using two-photon imaging.89 In this animal study, it was shown that the size of the perivascular space around the cortical veins was dynamically changed by a stimulation, which simulated a migraine aura.
Fig. 2.
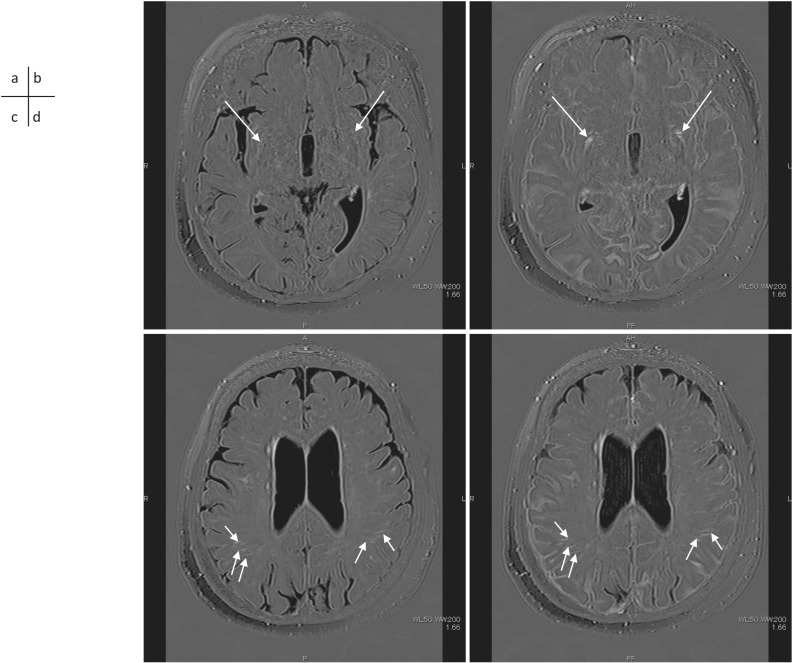
A 61-year-old man with a suspicion of endolymphatic hydrops. 3D-real inversion recovery (TR 15130/TE 549/TI 2700) images of the whole brain were obtained before (a), 5 mins (b), 4 hrs (c) and 24 hrs (d) after the IV-GBCA for the evaluation of endolymphatic hydrops. Perivenous enhancement appears at 5 mins after the IV-GBCA (b, arrows) and the enhancement spreads into the surrounding CSF space (c, arrows). The enhancement in the CSF space is markedly decreased at 24 hrs after the IV-GBCA (d, arrows). 3D-real IR images obtained before (e), 5 mins (f), 4 hrs (g) and 24 hrs (h) after the IV-GBCA at a more superior level are shown. A perivenous cyst (arrows) is visualized near the superior sagittal sinus. This cyst might be blocking the flow of the interstitial fluid in the peri-venous subpial space, or the cyst might have formed due to an obstruction of the subpial fluid flow by an unknown cause. CSF, cerebrospinal fluid; IV-GBCA, intravenous administration of a single dose of GBCA.
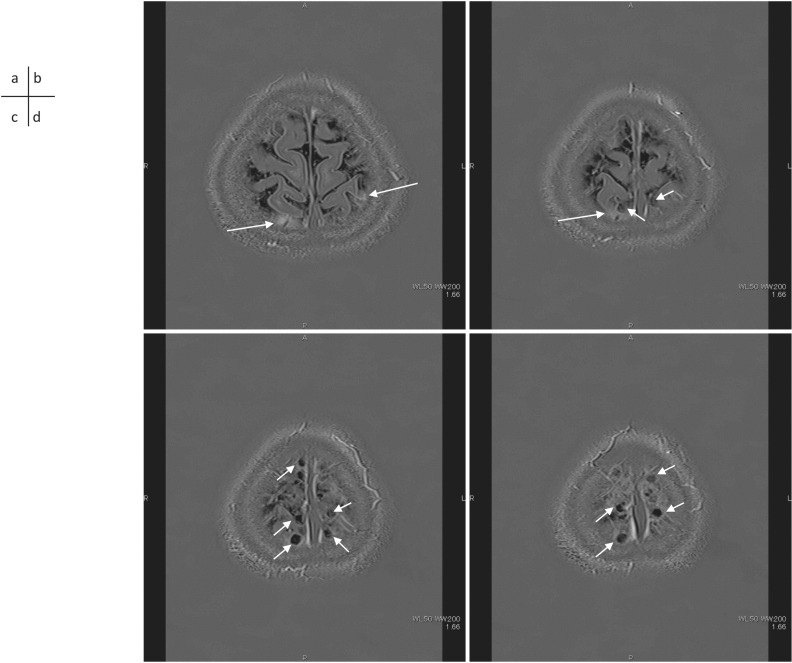
Cyst formation in contact with the subpial space around cortical veins near the superior sagittal sinus is often seen on MR images in adult humans. A correlation between the number and size of the cysts and the degree of leakage of GBCA from the subpial space around the cortical veins to the surrounding subarachnoid space has been reported90 (Figs. 2 and 3). Considering these findings, it was suggested that the flow of ISF in the subpial space around the cortical veins is obstructed in the cases with prominent leakage of GBCA into the subarachnoid space around the cortical veins. Cysts could be a cause or result of such obstruction. The findings of cysts and prominent leakage of GBCA into the peri-cortical venous areas may be a future imaging biomarker of glymphatic function. Further investigations are warranted.
