Abstract
One of the major issues in the surgical treatment of gliomas is the concern about maximizing the extent of resection while minimizing neurological impairment. Thus, surgical planning by carefully observing the relationship between the glioma infiltration area and eloquent area of the connecting fibers is crucial. Neurosurgeons usually detect an eloquent area by functional MRI and identify a connecting fiber by diffusion tensor imaging. However, during surgery, the accuracy of neuronavigation can be decreased due to brain shift, but the positional information may be updated by intraoperative MRI and the next steps can be planned accordingly. In addition, various intraoperative modalities may be used to guide surgery, including neurophysiological monitoring that provides real-time information (e.g., awake surgery, motor-evoked potentials, and sensory evoked potential); photodynamic diagnosis, which can identify high-grade glioma cells; and other imaging techniques that provide anatomical information during the surgery. In this review, we present the historical and current context of the intraoperative MRI and some related approaches for an audience active in the technical, clinical, and research areas of radiology, as well as mention important aspects regarding safety and types of devices.
Keywords: fluorescence guidance surgery, intraoperative magnetic resonance imaging, neurophysiological monitoring, surgical safety, ultrasound guidance surgery
Introduction
Glioma has an infiltrative nature, and neurosurgeons need to correctly identify the tumor margin to ensure maximum resection without affecting the surrounding areas of the eloquent cortex, such as Broca’s area, Wernicke’s area, the primary motor cortex, arcuate fasciculus, or the internal capsule. In general, the outcome of glioma surgery is strongly related to how much tissue is removed,1,2 and careful surgical planning is required. The UK National Institute for Health and Care Excellence guideline (NG99) for brain tumors (primary) and metastasis in adults (published July 11, 2018, last Update January 20213) includes the following information. With respect to the surgical expertise in the multidisciplinary team, one must include access to awake craniotomy with language and appropriate functional monitoring, intraoperative neurophysiological monitoring, and intraoperative imaging guidance. For technical considerations, if a suspected high-grade glioma with an enhanced lesion is possible, fluorescence-guided resection is offered as an adjunct to maximize resection. One can consider intraoperative MR (ioMR) imaging and intraoperative ultrasound (ioUS) imaging to facilitate achieving surgical resection of both low-grade and high-grade gliomas while preserving neurological function unless MRI is contraindicated. In addition, diffusion tensor imaging overlays with neuronavigation, which can contribute to minimizing the damage to functionally important fiber tracts during resection.
Neurosurgeons usually detect an eloquent area by functional MR imaging and identify connecting fibers by diffusion tensor imaging. Tumor grade is determined preoperatively by methionine positron emission tomography or MR spectroscopy. Neuronavigation information obtained from preoperative images processed through a computed reconstruction system4 guides the surgeon to the appropriate corridors into the surgical field.
During the neurosurgical procedure, the patient’s skull is fixed to the operating table to maintain the positional relationship between the neuronavigation information and the skull. However, once the dura mater is opened, cerebrospinal fluid leaks from the surgical field and, coupled with the removal of the glioma, can cause the brain to deform in all directions, leading to brain shift and misregistration of neuronavigation.5 The information can be updated by ioUS imaging, computed tomographic imaging, and ioMR throughout the surgical strategy of the next surgical steps.6–12 The neurophysiological status can also be monitored via awake craniotomy, motor-evoked potentials (MEPs), and somatosensory evoked potential (SEPs).13,14 Fluorescence-guidance surgery can identify the high-grade tumor cells in the excision margin under the surgical microscope.15–21 Scanners for ioMR have been adopted globally for aiding the surgical treatment of brain tumors, including glioma.
This review aims to deepen the understanding of ioMR imaging for those who are active in technical, clinical, and research areas of radiology. We present background information on the origin of ioMR imaging, describe the different types of theater layout that may be used to accommodate ioMR scanners and medical safety in magnetic fields, and, finally, we discuss how to use ioMR images for formulating surgical strategies.
History of Image-guided Surgery Using ioMR Imaging
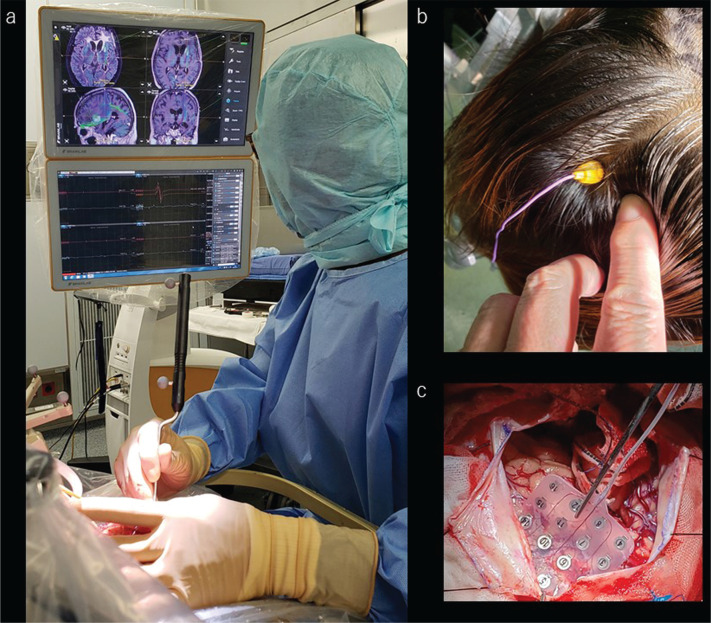
When talking about the history of ioMR imaging, Ferenc Andras Jolesz (May 21, 1946–December 31, 2014) must be mentioned. After graduating in 1971 from the Hungarian School of Medicine, Dr Jolesz completed a biomedical engineering and computer science research fellowship, as well as a Neurosurgery residency in his native Hungary before departing to Boston, MA, USA. There, he worked as a research fellow in the Department of Neurology at the Massachusetts General Hospital, as a resident in Diagnostic Radiology, and as a research fellow in Neuroradiology at Brigham and Women’s Hospital. He became the Director of the Division of Magnetic Resonance Imaging in 1988. Dr Jolesz was appointed as the first incumbent B Leonard Holman Chair in Radiology at the Harvard Medical School in 1998. At the same time, Peter McLaren Black was a Professor of Neurosurgery at the Brigham and Women’s Hospital, and, in 1993, Drs Jolesz and Black jointly introduced the first ioMR scanner within which they could work to remove a brain tumor (Fig. 1).6,22 This scanner, commercialized as GE Signa SP by GE Medical Systems (Milwaukee, WI, USA),6,23 provides rapid image processing and allows frameless stereotactic brain biopsy and real-time image-based intraoperative guidance. It consists of two coils arranged vertically (it is nicknamed the double donut because of its unique morphology), and the coils create a magnetic field of 0.5 Tesla (T). There is a space of 56 cm between the two coils, within which two surgeons may operate face to face, using MR conditional surgical instruments and a microscope to check the images projected on the monitor inside the coil.
Fig. 1.
Team that developed the first commercialized intraoperative MR scanner Dr. Jolesz (front center), Dr. Black (front right), and their colleagues at the world’s first intraoperative MR scanner room at Brigham and Women’s Hospital in Boston. Kindly provided by Dr. Black.
Other approaches to ioMR have been considered, and some have been developed commercially. For instance, in 1998, Steinmeier et al.24 described their experience with a 0.2 T MR scanner with the magnets placed horizontally, one above the other in a hamburger shape and a wide side opening to enable access for patient and physicians (Magnetom Open; Siemens, Erlangen, Germany). Hitachi produced a similar hamburger-shaped MR scanner.25 Sutherland et al.26 suspended a 1.5 T mobile high-field MR scanner with a 70-cm bore from the ceiling and moved it to the operating table using a ceiling-mounted rail system. Usually, the patients from operating room are taken to the heavy-weighted MR scanner; hence, the idea of moving the MR scanner itself is unique and distinctive. Martin et al.27 proposed a system in which the operating table and MR scanner were placed in a straight line. Patients are moved on to an MR conditional surgical tabletop plate that is slid into the MR scanner bore. The PoleStar ioMR imaging system (Odin Technologies, Yokneam, Israel, and Medtronic Surgical Navigation Technologies, Louisville, KY, USA) was developed by Hadani et al.28 This system is a low-field (0.15 T) compact system on a gantry that can be stored under the operating table and moved into place when needed during the surgery.
Why Do Neurosurgeons Need ioMR Images?
When intracranial pressure is elevated, brain bulging occurs immediately after craniotomy (Fig. 2). In addition, the brain shifts in various directions due to the progress of glioma resection, aspiration of the cerebrospinal fluid, expansion of the compressed brain, and increasing brain edema. This brain shift increases as the surgery progresses, and, consequently, the accuracy of neuronavigation based on preoperative MR images decreases.8,29–31 Therefore, neurosurgeons need to update and reregister images for neuronavigation throughout surgery, which is achieved with ioMR imaging.9,10 Moreover, ioMR images may be used to evaluate the percentage of the lesion that has been removed and might reveal unexpected remnants to surgeons (Fig. 3).6,32 Yet, identification of tumor remnants in the MR images is an important prognostic factor and guides decisions regarding the selection of adjuvant therapy.33 In addition, imaging can clarify the relationship between the remnant lesion and eloquent regions, connecting fibers, ventricular wall, major vessels, and so on,34,35 and can reveal unexpected vascular complications.36 However, it should be noted that there is a report that the hyperacute ischemic change could not be detected even by 3 T ioMR imaging.37 Therefore, one cannot exclusively rely on ioMR to determine possible complications.
Fig. 2.
Illustrative case of a right-temporal deep-seated glioma showing countermeasures against brain shift. a: Preoperative enhanced T1-weighted images show a ring-enhanced mass lesion with peritumoral edema. In this case, brain bulging after the craniotomy and brain shift are expected to occur during the surgery. b: The surgeon has uncapped the brain outside the lesion and immediately takes reference ioMR images for neuronavigation before brain shift occurs. c: The ioMR images after glioma removal show a nearly total removal. ioMR, intraoperative magnetic resonance.
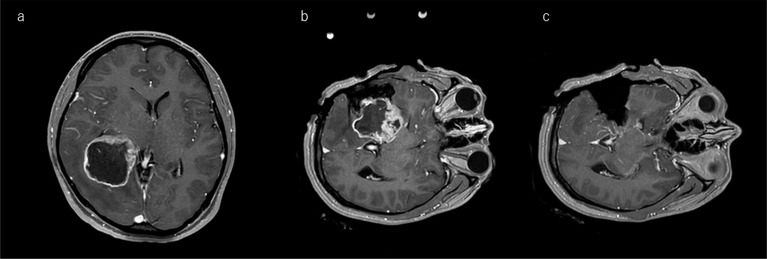
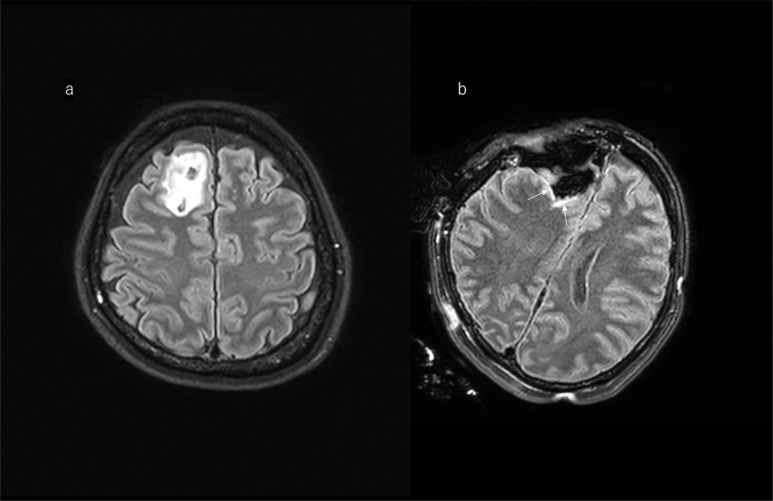
Fig. 3.
