Abstract
Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA; Gadoxetic acid; Gadoxetate disodium) is a hepatocyte-specific MR contrast agent. It acts as an extracellular contrast agent in the early phase after intravenous injection, and then is taken up by hepatocytes later. Using this contrast agent, we can evaluate the hemodynamics of the liver and liver tumors, and can therefore improve the detection and characterization of hepatocellular carcinoma (HCC). Gd-EOB-DTPA helps in the more accurate detection of hypervascular HCC than by other agents. In addition, Gd-EOB-DTPA can detect hypovascular HCC, which is an early stage of the multi-stage carcinogenesis, with a low signal in the hepatobiliary phase. In addition to tumor detection and characterization, Gd-EOB-DTPA contrast-enhanced MR imaging can be applied for liver function evaluation and prognoses evaluation. Thus, Gd-EOB-DTPA plays an important role in the diagnosis of HCC. However, we have to employ optimal imaging techniques to improve the diagnostic ability. In this review, we aimed to discuss the characteristics of the contrast media, optimal imaging techniques, diagnosis, and applications.
Keywords: magnetic resonance, liver, hepatocellular carcinoma, gadoxetic acid, xetate disodium
Introduction
Hepatocyte-specific MR contrast agents (Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid [Gd-EOB-DTPA]; Gadoxetic acid; Gadoxetate disodium) act as extracellular contrast agents (e.g. Gadopentetate dimeglumine, Gadolinium diethylenetriamine pentaacetic acid [Gd-DTPA], etc.) in the early phase after intravenous injection and are taken up by hepatocytes later. As it acts as an extracellular contrast agent in the early phase, it is possible to understand the hemodynamics of the liver and liver tumors, as well as extracellular contrast agents.1,2 In the late phase, Gd-EOB-DTPA is taken up by hepatocytes via a transporter expressed on the hepatocyte membrane. The transporter, organic anion transporting polypeptide (OATP) 1B3 (OATP8), is the main carrier of Gd-EOB-DTPA uptake; OATP1B1 and sodium taurocholate cotransporting peptide (NTCP) are also involved.3–5 It is excreted into the bile fluid with the help of multidrug resistance-associated protein (MRP) 2, which is expressed on the canalicular side of hepatocytes, and MRP3, which is expressed on the sinusoidal side.4 In fact, the contrast medium begins to be taken up by hepatocytes approximately 1 min after administration, and the hepatic parenchyma shows a high signal at approximately 15–20 min after the intravenous injection (hepatobiliary phase/hepatocellular phase). In the hepatobiliary phase, hepatic tumors that lack hepatocellular function are not enhanced, and sufficient tumor/liver contrast can be obtained.
Another liver-specific MR contrast agent is superparamagnetic iron oxide (SPIO), which is a nanoparticle that is taken up by Kupffer cells in the liver; SPIO can assess Kupffer cell function but cannot assess blood flow in the liver or tumors. In contrast, Gd-EOB-DTPA is characterized by its ability to evaluate blood flow as an extracellular contrast agent and hepatocellular function as a hepatocyte-specific contrast agent (Fig. 1).1–3,6–9
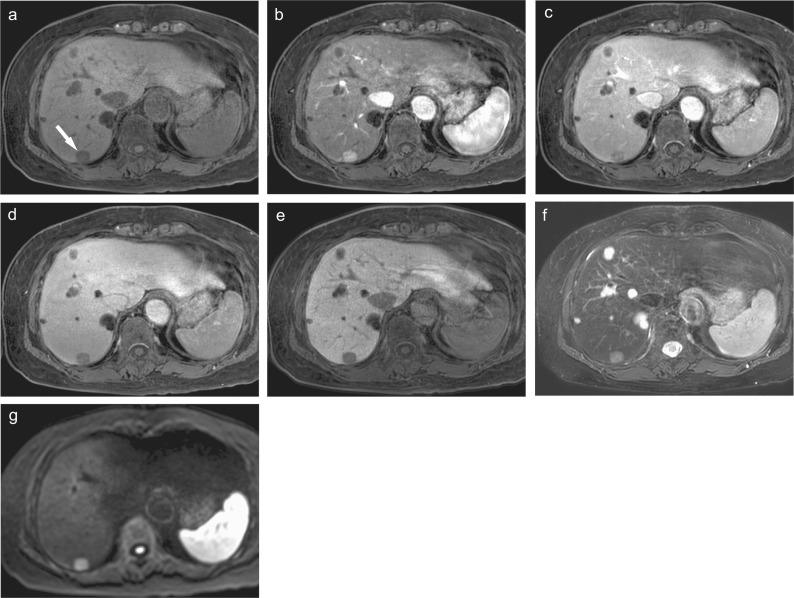
Fig. 1.
Gd-EOB-DTPA-enhanced MR images of hypervascular hepatocellular carcinoma. (a) Precontrast fat-suppressed 3D Fourier transformation T1-weighted MR image showing a small low-intensity lesion in the posterior lobe of the liver (arrow). (b) Arterial phase image showing the early enhanced tumor. (c) Portal venous phase and (d) transitional phase images showing the tumor as a hypointense area. (e) Hepatobiliary phase image showing a marked low-intensity tumor, suggesting the lack of hepatocellular function. (f) Fat-suppressed T2-weighted image and (g) diffusion-weighted image (b value = 800 s/mm2) showing the hyperintense tumor. These images show a characteristic finding of hypervascular hepatocellular carcinoma. Multiple cysts are also seen in the liver. Gd-EOB-DTPA, Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid.
Gd-EOB-DTPA can evaluate blood flow information and hepatocellular function, and is effective in the diagnosis of hepatocellular carcinoma (HCC). It is crucial to understand the characteristics of the contrast agent for ensuring early diagnosis of and treatment for HCC. In this review, the characteristics of contrast media, imaging techniques, diagnosis, and applications will be discussed.
Imaging Techniques Using Gd-EOB-DTPA Contrast-enhanced MR Imaging
Dynamic MR imaging, in which the whole liver is imaged sequentially with a rapid intravenous injection of a contrast medium, is essential for the characterization, detection, and staging of liver tumors. It is well known that dynamic MR imaging with an extracellular contrast agent is useful for the diagnosis of hypervascular HCC.10,11 Dynamic MR imaging with Gd-EOB-DTPA is also useful for the diagnosis of HCC (Fig. 1).8,9
Dynamic MR imaging
Arterial phase
Gd-EOB-DTPA has a high T1 relaxivity. Although the T1 relaxation of Gd-EOB-DTPA is about twice as that of extracellular Gd contrast agents, the total T1 shortening effect of Gd-EOB-DTPA is only about 1/2 because the Gd molar volume is 1/4. In addition, as the standard dose is 0.1 mL/kg, which is only half of that of extracellular Gd contrast agents, it is important to optimize the imaging method for each phase of dynamic MR imaging, especially the arterial phase, to evaluate the arterial enhancement of liver tumors.2,9 In a study of sequential changes of contrast enhancement in Gd-EOB-DTPA-enhanced MR imaging, the peak of hypervascular HCC enhancement occurred approximately 14 seconds after the arrival of the contrast medium to the aorta.12 This indicates that it is extremely effective to collect data near the center of the k-space at the peak of tumor enhancement approximately 14 seconds after the aortic arrival of the contrast medium to obtain good depiction of arterial enhancement of hypervascular HCC. The optimal imaging method for the arterial phase is highly dependent on the performance of the MR imaging system. To be able to capture the tumor peak enhancement at the optimal timing, the imaging protocol, such as the imaging time and the k-space data collection design of the imaging sequence, should be optimized considering the performance of the MR imaging system.