Fig. 3.
A 41-year-old woman with a suspicion of endolymphatic hydrops. 3D-real inversion images and recovery images (TR 15130/TE 549/TI 2700) obtained 4 hrs after an IV-GBCA. The enhancement by GBCA leakage spread into the surrounding CSF space (a, b, arrows). Perivenous cysts (b, c, d, short arrows) are visualized near the superior sagittal sinus. These perivenous cysts are located along the superior sagittal sinus. CSF, cerebrospinal fluid; IV-GBCA, intravenous administration of a single dose of GBCA.
Meningeal Lymphatics and the Parasagittal Dura
Meningeal lymphatics
The discovery of the meningeal lymphatic vessels, which were previously thought to be absent from the CNS, has attracted a great deal of research interest.14,15 The recent characterizations of the glymphatic and meningeal lymphatic systems in rodents and humans have facilitated the revaluation of the anatomical routes for CSF–ISF flow and the physiological role that these pathways play in CNS health. Some aspects of the glymphatic and meningeal lymphatic systems have been observed in humans. MRI scans after intrathecally administered contrast agents show that CSF flows along pathways that closely resemble the glymphatic system as outlined in rodents.5 Unlike dorsal meningeal lymphatic vessels, basal meningeal lymphatic vessels have lymphatic valves and capillaries located adjacent to the subarachnoid space in mice. It was also shown that basal meningeal lymphatic vessels are hotspots for the clearance of CSF macromolecules and that both meningeal lymphatic vessels’ integrity and CSF drainage are impaired with aging.91
Visualization of meningeal lymphatic vessels in humans and non-human primates has been reported using MR imaging after intravenous GBCA administration. Contrast enhanced T2-FLAIR and black blood T1 weighted images visualized meningeal lymphatic vessels along the superior sagittal sinus.16 Through a combination of appropriately positioned saturation bands and time-of-flight angiography sequences, MRI can resolve the direction of the flow within the vessels without the use of exogenous contrast agents. In six healthy volunteers, the lymphatic flow went from posterior to anterior, counter to the direction of the venous flow in the superior sagittal sinus. This strongly suggests that a large proportion of the CNS lymphatic flow in humans is directed through the cribriform plate.92
The recent discoveries of the brain’s glymphatic (glial–lymphatic) system and the meningeal lymphatic vessels have sparked considerable debate. It is important to note that several of the processes that guide the highly polarized fluid transport system still need to be defined. In particular, the mechanisms by which AQP4 channels support the transport of extracellular flow and the interlinkage of ISF clearance with meningeal and cervical lymphatic vessels are poorly understood.30
Research on the function of meningeal lymphatic vessels
To evaluate the relationship between impairment of the glymphatic system, meningeal lymphatic vessels, and aging, MR imaging before and at multiple time points (4.5 hrs, 15 hrs, and 39 hrs) after intrathecal administration of a contrast agent (gadodiamide) was obtained in 35 patients.93 They measured the signal intensity of putative meningeal lymphatics, brain, CSF, and cervical lymph nodes and evaluated the correlation between putative meningeal lymphatics and others. They hypothesized that the glymphatic pathway can be evaluated from the signal transition of CSF and brain parenchyma. In all patients, the signal of the glymphatic pathway and meningeal lymphatic vessels changed significantly after intrathecal injection of the contrast agent. The clearance from both the glymphatic pathway and the putative meningeal lymphatic vessels was related to aging significantly. The clearance from meningeal lymphatic vessels was significantly related to the clearance from the glymphatic pathway, and the clearance from the glymphatic pathway was significantly faster in patients with early filling of the meningeal lymphatic vessels than that in patients with late filing.93 It has been reported that in aged mammals, impaired function of the meningeal lymphatic vessels can lead to accelerated accumulation of toxic amyloid beta protein in the brain parenchyma, thus aggravating Alzheimer’s disease-related pathology.94