Illustrative case of tumor remnant in FLAIR image. a: A 47-year-old woman with high signal intensity in the right-temporal lobe on preoperative FLAIR MRI. b: The first intraoperative FLAIR image shows deep-seated tumor remnant, and the distance between remnant lesion and internal capsule was well identified. After identifying this relationship, the surgeon has chased the lesion more deeply. c: The arrow shows unexpected focal high signal intensity on the margin, which is suspected to be residual tumor requiring further resection when the patient is brought back to the operating room. This small amount of lesion is diagnosed as glioma by frozen-section pathological diagnosis. d: The postoperative images reached nearly total removal of the tumor. FLAIR, fluid-attenuated inversion recovery.
ioMR Imaging Theater Layout and Cost Performance
Initially, ioMR imaging systems used low-magnetic-field MR scanners.23–25,38–41 As the need for high-resolution and high-quality MR images increases, higher-field MR scanners have become mainstream.26,27,29,34,42–63 The use of ioMR imaging, however, has high initial costs and prolongs the operation time.64–69
Multi-theater layouts are generally used for high-magnetic-field MR scanners: in a single-theater type, the scanner is installed within the operating room to minimize the transfer distance to and fro the operating table;28,42,44,70,71 in a multi-theater type, the MR scanner is housed in separate room or an area that may be closed off when the scanner is not in use (Fig. 4).46–50,55,62,72 The latter multi-theater type may extend the transfer distance or require that the scanner is moved into the operating room.26
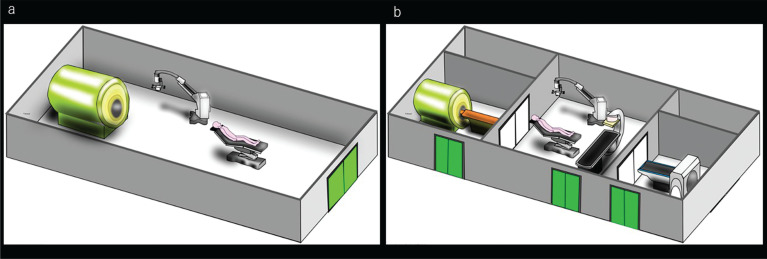
Fig. 4.
Two types of theater layouts of MR with operating system. a: A one theater type in which an ioMR scanner installed in the operating room, the surgical patient’s transfer distance is short, and ioMR imaging is completed in the operating room. However, an MR scanner installed in an operating room is mostly used to assist with surgery. The depreciation of the MR scanner depends on the turnover rate of surgery. b: A multi-theater type in which the operating room and imaging room are separated, and thus the surgical patient’s transfer distance is long. However, imaging equipment can be used for routine diagnostic imaging when performing surgery in the operating room. ioMR, intraoperative magnetic resonance.
The multi-theater type was pioneered by a German group. They used a 0.2 T MR scanner and the patient was moved to and fro the operating table by trolley.24,73 The approach was introduced so that the scanner could be used as a diagnostic device when not needed during surgery to ameliorate the high cost.49 In addition, since the separate MR scanner room is completely shielded, it prevents interference with magnetic fields, noise, radio waves, etc., in the operating theater. Therefore, as the throughput of imaging equipment increases and the multi-theater type has high-cost performance, the layout has been adopted in many hospitals,48,49,55,62,63,72,74,75 as sharing imaging equipment has a positive economic impact for hospitals. An increasing number of facilities are introducing imaging devices of different modalities, between which the patient is moved on a tabletop. The idea of using different modalities in this way began with the opening of a facility designated for endovascular treatment at the University of California in San Francisco, CA, USA, in 2001.76 When not in combined use, each device can be separated and used individually for routine diagnostic imaging to maximize cost performance by high throughput. The same idea was introduced to neurosurgical facilities in 2006 when Tokai University Hospital, Kanagawa, Japan, began to use a suite where MR and CT imaging and angiography could be performed. Shielded doors meant that the devices could be separated to use independently or in different combinations.49 In 2011, the Brigham and Women’s Hospital in Boston, MA, USA, launched its Advanced Multimodality Image-Guided Operating Suite (AMIGO) suite, which combines one operating area and three imaging areas for a 3 T MR, angiography, and positron emission tomography-CT.63,77–79 The suite has also facilitated innovative treatments using new technologies, such as laser thermoablation therapy, focused ultrasound therapy, MRI-assisted endoscopic surgery, and robotic surgery.78,80–85 Today, multimodality image-guided therapy has expanded not only to the neurosurgical field but also toward brachytherapy for gynecologic malignancies, skull base surgery for otorhinolaryngology, and image-guided-breast-conserving therapy.86–88
Safety in a Magnetic Field
Specifications that comply with the International Electrotechnical Commission 60601-2-33 standard are required for operating rooms in which ioMR imaging is performed and for MR gantry use.89 MR magnets produce a strong magnetic field. The attractive force on magnetic material largely depends on its mass and the strength and spatial gradient of the static magnetic field, and, therefore, great care must be taken when surgical instruments are present. As one of the measures for the safety zoning regarding the static magnetic field, some facilities employ floor markings that indicate strong magnetic fields to 5 Gauss (0.0005 T) (Fig. 5a). Furthermore, when devices, such as anesthesia machines, monitoring equipment, and infusion pumps, are in the vicinity of the MR scanner, it is necessary to pay close attention not only to displacement force to the equipment induced by the magnetic field gradient of the equipment by the magnetic field but also to possible malfunction of the medical equipment due to radiofrequency interference (Fig. 5b).90–92
Fig. 5.
Tips to ensure safety when using an ioMR system. a: The line (changes the color) on the floor of the MR scanner room indicates the 5 Gauss (0.0005 T) level and provides easy visual identification of the strong magnetic fields. b: Infusion pumps must be stored in the shielded box before bringing them into the MR scanner’s room. c: The patient’s body and all materials are cleared for MR bore size, and the neurosurgeon is checking the clearance using a specially ordered scale. ioMR, intraoperative magnetic resonance.
The American Society for Testing and Materials classifies articles related to MR in three categories.93 MR safe refers to products that are not conductors, metals, or magnetic (e.g., plastics), which are scientifically and physically safe in principle (i.e., not based on testing). MR conditional replaces the previous term MR compatible, which is deemed ambiguous, and refers to products that are judged to be nonhazardous based on testing under specific conditions, such as displacement, torque, spatial field gradient, time-varying magnetic field, heat generation due to the RF, and absorption rate. Further studies are required to assess risks of burns, current/voltage generation, noise, types of magnets, device arrangement (e.g. nerve stimulator leads), and interference between multiple devices (e.g. cardiac pacemaker and electrode).94–100 MR unsafe corresponds to materials that are dangerous in MR environments, such as surgical scissors and forceps.101–104
Preventing eddy current-induced complications in the patient
Consideration must be given to eddy currents, which are generated in nearby conductors, including the largest eddy current generated in an MR scanner, which is a shield panel placed inside the gradient coil, which is part of the MR scanner, and when there is ferrous, eddy current is not the only cause of heat generation but also there is so-called antenna effect and current inflow. The loop formed by the MR conductor is especially dangerous.105 It is also important to check the monitoring cables as care must be taken in order for it to not come into contact with each other or make loops, thus avoiding creation of eddy current. As the human body is a conductor, the patient’s body temperature increases due to the radiofrequency electromagnetic field, and in some cases, the induced eddy current can lead to burns.106–109 When placing the patient in position on the surgical table, skin-to-skin contact should be avoided, for instance by sandwiching a cushion between the knees, heels, and the arms and trunk. Finally, direct contact must be avoided between the skin and the MR gantry and monitoring cables (Fig. 5c). These may be new safety issues for operating room staff.
Training
With respect to safety management in operating rooms, the World Health Organization (WHO) has published a checklist that is widely used110 and has contributed to improving surgical safety.111,112 It is verbally administered and summarizes the minimum checks necessary to ensure the safety of patients when entering and leaving the operating room.113 Unfortunately, avoidable mistakes, such as craniotomy performed on the opposite side from the lesion, can still occur.114 The arguably first verbal checklist has been used for airline pilots since the 1930s, stipulating the simple operation checks to be performed aloud before or after performing one routine task, such as takeoff or landing.115
Multidisciplinary training
Medical personnel with various backgrounds are involved in ioMR-imaging-assisted surgery. To ensure the safety of patients and staff during the procedure, MR staff must be given physical and electrical safety education.103,104,116 All operating room staff, including surgeons, must also receive pathophysiological and physiological safety education.117 Finally, a key concept for ioMR imaging is a good communication between all staff. For instance, before making the first skin incisions, the WHO checklist suggests that everyone participating in the surgery verbally introduce themselves with their names and their roles.110 This has been shown to enhance communication during surgery.118 The final item on the WHO surgical safety checklist refers to obtaining confirmation of nonroutine steps, such as key concerns for the recovery and management of the patient from surgeons, nurses, and anesthetists. Some facilities are also trying to improve safety team building further by introducing briefing for procedure plans, manuals, and modified verbal checklists specific to ioMR-imaging-assisted surgery (Fig. 6a).74,75,92,119,120
Fig. 6.
Tips for preventing human error in the intraoperative MR system. a: Team briefing before starting the operation, where participants share the surgical process and precautions. b: Transferring the patient into or out of the MR scanner’s room is a dangerous time requiring full attention. c: An on-duty safety nurse is controlling every safety issue during the procedure.
In surgeries involving ioMR imaging, the usual workflow is interrupted, while the patient is moved (Fig. 6b).121 Therefore, to enable unfamiliar workflows to be performed smoothly, it is necessary for all involved staff, including neurosurgeons, circulating nurses, scrub nurses, neuromonitoring technicians, neuroradiologists, anesthesiologists, radiology technicians, and residents, to participate in discussions and simulations to standardize the processes and create a procedure manual.122,123
The role of a safety manager
A safety manager should be appointed to oversee safety during transfer of the patient between the MR scanner and operating table because, once surgery begins, neurosurgeons are devoted to surgical planning and procedure, anesthesiologists to anesthesia management, and radiologists to imaging quality.74,75,111,119,124 Some facilities appoint nurses who belong to sections other than the operating room and imaging staff to work as on-duty safety managers in order to objectively perform a series of operations from the standpoint of a third party (Fig. 6c).119,121
Key Issues for Interpretation of ioMR Images
FLAIR images
Low-grade gliomas generally appear as gadolinium-unenhanced lesions that are visible as high intensity lesions on T2-weighted images. Fluid attenuated inversion recovery (FLAIR) MR images can more clearly identify the tumor.125 Preoperative high intensity at the tumor edges on FLAIR MR imaging is generally due to peritumoral edema, although glioma cells within this region were detected, too.126,127
The liner FLAIR high-signal alterations on the margin of cavity (Fig. 7) visible on postsurgical MRI are rather due to surgical artifacts and should not be interpreted as a tumor remnant.128 Either way, for a better differential diagnosis (artifact vs tumor remnant), a comparison of FLAIR images before and after excision is recommended.
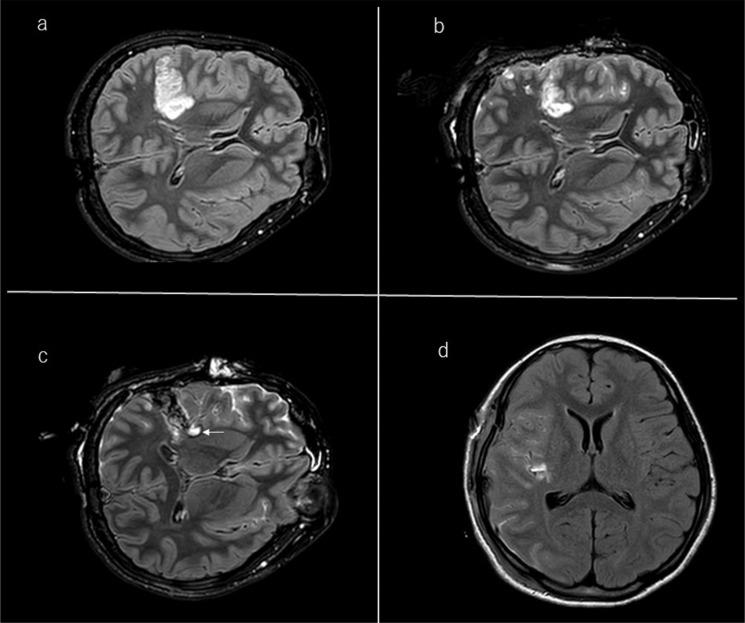
Fig. 7.
Illustrative case of a right frontal glioma. a: A 47-year-old woman with high signal intensity the right frontal lobe on pre-operative FLAIR image. b: Usually ioMR FLAIR images show a linear (like a border of the margin) high signal around the cavity; this should not be misdiagnosed as tumor remnant. FLAIR, fluid-attenuated inversion recovery; ioMR, intraoperative magnetic resonance.