As an imaging sequence, 3D Fourier transformation (3DFT) T1-weighted fast imaging with fat suppression is recommended.8,9 Although 3DFT imaging tends to require longer imaging time, some techniques, such as the parallel imaging, the sensitivity encoding (SENSE), and the compressed sensing techniques, enable us to shorten the imaging time and improve the spatial resolution.13,14 In dynamic MR imaging, it is essential to obtain T1-weighted images of the whole liver during breath holding. The 3DFT imaging method with fat suppression enables dynamic MR imaging with high spatial resolution (Fig. 1).
In recent years, ultra-fast multiple arterial phase imaging has become possible. The k-space information, which is the source of MR images, is composed of low-frequency components that contribute to image contrast and high-frequency components, which in turn contribute to spatial resolution. In keyhole imaging, low-frequency component data are collected continuously, and less frequently collected high-frequency component data are shared among time phases to improve temporal resolution. This method enables time-resolved imaging of the arterial phase while suppressing the decrease in spatial resolution. In addition, by sectioning the center of the k-space set and repeating short imaging sessions, as well as using the view sharing method, it is possible to further improve the temporal resolution while reducing the effects of body movement during dynamic imaging (Fig. 2). These fast imaging techniques are particularly effective in 3-Tesla (3T) MR imaging systems that have a high SNR, allowing whole liver dynamic MR imaging, with a temporal resolution of 1–2 seconds, to be performed with a single breath-hold.15 More recently, free-breathing imaging methods using techniques such as stack-of-stars k-space sampling and golden-angle radial sparse parallel (GRASP) reconstruction have been developed, and the usefulness of dynamic imaging without breath-holding has been studied.16
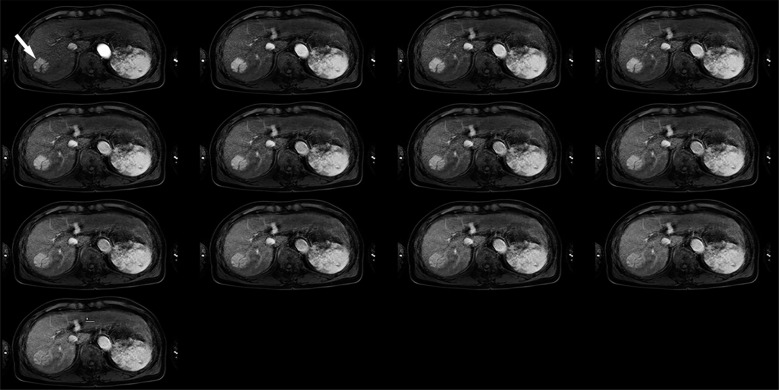
Fig. 2.
Hypervascular hepatocellular carcinoma (arrow) in the right lobe of the liver. A 3D Fourier transformation gradient-echo sequence with a parallel imaging and keyhole data sampling techniques can show sequential enhancement of hypervascular hepatocellular carcinoma during the 20-second breath-holding without severe artifacts caused by respiratory motion.
Portal venous phase and transitional phase
The portal venous phase imaging is performed approximately 60 seconds after the intravenous administration of Gd-EOB-DTPA, and transitional phase imaging after 120–180 seconds. Since Gd-EOB-DTPA is taken up by hepatocytes up to 1.5 min after the intravenous administration,9,17 the portal and equilibrium phases of extracellular contrast-enhanced MR imaging or dynamic CT do not correspond to the portal and transitional phases of Gd-EOB-DTPA contrast-enhanced MR imaging. Since this difference is more pronounced 120–180 seconds than 60–80 seconds after the intravenous administration, the term equilibrium phase is not appropriate for the 180-second post-contrast phase of Gd-EOB-DTPA contrast-enhanced MRI; instead, the term transitional phase is recommended (Fig. 3). In the diagnosis of hypervascular HCC using dynamic MR imaging with extracellular contrast medium and dynamic CT, washout of the contrast medium during portal and equilibrium phases is an important diagnostic finding. However, it should be noted that the portal and transitional phases of Gd-EOB-DTPA contrast-enhanced MR imaging and extracellular contrast-enhanced MR imaging are different. According to the current version of Liver Imaging Reporting and Data System (LI-RADS), washout should be evaluated only in the portal phase for Gd-EOB-DTPA contrast-enhanced MR imaging.18
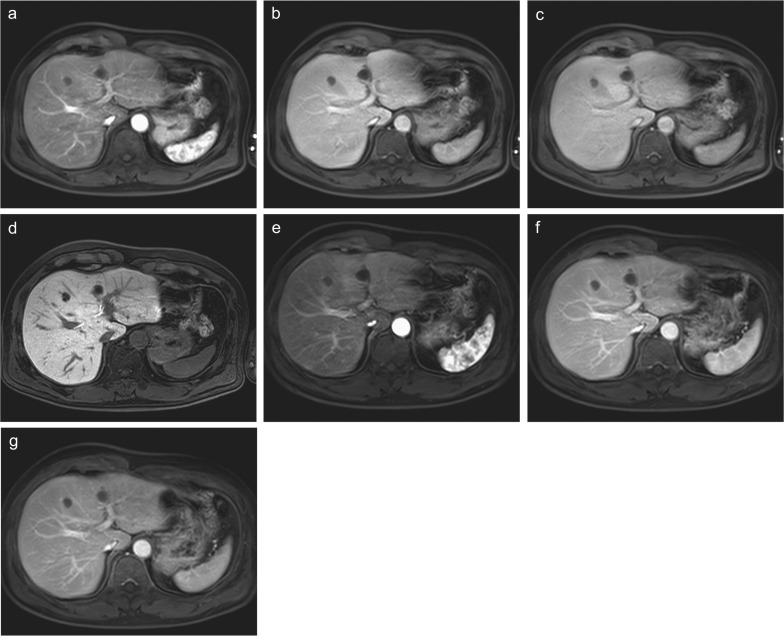
Fig. 3.
A comparison of dynamic MR images between Gd-EOB-DTPA enhancement and extracellular Gd contrast enhancement in the same patient. Gd-EOB-DTPA-enhanced (a) arterial, (b) portal venous, (c) transitional, and (d) hepatobiliary phase images and extracellular Gd contrast-enhanced (e) arterial, (f) portal venous, and (g) equilibrium phase images are shown. Although arterial phase images are similar between the images using two contrast agents, the contrast between the liver parenchyma and vasculatures is better in (f) extracellular Gd contrast-enhanced images than in (b) Gd-EOB-DTPA-enhanced images in the portal venous phase. The difference is more prominent between (g) extracellular Gd contrast-enhanced equilibrium phase image and (c) Gd-EOB-DTPA-enhanced transitional phase image probably due to slight uptake of Gd-EOB-DTPA into hepatocytes. The hepatic parenchyma shows a high signal and the vasculature shows a low signal in (d) Gd-EOB-DTPA-enhanced hepatobiliary phase. Gd-EOB-DTPA, Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid.
Hepatobiliary phase
In hepatobiliary phase imaging, which is performed 15–20 min after the intravenous Gd-EOB-DTPA administration, the presence or absence of the contrast agent uptake by the tumor is an important finding. The hepatic parenchyma with hepatocellular function shows a high signal, whereas tumors without hepatocellular function show a low signal, resulting in increased liver-tumor contrast and improved tumor delineation.1,2,8,9 However, as hepatic damage progresses due to chronic liver diseases, it becomes difficult to obtain sufficient signal elevation of the liver parenchyma even after waiting more than 20 min after administration; thus, the ability to depict tumors decreases. The degree of hepatic parenchymal enhancement correlates with total bilirubin levels and indocyanine green (ICG) values.19,20 This phenomenon could be used to evaluate liver function.19–21
In an evaluation of metastatic liver tumors, the liver function of the patient is often good. Therefore, even images taken 15 min after the Gd-EOB-DTPA administration show sufficient Gd-EOB-DTPA uptake into the background liver, which is sufficient for hepatobiliary phase imaging.22–24
Artifacts and countermeasures
In dynamic MR imaging with Gd-EOB-DTPA, artifacts such as truncation, marginal high intensity, blurring, and motion artifacts can degrade image quality, especially in the arterial phase.