Recently, meningeal lymphatic vessels have been presumed to play an important role in the development of neurodegenerative diseases such as Alzheimer’s disease, via dynamic fluid excretion in the brain and the accumulation and aggregation of amyloid-β.94 The meningeal lymphatics drain extravasated erythrocytes from the CSF into the cervical lymph nodes after subarachnoid hemorrhage. Modulation of this drainage may offer therapeutic approaches to alleviate subarachnoid hemorrhage severity.95 It is also shown that subdural hematoma was absorbed through the meningeal lymphatic vessel drainage pathway and that hematomas could inhibit the function of meningeal lymphatic vessels.96
In the search for the T-cell gateways into and out of the meninges, functional lymphatic vessels lining the dural sinuses were discovered. These structures express all of the molecular hallmarks of lymphatic endothelial cells, are able to carry both fluid and immune cells from the CSF, and are connected to the deep cervical lymph nodes.15 Dorsal meningeal lymphatic vessels undergo extensive remodeling in mice with intracranial gliomas or metastatic melanomas and are essential to generate an efficient immune response against brain tumors.97 Immune cells in meningeal tissues provide immune surveillance in the brain. Meningeal lymphatics and their direct connection with the cervical lymph nodes have been shown to impact CNS immune cell homeostasis.98
Concept of parasagittal dura
The absorption of CSF has been thought to occur primarily by means of arachnoid granulations in the superior sagittal sinus and the lacunae laterales in the parasagittal dura. The concept of “parasagittal dura” as the site of the absorption of CSF had been reported.99 Extensive networks of intradural channels from 0.02 to 2.0 mm in diameter were noted in all of the examined cadaver specimens. The channels were connected to the superior sagittal sinus at intervals along the side wall, or drained directly into the lacunae laterales, which extended up to 3 cm from the midline. It was suggested that these channels may represent a pathway for the flow of CSF from the arachnoid granulations to the superior sagittal sinus.
Recently, it has been reported that intrathecally injected GBCA escaped from the CSF into the parasagittal dura along the superior sagittal sinus at areas near the entry of the cortical cerebral veins in human patients. These findings demonstrate that trans-arachnoid molecular passage does occur, and suggest that the parasagittal dura may serve as a bridging link between human brain parenchyma and dural lymphatic vessels.100 The findings are consistent with the aforementioned report using intravenous GBCA administration, in which the connection from the peri-cortical venous subpial space to the meningeal lymphatic vessels appeared to be the drainage pathway for brain ISF.88 The parasagittal dura contains a network of intradural channels, coalescing into the lateral lacunae medially, and in close relation to a dense carpet of intradural arachnoid granulations. The parasagittal dura is most prominent at the level where the cortical veins enter the superior sagittal sinus.100 It has been reported in the pig that the gaps and fissures in the dura mater adjacent to the superior sagittal sinus may be intradural channels in the parasagittal dura and may function as the CSF drainage pathway.101 These gaps have a reticular conglomerate consisting of endothelial cells, which resemble lymphatic linings. Furthermore, immunohistochemistry and immunoelectron microscopy showed that they express molecules specific to lymphatic endothelial cells.101