Enhanced T1-weighted image
Several studies have reported thin linear new enhancements around the surgical margin seen during or immediately after surgery on T1-weighted images.129–131 These anomalous enhancements are caused by disruption of the blood‒brain barrier or bleeding caused by surgical intervention or contrast leaking into the tumor cavity, and should not be confused with residual tumor.132 The preoperative enhanced lesion and the intraoperatively and immediately postoperative occurring enhanced region must be carefully compared. These transient surgically induced enhanced lesions diminish soon after surgery.53,131,133
Preventing susceptibility artifacts on ioMR images
Good image quality is obtained from high-field MR machines when used intraoperatively, but susceptibility to artifacts can negatively influence the quality of the images (Fig. 8a). Diffusion-weighted imaging can be used to detect neural fibers and ischemia, but it is very sensitive to artifacts, especially air bubbles at the surgical site.134 Filling of the tumor cavity with irrigation fluid may help to prevent such artifacts (Fig. 8b) and enable adequate positioning of the patient’s head with respect to the MR isocenter.135
Fig. 8.
Illustrative case of a right frontal glioma. a: A 47-year-old woman with the right frontal lobe glioma. Diffusion-weighted ioMR imaging shows a minimal susceptibility artifact around the cavity. Note also that some artifacts related to the head pin were identified in both occipital lobes (arrows). b: After removing the tumor, the surgeon decides to take intraoperative MR images. Before moving the patient into the intraoperative MR scanner, large enough surgical gauze (with X-ray-enhanced fiber containing polypropylene, barium sulphate, and polyester, which does not affect MR images) is placed into the tumor-removed cavity. It is filled with fluid so that it will not collapse the cavity, and this step prevents the cavity wall falling inward. The cavity is filled with irrigation fluid preventing air bubbles, which can induce susceptibility artifacts on ioMR images. ioMR, intraoperative magnetic resonance.
Additional Intraoperative Modalities
Neurophysiological monitoring
As ioMR images cannot be updated frequently, neurosurgeons need to use other forms of intraoperative monitoring, such as MEP and SEP (Fig. 9), in order to avoid damage to the surrounding brain. Evoked potentials can identify both location and function of cortical and subcortical connections.13 MEP uses transcranial and transcortical stimulation of the primary motor cortex to elicit evoked electromyograms of muscles in the extremities.
Fig. 9.
Neuronavigation, motor-evoked potentials, and somatosensory evoked potentials provide real-time anatomical and neurophysiological information to the surgeons. a: The surgeon is handling a pointer device and touches the surgical field; the navigation monitor shows the exact position on the upper monitor. The lower monitor shows the motor-evoked potential. b: The evoked potential electrode is screwed into the scalp for transcranial motor-evoked potential monitoring. c: The monitoring electrode is slipped underneath the dura mater for testing of somatosensory and motor-evoked potentials through the cortical surface.
Direct subcortical stimulation (during the dissection) of the tumor cavity wall can then be used to infer the distance to the corticospinal tract by means of the degree of response to the stimulation intensity,14,29 along with the neuronavigation data.
While doing this, careful attention must be paid to white-matter-fiber tract shift, following craniotomy and durotomy and during lesion resection.136
During surgery, intermittent stimulation of the motor cortex is performed when the MEP signal decreases, and subcortical stimulation is performed there to expand the resection range and approach the corticospinal tract.14,31,40 A correlation has been reported between subcortical stimulation and the distance to the corticospinal tract, where 10 mA corresponds to roughly 10 mm and 5 mA to 5 mm.137 When there is no response after stimulation at 10 mA, the area is safe for deeper removal. If there is a weak response at 5 mA, the removal plan is close to the corticospinal tract. According to Kamada et al., 1.8 mA could be considered as the electrical threshold of the corticospinal tract.137 SEP is a reliable method for identifying the central sulcus in phase reversal and is used to identify the primary motor cortex in the first step of surgery.138
Awake craniotomy
The language-dominant hemisphere has important language networks, and, therefore, simple neurofunctional monitoring is insufficient. Awake craniotomy and language mapping with electrical stimulation are being applied with increasing frequency to avoid postoperative language dysfunction when tumors are located close to the eloquent area associated with language.139–144 Surgical accuracy may be improved by combining awake craniotomy and ioMR imaging.142,145–147 When awake craniotomy is performed, special anesthetic management is required and must be performed by an expert anesthesiologist.145 In addition, language tasks might be performed by speech therapists and psychologists before and after awake craniotomy.148 The patient should be fully informed about the benefits and risks of the procedure because their cooperation is needed, and they should not be significantly distressed by the awake craniotomy.146,147,149,150 A systematic review showed that the use of awake craniotomy with electrical stimulation during glioma resection is associated with lower risks of long-term neurological and language deficits and a higher extent of glioma resection, leading to shorter hospital stay.140
Awake surgery is a useful method when the tumor is located in the language-dominant hemisphere.141,142 Full anesthesia is initially induced before craniotomy.58 After the craniotomy, the dura mater is blocked with local anesthesia, the dura is opened, and the patient is gradually awakened. Electrical stimulation and awake testing are performed to detect the language functioning area.58 When surgeons decide to conduct ioMR imaging, general anesthesia is reapplied according to the regular operating room technique.58 This procedure is called the asleep-awake-asleep anesthetic technique.151
Intraoperative fluorescence guidance under excitation light
Radical resection of glioma can be obtained only in a low percentage of cases due to glioma infiltration into both eloquent cortex and subcortical regions.17,152–154 There is also difficulty in intraoperatively distinguishing the respectable glioma tissue at the margin of the resection even in noneloquent areas.155 Photodynamic detection, which is the use of photosensitive materials that enhance tumor visualization by fluorescence, has been proposed during the removal of glioma.17 Fluorescence-guided surgery using 5-aminolevulinic acid (5-ALA) is highly specific for the detection of high-grade glioma on the surgical field. Using fluorescence real time guided surgery by 5-ALA is a tool for neurosurgeons in identifying high-grade glioma that can be visually recognized simultaneously under the surgical microscope. Therefore, fluorescence-guided surgery by 5-ALA provides navigation in the right resection area during the surgery.15 5-ALA is a building block in the heme synthesis pathway that is naturally converted to protoporphyrin IX, a fluorescent molecule that accumulates in glioma tissue due to local disruption of the blood‒brain barrier and increased synthesis by tumor cells.15,16 5-ALA mostly accumulates in high-grade tumors (WHO Grades III or IV) and emits fluorescence in real time. When the tumor is irradiated with excitation light, protoporphyrin IX fluorescence can be intraoperatively visualized with special filter for the operating microscope, resulting in red at the tumor core and pink at the margins where concentrations are lower15,16 and may be used to guide the excision area.15,16,18,40,156–164 In addition, the alternative option of fluorescence-guided surgery is sodium fluorescein. Sodium fluorescein is a dye that accumulates in high-grade glioma due to their disruption of the blood–brain barrier. It is administered by intravenous injection during surgery and, with the use of a special filter in the operating microscope, results in yellow appearance of the tumor compared to pink appearance of the normal brain tissue.18–21 A limitation of the above method is the lack of fluorescence in the majority of low-grade glioma. Moreover, deeper seated glioma tissues might fail to be detected. However, fluorescence-guided surgery is not limited by brain shift or navigation inaccuracy; hence, it is a suitable tool to achieve gross total resection of high-grade glioma.165–167 Several randomized controlled trials have shown that 5-ALA photodynamic diagnosis is beneficial with respect to indicating resection margins, which improves progression-free survival when compared with standard surgery, although the overall survival is not improved.168,169 Sodium fluorescence is limited to small cohort studies without uniform results.167,170
Intraoperative ultrasound
IoUS, including microbubble contrast-enhanced and 3D ioUS, provides simultaneous visualization of tumor with the information of surrounding structures. Gliomata appear hypoechoic on ioUS, and this characteristic can be a reliable method to navigate toward glioma during surgical procedure.5,11,157,171–183 The use of ioUS is gaining popularity due to accuracy in localizing glioma, evaluating the extent of resection and cost-effectiveness.12 In particular, ioUS may be used to provide information on brain shift. The application of ultrasound–MR image fusion can improve the total resection rate of glioma, thus playing an important role in clinical practice.184 Its use is expected to further increase as software development progresses.5,171,176,185 Limitations of ioUS are the necessary training of the personnel in order to create good quality images, as well as problems with artifacts due to bone, blood, and hemostatic materials.186 However, ioUS can still serve as a cheaper alternative to ioMR and is easy to handle.18 A meta-analysis showed that ioUS is effective for assessing resection of diffuse glioma, but that accuracy is greater for low-grade glioma than high-grade lesions. Accuracy might be affected in patients who have undergone previous treatment, particularly radiation therapy, or by surgical artifacts (e.g., blood clots or hemostatic agents) or small tumor remnants (generally < 5 mL).12
Since ioMR images cannot be frequently updated, both fluorescence and ioUS without time lag guide the neurosurgeon to the right corridors into the glioma resection area during the surgery. Therefore, ioUS and fluorescence guide surgery complete MRI in this procedure.
Changing the Surgical Strategy for Shifting to Adjuvant Therapy without Chasing the Lesion
Neurosurgeons use several modalities, such as MEP, neuronavigation, fluorescence, and ioUS, to obtain simultaneous information in order to perform maximum tumor resection while preserving nerve function, to evaluate their surgical procedures, and to make decisions to move forward to the next surgical step. This contributes to improving their skills and, of course, patient outcomes.
Occasionally, the surgeon changes the surgical strategy based on the progress of the surgery and the ioMR images. Carefully reviewing the surgical steps and neuronavigation monitoring, neurosurgeons may decide to not chase the lesion deeper, and then treatment of the remaining lesion may include postoperative radiation 2 Gy per day, 5 days a week for a total 60 Gy, and, at the same time as radiation, temozolomide (chemotherapeutic agent) administered orally, 75 mg/m2 of body surface area per day, 7 days a week for high-grade glioma. Following 6 weeks of radiation and oral temozolomide, followed by six cycles of adjuvant temozolomide (150–200 mg per square meter for 5 days during each 28-day cycle)187, bevacizumab is administered intravenously at a dose of 10 mg/kg once every 2 weeks for high-grade glioma.188–193 The newly developed U.S. Food and Drug Administration-approved Optune transducer array (Novocure, Haifa, Israel) is an noninvasive regional therapy that aims to inhibit the growth of glioblastoma multiforme cells via the use of alternating electric fields.194–199
Photodynamic therapy
Several studies have revealed that 80%–90% of local recurrence within 2 cm of the original margin has appeared in high-grade glioma patient.200–202 Therefore, local control after surgery by adjuvant therapy delivered intraoperatively could potentially improve patient’s overall survival.201,203–205 Intraoperative photodynamic therapy using 5-ALA has potentially permitted targeting of residual glioma cells at infiltrative margin after fluorescein-guided surgery and is used worldwide.60,206–209 Photodynamic therapy actually relies on a photochemical reaction occurring after the laser light activation of the photosensitive 5-ALA metabolite, protoporphyrin IX, which results in the release of free radicals, including singlet oxygen species.206 The intracellular accumulation of protoporphyrin IX and free radicals can lead to a very local tumor cytotoxic effect sparing normal cells.207–209 On the other hand, the alternative option of photodynamic therapy for glioma by means of talaporfin sodium is mainly used in Japan.210–212
Interstitial chemotherapy
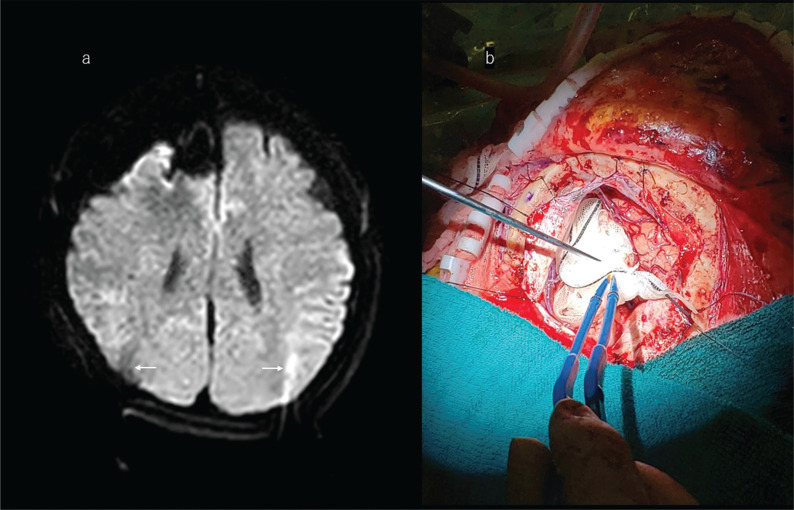
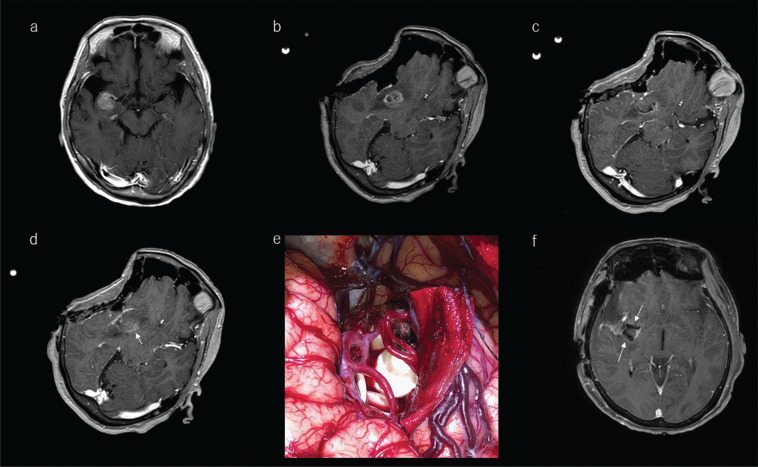
If excision is stopped because maximum safe resection has been reached, but ioMR images confirm remnant lesion that has infiltrated into eloquent regions, surgeons have the option of implanting carmustine-impregnated wafers in the tumor cavity. The indication for implantation is a diagnosis of malignant glioma by frozen-section pathological diagnosis during surgery. Biodegradable carmustine-impregnated wafers are the only approved interstitial chemotherapy for newly diagnosed malignant glioma and recurrent glioblastoma (Fig. 10).213–221 The drug is able to penetrate the blood‒brain barrier at the site of delivery.222 The wafers are placed on the surface of the tumor cavity and slowly release carmustine over 5 days, during which the drug infiltrates into brain parenchyma to around 6 mm. Of note, carmustine induces localized brain edema that may be seen on postoperative images and should not be confused with tumor remnant.223 Carmustine-impregnated wafers improve survival compared with placebo without increased incidence of adverse events.224
Fig. 10.