Truncation artifacts are more likely to be seen in the arterial phase of Gd-EOB-DTPA contrast-enhanced MR imaging. This is because the aortic enhancement time in the arterial phase is shorter when using Gd-EOB-DTPA than when using extracellular Gd contrast agents, and this leads to large signal changes during k-space data acquisition. To improve the truncation artifact, it is effective to extend the enhancement time in the arterial phase by slowing down the infusion rate to 1–2 mL/s and to complete imaging, while the signal change in the aorta is small, i.e., in a short time (high speed), or to increase the spatial resolution in the phase encode direction.25
Marginal high intensity and blurring are artifacts that are often observed when centric ordered k-space data collection is employed. In this method, data are collected starting from the center of the k-space when the fat signal passes the null point after the inversion recovery (IR) pulse. In contrast, the data collection of the periphery of the k-space (high-frequency component) is performed later. By the time the high-frequency component data are collected, the fat signal that was knocked down using the IR pulse recovers considerably. Since the high-frequency component is a signal that emphasizes the sharpness of images, the signal at the marginal regions, such as the edge of the liver, becomes high. This is called marginal high intensity artifact (Fig. 4). Besides, in centric ordering, blurring due to an unstable steady state may occur because k-space centric data acquisition starts immediately after the spins are excited. When those artifacts are conspicuous, we recommend the choice of sequential ordering for k-space data acquisition to reduce artifacts.13
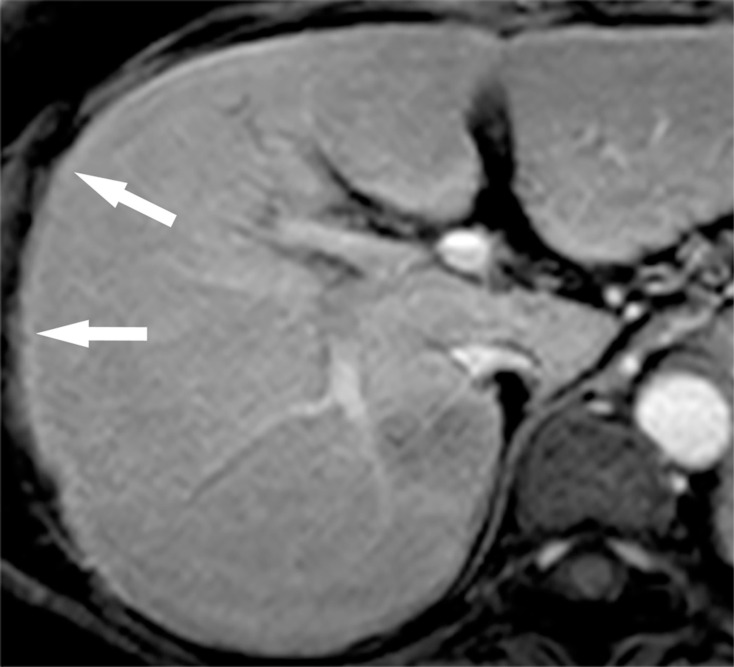
Fig. 4.
Gd-EOB-DTPA-enhanced arterial phase image. High-intensity band is seen at the margin of the liver (arrows). This marginal high intensity is an artifact that is sometimes observed when k-space data collection is centrally ordered. Gd-EOB-DTPA, Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid.
In Gd-EOB-DTPA contrast-enhanced MR imaging, transient severe motion artifacts in the arterial phase are known to occur frequently.26 Compared with other extracellular contrast agents, Gd-EOB-DTPA is reported to cause a higher frequency of image degradation owing to shorter patient breath-hold times and greater respiratory motion during the arterial phase. This artifact reportedly occurs in 5% to 18% of patients. Several methods have been proposed to reduce the motion artifacts that are frequently seen in the arterial phase. These include shortening the imaging time, using a multi-arterial phase, using a diluted contrast agent, and performing breath-hold training before the scan.27
Imaging techniques other than dynamic imaging
In addition to dynamic imaging, it is important to use T1-weighted images, T2-weighted images, and diffusion-weighted images to obtain higher diagnostic performance.
Unenhanced T1-weighted image
For T1-weighted images, in-phase and out-of-phase gradient-echo images are obtained during breath-holding. This can assess the fat content in the liver nodules, such as lipoma, angiomyolipoma, adenoma, and fatty degenerated well-differentiated HCC, and is useful for HCC diagnosis.
T2-weighted image and diffusion-weighted image
Gd-EOB-DTPA has a strong T1-shortening effect but a weak T2-shortening effect. After the administration of Gd-EOB-DTPA, the contrast agent migrates to the liver parenchyma and bile, and its T2*-shortening effect causes mild signal loss in the liver parenchyma on T2-weighted and diffusion-weighted images. Therefore, the tumor and liver parenchyma contrast are improved on T2-weighted image; thus, imaging after Gd-EOB-DTPA contrast is not considered a problem.28,29
Heavily T2-weighted image
Heavily T2-weighted images are used in MR cholangiopancreatography (MRCP) and should be obtained before or within 1.5 min after Gd-EOB-DTPA administration. After 1.5 min of contrast administration, Gd-EOB-DTPA excretes into the bile, shortening the T2 value of the bile and decreasing the signal of the bile ducts. Therefore, the image quality of MRCP is degraded.30
T1 mapping
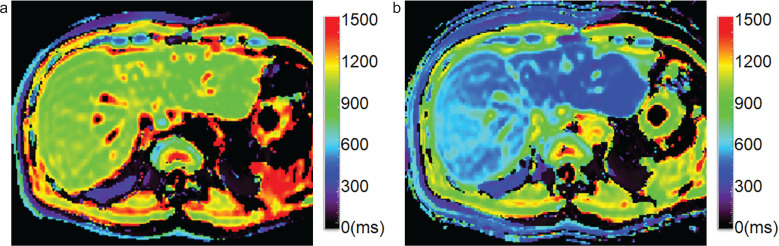
T1 mapping was developed as a method to measure T1 values, which are specific to tissues, and is useful for the quantitative evaluation of the degree of contrast enhancement (Fig. 5). To obtain T1 mapping of tissues, image data obtained using the Look-Locker sequence are used. This sequence is based on the IR method, in which data are collected continuously during the longitudinal relaxation after the IR pulse, and T1 values can be measured.31 Since it is possible to quantitatively evaluate the kinetics of Gd-EOB-DTPA uptake and excretion into bile, it is expected to be applied to evaluate hepatocyte function.19,20,32
Fig. 5.
T1 mapping images after intraarterial chemotherapy to the right lobe of the liver. T1 mapping images calculated using the Look-Locker sequence (TR, 12 ms; TE, 1.7 ms; flip angle, 7 deg) obtained (a) before Gd-EOB-DTPA administration and (b) 18 min after administration. Precontrast T1 relaxation times were 937.1 and 878.1 ms for the right and left lobe of the liver, respectively. Post-contrast times were 495.8 and 350.2 ms for the right and left lobe, respectively. The reduction rates of T1 relaxation time were 47.1% and 60.1%, respectively. This patient underwent arterial injection chemotherapy before MR imaging. Anti-cancer drug was injected only into the right hepatic artery. The reduction rate is higher in the left lobe than in the right lobe, suggesting better hepatocyte function in the left lobe of the liver. Gd-EOB-DTPA, Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid.
Magnetic field strength
In recent years, there has been a rapid increase in the clinical use of 3T MR imaging. Compared to 1.5T systems, 3T systems have sufficient SNR improvement. As mentioned above, Gd-EOB-DTPA contrast-enhanced MR imaging requires high-speed imaging with high spatial resolution. Both of these conditions are unfavorable to SNR, but 3T MR imaging ensures sufficient SNR to achieve high-speed and high spatial resolution imaging.