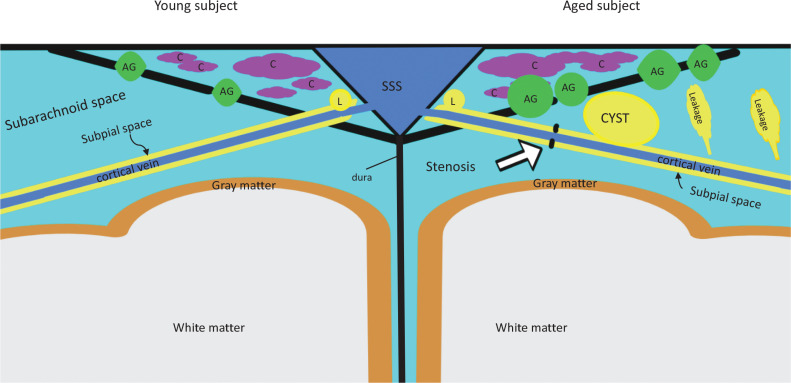
Taken together the recent findings in MR imaging, studies regarding the parasagittal dura, either by intrathecal or intravenous administration of GBCA,86,88,90,100 indicate that the parasagittal dura might serve a filtering function for ISF at the outlet of the glymphatic system in the CNS to systemic venous circulation and the lymphatic system. Aging and deterioration of this filtering function might be key to reveal the mystery of neurodegenerative diseases. Considering the results of recent research together, we speculate the structure of parasagittal dural area as the schematic diagram shown as Fig. 4. Meanwhile, it is important in the study of the glymphatic system to elucidate the detailed anatomy of the connections from the perivenous space around the veins in the brain, to the subpial space around the cortical veins, and finally, to meningeal lymphatic vessels.84–87 Also, it is crucial to reveal the relationship between cyst formation in the vicinity of these connections and the stagnation of ISF flow.90 These studies will help us to understand the glymphatic system and the kinetics of drugs in the CNS. It will contribute to the understanding and furthermore to the targeting of treatments for glymphatic system dysfunction.
Fig. 4.
Schematic diagram for the parasagittal dural area with our speculation from recent publications. The left side of the diagram shows the state in younger subjects and the right side shows the state in older subjects. Subpial space around the cortical vein continues from the perivenous space of the brain, draining interstitial fluid. The subpial space connects to meningeal lymphatics along SSS. In the aged subjects, stenosis of subpial space (open arrow) might cause the cyst formation near parasagittal dural area. Also, note that in the aged subjects, leakage from subpial space into subarachnoid space increases. The cyst formation correlates with increased leakage from subpial space. Arachnoi granulation increases in number and in size by aging. In the parasagittal dura (triangular area with many channels along SSS), the channels that contain cerebrospinal fluid and interstitial fluid, exist. Channels might be a part of lateral lacunae. The channels are connected to SSS and might be connected to arachnoid granulations. It is unknown if the channels are connected directly to meningeal lymphatics. AG, arachnoid granulation; C, channels; L, lymphatics; SSS, superior sagittal sinus.
Conclusions
No current MR imaging methodology has yet been able to definitively visualize the glymphatic system in humans. However, a method might be established in the future after various techniques are combined and examined. In addition, a less invasive MRI method of intravenous GBCA could be used to obtain larger scale cohort data than intrathecal GBCA, integrated with other biometric data. Then, we might be able to develop a system that does not require MRI in the future. Biometric features, which can be obtained during daily living at home by wearable devices, might be established to interrogate the function of the glymphatic system. Rather than the glymphatic system assessment by MR imaging, these biometric features would be immediately useful for individual patient diagnosis. We believe that the creation of such an infrastructure would be very useful in the future.
Footnotes
Conflicts of Interest
Shinji Naganawa declares that he has no conflicts of interest regarding this manuscript.
Toshiaki Taoka is the professor in the Department of Innovative Biomedical Visualization (iBMV), which is financially supported by CANON MEDICAL SYSTEMS CORPORATION.