Illustrative case of a 72-year-old woman with high-grade glioma treated intraoperatively with a carmustine-impregnated wafer implanted into the tumor cavity. a: Preoperative T1-weighted gadolinium-enhanced MRI showed a lesion located at the anterior part of the right insula. b: After craniotomy, marked brain shift was noted related to drainage of cerebrospinal fluid from the Sylvian fissure. The surgeon decided to use these ioMR images as reference images in the neuronavigation system. c: The ioMR image seemed to indicate the removal of the enhanced lesion. d: One slice above the ioMR image in c showed a small volume of enhanced lesion (arrow). The surgeons decided to leave this remnant in place because it has crossed the pyramidal tract, and to treat it with adjuvant therapy. e: Biodegradable carmustine-impregnated wafers (white materials) were placed in the tumor cavity. f: The carmustine-impregnated wafers appeared with low-intensity signal on the postoperative MR image (arrows). ioMR, intraoperative magnetic resonance.
Interstitial chemotherapy and photodynamic therapy are useful as a bridge between surgery and standard postoperative radiation and chemotherapy for high-grade glioma.220
The Impact of Intraoperative Imaging on Brain Tumor Surgery
Kubben et al.,64 in a 2014 randomized trial of ultra-low-field ioMR in glioblastoma resection, found no advantage with respect to the extent of resection, clinical performance, or survival when compared with conventional neuronavigation-guided glioblastoma resection. Moreover, they found that ultra-low-field ioMR imaging was not cost effective compared with conventional neuronavigation. In contrast, Fountain et al.169 published a meta-analysis (including several RCTs, such as those reported by Senft et al.225 and Willems et al.4), which revealed that ioMR imaging might help to maximize the extent of resection in patients with high-grade glioma, although this conclusion was based on low-certainty evidence. This supported the findings of a previous review by Jenkinson et al.226 In a recent meta-analysis report by Lo et al.,227 the usage of ioMR imaging led to improved gross total resection of gliomas, but no benefits were seen for progression-free or overall survival.
Golub et al.163 performed a network meta-analysis, which showed that ioMR imaging is superior to conventional neuronavigation for achieving gross total resection of high-grade gliomas. Wu et al. performed a randomized, triple-blind, parallel, controlled trial using 3.0 T ioMR imaging and reported clinical utility for safe maximum resection in glioma surgery.45 Shah et al. reported in a retrospective multicenter registry comparative study of patients with newly diagnosed glioblastoma that ioMR imaging increased the gross total resection, which in turn was associated with improved overall survival after adjustment for other prognostic factors.228 However, ioMR imaging was not an independent predictor of overall survival in multivariate analysis.228
Another significant aspect of the ioMR imaging procedure concerns a possible increase in surgical site infection because craniotomy patients need to be moved into unsterilized diagnostic MR scanner. We were able to identify only two reports dealing with this issue, where it was noted that the rate of surgical site infection and the frequency of new neurologic deficits after ioMR image-guided surgery were within the normal range of pediatric neuro-oncologic surgery,229 as well as wide age ranged (1–84 years old) at the multi-theater type system.62
Overall, ioMR imaging seems to have improved the safety and increased the amount of tumor resected in patients with glioma, but the certainty of the evidence is low. There is no consensus on outcomes, such as survival. Therefore, the long-term outcomes remain unclear and additional studies are necessary. Network analyses have not been possible due to the identified adverse events, and even the existing information was incomplete and suggestive of significant reporting bias (very low-certainty evidence). Overall, the proportion of reported events was low in most trials, and even the survival outcomes were not adequately reported. The existing data regarding the quality of life are also insufficient and biased in order to extract valuable knowledge from it.169
Conclusion
In the treatment of glioma, which infiltrates into the brain parenchyma, it is important to remove as much as possible the tumor while preserving neurological function. The diagnosis of glioma is based on MR images, and, therefore, the use of intraoperative MRI can help neurosurgeons understand how much of the tumor has been removed, how far the excision site is from eloquent regions, and how to correct brain shift in the neuronavigation system. The use of multiple intraoperative imaging devices, various neurophysiological monitors, and photodynamic diagnosis, increases the likelihood of achieving maximum extent of resection while preserving neurological function. The layout of the MR equipment and operating room is important in order to achieve the best results and, taking into consideration the cost of MR and other imaging machines, can contribute to improving the cost-effectiveness. Important aspects to consider are the safety of patients and staff during intraoperative MRI. The usual operation procedure is interrupted, the patient must be moved, and multidisciplinary training is required to minimize the associated risks. It is imperative to design a manual and assign a safety manager.
When intraoperative MRI indicates that excision should not continue because of neurofunctional risks, the availability of indwelling chemotherapeutic agents and effective postoperative radiation therapy means that some therapeutic effect can still be expected even with tumor remnants. However, the field is evolving, and neurosurgeons continue trying to maximize tumor resection while preserving neurological function. Since the correction of brain shift by combined use of intraoperative ultrasound can be performed in real time during surgery, and it is also useful for the confirmation of tumor remnant or eloquent area of the connecting fibers, we are confident that the combined use of intraoperative neuronavigation, neuromonitoring, and multimodality imaging-assisted surgery has the potential to contribute to significant developments in glioma surgery in the future.
Acknowledgment
The authors would like to express their utmost gratitude to Takatoshi Sorimachi for creating the diagrammatic illustration shown in Figure 4.
The development and implementation of intraoperative MRI in our hospital involves not only neurosurgeons and anesthesiologists but also multidisciplinary paramedics. Herewith, we would like to introduce and acknowledge them for their contributions.
For intraoperative MRI, the cooperation of a radiological technologist is indispensable for capturing the vivid images as required by surgeons. Thus, we are thankful to the following radiological technologists: Tomohiko Horie (Manager of the Department of Radiology), Susumu Takano (Head of the MRI Department) and Takashi Baba (Vice Head of the MRI Department).
We are also grateful to the intraoperative MRI clinical engineers involved in managing the operating room equipment, moving anesthesia machines and operating tables, transporting patients under general anesthesia, and intraoperative monitoring, Misako Shirasu, Shota Yamaguchi, Wataru Matsumoto, and Yusuke Komiya, as well as to Saori Hirata (central operating room nurse) and Shingo Nishida (intensive care unit nurse).
Last but not the least, we are thankful to the safety management nurses (also called core nurses) Toru Anzai, Tsuyoshi Yoshida, Junpei Netsu, Tomonori Miyakawa, Kentaro Kikuchi, Maya Ishida, and Yasuaki Torii, and to the other medical staff involved in intraoperative MRI, who were not mentioned above.
We would like to thank Enago (www.enago.com) for the English language editing.
This work was supported by Grant-in-Aid for Scientific Research (B-17H04307) by Japan Society for the Promotion of Science.