Diagnosis of Hepatocellular Carcinoma
Based on hemodynamics, HCC can be divided into hypervascular or hypovascular HCC. Typical hypervascular HCC is relatively easy to diagnose because it is enhanced in the arterial phase of dynamic MR imaging and shows washout of the contrast medium in the portal and equilibrium phases (Fig. 1). However, hypovascular HCC without arterial enhancement may be difficult to detect and diagnose.
Hypervascular hepatocellular carcinoma
Hypervascular HCC is enhanced in the arterial phase and shows washout later. According to reports on the detection sensitivity of hypervascular HCC, Gd-EOB-DTPA contrast-enhanced MR imaging is better than dynamic CT or dynamic MR imaging using extracellular contrast agents.33,34 However, Gd-EOB-DTPA contrast-enhanced MR imaging may be less diagnostic in patients with poor liver function or obstructive jaundice because contrast uptake decreases in such conditions. Angiography-assisted CT, which is invasive, should not only be performed for diagnostic purposes but also in conjunction with endovascular treatment procedures, such as transcatheter arterial chemoembolization.
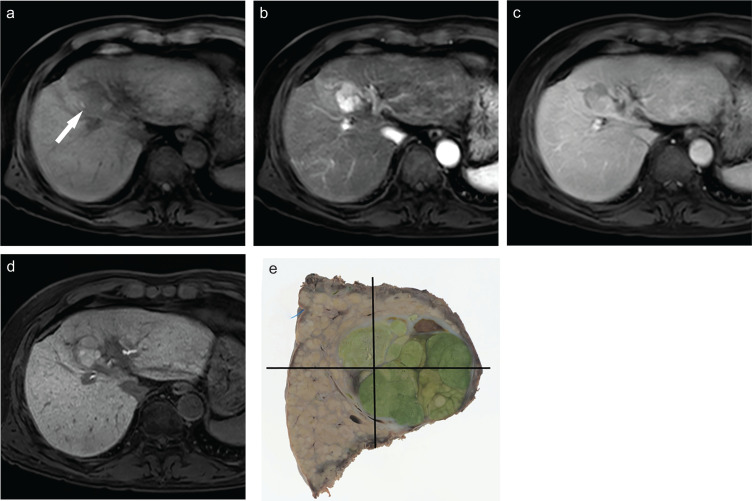
In some HCCs, bile is produced in the tumor and excreted immaturely; thus, the tumor shows a deep greenish color after formalin fixation and is called green hepatoma.3–5 These types of HCC take up Gd-EOB-DTPA through the OATP1 transporter but accumulate Gd-EOB-DTPA in the small bile ducts and pseudogland owing to an immature excretory system. These tumors are usually hypervascular and moderately differentiated HCC and are characterized by enhancement in both the arterial and hepatobiliary phases (Fig. 6),4,5 with a relatively good prognosis.35
Fig. 6.
Moderately differentiated hepatocellular carcinoma. (a) Precontrast MR image showing a hypointense nodule (arrow). (b) Gd-EOB-DTPA-enhanced arterial phase image showing the tumor as a hypervascular one. (c) Gd-EOB-DTPA-enhanced transitional phase image. (d) Hepatobiliary phase image showing that the tumor that was more enhanced than the liver parenchyma. (e) Image showing the tumor that has a diffuse greenish color on the cut surface. These types of tumor take up Gd-EOB-DTPA through the OATP1 transporter but accumulate Gd-EOB-DTPA in the small bile ducts and pseudogland owing to an immature excretory system. Gd-EOB-DTPA, Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid; OATP, organic anion transporting polypeptide.
Hypovascular hepatocellular carcinoma
The ability of Gd-EOB-DTPA contrast-enhanced MR imaging to detect hypovascular HCC is superior to that of dynamic CT, extracellular contrast-enhanced dynamic MR imaging, and SPIO contrast-enhanced MR imaging. Besides, it is comparable to or better than CT during arterial portography (CTAP) and CT during hepatic arteriography (CTHA) (Fig. 7).7,36
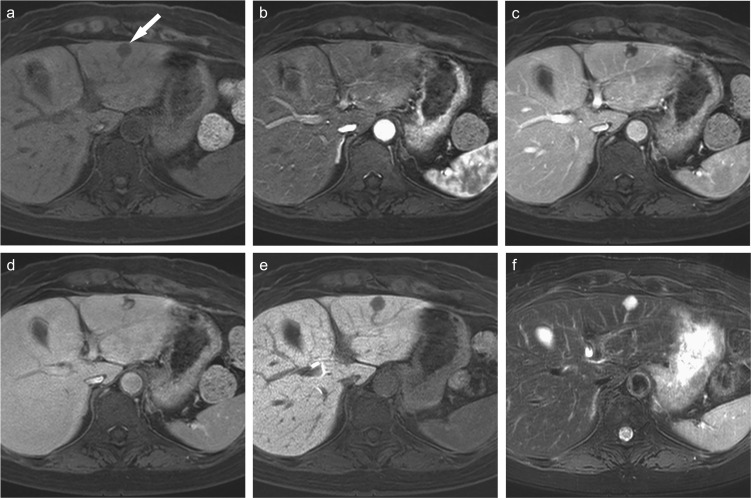
Fig. 7.
Pathologically confirmed well-differentiated hepatocellular carcinoma. (a) Precontrast fat-suppressed 3D Fourier transformation T1-weighted MR image showing a lesion in the lateral segment (arrow) that is almost iso-intense compared to the surrounding liver parenchyma. (Reprinted, with permission, from reference #7). Gd-EOB-DTPA-enhanced (b) arterial phase and (c) transitional phase images showing the tumor as iso- and hypointense nodules, respectively (arrows). (d) Hepatobiliary phase image showing a hypointense nodule (arrow). (e) Unenhanced T2*-weighted gradient-echo image showing the lesion as an iso-intense area compared to the surrounding liver parenchyma. (f) Superparamagnetic iron oxide (ferucarbotran)-enhanced T2*-weighted gradient-echo image (TR/TE/flip angle, 200/14/50 deg) obtained approximately 15 min after the injection (Kupffer phase) showing the nodule as an iso-intense area, indicating the similarity of intramodular Kupffer cell function between the nodule and the surrounding liver parenchyma. (g) CT during hepatic arteriography shows iso-attenuation, and (h) CT during arterial portography shows a slight low-attenuation compared to the surrounding liver parenchyma (arrow). Gd-EOB-DTPA, Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid.
Most hypovascular HCCs are well-differentiated HCCs. In well-differentiated HCCs, the tumor may take up SPIO owing to the presence of Kupffer cells in the tumor, which may reduce the ability of SPIO contrast-enhanced MR imaging to detect tumors.3,37,38 As for Sonazoid contrast-enhanced ultrasound, the ability to detect hypovascular HCC is limited, as is the case with SPIO, because Sonazoid is taken up by Kupffer cells in the post-vascular phase. In the process of multistep carcinogenesis, as the degree of tumor differentiation progresses from low to high grade, there is a progressive decrease in Sonazoid uptake into the lesion in the post-vascular phase. However, this reduction in uptake appears later than the reduction in Gd-EOB-DTPA uptake in the hepatobiliary phase. Thus, Gd-EOB-DTPA contrast-enhanced MR imaging is reportedly more effective than Sonazoid contrast-enhanced ultrasonography in detecting well-differentiated hypovascular HCC.39 In our past study, decreased Gd-EOB-DTPA uptake was observed in well-differentiated HCC and was not significantly different from the decreased uptake in moderately or poorly differentiated HCC. Gd-EOB-DTPA contrast-enhanced MR imaging is useful in delineating lesions regardless of the differentiation of HCC.36
In patients with chronic liver disease, hypovascular nodules, which are depicted only in the hepatobiliary phase of Gd-EOB-DTPA contrast-enhanced MR imaging, are often observed. The term “hepatobiliary phase (HBP) hypointense nodule without arterial phase hyperenhancement (APHE)” has recently been proposed for these nodules.40 The clinical question is how to deal with these nodules. A certain percentage of these nodules contain precancerous lesions or HCC, and the 2-years and 3-years cumulative incidence rates of transformation to hypervascular HCC are 11.1% and 15.9%, respectively.41 In another report, the 1-year and 2-years cumulative incidence rates were 19% and 45%, respectively.42 Imaging findings of hepatobiliary phase hypointense nodule without arterial phase hyperenhancement that is likely to transform into hypervascular HCC include a high signal on T2-weighted and diffusion-weighted images, growth rate of ≥ 1.8 × 10-3 /day (tumor volume doubling time of ≤ 542 days), presence of fatty components, and a diameter of ≥ 10–15 mm at the time of detection.43–46 In such cases, it is recommended that biopsy should be performed if the tumor is hypovascular, and early treatment be initiated, if necessary.