References
- 1.Jessen NA, Munk AS, Lundgaard I, et al. The Glymphatic system: A beginner’s guide. Neurochem Res 2015; 40:2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013; 123:1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012; 4:147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 2014; 34:16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol 2018; 17:1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albargothy NJ, Johnston DA, MacGregor-Sharp M, et al. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol 2018; 136:139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasschaert M, Weller RO, Schroeder JA, et al. Retention of gadolinium in brain parenchyma: Pathways for speciation, access, and distribution. A critical review. J Magn Reson Imaging 2020; 52:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nedergaard M, Goldman SA. BRAIN DRAIN. Sci Am 2016; 314:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves BC, Karimy JK, Kundishora AJ, et al. Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol Med 2020; 26:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eide PK, Ringstad G. In Vivo imaging of molecular clearance from human entorhinal cortex: A possible method for preclinical testing of dementia. Gerontol Geriatr Med 2019; 5:2333721419889739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Lou N, Eberhardt A, et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci Transl Med 2020; 12:eaaw3210. 10.1126/scitranslmed.aaw3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wostyn P, Killer HE, De Deyn PP. Glymphatic stasis at the site of the lamina cribrosa as a potential mechanism underlying open-angle glaucoma. Clin Exp Ophthalmol 2017; 45:539–547. [DOI] [PubMed] [Google Scholar]
- 14.Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015; 212:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Absinta M, Ha S-K, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. elife 2017; 6:e29738. 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eide PK, Vatnehol SAS, Emblem KE, et al. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep 2018; 8:7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanda T, Oba H, Toyoda K, et al. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol 2016; 34:3–9. [DOI] [PubMed] [Google Scholar]
- 19.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276:228–232. [DOI] [PubMed] [Google Scholar]
- 20.Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270:834–841. [DOI] [PubMed] [Google Scholar]
- 21.Naganawa S, Nakane T, Kawai H, et al. Gd-based contrast enhancement of the perivascular spaces in the Basal Ganglia. Magn Reson Med Sci 2017; 16:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naganawa S, Suzuki K, Yamazaki M, et al. Serial scans in healthy volunteers following intravenous administration of gadoteridol: time course of contrast enhancement in various cranial fluid spaces. Magn Reson Med Sci 2014; 13:7–13. [DOI] [PubMed] [Google Scholar]
- 23.Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open 2015; 4:2058460115609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzano DA, Pellis Z, DeRiggi L. Fatal gadolinium-induced encephalopathy following accidental intrathecal administration: a case report and a comprehensive evidence-based review. Reg Anesth Pain Med 2019; 44:721-729. 10.1136/rapm-2019-100422 [DOI] [PubMed] [Google Scholar]
- 25.Kapoor R, Liu J, Devasenapathy A, et al. Gadolinium encephalopathy after intrathecal gadolinium injection. Pain Physician 2010; 13:E321–326. [PubMed] [Google Scholar]
- 26.Li L, Gao FQ, Zhang B, et al. Overdosage of intrathecal gadolinium and neurological response. Clin Radiol 2008; 63:1063–1068. [DOI] [PubMed] [Google Scholar]
- 27.Safriel Y, Ang R, Ali M. Gadolinium use in spine pain management procedures for patients with contrast allergies: results in 527 procedures. Cardiovasc Intervent Radiol 2008; 31:325–331. [DOI] [PubMed] [Google Scholar]
- 28.Öner AY, Barutcu B, Aykol Ş, et al. Intrathecal contrast-enhanced magnetic resonance imaging-related brain signal changes: Residual gadolinium deposition? Invest Radiol 2017; 52:195–197. [DOI] [PubMed] [Google Scholar]
- 29.Taoka T, Naganawa S. Gadolinium-based contrast media, cerebrospinal fluid and the glymphatic system: possible mechanisms for the deposition of gadolinium in the brain. Magn Reson Med Sci 2018; 17:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mestre H, Mori Y, Nedergaard M. The Brain’s glymphatic system: Current controversies. Trends Neurosci. 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benveniste H, Liu X, Koundal S, et al. The glymphatic system and waste clearance with brain aging: A review. Gerontology 2019; 65:106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith AJ, Verkman AS. The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB J 2018; 32:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AJ, Yao X, Dix JA, et al. Test of the 'glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. elife 2017; 6:e27679. 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taoka T, Naganawa S. Imaging for central nervous system (CNS) interstitial fluidopathy: disorders with impaired interstitial fluid dynamics. Jpn J Radiol 2020. July 11. 10.1007/s11604-020-01017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal N, Contarino C, Toro E. Neurofluids: A holistic approach to their physiology, interactive dynamics and clinical implications for neurological diseases. Veins Lymphatics 2019; 8:49. [Google Scholar]