Informed Consent
All figures were taken with the consent of the patients, with the explanation and consent form obtained from the Internal Review Board of our hospital (19R-299).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 2008; 26:1338–1345. [DOI] [PubMed] [Google Scholar]
- 2.Brown TJ, Brennan MC, Li M, et al. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol 2016; 2:1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence. Brain tumours (primary) and brain metastases in adults. NICE guideline [NG99], 2018. https://www.nice.org.uk/guidance/ng99. (Accessed: September 26, 2021) [PubMed]
- 4.Willems PW, Taphoorn MJ, Burger H, et al. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg 2006; 104:360–368. [DOI] [PubMed] [Google Scholar]
- 5.Gerard IJ, Kersten-Oertel M, Hall JA, et al. Brain Shift in Neuronavigation of Brain Tumors: An Updated Review of Intra-Operative Ultrasound Applications. Front Oncol 2020; 10:618837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolesz FA, Blumenfeld SM. Interventional use of magnetic resonance imaging. Magn Reson Q 1994; 10:85–96. [PubMed] [Google Scholar]
- 7.Nabavi A, Black PM, Gering DT, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery 2001; 48:787–797; discussion 797-798. [DOI] [PubMed] [Google Scholar]
- 8.Riva M, Hiepe P, Frommert M, et al. Intraoperative Computed Tomography and Finite Element Modelling for Multimodal Image Fusion in Brain Surgery. Oper Neurosurg (Hagerstown) 2020; 18:531–541. [DOI] [PubMed] [Google Scholar]
- 9.Kuhnt D, Bauer MH, Nimsky C. Brain shift compensation and neurosurgical image fusion using intraoperative MRI: current status and future challenges. Crit Rev Biomed Eng 2012; 40:175–185. [DOI] [PubMed] [Google Scholar]
- 10.Yahanda AT, Chicoine MR. Intraoperative MRI for Glioma Surgery: Present Overview and Future Directions. World Neurosurg 2021; 149:267–268. [DOI] [PubMed] [Google Scholar]
- 11.Iversen DH, Wein W, Lindseth F, et al. Automatic Intraoperative Correction of Brain Shift for Accurate Neuronavigation. World Neurosurg 2018; 120:e1071–e1078. [DOI] [PubMed] [Google Scholar]
- 12.Trevisi G, Barbone P, Treglia G, et al. Reliability of intraoperative ultrasound in detecting tumor residual after brain diffuse glioma surgery: a systematic review and meta-analysis. Neurosurg Rev 2020; 43:1221–1233. [DOI] [PubMed] [Google Scholar]
- 13.Hamer RP, Jain S, Teo C, et al. Optimizing the onco-functional balance in supratentorial brain tumour surgery: A single institution’s initial experience with intraoperative cortico-subcortical mapping and monitoring in Singapore. J Clin Neurosci 2020; 79:224–230. [DOI] [PubMed] [Google Scholar]
- 14.Lim J, Park Y, Ahn JW, et al. Maximal surgical resection and adjuvant surgical technique to prolong the survival of adult patients with thalamic glioblastoma. PLoS One 2021; 16:e0244325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orillac C, Stummer W, Orringer DA. Fluorescence Guidance and Intraoperative Adjuvants to Maximize Extent of Resection. Neurosurgery 2021; 89:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coburger J, Wirtz CR. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: a systematic review. J Neurooncol 2019; 141:533–546. [DOI] [PubMed] [Google Scholar]
- 17.Acerbi F, Broggi M, Schebesch KM, et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin Cancer Res 2018; 24:52–61. [DOI] [PubMed] [Google Scholar]
- 18.Verburg N, de Witt Hamer PC. State-of-the-art imaging for glioma surgery. Neurosurg Rev 2021; 44:1331–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manoharan R, Parkinson J. Sodium Fluorescein in Brain Tumor Surgery: Assessing Relative Fluorescence Intensity at Tumor Margins. Asian J Neurosurg 2020; 15:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamamcıoğlu MK, Akçakaya MO, Göker B, et al. The use of the YELLOW 560 nm surgical microscope filter for sodium fluorescein-guided resection of brain tumors: Our preliminary results in a series of 28 patients. Clin Neurol Neurosurg 2016; 143:39–45. [DOI] [PubMed] [Google Scholar]
- 21.Zhang N, Tian H, Huang D, et al. Sodium Fluorescein-Guided Resection under the YELLOW 560 nm Surgical Microscope Filter in Malignant Gliomas: Our First 38 Cases Experience. Biomed Res Int 2017; 2017:7865747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittal S, Black PM. Intraoperative magnetic resonance imaging in neurosurgery: the Brigham concept. Acta Neurochir Suppl 2006; 98:77–86. [DOI] [PubMed] [Google Scholar]
- 23.Black PM, Moriarty T, Alexander E, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery 1997; 41:831–842; discussion 842-845. [DOI] [PubMed] [Google Scholar]
- 24.Steinmeier R, Fahlbusch R, Ganslandt O, et al. Intraoperative magnetic resonance imaging with the magnetom open scanner: concepts, neurosurgical indications, and procedures: a preliminary report. Neurosurgery 1998; 43:739–747; discussion 747-748. [DOI] [PubMed] [Google Scholar]
- 25.Darakchiev BJ, Tew JM, Bohinski RJ, et al. Adaptation of a standard low-field (0.3-T) system to the operating room: focus on pituitary adenomas. Neurosurg Clin N Am 2005; 16:155–164. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland GR, Kaibara T, Louw D, et al. A mobile high-field magnetic resonance system for neurosurgery. J Neurosurg 1999; 91:804–813. [DOI] [PubMed] [Google Scholar]
- 27.Martin AJ, Hall WA, Liu H, et al. Brain tumor resection: intraoperative monitoring with high-field-strength MR imaging-initial results. Radiology 2000; 215:221–228. [DOI] [PubMed] [Google Scholar]
- 28.Hadani M, Spiegelman R, Feldman Z, et al. Novel, compact, intraoperative magnetic resonance imaging-guided system for conventional neurosurgical operating rooms. Neurosurgery 2001; 48:799–807; discussion 807–809. [DOI] [PubMed] [Google Scholar]
- 29.Ille S, Schroeder A, Wagner A, et al. Intraoperative MRI-based elastic fusion for anatomically accurate tractography of the corticospinal tract: correlation with intraoperative neuromonitoring and clinical status. Neurosurg Focus 2021; 50:E9. [DOI] [PubMed] [Google Scholar]
- 30.Tomasi SO, Umana GE, Scalia G, et al. Importance of Veins for Neurosurgery as Landmarks Against Brain Shifting Phenomenon: An Anatomical and 3D-MPRAGE MR Reconstruction of Superficial Cortical Veins. Front Neuroanat 2020; 14:596167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krivosheya D, Rao G, Tummala S, et al. Impact of Multi-modality Monitoring Using Direct Electrical Stimulation to Determine Corticospinal Tract Shift and Integrity in Tumors using the Intraoperative MRI. J Neurol Surg A Cent Eur Neurosurg 2021; 82:375–380. [DOI] [PubMed] [Google Scholar]
- 32.Özduman K, Yıldız E, Dinçer A, et al. Using intraoperative dynamic contrast-enhanced T1-weighted MRI to identify residual tumor in glioblastoma surgery. J Neurosurg 2014; 120:60–66. [DOI] [PubMed] [Google Scholar]
- 33.Albert FK, Forsting M, Sartor K, et al. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 1994; 34:45–60; discussion 60–61. [DOI] [PubMed] [Google Scholar]
- 34.Leroy HA, Delmaire C, Le Rhun E, et al. High-field intraoperative MRI in glioma surgery: A prospective study with volumetric analysis of extent of resection and functional outcome. Neurochirurgie 2018; 64:155–160. [DOI] [PubMed] [Google Scholar]
- 35.Jolesz FA, Nabavi A, Kikinis R. Integration of interventional MRI with computer-assisted surgery. J Magn Reson Imaging 2001; 13:69–77. [DOI] [PubMed] [Google Scholar]
- 36.Saint-Martin C, Apuzzo S, Salman A, et al. Hyperacute Infarct on Intraoperative Diffusion Imaging of Pediatric Brain Tumor Surgery. Can J Neurol Sci 2019; 46:550–558. [DOI] [PubMed] [Google Scholar]
- 37.Voglis S, Hiller A, Hofer AS, et al. Failure of diffusion-weighted imaging in intraoperative 3 Tesla MRI to identify hyperacute strokes during glioma surgery. Sci Rep 2021; 11:16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohinski RJ, Kokkino AK, Warnick RE, et al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection. Neurosurgery 2001; 48:731–742; discussion 742-744. [DOI] [PubMed] [Google Scholar]
- 39.Hirai N, Kosaka A, Kawamata T, et al. Image-guided neurosurgery system integrating AR-based navigation and open-MRI monitoring. Comput Aided Surg 2005; 10:59–71. [DOI] [PubMed] [Google Scholar]
- 40.Saito T, Muragaki Y, Tamura M, et al. Correlation between localization of supratentorial glioma to the precentral gyrus and difficulty in identification of the motor area during awake craniotomy. J Neurosurg 2020; 134:1490–1499. [DOI] [PubMed] [Google Scholar]
- 41.Ogiwara T, Goto T, Fujii Y, et al. Endoscopic Endonasal Approach in the Smart Cyber Operating Theater (SCOT): Preliminary Clinical Application. World Neurosurg 2021; 147:e533–e537. [DOI] [PubMed] [Google Scholar]
- 42.Nimsky C, Ganslandt O, von Keller B, et al. Preliminary experience in glioma surgery with intraoperative high-field MRI. Acta Neurochir Suppl 2003; 88:21–29. [DOI] [PubMed] [Google Scholar]
- 43.Hall WA, Liu H, Martin AJ, et al. Safety, efficacy, and functionality of high-field strength interventional magnetic resonance imaging for neurosurgery. Neurosurgery 2000; 46:632–641; discussion 641-642. [DOI] [PubMed] [Google Scholar]
- 44.Hall WA, Galicich W, Bergman T, et al. 3-Tesla intraoperative MR imaging for neurosurgery. J Neurooncol 2006; 77:297–303. [DOI] [PubMed] [Google Scholar]
- 45.Wu JS, Gong X, Song YY, et al. 3.0-T intraoperative magnetic resonance imaging-guided resection in cerebral glioma surgery: interim analysis of a prospective, randomized, triple-blind, parallel-controlled trial. Neurosurgery 2014; 61 Suppl 1:145–154. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Chen X, Zhao Y, et al. Impact of intraoperative magnetic resonance imaging and functional neuronavigation on surgical outcome in patients with gliomas involving language areas. Neurosurg Rev 2015; 38:319–330; discussion 330. [DOI] [PubMed] [Google Scholar]
- 47.Roder C, Bisdas S, Ebner FH, et al. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 2014; 40:297–304. [DOI] [PubMed] [Google Scholar]
- 48.Tanji M, Kataoka H, Kikuchi M, et al. Impact of Intraoperative 3-Tesla MRI on Endonasal Endoscopic Pituitary Adenoma Resection and a Proposed New Scoring System for Predicting the Utility of Intraoperative MRI. Neurol Med Chir (Tokyo) 2020; 60:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumae M, Koizumi J, Fukuyama H, et al. World’s first magnetic resonance imaging/x-ray/operating room suite: a significant milestone in the improvement of neurosurgical diagnosis and treatment. J Neurosurg 2007; 107:266–273. [DOI] [PubMed] [Google Scholar]
- 50.Eid H, Crevier-Sorbo G, Moreau JT, et al. Eight-Year Experience With 3-T Intraoperative MRI Integration in Focal Pediatric Epilepsy Surgery: Impact on Extent of Resection, Residual Volumes, and Seizure Outcomes. AJR Am J Roentgenol 2020; 214:1343–1351. [DOI] [PubMed] [Google Scholar]
- 51.Avula S, Jaspan T, Pizer B, et al. Comparison of intraoperative and post-operative 3-T MRI performed at 24-72 h following brain tumour resection in children. Neuroradiology 2021; 63:1367–1376. [DOI] [PubMed] [Google Scholar]
- 52.Multani KM, Balasubramaniam A, Rajesh BJ, et al. Utility and pitfalls of high field 3 tesla intraoperative MRI in neurosurgery: A single centre experience of 100 cases. Neurol India 2020; 68:413–418. [DOI] [PubMed] [Google Scholar]
- 53.Miskin N, Unadkat P, Carlton ME, et al. Frequency and Evolution of New Postoperative Enhancement on 3 Tesla Intraoperative and Early Postoperative Magnetic Resonance Imaging. Neurosurgery 2020; 87:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coburger J, Wirtz CR, König RW. Impact of extent of resection and recurrent surgery on clinical outcome and overall survival in a consecutive series of 170 patients for glioblastoma in intraoperative high field magnetic resonance imaging. J Neurosurg Sci 2017; 61:233–244. [DOI] [PubMed] [Google Scholar]
- 55.Fomekong E, Duprez T, Docquier MA, et al. Intraoperative 3T MRI for pituitary macroadenoma resection: Initial experience in 73 consecutive patients. Clin Neurol Neurosurg 2014; 126:143–149. [DOI] [PubMed] [Google Scholar]
- 56.Fujita Y, Kohta M, Sasayama T, et al. Intraoperative 3-T Magnetic Resonance Spectroscopy for Detection of Proliferative Remnants of Glioma. World Neurosurg 2020; 137:149–157. [DOI] [PubMed] [Google Scholar]