LI-RADS
Recently, LI-RADS was created to universalize the diagnostic imaging of the liver. The LI-RADS includes contrast-enhanced CT and MR imaging (extracellular contrast or hepatocyte-specific contrast) for patients at high risk for HCC, including adult liver transplant candidates and transplant recipients.47,48
The evaluation based on LI-RADS begins with the description of the major findings that are suggestive of HCC on dynamic CT or MR imaging. These are called major imaging features and include the three imaging findings of nonrim APHE, washout, and capsule, as well as tumor size and change in size over time. In addition to the major imaging features, the LI-RADS includes a set of ancillary imaging features. There are three ancillary imaging features: a) favoring HCC in particular, b) favoring malignancy, and c) favoring benignity. Favoring HCC in particular does not include anything related to Gd-EOB-DTPA. Favoring malignancy is a common finding in hepatic malignancies including HCC, and the imaging findings related to Gd-EOB-DTPA contrast-enhanced MR imaging include transitional phase hypointensity and hepatobiliary phase hypointensity. Favoring benignity is a finding suggestive of benignity, and the imaging findings related to Gd-EOB-DTPA contrast-enhanced MR imaging include EOB-MRI hepatobiliary phase isointensity.
A category of malignancies other than the typical HCC is an LR-M criterion, which includes atypical HCCs, combined hepatocellular–cholangiocellular carcinoma, and cholangiocellular carcinoma. In addition, abscesses and sclerosing hemangiomas may also be included. Targetoid appearance includes rim APHE, which is a spatially defined subtype of APHE in which arterial phase enhancement is most pronounced in observation periphery and peripheral washout, which is spatially defined subtype of washout in which apparent washout is most pronounced at the periphery. In terms of imaging findings related to Gd-EOB-DTPA contrast-enhanced MR imaging, targetoid transitional phase or hepatobiliary phase appearance is described.
Differential diagnosis
Points of differential diagnosis between HCC and other focal liver lesions on Gd-EOB-DTPA contrast-enhanced MR imaging are described.
Hemangioma
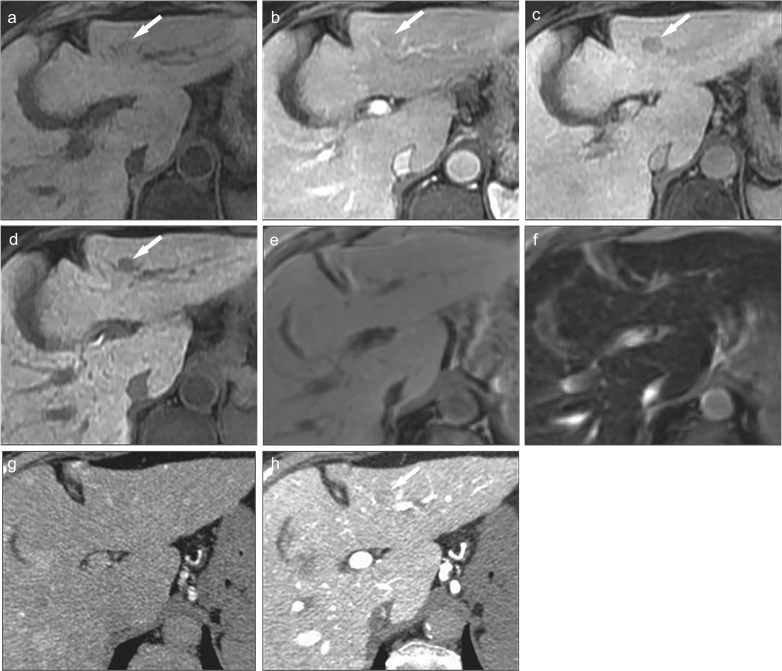
In extracellular contrast-enhanced dynamic MR imaging, hepatic hemangioma usually shows prolonged enhancement and appears as a high-signal lesion in the equilibrium phase. In contrast, hepatic hemangiomas do not show prolonged enhancement and appears as a low-signal lesion on Gd-EOB-DTPA contrast-enhanced hepatobiliary phase images. This is due to the faster disappearance of Gd-EOB-DTPA from the blood pool than of extracellular Gd contrast agents, and this should be considered when evaluating Gd-EOB-DTPA contrast-enhanced MR imaging. Therefore, it is important to detect characteristic findings in the arterial phase, such as peripheral globular enhancement, and a relatively strong high signal on T2-weighted images (Fig. 8).49 However, it must be noted that some hemangiomas show early total enhancement on Gd-EOB-DTPA contrast-enhanced arterial or portal phase images.50
Fig. 8.
Hemangioma of the liver. (a) Precontrast fat-suppressed 3D Fourier transformation T1-weighted MR image showing a hypointense small lesion in the lateral segment (arrow). Gd-EOB-DTPA-enhanced (b) arterial, (b) portal venous, and (d) transitional phase images showing peripheral globular enhancement pattern in the tumor. Gd-EOB-DTPA-enhanced (e) hepatobiliary phase image showing a hypointense nodule. Hemangiomas do not show prolonged enhancement that is usually shown in extracellular Gd contrast-enhanced MR imaging. (f) Fat-suppressed T2-weighted MR image showing the tumor as a strongly hyperintense tumor. It is important to detect characteristic findings such as peripheral globular enhancement, and a relatively stronger high signal on T2-weighted images to differentiate hepatocellular carcinoma from hemangioma. Gd-EOB-DTPA, Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid.
Arterioportal shunt (AP shunt)
Tissue-specific contrast agents, such as Gd-EOB-DTPA, do not show decreased uptake in the area with an AP shunt and are very useful in differentiating it from HCC. However, in rare cases, decreased uptake may occur.51
Focal nodular hyperplasia (FNH)
FNH is a typical hypervascular benign tumor that arises in the normal liver. Typical imaging findings include central scarring, a central artery, spoke wheel-like centrifugal flow, and the presence of Kupffer cells. Due to its hepatocellular function, Gd-EOB-DTPA is taken up by the tumor in the hepatobiliary phase.52,53
Nodular lesions associated with liver cirrhosis (FNH-like nodule)
Benign hyperplastic nodules may be seen in the liver of patients with chronic alcoholic liver disease and other conditions. In addition, hypervascular nodules have been reported in conditions with hemodynamic changes in the liver such as Budd–Chiari syndrome. Pathologically, these nodules are similar to FNH and are collectively referred to as FNH-like nodules.54 Most of these nodules show enhancement in the arterial phase and ring-like uptake of Gd-EOB-DTPA in the hepatobiliary phase.