- 36.Taoka T, Naganawa. S. Neurofluid dynamics and the glymphatic system: A neuroimaging perspective. Korean J Radiol 2020; 21:1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hladky SB, Barrand MA. Is solute movement within the extracellular spaces of brain gray matter brought about primarily by diffusion or flow? A commentary on “Analysis of convective and diffusive transport in the brain interstitium” Fluids and Barriers of the CNS (2019) 16:6 by L. Ray, J.J. Iliff and J.J. Heys. Fluids Barriers CNS 2019; 16:24. 10.1186/s12987-019-0141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Croci M, Vinje V, Rognes ME. Uncertainty quantification of parenchymal tracer distribution using random diffusion and convective velocity fields. Fluids Barriers CNS 2019; 16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benveniste H, Heerdt PM, Fontes M, et al. Glymphatic system function in relation to anesthesia and sleep states. Anesth Analg 2019; 128:747–758. [DOI] [PubMed] [Google Scholar]
- 40.Hablitz LM, Vinitsky HS, Sun Q, et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv 2019; 5:eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kedarasetti RT, Drew PJ, Costanzo F.. Arterial pulsations drive oscillatory flow of CSF but not directional pumping. Sci Rep 2020; 10:10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiviniemi V, Wang X, Korhonen V, et al. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 2016; 36:1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Besteher B, Chung H-Y, Mayer TE, et al. Acute encephalopathy and cardiac arrest induced by intrathecal gadolinium administration. Clin Neuroradiol 2020; 30:629–631. 10.1007/s00062-019-00845-6 [DOI] [PubMed] [Google Scholar]
- 44.Ringstad G, Valnes LM, Dale AM, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 2018; 3:e121537. 10.1172/jci.insight.121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017; 140:2691–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: A glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab 2019; 39:1355–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He FF, Li L, Liu MJ, et al. Targeted epidural blood patch treatment for refractory spontaneous intracranial hypotension in China. J Neurol Surg B Skull Base 2018; 79:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edeklev CS, Halvorsen M, Løvland G, et al. Intrathecal use of gadobutrol for glymphatic MR imaging: Prospective safety study of 100 patients. AJNR Am J Neuroradiol 2019; 40:1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dyke JP, Xu HS, Verma A, et al. MRI characterization of early CNS transport kinetics post intrathecal gadolinium injection: Trends of subarachnoid and parenchymal distribution in healthy volunteers. Clin Imaging 2020; 68:1–6. [DOI] [PubMed] [Google Scholar]
- 50.Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol 2017; 35:172–178. [DOI] [PubMed] [Google Scholar]
- 51.Yokota H, Vijayasarathi A, Cekic M, et al. Diagnostic performance of glymphatic system evaluation using diffusion tensor imaging in idiopathic normal pressure hydrocephalus and mimickers. Curr Gerontol Geriatr Res 2019; 2019:5675014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Debacker C, Djemai B, Ciobanu L, et al. Diffusion MRI reveals in vivo and non-invasively changes in astrocyte function induced by an aquaporin-4 inhibitor. PLoS One 2020; 15:e0229702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obata T, Kershaw J, Tachibana Y, et al. Comparison of diffusion-weighted MRI and anti-Stokes Raman scattering (CARS) measurements of the inter-compartmental exchange-time of water in expression-controlled aquaporin-4 cells. Sci Rep 2018; 8:17954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison IF, Siow B, Akilo AB, et al. Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. elife 2018; 7:e34028. 10.7554/eLife.34028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magdoom KN, Zeinomar A, Lonser RR, et al. Phase contrast MRI of creeping flows using stimulated echo. J Magn Reson 2019; 299:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans PG, Sokolska M, Alves A, et al. Non-invasive MRI of blood-cerebrospinal fluid barrier function. Nat Commun 2020; 11:2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yatsushiro S, Sunohara S, Hayashi N, et al. Cardiac-driven pulsatile motion of intracranial cerebrospinal fluid visualized based on a correlation mapping technique. Magn Reson Med Sci 2018; 17:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horie T, Kajihara N, Saito H, et al. Visualization of cerebrospinal fluid motion in the whole brain using three-dimensional dynamic improved motion-sensitized driven-equilibrium steady-state free precession. Magn Reson Med Sci 2021; 20:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taoka T, Naganawa S, Kawai H, et al. Can low b value diffusion weighted imaging evaluate the character of cerebrospinal fluid dynamics? Jpn J Radiol 2019; 37:135–144. [DOI] [PubMed] [Google Scholar]