- 57.Huntoon K, Makary MS, Damante M, et al. Intraoperative 3 T MRI is more correlative to residual disease extent than early postoperative MRI. J Neurooncol 2021; 154:345–351. [DOI] [PubMed] [Google Scholar]
- 58.Maldaun MV, Khawja SN, Levine NB, et al. Awake craniotomy for gliomas in a high-field intraoperative magnetic resonance imaging suite: analysis of 42 cases. J Neurosurg 2014; 121:810–817. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa E, Sugii N, Matsuda M, et al. Maximum resection and immunotherapy improve glioblastoma patient survival: a retrospective single-institution prognostic analysis. BMC Neurol 2021; 21:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vermandel M, Dupont C, Lecomte F, et al. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: a preliminary analysis of the INDYGO clinical trial. J Neurooncol 2021; 152:501–514. [DOI] [PubMed] [Google Scholar]
- 61.Cornaz F, Neidert MC, Piccirelli M, et al. Compatibility of intraoperative 3T MR imaging and intraoperative neurophysiological monitoring. Clin Neurophysiol 2015; 126:218–220. [DOI] [PubMed] [Google Scholar]
- 62.Dinevski N, Sarnthein J, Vasella F, et al. Postoperative Neurosurgical Infection Rates After Shared-Resource Intraoperative Magnetic Resonance Imaging: A Single-Center Experience with 195 Cases. World Neurosurg 2017; 103:275–282. [DOI] [PubMed] [Google Scholar]
- 63.Mislow JM, Golby AJ, Black PM. Origins of intraoperative MRI. Neurosurg Clin N Am 2009; 20:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kubben PL, Scholtes F, Schijns OE, et al. Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: A randomized controlled trial. Surg Neurol Int 2014; 5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makary M, Chiocca EA, Erminy N, et al. Clinical and economic outcomes of low-field intraoperative MRI-guided tumor resection neurosurgery. J Magn Reson Imaging 2011; 34:1022–1030. [DOI] [PubMed] [Google Scholar]
- 66.Ning MS, Venkatesan AM, Stafford RJ, et al. Developing an intraoperative 3T MRI-guided brachytherapy program within a diagnostic imaging suite: Methods, process workflow, and value-based analysis. Brachytherapy 2020; 19:427–437. [DOI] [PubMed] [Google Scholar]
- 67.Bettmann MA. Intraoperative MRI for Treatment of High-Grade Glioma: Is It Cost-effective? Radiology 2019; 291:698–699. [DOI] [PubMed] [Google Scholar]
- 68.Abraham P, Sarkar R, Brandel MG, et al. Cost-effectiveness of Intraoperative MRI for Treatment of High-Grade Gliomas. Radiology 2019; 291:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eljamel MS, Mahboob SO. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis Photodyn Ther 2016; 16:35–43. [DOI] [PubMed] [Google Scholar]
- 70.Nimsky C, Ganslandt O, Fahlbusch R. 1.5 T: intraoperative imaging beyond standard anatomic imaging. Neurosurg Clin N Am 2005; 16:185–200. [DOI] [PubMed] [Google Scholar]
- 71.Hall WA, Truwit CL. Intraoperative MR-guided neurosurgery. J Magn Reson Imaging 2008; 27:368–375. [DOI] [PubMed] [Google Scholar]
- 72.Azmi H, Gibbons M, DeVito MC, et al. The interventional magnetic resonance imaging suite: Experience in the design, development, and implementation in a pre-existing radiology space and review of concepts. Surg Neurol Int 2019; 10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tronnier VM, Wirtz CR, Knauth M, et al. Intraoperative diagnostic and interventional magnetic resonance imaging in neurosurgery. Neurosurgery 1997; 40:891–900; discussion 900–902. [DOI] [PubMed] [Google Scholar]
- 74.Stienen MN, Fierstra J, Pangalu A, et al. The Zurich Checklist for Safety in the Intraoperative Magnetic Resonance Imaging Suite: Technical Note. Oper Neurosurg (Hagerstown) 2019; 16:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rahmathulla G, Recinos PF, Traul DE, et al. Surgical briefings, checklists, and the creation of an environment of safety in the neurosurgical intraoperative magnetic resonance imaging suite. Neurosurg Focus 2012; 33:E12. [DOI] [PubMed] [Google Scholar]
- 76.Henk CB, Higgins CB, Saeed M. Endovascular interventional MRI. J Magn Reson Imaging 2005; 22:451–460. [DOI] [PubMed] [Google Scholar]
- 77.Tempany CM, Jayender J, Kapur T, et al. Multimodal imaging for improved diagnosis and treatment of cancers. Cancer 2015; 121:817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jolesz FA. Intraoperative imaging in neurosurgery: where will the future take us? Acta Neurochir Suppl 2011; 109:21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jolesz FA, Bleier AR, Jakab P, et al. MR imaging of laser-tissue interactions. Radiology 1988; 168:249–253. [DOI] [PubMed] [Google Scholar]
- 80.Patel NA, Li G, Shang W, et al. System Integration and Preliminary Clinical Evaluation of a Robotic System for MRI-Guided Transperineal Prostate Biopsy. J Med Robot Res 2019; 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schulze PC, Vitzthum HE, Goldammer A, et al. Laser-induced thermotherapy of neoplastic lesions in the brain—underlying tissue alterations, MRI-monitoring and clinical applicability. Acta Neurochir (Wien) 2004; 146:803–812. [DOI] [PubMed] [Google Scholar]
- 82.Zaidi HA, De Los Reyes K, Barkhoudarian G, et al. The utility of high-resolution intraoperative MRI in endoscopic transsphenoidal surgery for pituitary macroadenomas: early experience in the Advanced Multimodality Image Guided Operating suite. Neurosurg Focus 2016; 40:E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeong H, Im JJ, Park JS, et al. A pilot clinical study of low-intensity transcranial focused ultrasound in Alzheimer’s disease. Ultrasonography 2021; 40:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kettenbach J, Silverman SG, Hata N, et al. Monitoring and visualization techniques for MR-guided laser ablations in an open MR system. J Magn Reson Imaging 1998; 8:933–943. [DOI] [PubMed] [Google Scholar]
- 85.Kuroda K. MR techniques for guiding high-intensity focused ultrasound (HIFU) treatments. J Magn Reson Imaging 2018; 47:316–331. [DOI] [PubMed] [Google Scholar]
- 86.Kapur T, Egger J, Damato A, et al. 3-T MR-guided brachytherapy for gynecologic malignancies. Magn Reson Imaging 2012; 30:1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Golshan M, Sagara Y, Wexelman B, et al. Pilot study to evaluate feasibility of image-guided breast-conserving therapy in the advanced multimodal image-guided operating (AMIGO) suite. Ann Surg Oncol 2014; 21:3356–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ibn Essayed W, Almefty KK, Al-Mefty O. Recurrent Chordoma Resection in the Advanced Multimodality Image Guided Operating Suite: 2-Dimensional Operative Video. Oper Neurosurg (Hagerstown) 2021; 20:E344–E345. [DOI] [PubMed] [Google Scholar]
- 89.Nakai T, Kamiya N, Sone M, et al. A survey analysis of acoustic trauma related to MR scans. Magn Reson Med Sci 2012; 11:253–264. [DOI] [PubMed] [Google Scholar]
- 90.Gandhe RU, Bhave CP. Intraoperative magnetic resonance imaging for neurosurgery - An anaesthesiologist’s challenge. Indian J Anaesth 2018; 62:411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Practice advisory on anesthetic care for magnetic resonance imaging: an updated report by the american society of anesthesiologists task force on anesthetic care for magnetic resonance imaging. Anesthesiology 2015; 122:495–520. [DOI] [PubMed] [Google Scholar]
- 92.Berkow LC. Anesthetic management and human factors in the intraoperative MRI environment. Curr Opin Anaesthesiol 2016; 29:563–567. [DOI] [PubMed] [Google Scholar]
- 93.Delfino JG, Woods TO. New developments in standards for MRI safety testing of medical devices. Curr Radiol Rep 2016; 4:1–9. [Google Scholar]
- 94.Ryan JW, Murray AS, Gilligan PJ, et al. MRI safety management in patients with cardiac implantable electronic devices: Utilizing failure mode and effects analysis for risk optimization. Int J Qual Health Care 2020; 32:431–437. [DOI] [PubMed] [Google Scholar]
- 95.Peedicail JS, Poulin T, Scott JN, et al. Calgary Comprehensive Epilepsy Program Collaborators. Clinical safety of intracranial EEG electrodes in MRI at 1.5 T and 3 T: a single-center experience and literature review. Neuroradiology 2021; 63:1669–1678. [DOI] [PubMed] [Google Scholar]
- 96.Bhusal B, Stockmann J, Guerin B, et al. Safety and image quality at 7T MRI for deep brain stimulation systems: Ex vivo study with lead-only and full-systems. PLoS One 2021; 16:e0257077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhusal B, Nguyen BT, Sanpitak PP, et al. Effect of Device Configuration and Patient’s Body Composition on the RF Heating and Nonsusceptibility Artifact of Deep Brain Stimulation Implants During MRI at 1.5T and 3T. J Magn Reson Imaging 2021; 53:599–610. [DOI] [PubMed] [Google Scholar]
- 98.Kazemivalipour E, Bhusal B, Vu J, et al. Vertical open-bore MRI scanners generate significantly less radiofrequency heating around implanted leads: A study of deep brain stimulation implants in 1.2T OASIS scanners versus 1.5T horizontal systems. Magn Reson Med 2021; 86:1560–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kazemivalipour E, Vu J, Lin S, et al. RF heating of deep brain stimulation implants during MRI in 1.2 T vertical scanners versus 1.5 T horizontal systems: A simulation study with realistic lead configurations. Annu Int Conf IEEE Eng Med Biol Soc 2020; 2020:6143–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seo HC, Lee Y, Joo S. A simple apparatus for safety assessment of magnetically induced torque on active implantable medical devices (AIMDs) under 1.5 T and 3.0 T MRI. MAGMA 2021; 34:767–774. [DOI] [PubMed] [Google Scholar]
- 101.Caraiani C, Petresc B, Dong Y, et al. Contraindications and adverse effects in abdominal imaging. Med Ultrason 2019; 21:456–463. [DOI] [PubMed] [Google Scholar]
- 102.Watson RE, Edmonson HA. MR Safety: Active Implanted Electronic Devices. Magn Reson Imaging Clin N Am 2020; 28:549–558. [DOI] [PubMed] [Google Scholar]
- 103.Tsai LL, Grant AK, Mortele KJ, et al. A Practical Guide to MR Imaging Safety: What Radiologists Need to Know. Radiographics 2015; 35:1722–1737. [DOI] [PubMed] [Google Scholar]
- 104.Jaimes C, Biaggotti D, Sreedher G, et al. Magnetic resonance imaging in children with implants. Pediatr Radiol 2021; 51:748–759. [DOI] [PubMed] [Google Scholar]
- 105.Breitkopf M, Bisdas S, Liebsch M, et al. Safety, Utility, and Clinical Results of Continuous Intraoperative Electrophysiologic Monitoring in 1.5T iMRI-Guided Surgery. World Neurosurg 2017; 106:198–205. [DOI] [PubMed] [Google Scholar]
- 106.Tagell L, Alcheikh A, Jurevics R, et al. Thigh burn - A magnetic resonance imaging (MRI) related adverse event. Radiol Case Rep 2020; 15:2569–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dempsey MF, Condon B. Thermal injuries associated with MRI. Clin Radiol 2001; 56:457–465. [DOI] [PubMed] [Google Scholar]
- 108.Sarnthein J, Lüchinger R, Piccirelli M, et al. Prevalence of Complications in Intraoperative Magnetic Resonance Imaging Combined with Neurophysiologic Monitoring. World Neurosurg 2016; 93:168–174. [DOI] [PubMed] [Google Scholar]
- 109.Winter L, Seifert F, Zilberti L, et al. MRI-Related Heating of Implants and Devices: A Review. J Magn Reson Imaging 2021; 53:1646–1665. [DOI] [PubMed] [Google Scholar]
- 110.Mahajan RP. The WHO surgical checklist. Best Pract Res Clin Anaesthesiol 2011; 25:161–168. [DOI] [PubMed] [Google Scholar]
- 111.McLaughlin N, Winograd D, Chung HR, et al. University of California, Los Angeles, surgical time-out process: evolution, challenges, and future perspective. Neurosurg Focus 2012; 33:E5. [DOI] [PubMed] [Google Scholar]
- 112.Wong JM, Perry WRG, Greenberg Y, et al. Integrating Cerebrospinal Fluid Shunt Quality Checks into the World Health Organization’s Safe Surgery Checklist: A Pilot Study. World Neurosurg 2016; 92:491-498.e3. [DOI] [PubMed] [Google Scholar]
- 113.Weiser TG, Haynes AB, Lashoher A, et al. Perspectives in quality: designing the WHO Surgical Safety Checklist. Int J Qual Health Care 2010; 22:365–370. [DOI] [PubMed] [Google Scholar]
- 114.Cohen FL, Mendelsohn D, Bernstein M. Wrong-site craniotomy: analysis of 35 cases and systems for prevention. J Neurosurg 2010; 113:461–473. [DOI] [PubMed] [Google Scholar]
- 115.Hart EM, Owen H. Errors and omissions in anesthesia: a pilot study using a pilot’s checklist. Anesth Analg 2005; 101:246–50, table of contents. [DOI] [PubMed] [Google Scholar]
- 116.Kimbrell V. Elements of Effective Patient Screening to Improve Safety in MRI. Magn Reson Imaging Clin N Am 2020; 28:489–496. [DOI] [PubMed] [Google Scholar]