Hepatocelullar adenoma (HCA)
According to the 2010 WHO classification, HCA is classified into four molecular biological types (hepatocyte nuclear factor 1α-inactivated type, β-catenin-activated type, inflammatory type, and unclassified type).55 Among these, the inflammatory type is the most common and is associated with fatty liver, heavy alcohol consumption, and obesity. The hepatocyte nuclear factor 1α-inactivated type and inflammatory type do not have Gd-EOB-DTPA uptake in the hepatocyte phase, but the β-catenin-activated type shows hyperenhancement in the hepatocyte phase. β-catenin-activated type has the potential for malignant transformation.56,57
Cholangiocellular carcinoma
The imaging findings of cholangiocellular carcinoma are similar to those of metastatic adenocarcinoma, with enhancement of the margins in the arterial phase of dynamic CT or MR imaging and delayed contrast enhancement in the central area with strong fibrosis in the late phase. It may also be accompanied by the dilatation of the peripheral intrahepatic bile ducts. Cholangiocellular carcinoma associated with chronic liver disease has been reported to have a high frequency of early enhancement, and it is important to differentiate it from HCC.58 In general, cholangiocellular carcinoma is differentiated by the absence of a capsule, the presence of dilated peripheral bile ducts, and a delayed enhancement due to the presence of fibrous stroma. Hepatobiliary phase Gd-EOB-DTPA contrast-enhanced MR image shows a low signal at the margins where tumor cells are abundant and a high signal at the center where the contrast agent remains owing to fibrosis, showing a target sign. This is considered to be an ancillary feature in LI-RADS.59
Metastatic liver cancer
Metastatic liver cancer is clearly visualized as a hypoenhanced nodule in the hepatobiliary phase. Gd-EOB-DTPA has a good ability to detect metastatic liver tumors,1,60 which can influence the choice of treatment.23
Prognostic and Hepatic Function Assessment Using Gd-EOB-DTPA MR Imaging
Functional assessment using Gd-EOB-DTPA MR imaging includes prognosis prediction based on tumor grade and assessment of regional liver function.
Prognostic evaluation
Hypervascular HCC with a high signal intensity in the hepatobiliary phase is sometimes observed. These tumors are usually hypervascular and moderately differentiated HCC (Fig. 6).4,5 This type of HCC is called green hepatoma and has been reported to have a relatively good prognosis.61
In addition, microvascular invasion (MVI) is associated with the prognosis of patients with HCC, and arterial and hepatobiliary phase Gd-EOB-DTPA contrast-enhanced MR imaging will be effective for the evaluation since they can clearly depict the arterial peritumoral enhancement, non-smooth tumor margin, and peritumoral hypointensity on the hepatobiliary phase as the risk factors of MVI.62–65
Gd-EOB-DTPA contrast-enhanced MR imaging is also useful in predicting post-hepatectomy liver failure. When liver function is heterogeneous owing to portal vein invasion or other factors, a combination of volumetry and liver function quantification using Gd-EOB-DTPA is reportedly useful.66–69
Resistance to immune checkpoint inhibitor therapy in HCCs with WNT/β-catenin mutations is recently reported. Since HCCs with WNT/β-catenin mutations show an iso-high intensity in the hepatobiliary phase, Gd-EOB-DTPA contrast-enhanced MR imaging may predict WNT/β-catenin mutation and resistance to immune checkpoint inhibitor therapy in HCC.70
Quantification of liver function using Gd-EOB-DTPA contrast-enhanced MR imaging
Liver functional assessment is important for the prediction of postoperative liver failure and prognosis. These have been heretofore predicted following serum testing with the ICG test and asialoscintigraphy (99mTc-GSA). As mentioned above, 50% of Gd-EOB-DTPA is taken up by hepatocytes via the OATP 1B1/B3 transporter and excreted via the MRP 2 transporter after administration. Taking advantage of this feature, many studies have been conducted on its usefulness in the evaluation of liver function. The kinetics of hepatocyte uptake and excretion of Gd-EOB-DTPA are affected by the hepatocyte function.19,20,32 The degree of hepatic parenchymal enhancement correlates with total bilirubin and ICG values.19 When hepatic damage is severe, it is difficult to obtain a hepatic signal elevation even after waiting for > 20 min after administration. This could be used to evaluate liver function based on the degree of contrast enhancement of the liver. In particular, the possibility of assessing segmental liver function is promising.19,20,32
Gd-EOB-DTPA contrast-enhanced MR imaging is mainly used to evaluate liver function by measuring the contrast effect in the liver parenchyma. However, it is often difficult to quantitatively evaluate the degree of enhancement on MR imaging because the signal intensity varies depending on various conditions, such as the presence or absence of tuning before imaging, even with the same imaging method. The method of using the signal intensity ratio between the liver and spleen or muscle is the simplest evaluation technique because it does not require a special imaging sequence. However, because signal intensity depends on the model and parameters, reproducibility is challenging.71 Unlike signal intensity, MR relaxation time (MR relaxometry) is an intrinsic value and is an ideal variable to evaluate liver function.32,72 In our study, we showed that the uptake of Gd-EOB-DTPA into hepatocytes clearly decreased when liver function was severely reduced. This may reflect a decrease in liver function owing to a decrease in the number of hepatocytes and, subsequently, a decrease in transporter function.72 Similarly, a strong correlation between changes in the T1 relaxometry and the extent of liver disease, expressed by the model for end-stage liver disease (MELD) score, was reported.73 Kamimura et al. also showed that indices using T1 values correlated more highly with the ICG removal rate than indices using the liver–spleen or liver–muscle signal ratio.74
Gd-EOB-DTPA contrast-enhanced MR imaging has excellent spatial resolution and can quantitatively evaluate partial hepatic function and is expected to be applied to daily clinical practice (Fig. 5).
Conclusion
Gd-EOB-DTPA, a hepatocyte-specific contrast agent, helps in the more accurate detection of hypervascular HCC than by other agents. In addition, Gd-EOB-DTPA can detect hypovascular HCC, which is an early stage of the multi-stage carcinogenesis, with a low signal in the hepatobiliary phase. In addition to tumor detection and characterization, Gd-EOB-DTPA contrast-enhanced MR imaging can be applied for liver function evaluation and prognoses evaluation. Thus, Gd-EOB-DTPA plays an important role in the diagnosis of HCC.