- 60.Matsumae M, Kuroda K, Yatsushiro S, et al. Changing the currently held concept of cerebrospinal fluid dynamics based on shared findings of cerebrospinal fluid motion in the cranial cavity using various types of magnetic resonance imaging techniques. Neurol Med Chir (Tokyo) 2019; 59:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada S, Miyazaki M, Kanazawa H, et al. Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology 2008; 249:644–652. [DOI] [PubMed] [Google Scholar]
- 62.Yamada S, Miyazaki M, Yamashita Y, et al. Influence of respiration on cerebrospinal fluid movement using magnetic resonance spin labeling. Fluids Barriers CNS 2013; 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shibukawa S, Miyati T, Niwa T, et al. Time-spatial Labeling Inversion Pulse (Time-SLIP) with pencil beam pulse: A selective labeling technique for observing cerebrospinal fluid flow dynamics. Magn Reson Med Sci 2018; 17:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mestre H, Hablitz LM, Xavier AL, et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. elife 2018; 7:e40070. 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DiNuzzo M, Nedergaard M. Brain energetics during the sleep-wake cycle. Curr Opin Neurobiol 2017; 47:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019; 366:628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Demiral ŞB, Tomasi D, Sarlls J, et al. Apparent diffusion coefficient changes in human brain during sleep - Does it inform on the existence of a glymphatic system? Neuroimage 2019; 185:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taoka T, Jost G, Frenzel T, et al. Impact of the glymphatic system on the kinetic and distribution of gadodiamide in the rat brain: Observations by dynamic MRI and effect of circadian rhythm on tissue gadolinium concentrations. Invest Radiol 2018; 53:529–534. [DOI] [PubMed] [Google Scholar]
- 69.Naganawa S, Nakane T, Kawai H, et al. Differences in signal intensity and enhancement on MR images of the perivascular spaces in the Basal Ganglia versus those in white matter. Magn Reson Med Sci 2018; 17:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oshio K, Yui M, Shimizu S, et al. The spatial distribution of water components with similar T2 may provide insight into pathways for large molecule transportation in the brain. Magn Reson Med Sci 2021; 20:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y, Dai Z, Fan R, et al. Glymphatic system visualized by chemical-exchange-saturation-transfer magnetic resonance imaging. ACS Chem Neurosci 2020; 11:1978–1984. [DOI] [PubMed] [Google Scholar]
- 72.Kudo K, Harada T, Kameda H, et al. Indirect proton MR imaging and kinetic analysis of 17O-labeled water tracer in the brain. Magn Reson Med Sci 2018; 17:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jost G, Frenzel T, Lohrke J, et al. Penetration and distribution of gadolinium-based contrast agents into the cerebrospinal fluid in healthy rats: a potential pathway of entry into the brain tissue. Eur Radiol 2017; 27:2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berger F, Kubik-Huch RA, Niemann T, et al. Gadolinium distribution in cerebrospinal fluid after administration of a gadolinium-based MR contrast agent in humans. Radiology 2018; 288:703–709. [DOI] [PubMed] [Google Scholar]
- 75.Nehra AK, McDonald RJ, Bluhm AM, et al. Accumulation of gadolinium in human cerebrospinal fluid after gadobutrol-enhanced MR imaging: A prospective observational cohort study. Radiology 2018; 288:416–423. [DOI] [PubMed] [Google Scholar]
- 76.Naganawa S, Kawai H, Sone M, et al. Increased sensitivity to low concentration gadolinium contrast by optimized heavily T2-weighted 3D-FLAIR to visualize endolymphatic space. Magn Reson Med Sci 2010; 9:73–80. [DOI] [PubMed] [Google Scholar]
- 77.Naganawa S, Yamazaki M, Kawai H, et al. Visualization of endolymphatic hydrops in Ménière’s disease with single-dose intravenous gadolinium-based contrast media using heavily T(2)-weighted 3D-FLAIR. Magn Reson Med Sci 2010; 9:237–242. [DOI] [PubMed] [Google Scholar]