- 117.Kettenbach J, Kacher DF, Kanan AR, et al. Intraoperative and interventional MRI: recommendations for a safe environment. Minim Invasive Ther Allied Technol 2006; 15:53–64. [DOI] [PubMed] [Google Scholar]
- 118.Calland JF, Turrentine FE, Guerlain S, et al. The surgical safety checklist: lessons learned during implementation. Am Surg 2011; 77:1131–1137. [PubMed] [Google Scholar]
- 119.Matsumae M, Nakajima Y, Morikawa E, et al. Improving patient safety in the intra-operative MRI suite using an on-duty safety nurse, safety manual and checklist. Acta Neurochir Suppl 2011; 109:219–222. [DOI] [PubMed] [Google Scholar]
- 120.White MJ, Thornton JS, Hawkes DJ, et al. Design, operation, and safety of single-room interventional MRI suites: practical experience from two centers. J Magn Reson Imaging 2015; 41:34–43. [DOI] [PubMed] [Google Scholar]
- 121.Hemingway M, Kilfoyle M. Safety planning for intraoperative magnetic resonance imaging. AORN J 2013; 98:508–524. [DOI] [PubMed] [Google Scholar]
- 122.Fourcade A, Blache JL, Grenier C, et al. Barriers to staff adoption of a surgical safety checklist. BMJ Qual Saf 2012; 21:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schroeck H, Welch TL, Rovner MS, et al. Anesthetic challenges and outcomes for procedures in the intraoperative magnetic resonance imaging suite: A systematic review. J Clin Anesth 2019; 54:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Laochamroonvorapongse D, Theard MA, Yahanda AT, et al. Intraoperative MRI for Adult and Pediatric Neurosurgery. Anesthesiol Clin 2021; 39:211–225. [DOI] [PubMed] [Google Scholar]
- 125.Fouke SJ, Benzinger T, Gibson D, et al. The role of imaging in the management of adults with diffuse low grade glioma: A systematic review and evidence-based clinical practice guideline. J Neurooncol 2015; 125:457–479. [DOI] [PubMed] [Google Scholar]
- 126.Price SJ, Jena R, Burnet NG, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol 2006; 27:1969–1974. [PMC free article] [PubMed] [Google Scholar]
- 127.Gill BJ, Pisapia DJ, Malone HR, et al. MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proc Natl Acad Sci U S A 2014; 111:12550–12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Edjlali M, Ploton L, Maurage CA, et al. Intraoperative MRI and FLAIR Analysis: Implications for low-grade glioma surgery. J Neuroradiol 2021; 48:61–64. [DOI] [PubMed] [Google Scholar]
- 129.Walker M, Khawar S, Shaibani A, et al. Gadolinium leakage into the surgical bed mimicking residual enhancement following spinal cord surgery. Case report. J Neurosurg 2004; 100:291–294. [DOI] [PubMed] [Google Scholar]
- 130.Zaidi HA, Chowdhry SA, Wilson DA, et al. The dilemma of early postoperative magnetic resonance imaging: when efficiency compromises accuracy: case report. Neurosurgery 2014; 74:E335–340; discussion E340. [DOI] [PubMed] [Google Scholar]
- 131.Rykkje AM, Li D, Skjøth-Rasmussen J, et al. Surgically Induced Contrast Enhancements on Intraoperative and Early Postoperative MRI Following High-Grade Glioma Surgery: A Systematic Review. Diagnostics (Basel) 2021; 11:1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heßelmann V, Mager AK, Goetz C, et al. Accuracy of High-Field Intraoperative MRI in the Detectability of Residual Tumor in Glioma Grade IV Resections. Rofo 2017; 189:519–526. [DOI] [PubMed] [Google Scholar]
- 133.Knauth M, Aras N, Wirtz CR, et al. Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR Am J Neuroradiol 1999; 20:1547–1553. [PMC free article] [PubMed] [Google Scholar]
- 134.Masuda Y, Akutsu H, Ishikawa E, et al. Evaluation of the extent of resection and detection of ischemic lesions with intraoperative MRI in glioma surgery: is intraoperative MRI superior to early postoperative MRI? J Neurosurg 2018; 131:209–216. [DOI] [PubMed] [Google Scholar]
- 135.Roder C, Haas P, Tatagiba M, et al. Technical limitations and pitfalls of diffusion-weighted imaging in intraoperative high-field MRI. Neurosurg Rev 2021; 44:327–334. [DOI] [PubMed] [Google Scholar]
- 136.Yang JY, Beare R, Seal ML, et al. A systematic evaluation of intraoperative white matter tract shift in pediatric epilepsy surgery using high-field MRI and probabilistic high angular resolution diffusion imaging tractography. J Neurosurg Pediatr 2017; 19:592–605. [DOI] [PubMed] [Google Scholar]
- 137.Kamada K, Todo T, Ota T, et al. The motor-evoked potential threshold evaluated by tractography and electrical stimulation. J Neurosurg 2009; 111:785–795. [DOI] [PubMed] [Google Scholar]
- 138.Hsieh JC, Lee TY, Shih YH, et al. Demarcation and localization of primary sensor and motor areas in human cortex by cortical somatosensory. Evoked potential (Co-SEP) during operation in surgery for epilepsy and intracranial tumor. Ma Zui Xue Za Zhi 1990; 28:285–293. [PubMed] [Google Scholar]
- 139.Skrap M, Mondani M, Tomasino B, et al. Surgery of insular nonenhancing gliomas: volumetric analysis of tumoral resection, clinical outcome, and survival in a consecutive series of 66 cases. Neurosurgery 2012; 70:1081–1093; discussion 1093-1094. [DOI] [PubMed] [Google Scholar]
- 140.Bu LH, Zhang J, Lu JF, et al. Glioma surgery with awake language mapping versus generalized anesthesia: a systematic review. Neurosurg Rev 2021; 44:1997–2011. [DOI] [PubMed] [Google Scholar]
- 141.Verst SM, de Castro I, Scappini-Junior W, et al. Methodology for creating and validating object naming and semantic tests used by Verst-Maldaun Language Assessment during awake craniotomies. Clin Neurol Neurosurg 2021; 202:106485. [DOI] [PubMed] [Google Scholar]
- 142.Camporeze B, Galafassi G, Caggiano C, et al. Is awake craniotomy an ideal technique for surgical resection of tumors involving the motor area? Analysis of functional outcomes. J Neurosurg Sci 2021; 65:81–84. [DOI] [PubMed] [Google Scholar]
- 143.Bährend I, Muench MR, Schneider H, et al. Incidence and linguistic quality of speech errors: a comparison of preoperative transcranial magnetic stimulation and intraoperative direct cortex stimulation. J Neurosurg 2020; 134:1409–1418. [DOI] [PubMed] [Google Scholar]
- 144.Voets NL, Pretorius P, Birch MD, et al. Diffusion tractography for awake craniotomy: accuracy and factors affecting specificity. J Neurooncol 2021; 153:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peruzzi P, Puente E, Bergese S, et al. Intraoperative MRI (ioMRI) in the setting of awake craniotomies for supratentorial glioma resection. Acta Neurochir Suppl 2011; 109:43–48. [DOI] [PubMed] [Google Scholar]
- 146.Aleem Bhatti AU, Jakhrani NK, Parekh MA. Awake Craniotomy with Noninvasive Brain Mapping by 3-Tesla Functional Magnetic Resonance Imaging for Excision of Low-grade Glioma: A Case of a Young Patient from Pakistan. Asian J Neurosurg 2018; 13:471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chowdhury T, Zeiler FA, Singh N, et al. Awake Craniotomy Under 3-Tesla Intraoperative Magnetic Resonance Imaging: A Retrospective Descriptive Report and Canadian Institutional Experience. J Neurosurg Anesthesiol. 2022; 34:e46–e51. [DOI] [PubMed] [Google Scholar]
- 148.Zarino B, Sirtori MA, Meschini T, et al. Insular lobe surgery and cognitive impairment in gliomas operated with intraoperative neurophysiological monitoring. Acta Neurochir (Wien) 2021; 163:1279–1289. [DOI] [PubMed] [Google Scholar]
- 149.Leon-Rojas JE, Ekert JO, Kirkman MA, et al. Experience with awake throughout craniotomy in tumour surgery: technique and outcomes of a prospective, consecutive case series with patient perception data. Acta Neurochir (Wien) 2020; 162:3055–3065. [DOI] [PubMed] [Google Scholar]
- 150.Chowdhury T, Singh GP, Zeiler FA, et al. Anesthesia for Awake Craniotomy for Brain Tumors in an Intraoperative MRI Suite: Challenges and Evidence. Front Oncol 2018; 8:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huncke K, Van de Wiele B, Fried I, et al. The asleep-awake-asleep anesthetic technique for intraoperative language mapping. Neurosurgery 1998; 42:1312–1316; discussion 1316–1317. [DOI] [PubMed] [Google Scholar]
- 152.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001; 95:190–198. [DOI] [PubMed] [Google Scholar]
- 153.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 2009; 110:156–162. [DOI] [PubMed] [Google Scholar]
- 154.Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 2011; 115:3–8. [DOI] [PubMed] [Google Scholar]
- 155.Tonn JC, Stummer W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: practical use, risks, and pitfalls. Clin Neurosurg 2008; 55:20–26. [PubMed] [Google Scholar]
- 156.Tsugu A, Ishizaka H, Mizokami Y, et al. Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg 2011; 76:120–127. [DOI] [PubMed] [Google Scholar]
- 157.Della Pepa GM, Ius T, La Rocca G, et al. 5-Aminolevulinic Acid and Contrast-Enhanced Ultrasound: The Combination of the Two Techniques to Optimize the Extent of Resection in Glioblastoma Surgery. Neurosurgery 2020; 86:E529–E540. [DOI] [PubMed] [Google Scholar]
- 158.Barbagallo GMV, Certo F, Di Gregorio S, et al. Recurrent high-grade glioma surgery: a multimodal intraoperative protocol to safely increase extent of tumor resection and analysis of its impact on patient outcome. Neurosurg Focus 2021; 50:E20. [DOI] [PubMed] [Google Scholar]
- 159.Yoneyama T, Watanabe T, Tamai S, et al. Bright spot analysis for photodynamic diagnosis of brain tumors using confocal microscopy. Photodiagnosis Photodyn Ther 2019; 25:463–471. [DOI] [PubMed] [Google Scholar]
- 160.Hosmann A, Millesi M, Wadiura LI, et al. 5-ALA Fluorescence Is a Powerful Prognostic Marker during Surgery of Low-Grade Gliomas (WHO Grade II)-Experience at Two Specialized Centers. Cancers (Basel) 2021; 13:2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vogelbaum MA, Kroll D, Etame A, et al. A Prospective Validation Study of the First 3D Digital Exoscope for Visualization of 5-ALA-Induced Fluorescence in High-Grade Gliomas. World Neurosurg 2021; 149:e498–e503. [DOI] [PubMed] [Google Scholar]
- 162.Ontario Health (Quality). 5-Aminolevulinic Acid Hydrochloride (5-ALA)-Guided Surgical Resection of High-Grade Gliomas: A Health Technology Assessment. Ont Health Technol Assess Ser 2020; 20:1–92. [PMC free article] [PubMed] [Google Scholar]
- 163.Golub D, Hyde J, Dogra S, et al. Intraoperative MRI versus 5-ALA in high-grade glioma resection: a network meta-analysis. J Neurosurg. 2020. Feb 21. doi: 10.3171/2019.12.JNS191203. [Epub ahead of print. PMID: 32084631] [DOI] [PubMed] [Google Scholar]
- 164.Maragkos GA, Schüpper AJ, Lakomkin N, et al. Fluorescence-Guided High-Grade Glioma Surgery More Than Four Hours After 5-Aminolevulinic Acid Administration. Front Neurol 2021; 12:644804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hauser SB, Kockro RA, Actor B, et al. Combining 5-Aminolevulinic Acid Fluorescence and Intraoperative Magnetic Resonance Imaging in Glioblastoma Surgery: A Histology-Based Evaluation. Neurosurgery 2016; 78:475–483. [DOI] [PubMed] [Google Scholar]
- 166.Roberts DW, Valdés PA, Harris BT, et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg 2011; 114:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yano H, Nakayama N, Ohe N, et al. Pathological analysis of the surgical margins of resected glioblastomas excised using photodynamic visualization with both 5-aminolevulinic acid and fluorescein sodium. J Neurooncol 2017; 133:389–397. [DOI] [PubMed] [Google Scholar]
- 168.Stummer W, Pichlmeier U, Meinel T, et al. ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006; 7:392–401. [DOI] [PubMed] [Google Scholar]
- 169.Fountain DM, Bryant A, Barone DG, et al. Intraoperative imaging technology to maximise extent of resection for glioma: a network meta-analysis. Cochrane Database Syst Rev 2021; 1:CD013630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Della Puppa A, Munari M, Gardiman MP, et al. Combined Fluorescence Using 5-Aminolevulinic Acid and Fluorescein Sodium at Glioblastoma Border: Intraoperative Findings and Histopathologic Data About 3 Newly Diagnosed Consecutive Cases. World Neurosurg 2019; 122:e856–e863. [DOI] [PubMed] [Google Scholar]
- 171.Canalini L, Klein J, Miller D, et al. Enhanced registration of ultrasound volumes by segmentation of resection cavity in neurosurgical procedures. Int J Comput Assist Radiol Surg 2020; 15:1963–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bø HK, Solheim O, Kvistad KA, et al. Intraoperative 3D ultrasound-guided resection of diffuse low-grade gliomas: radiological and clinical results. J Neurosurg 2019; 132:518–529. [DOI] [PubMed] [Google Scholar]