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Huppertz A, Balzer T, Blakeborough A, et al. European EOB study group. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 2004; 230:266–275. [DOI] [PubMed] [Google Scholar]
- 2.Jung G, Breuer J, Poll LW, et al. Imaging characteristics of hepatocellular carcinoma using the hepatobiliary contrast agent Gd-EOB-DTPA. Acta Radiol 2006; 47:15–23. [DOI] [PubMed] [Google Scholar]
- 3.Narita M, Hatano E, Arizono S, et al. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol 2009; 44:793–798. [DOI] [PubMed] [Google Scholar]
- 4.Kitao A, Zen Y, Matsui O, et al. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging—correlation with molecular transporters and histopathologic features. Radiology 2010; 256:817–826. [DOI] [PubMed] [Google Scholar]
- 5.Tsuboyama T, Onishi H, Kim T, et al. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging—correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology 2010; 255:824–833. [DOI] [PubMed] [Google Scholar]
- 6.Park G, Kim YK, Kim CS, et al. Diagnostic efficacy of gadoxetic acid-enhanced MRI in the detection of hepatocellular carcinomas: comparison with gadopentetate dimeglumine. Br J Radiol 2010; 83:1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada M, Imai Y, Kim T, et al. Comparison of enhancement patterns of histologically confirmed hepatocellular carcinoma between gadoxetate- and ferucarbotran-enhanced magnetic resonance imaging. J Magn Reson Imaging 2010; 32:903–913. [DOI] [PubMed] [Google Scholar]
- 8.Huppertz A, Haraida S, Kraus A, et al. Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT—initial observations. Radiology 2005; 234:468–478. [DOI] [PubMed] [Google Scholar]
- 9.Vogl TJ, Kümmel S, Hammerstingl R, et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 1996; 200:59–67. [DOI] [PubMed] [Google Scholar]
- 10.Kim T, Murakami T, Oi H, et al. Detection of hypervascular hepatocellular carcinoma by dynamic MRI and dynamic spiral CT. J Comput Assist Tomogr 1995; 19:948–954. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimitsu K, Honda H, Jimi M, et al. Correlation of three-dimensional gradient echo dynamic MR imaging with CT during hepatic arteriography in patients with hypervascular hepatocellular carcinomas: preliminary clinical experience. J Magn Reson Imaging 2001; 13:258–262. [DOI] [PubMed] [Google Scholar]
- 12.Kagawa Y, Okada M, Kumano S, et al. Optimal scanning protocol of arterial dominant phase for hypervascular hepatocellular carcinoma with gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MR. J Magn Reson Imaging 2011; 33:864–872. [DOI] [PubMed] [Google Scholar]
- 13.Kim KA, Herigault G, Kim MJ, et al. Three-dimensional contrast-enhanced hepatic MR imaging: comparison between a centric technique and a linear approach with partial Fourier along both slice and phase directions. J Magn Reson Imaging 2011; 33:160–166. [DOI] [PubMed] [Google Scholar]
- 14.Chandarana H, Feng L, Block TK, et al. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol 2013; 48:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coenegrachts K, Ghekiere J, Denolin V, et al. Perfusion maps of the whole liver based on high temporal and spatial resolution contrast-enhanced MRI (4D THRIVE): feasibility and initial results in focal liver lesions. Eur J Radiol 2010; 74:529–535. [DOI] [PubMed] [Google Scholar]
- 16.Kim YC, Min JH, Kim YK, et al. Intra-individual comparison of gadolinium-enhanced MRI using pseudo-golden-angle radial acquisition with gadoxetic acid-enhanced MRI for diagnosis of HCCs using LI-RADS. Eur Radiol 2019; 29:2058–2068. [DOI] [PubMed] [Google Scholar]
- 17.Hamm B, Staks T, Mühler A, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 1995; 195:785–792. [DOI] [PubMed] [Google Scholar]
- 18.American College of Radiology . LI-RADS version 2018. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. (Accessed: Jun 1, 2021)
- 19.Kim T, Murakami T, Hasuike Y, et al. Experimental hepatic dysfunction: evaluation by MRI with Gd-EOB-DTPA. J Magn Reson Imaging 1997; 7:683–688. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda N, Okada M, Murakami T. Potential of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) for differential diagnosis of nonalcoholic steatohepatitis and fatty liver in rats using magnetic resonance imaging. Invest Radiol 2007; 42:242–247. [DOI] [PubMed] [Google Scholar]
- 21.Tsuda N, Okada M, Murakami T. New proposal for the staging of nonalcoholic steatohepatitis: evaluation of liver fibrosis on Gd-EOB-DTPA-enhanced MRI. Eur J Radiol 2010; 73:137–142. [DOI] [PubMed] [Google Scholar]
- 22.Sofue K, Tsurusaki M, Tokue H, et al. Gd-EOB-DTPA-enhanced 3.0 T MR imaging: quantitative and qualitative comparison of hepatocyte-phase images obtained 10 min and 20 min after injection for the detection of liver metastases from colorectal carcinoma. Eur Radiol 2011; 21:2336–2343. [DOI] [PubMed] [Google Scholar]
- 23.Sofue K, Tsurusaki M, Murakami T, et al. Does Gadoxetic acid-enhanced 3.0T MRI in addition to 64-detector-row contrast-enhanced CT provide better diagnostic performance and change the therapeutic strategy for the preoperative evaluation of colorectal liver metastases? Eur Radiol 2014; 24:2532–2539. [DOI] [PubMed] [Google Scholar]
- 24.Okada M, Murakami T, Kuwatsuru R, et al. Biochemical and clinical predictive approach and time point analysis of hepatobiliary phase liver enhancement on Gd-EOB-DTPA-enhanced MR images: a multicenter study. Radiology 2016; 281:474–483. [DOI] [PubMed] [Google Scholar]
- 25.Sofue K, Marin D, Jaffe TA, et al. Can combining triple-arterial phase acquisition with fluoroscopic triggering provide both optimal early and late hepatic arterial phase images during gadoxetic acid-enhanced MRI? J Magn Reson Imaging 2016; 43:1073–1081. [DOI] [PubMed] [Google Scholar]
- 26.Davenport MS, Viglianti BL, Al-Hawary MM, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 2013; 266:452–461. [DOI] [PubMed] [Google Scholar]
- 27.Kromrey ML, Hori M, Goshima S, et al. Gadoxetate disodium-related event during image acquisition: a prospective multi-institutional study for better MR practice. Eur Radiol 2020; 30:281–290. [DOI] [PubMed] [Google Scholar]
- 28.Saito K, Araki Y, Park J, et al. Effect of Gd-EOB-DTPA on T2-weighted and diffusion-weighted images for the diagnosis of hepatocellular carcinoma. J Magn Reson Imaging 2010; 32:229–234. [DOI] [PubMed] [Google Scholar]
- 29.Choi JS, Kim MJ, Choi JY, et al. Diffusion-weighted MR imaging of liver on 3.0-Tesla system: effect of intravenous administration of gadoxetic acid disodium. Eur Radiol 2010; 20:1052–1060. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Ohmoto T, Saito T, et al. Effects of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid on T2-weighted MRCP. Magn Reson Med Sci 2009; 8:143–148. [DOI] [PubMed] [Google Scholar]
- 31.Shin W, Gu H, Yang Y. Fast high-resolution T1 mapping using inversion-recovery Look-Locker echo-planar imaging at steady state: optimization for accuracy and reliability. Magn Reson Med 2009; 61:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsube T, Okada M, Kumano S, et al. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Invest Radiol 2011; 46:277–283. [DOI] [PubMed] [Google Scholar]
- 33.Besa C, Kakite S, Cooper N, et al. Comparison of gadoxetic acid and gadopentetate dimeglumine-enhanced MRI for HCC detection: prospective crossover study at 3 T. Acta Radiol Open 2015; 4:2047981614561285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue T, Kudo M, Komuta M, et al. Assessment of Gd-EOB-DTPA-enhanced MRI for HCC and dysplastic nodules and comparison of detection sensitivity versus MDCT. J Gastroenterol 2012; 47:1036–1047. [DOI] [PubMed] [Google Scholar]
- 35.Kitao A, Matsui O, Yoneda N, et al. Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology 2012; 265:780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kogita S, Imai Y, Okada M, et al. Gd-EOB-DTPA-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol 2010; 20:2405–2413. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka M, Nakashima O, Wada Y, et al. Pathomorphological study of Kupffer cells in hepatocellular carcinoma and hyperplastic nodular lesions in the liver. Hepatology 1996; 24:807–812. [DOI] [PubMed] [Google Scholar]