- 78.Ohashi T, Naganawa S, Takeuchi A, et al. Quantification of endolymphatic space volume after intravenous administration of a single dose of gadolinium-based contrast agent: 3D-real inversion recovery versus HYDROPS-Mi2. Magn Reson Med Sci 2020; 19:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naganawa S, Yamazaki M, Kawai H, et al. Visualization of endolymphatic hydrops in Ménière’s disease after intravenous administration of single-dose gadodiamide at 1.5T. Magn Reson Med Sci 2013; 12:137–139. [DOI] [PubMed] [Google Scholar]
- 80.Naganawa S, Kawai H, Taoka T, et al. Improved 3D-real inversion recovery: A robust imaging technique for endolymphatic hydrops after intravenous administration of gadolinium. Magn Reson Med Sci 2019; 18:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Naganawa S, Kawai H, Taoka T, et al. Improved HYDROPS: Imaging of endolymphatic hydrops after intravenous administration of gadolinium. Magn Reson Med Sci 2017; 16:357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kato Y, Bokura K, Taoka T, et al. Increased signal intensity of low-concentration gadolinium contrast agent by longer repetition time in heavily T2-weighted-3D-FLAIR. Jpn J Radiol 2019; 37:431–435. [DOI] [PubMed] [Google Scholar]
- 83.Naganawa S, Nakamich R, Ichikawa K, et al. MR imaging of endolymphatic hydrops: Utility of iHYDROPS-Mi2 combined with deep learning reconstruction denoising. Mang Reson Med Sci 2021; 20:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naganawa S, Nakane T, Kawai H, et al. Detection of IV-gadolinium leakage from the cortical veins into the CSF using MR fingerprinting. Magn Reson Med Sci 2020; 19:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Naganawa S, Ito R, Kato Y, et al. Intracranial distribution of intravenously administered gadolinium-based contrast agent over a period of 24 hours: Evaluation with 3D-real IR imaging and MR fingerprinting. Magn Reson Med Sci 2021; 20:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naganawa S, Ito R, Kawai H, et al. Confirmation of age-dependence in the leakage of contrast medium around the cortical veins into cerebrospinal fluid after intravenous administration of gadolinium-based contrast agent. Magn Reson Med Sci 2020; 19:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naganawa S, Nakane T, Kawai H, et al. Age dependence of gadolinium leakage from the cortical veins into the cerebrospinal fluid assessed with whole brain 3D-real inversion recovery MR imaging. Magn Reson Med Sci 2019; 18:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naganawa S, Ito R, Taoka T, et al. The space between the pial sheath and the cortical venous wall may connect to the meningeal lymphatics. Magn Reson Med Sci 2020; 19:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schain AJ, Melo-Carrillo A, Strassman AM, et al. Cortical spreading depression closes paravascular space and impairs glymphatic flow: Implications for migraine headache. J Neurosci 2017; 37:2904–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naganawa S, Ito R, Nakamichi R, et al. Relationship between parasagittal perivenous cysts and leakage of gadolinium-based contrast agents into the subarachnoid space around the cortical veins after intravenous administration. Magn Reson Med Sci 2021; 20:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahn JH, Cho H, Kim J-H, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019; 572:62–66. [DOI] [PubMed] [Google Scholar]
- 92.Kuo PH, Stuehm C, Squire S, et al. Meningeal lymphatic vessel flow runs countercurrent to venous flow in the superior sagittal sinus of the human brain. Tomography 2018; 4:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Y, Cai J, Zhang W, et al. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann Neurol 2020; 87:357–369. [DOI] [PubMed] [Google Scholar]
- 94.Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system: A new player in neurophysiology. Neuron 2018; 100:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen J, Wang L, Xu H, et al. Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat Commun 2020; 11:3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X, Gao C, Yuan J, et al. Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol Commun 2020; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu X, Deng Q, Ma L, et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res 2020; 30:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hershenhouse KS, Shauly O, Gould DJ, et al. Meningeal lymphatics: A review and future directions from a clinical perspective. Neurosci Insights 2019; 14:1179069519889027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fox RJ, Walji AH, Mielke B, et al. Anatomic details of intradural channels in the parasagittal dura: a possible pathway for flow of cerebrospinal fluid. Neurosurgery 1996; 39:84–91. [DOI] [PubMed] [Google Scholar]
- 100.Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun 2020; 11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kutomi O, Takeda S. Identification of lymphatic endothelium in cranial arachnoid granulation-like dural gap. Microscopy 2020; 69:391–400. [DOI] [PubMed] [Google Scholar]