- 173.Unsgård G, Lindseth F. 3D ultrasound-guided resection of low-grade gliomas: principles and clinical examples. Neurosurg Focus 2019; 47:E9. [DOI] [PubMed] [Google Scholar]
- 174.Cepeda S, García-García S, Velasco-Casares M, et al. Is There a Relationship between the Elasticity of Brain Tumors, Changes in Diffusion Tensor Imaging, and Histological Findings? A Pilot Study Using Intraoperative Ultrasound Elastography. Brain Sci 2021; 11:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yeole U, Singh V, Mishra A, et al. Navigated intraoperative ultrasonography for brain tumors: a pictorial essay on the technique, its utility, and its benefits in neuro-oncology. Ultrasonography 2020; 39:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nitsch J, Klein J, Dammann P, et al. Automatic and efficient MRI-US segmentations for improving intraoperative image fusion in image-guided neurosurgery. Neuroimage Clin 2019; 22:101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Coburger J, Scheuerle A, Kapapa T, et al. Sensitivity and specificity of linear array intraoperative ultrasound in glioblastoma surgery: a comparative study with high field intraoperative MRI and conventional sector array ultrasound. Neurosurg Rev 2015; 38:499–509; discussion 509. [DOI] [PubMed] [Google Scholar]
- 178.Bastos DCA, Juvekar P, Tie Y, et al. Challenges and Opportunities of Intraoperative 3D Ultrasound With Neuronavigation in Relation to Intraoperative MRI. Front Oncol 2021; 11:656519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shi J, Zhang Y, Yao B, et al. Application of Multiparametric Intraoperative Ultrasound in Glioma Surgery. Biomed Res Int 2021; 2021:6651726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Carton FX, Chabanas M, Le Lann F, et al. Automatic segmentation of brain tumor resections in intraoperative ultrasound images using U-Net. J Med Imaging (Bellingham) 2020; 7:031503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mursch K, Scholz M, Brück W, Behnke-Mursch J. The value of intraoperative ultrasonography during the resection of relapsed irradiated malignant gliomas in the brain. Ultrasonography 2017; 36:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Saß B, Pojskic M, Zivkovic D, et al. Utilizing Intraoperative Navigated 3D Color Doppler Ultrasound in Glioma Surgery. Front Oncol 2021; 11:656020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Policicchio D, Ticca S, Dipellegrini G, et al. Multimodal Surgical Management of Cerebral Lesions in Motor-Eloquent Areas Combining Intraoperative 3D Ultrasound with Neurophysiological Mapping. J Neurol Surg A Cent Eur Neurosurg 2021; 82:344–356. [DOI] [PubMed] [Google Scholar]
- 184.Hu X, Xu R, Ding H, et al. The total resection rate of glioma can be improved by the application of US-MRI fusion combined with contrast-enhanced ultrasound. Clin Neurol Neurosurg 2021; 208:106892. [DOI] [PubMed] [Google Scholar]
- 185.Xiao Y, Rivaz H, Chabanas M, et al. Evaluation of MRI to Ultrasound Registration Methods for Brain Shift Correction: The CuRIOUS2018 Challenge. IEEE Trans Med Imaging 2020; 39:777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sastry R, Bi WL, Pieper S, et al. Applications of Ultrasound in the Resection of Brain Tumors. J Neuroimaging 2017; 27:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352:987–996. [DOI] [PubMed] [Google Scholar]
- 188.Blumenthal DT, Mendel L, Bokstein F. The optimal regimen of bevacizumab for recurrent glioblastoma: does dose matter? J Neurooncol 2016; 127:493–502. [DOI] [PubMed] [Google Scholar]
- 189.Yamaguchi S, Motegi H, Ishi Y, et al. Clinical Outcome of Cytoreductive Surgery Prior to Bevacizumab for Patients with Recurrent Glioblastoma: A Single-center Retrospective Analysis. Neurol Med Chir (Tokyo) 2021; 61:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yonezawa H, Ohno M, Igaki H, et al. Outcomes of salvage fractionated re-irradiation combined with bevacizumab for recurrent high-grade gliomas that progressed after bevacizumab treatment*. Jpn J Clin Oncol 2021; 51:1028–1035. [DOI] [PubMed] [Google Scholar]
- 191.Detti B, Scoccianti S, Teriaca MA, et al. Bevacizumab in recurrent high-grade glioma: a single institution retrospective analysis on 92 patients. Radiol Med 2021; 126:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takigawa K, Hata N, Michiwaki Y, et al. Volumetric study reveals the relationship between outcome and early radiographic response during bevacizumab-containing chemoradiotherapy for unresectable glioblastoma. J Neurooncol 2021; 154:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kulinich DP, Sheppard JP, Nguyen T, et al. Radiotherapy versus combination radiotherapy-bevacizumab for the treatment of recurrent high-grade glioma: a systematic review. Acta Neurochir (Wien) 2021; 163:1921–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kinzel A, Ambrogi M, Varshaver M, et al. Tumor Treating Fields for Glioblastoma Treatment: Patient Satisfaction and Compliance With the Second-Generation Optune® System. Clin Med Insights Oncol 2019; 13:1179554918825449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chan AK, Birk HS, Winkler EA, et al. Stability of Programmable Shunt Valve Settings with Simultaneous Use of the Optune Transducer Array: A Case Report. Cureus 2016; 8:e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Murphy J, Bowers ME, Barron L. Optune®: Practical Nursing Applications. Clin J Oncol Nurs 2016; 20:S14–19. [DOI] [PubMed] [Google Scholar]
- 197.Doyle SP, Gurbani SS, Ross AS, et al. The role of erlotinib and the Optune device in a patient with an epidermal growth factor receptor viii amplified glioblastoma. Oxf Med Case Reports 2018; 2018:omy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Walker BC, Adhikari S, Mittal S. Therapeutic potential of curcumin for the treatment of malignant gliomas, In: Debinski W, ed. Gliomas. Brisbane:Exon Publications, 2021; 139–149. [PubMed] [Google Scholar]
- 199.Walker BC, Mittal S. Antitumor Activity of Curcumin in Glioblastoma. Int J Mol Sci 2020; 21:9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ahmed R, Oborski MJ, Hwang M, et al. Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag Res 2014; 6:149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sharma RR, Singh DP, Pathak A, et al. Local control of high-grade gliomas with limited volume irradiation versus whole brain irradiation. Neurol India 2003; 51:512–517. [PubMed] [Google Scholar]
- 202.Kadiyala P, Li D, Nuñez FM, et al. High-Density Lipoprotein-Mimicking Nanodiscs for Chemo-immunotherapy against Glioblastoma Multiforme. ACS Nano 2019; 13:1365–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Petrecca K, Guiot MC, Panet-Raymond V, et al. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J Neurooncol 2013; 111:19–23. [DOI] [PubMed] [Google Scholar]
- 204.Zhu Y, Jia J, Zhao G, et al. Multi-responsive nanofibers composite gel for local drug delivery to inhibit recurrence of glioma after operation. J Nanobiotechnology 2021; 19:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhao G, Jia J, Wang L, et al. Local Delivery of Minocycline and Vorinostat Targets the Tumor Microenvironment to Inhibit the Recurrence of Glioma. Onco Targets Ther 2020; 13:11397–11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bacellar IO, Tsubone TM, Pavani C, et al. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int J Mol Sci 2015; 16:20523–20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Valdés PA, Kim A, Brantsch M, et al. δ-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: the need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro Oncol 2011; 13:846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Johansson A, Faber F, Kniebühler G, et al. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg Med 2013; 45:225–234. [DOI] [PubMed] [Google Scholar]
- 209.Johansson A, Palte G, Schnell O, et al. 5-Aminolevulinic acid-induced protoporphyrin IX levels in tissue of human malignant brain tumors. Photochem Photobiol 2010; 86:1373–1378. [DOI] [PubMed] [Google Scholar]
- 210.Fukami S, Akimoto J, Nagai K, et al. Clinical application of the mirror irradiation technique in photodynamic therapy for malignant glioma. Photodiagnosis Photodyn Ther 2020; 31:101956. [DOI] [PubMed] [Google Scholar]
- 211.Akimoto J, Fukami S, Ichikawa M, et al. Intraoperative Photodiagnosis for Malignant Glioma Using Photosensitizer Talaporfin Sodium. Front Surg 2019; 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shimizu K, Nitta M, Komori T, et al. Intraoperative Photodynamic Diagnosis Using Talaporfin Sodium Simultaneously Applied for Photodynamic Therapy against Malignant Glioma: A Prospective Clinical Study. Front Neurol 2018; 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bock HC, Puchner MJ, Lohmann F, et al. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev 2010; 33:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kadota T, Saito R, Kumabe T, et al. A multicenter randomized phase III study for newly diagnosed maximally resected glioblastoma comparing carmustine wafer implantation followed by chemoradiotherapy with temozolomide with chemoradiotherapy alone; Japan Clinical Oncology Group Study JCOG1703 (MACS study). Jpn J Clin Oncol 2019; 49:1172–1175. [DOI] [PubMed] [Google Scholar]
- 215.Champeaux C, Weller J. Implantation of carmustine wafers (Gliadel®) for high-grade glioma treatment. A 9-year nationwide retrospective study. J Neurooncol 2020; 147:159–169. [DOI] [PubMed] [Google Scholar]
- 216.Bettag C, Hussein A, Sachkova A, et al. Implantation of Carmustine wafers after resection of malignant glioma with and without opening of the ventricular system. J Neurooncol 2021; 153:519–525. [DOI] [PubMed] [Google Scholar]
- 217.Xiao ZZ, Wang ZF, Lan T, et al. Carmustine as a Supplementary Therapeutic Option for Glioblastoma: A Systematic Review and Meta-Analysis. Front Neurol 2020; 11:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yerram P, Reiss SN, Modelevsky L, et al. Evaluation of toxicity of carmustine with or without bevacizumab in patients with recurrent or progressive high grade gliomas. J Neurooncol 2019; 145:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.La Rocca RV, Rezazadeh A. Carmustine-impregnated wafers and their impact in the management of high-grade glioma. Expert Opin Pharmacother 2011; 12:1325–1332. [DOI] [PubMed] [Google Scholar]
- 220.Fisher JP, Adamson DC. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021; 9:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shibahara I, Miyasaka K, Sekiguchi A, et al. Long-term follow-up after BCNU wafer implantation in patients with newly diagnosed glioblastoma. J Clin Neurosci 2021; 86:202–210. [DOI] [PubMed] [Google Scholar]
- 222.Fung LK, Ewend MG, Sills A, et al. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res 1998; 58:672–684. [PubMed] [Google Scholar]
- 223.Nishikawa R, Iwata H, Sakata Y, et al. Safety of Gliadel Implant for Malignant Glioma: Report of Postmarketing Surveillance in Japan. Neurol Med Chir (Tokyo) 2021; 61: 536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hart MG, Grant R, Garside R, et al. Chemotherapy wafers for high grade glioma. Cochrane Database Syst Rev 2011; 2011:CD007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Senft C, Bink A, Franz K, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 2011; 12:997–1003. [DOI] [PubMed] [Google Scholar]
- 226.Jenkinson MD, Barone DG, Bryant A, et al. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst Rev 2018; 1:CD012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lo YT, Lee H, Shui C, et al. Intraoperative Magnetic Resonance Imaging for Low-Grade and High-Grade Gliomas: What Is the Evidence? A Meta-Analysis. World Neurosurg 2021; 149:232–243.e3. [DOI] [PubMed] [Google Scholar]
- 228.Shah AS, Sylvester PT, Yahanda AT, et al. Intraoperative MRI for newly diagnosed supratentorial glioblastoma: a multicenter-registry comparative study to conventional surgery. J Neurosurg. 2020. Oct 9. doi: 10.3171/2020.6.JNS19287. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 229.Wach J, Banat M, Borger V, et al. Intraoperative MRI-guided Resection in Pediatric Brain Tumor Surgery: A Meta-analysis of Extent of Resection and Safety Outcomes. J Neurol Surg A Cent Eur Neurosurg 2021; 82:64–74. [DOI] [PubMed] [Google Scholar]