- 38.Imai Y, Murakami T, Hori M, et al. Hypervascular hepatocellular carcinoma: combined dynamic MDCT and SPIO-enhanced MRI versus combined CTHA and CTAP. Hepatol Res 2008; 38:147–158. [DOI] [PubMed] [Google Scholar]
- 39.Ohama H, Imai Y, Nakashima O, et al. Images of Sonazoid-enhanced ultrasonography in multistep hepatocarcinogenesis: comparison with Gd-EOB-DTPA-enhanced MRI. J Gastroenterol 2014; 49:1081–1093. [DOI] [PubMed] [Google Scholar]
- 40.Motosugi U, Murakami T, Lee JM, et al. LI-RADS HBA working group. Recommendation for terminology: nodules without arterial phase hyperenhancement and with hepatobiliary phase hypointensity in chronic liver disease. J Magn Reson Imaging 2018; 48:1169–1171. [DOI] [PubMed] [Google Scholar]
- 41.Akai H, Matsuda I, Kiryu S, et al. Fate of hypointense lesions on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Eur J Radiol 2012; 81:2973–2977. [DOI] [PubMed] [Google Scholar]
- 42.Inoue T, Hyodo T, Murakami T, et al. Hypovascular hepatic nodules showing hypointense on the hepatobiliary-phase image of Gd-EOB-DTPA-enhanced MRI to develop a hypervascular hepatocellular carcinoma: a nationwide retrospective study on their natural course and risk factors. Dig Dis 2013; 31:472–479. [DOI] [PubMed] [Google Scholar]
- 43.Kumada T, Toyoda H, Tada T, et al. Evolution of hypointense hepatocellular nodules observed only in the hepatobiliary phase of gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol 2011; 197:58–63. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi S, Matsui O, Gabata T, et al. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic Acid-enhanced magnetic resonance imaging findings of borderline lesions at high risk for progression to hypervascular classic hepatocellular carcinoma. J Comput Assist Tomogr 2011; 35:181–186. [DOI] [PubMed] [Google Scholar]
- 45.Yu JS, Chung JJ, Kim JH, et al. Fat-containing nodules in the cirrhotic liver: chemical shift MRI features and clinical implications. AJR Am J Roentgenol 2007; 188:1009–1016. [DOI] [PubMed] [Google Scholar]
- 46.Hyodo T, Murakami T, Imai Y, et al. Hypovascular nodules in patients with chronic liver disease: risk factors for development of hypervascular hepatocellular carcinoma. Radiology 2013; 266:480–490. [DOI] [PubMed] [Google Scholar]
- 47.Ludwig DR, Fraum TJ, Cannella R, et al. Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of liver imaging reporting and data system v2018. Abdom Radiol (NY) 2019; 44:2116–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alhasan A, Cerny M, Olivié D, et al. LI-RADS for CT diagnosis of hepatocellular carcinoma: performance of major and ancillary features. Abdom Radiol (NY) 2019; 44:517–528. [DOI] [PubMed] [Google Scholar]
- 49.Gupta RT, Marin D, Boll DT, et al. Hepatic hemangiomas: difference in enhancement pattern on 3T MR imaging with gadobenate dimeglumine versus gadoxetate disodium. Eur J Radiol 2012; 81:2457–2462. [DOI] [PubMed] [Google Scholar]
- 50.Goshima S, Kanematsu M, Watanabe H, et al. Hepatic hemangioma and metastasis: differentiation with gadoxetate disodium-enhanced 3-T MRI. AJR Am J Roentgenol 2010; 195:941–946. [DOI] [PubMed] [Google Scholar]
- 51.Motosugi U, Ichikawa T, Sou H, et al. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology 2010; 256:151–158. [DOI] [PubMed] [Google Scholar]
- 52.Zech CJ, Grazioli L, Breuer J, et al. Diagnostic performance and description of morphological features of focal nodular hyperplasia in Gd-EOB-DTPA-enhanced liver magnetic resonance imaging: results of a multicenter trial. Invest Radiol 2008; 43:504–511. [DOI] [PubMed] [Google Scholar]
- 53.Suh CH, Kim KW, Kim GY, et al. The diagnostic value of Gd-EOB-DTPA-MRI for the diagnosis of focal nodular hyperplasia: a systematic review and meta-analysis. Eur Radiol 2015; 25:950–960. [DOI] [PubMed] [Google Scholar]
- 54.Nakashima O, Kurogi M, Yamaguchi R, et al. Unique hypervascular nodules in alcoholic liver cirrhosis: identical to focal nodular hyperplasia-like nodules? J Hepatol 2004; 41:992–998. [DOI] [PubMed] [Google Scholar]
- 55.Bosman FT, World Health Organization, International agency for research on cancer. WHO classification of tumours of the digestive system, In: World Health Organization classification of tumours. 4th ed. Lyon:International Agency for Research on Cancer, 2010. [Google Scholar]
- 56.Grazioli L, Federle MP, Ichikawa T, et al. Liver adenomatosis: clinical, histopathologic, and imaging findings in 15 patients. Radiology 2000; 216:395–402. [DOI] [PubMed] [Google Scholar]
- 57.Grazioli L, Federle MP, Brancatelli G, et al. Hepatic adenomas: imaging and pathologic findings. Radiographics 2001; 21:877–892; discussion 892–894. [DOI] [PubMed] [Google Scholar]
- 58.Kim SA, Lee JM, Lee KB, et al. Intrahepatic mass-forming cholangiocarcinomas: enhancement patterns at multiphasic CT, with special emphasis on arterial enhancement pattern—correlation with clinicopathologic findings. Radiology 2011; 260:148–157. [DOI] [PubMed] [Google Scholar]
- 59.Kim SH, Lee CH, Kim BH, et al. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr 2012; 36:704–709. [DOI] [PubMed] [Google Scholar]
- 60.Zech CJ, Herrmann KA, Reiser MF, et al. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci 2007; 6:43–52. [DOI] [PubMed] [Google Scholar]
- 61.Kitao A, Matsui O, Yoneda N, et al. Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: molecular and genetic background. Eur Radiol 2020; 30:3438–3447. [DOI] [PubMed] [Google Scholar]
- 62.Lee S, Kim SH, Lee JE, et al. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 2017; 67:526–534. [DOI] [PubMed] [Google Scholar]
- 63.Lee S, Kang TW, Song KD, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg 2021; 273:564–571. [DOI] [PubMed] [Google Scholar]
- 64.Yang L, Gu D, Wei J, et al. A radiomics nomogram for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Liver Cancer 2019; 8:373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee S, Kim KW, Jeong WK, et al. Gadoxetic acid-enhanced MRI as a predictor of recurrence of HCC after liver transplantation. Eur Radiol 2020; 30:987–995. [DOI] [PubMed] [Google Scholar]
- 66.Chuang YH, Ou HY, Lazo MZ, et al. Predicting post-hepatectomy liver failure by combined volumetric, functional MR image and laboratory analysis. Liver Int 2018; 38:868–874. [DOI] [PubMed] [Google Scholar]
- 67.Kim DK, Choi JI, Choi MH, et al. Prediction of posthepatectomy liver failure: MRI with hepatocyte-specific contrast agent versus indocyanine green clearance test. AJR Am J Roentgenol 2018; 211:580–587. [DOI] [PubMed] [Google Scholar]
- 68.Asenbaum U, Kaczirek K, Ba-Ssalamah A, et al. Post-hepatectomy liver failure after major hepatic surgery: not only size matters. Eur Radiol 2018; 28:4748–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsujita Y, Sofue K, Komatsu S, et al. Prediction of post-hepatectomy liver failure using gadoxetic acid-enhanced magnetic resonance imaging for hepatocellular carcinoma with portal vein invasion. Eur J Radiol 2020; 130:109189. [DOI] [PubMed] [Google Scholar]
- 70.Kudo M. Gd-EOB-DTPA-MRI could predict WNT/β-Catenin mutation and resistance to immune checkpoint inhibitor therapy in hepatocellular carcinoma. Liver Cancer 2020; 9:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada A, Hara T, Li F, et al. Quantitative evaluation of liver function with use of gadoxetate disodium-enhanced MR imaging. Radiology 2011; 260:727–733. [DOI] [PubMed] [Google Scholar]
- 72.Sofue K, Zhong X, Nickel MD, et al. Stability of liver proton density fat fraction and changes in R 2* measurements induced by administering gadoxetic acid at 3T MRI. Abdom Radiol (NY) 2016; 41:1555–1564. [DOI] [PubMed] [Google Scholar]
- 73.Haimerl M, Verloh N, Fellner C, et al. MRI-based estimation of liver function: Gd-EOB-DTPA-enhanced T1 relaxometry of 3T vs. the MELD score. Sci Rep 2014; 4:5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamimura K, Fukukura Y, Yoneyama T, et al. Quantitative evaluation of liver function with T1 relaxation time index on Gd-EOB-DTPA-enhanced MRI: comparison with signal intensity-based indices. J Magn Reson Imaging 2014; 40:884the MELD score. 889. [DOI] [PubMed] [Google Scholar]