Abstract
The effects of ozone at 0.25, 0.40, and 1.00 ppm on Listeria monocytogenes were evaluated in distilled water and phosphate-buffered saline. Differences in sensitivity to ozone were found to exist among the six strains examined. Greater cell death was found following exposure at lower temperatures. Early stationary-phase cells were less sensitive to ozone than mid-exponential- and late stationary-phase cells. Ozonation at 1.00 ppm of cabbage inoculated with L. monocytogenes effectively inactivated all cells after 5 min. The abilities of in vivo catalase and superoxide dismutase to protect the cells from ozone were also examined. Three listerial test strains were inactivated rapidly upon exposure to ozone. Both catalase and superoxide dismutase were found to protect listerial cells from ozone attack, with superoxide dismutase being more important than catalase in this protection.
Listeria monocytogenes is a ubiquitous, gram-positive, aerobic to facultative anaerobic bacterium. It is the causative agent of the disease listeriosis and was discovered almost 90 years ago (6, 10). In 1981, it was recognized as an important foodborne pathogen. L. monocytogenes causes a high percentage of the fatalities due to foodborne disease, exceeding even Clostridium botulinum and Salmonella. It has been suggested that listeriosis may be the leading fatal foodborne disease in the United States (8). Consumption of foods contaminated with L. monocytogenes can cause both sporadic illness and foodborne disease outbreaks. The annual rate of listeriosis incidence is 0.7 case per 100,000 persons. The rate is three times higher for persons over 70 years old and is 17 times higher for pregnant women (30). The overall fatality rate for recent outbreaks is 33% (23, 25).
Ozone (O3) is a powerful oxidizing agent, with an oxidation potential of 2.07 V in alkaline solution, second only to fluorine (28). Dominquez et al. (5) found that ozone was more effective than chlorine in the destruction of Legionella pneumophila. Herbold et al. (14) showed that ozone inactivation of hepatitis A virus and Escherichia coli was faster at 10°C than at 20°C. Sugita et al. (31) found that ozone was highly effective in the destruction of Enterococcus seriolicida, Vibrio anguillarum, and Pasteurella piscicida in seawater. Ozone is a protoplasm oxidant, and its bactericidal action is extremely rapid. Approximately 10 min was the critical time for all of the microorganisms tested with an O3 concentration of 0.18 ppm. Ozone may replace chlorine as a common sanitizing agent in the food industry.
The ability of ozone to kill Salmonella typhi, Vibrio cholerae, and Bacillus anthracis was demonstrated as early as 1892. Sterilization of drinking water showed the destruction of all pathogens and saprophytic microbes encountered in water even when it was heavily contaminated (30). Whiteside and Hassan (35) demonstrated the effectiveness of ozone at causing inhibition of growth and loss of viability of E. coli. The critical dose for E. coli was between 0.40 and 0.50 ppm (32). Komanapalli and Lau (20) found that the E. coli membrane was the primary site of attack by ozone, with other cell sites subsequently damaged. This group proposed that sulfhydryl groups in the membrane were the primary targets (21). The proposed killing mechanism of ozone is as follows. Unsaturated lipids are prominent constituents of the cytoplasmic membrane. Upon exposure to ozone, the olefinic bonds are attacked to form an ozonide. This action starts the destruction of the cell's ability to function and may even be sufficient to cause the death of weaker cells. This ozonide has a high oxidation potential, is unstable, and exerts its own disinfecting action by attacking enzymes, sulfhydryl groupings, or aldehydes, releasing peroxyl compounds, which are also disinfectants. Finally, the cell is lysed and the cytoplasm is dispersed. Thus, the action of ozone is characterized by the proliferation of many other oxidizing substances which can compete with or supplement the action of ozone to destroy critical sites within the cell or to generally oxidize protoplasm. This cascade effect is unique to ozone and its decomposition products (32). Ozone has been found to cause single- and double-stranded DNA breaks in E. coli (13).
This study examined the effects of ozone on L. monocytogenes in different phases of growth and at different temperatures. We also evaluated the use of ozone on cabbage inoculated with L. monocytogenes. Another main objective of this study was to examine the role of in vivo catalase (CA) and superoxide dismutase (SOD) in protecting the cell from ozone.
MATERIALS AND METHODS
Bacterial strains.
L. monocytogenes strains 19112 and 7644 were obtained from the American Type Culture Collection, Manassas, Va.; strains 10403S and SLCC 5764 were obtained from Daniel A. Portnoy, University of Pennsylvania, Philadelphia; strain Scott A was obtained from Larry Beuchat, University of Georgia Experimental Station, Experiment; and strain LO28 was obtained from J. Claudio Pérez-Diaz, Madrid, Spain.
L. monocytogenes 10403S produces both CA and SOD (CA+ SOD+); L. monocytogenes strain 1370 (CA− SOD+) was obtained from J. T. von Dissel, Department of Infectious Diseases and General Internal Medicine, University Hospital, Leiden, The Netherlands; and L. monocytogenes strain DHL1 (CA+ SOD−) was constructed from 10403S by insertional inactivation of sodA with plasmid pSODM. The recombinant plasmid pSODM was constructed by Jürgen Kreft (Biozentrum Lehrstuhl für Mikrobiologie, Am Hubland, Germany) by ligating about 400 bp from the N terminus of L. monocytogenes sodA into plasmid pLSV1 (J. Kreft, personal communication). The sodA fragment was inserted between the EcoRI and BamHI sites on the vector.
Growth conditions.
Frozen stocks of the cultures were prepared by inoculating 10 ml of tryptic soy broth (TSB; Fisher Scientific, Pittsburgh, Pa.) with 0.1 ml of an overnight stationary-phase inoculum. These tubes were then vortexed, frozen, and stored at −20°C. As needed, stocks were thawed and inoculated into 250-ml Erlenmeyer flasks containing 90 ml of TSB and grown at 37°C in a gyratory shaking water bath (New Brunswick Scientific, Edison, N.J.) to early stationary phase as determined by growth curves obtained by using a DU-40 spectrophotometer (Beckman, Irvine, Calif.). Mid-exponential phase was defined as an optical density at 600 nm (OD600) of 0.6 to 0.7; early stationary phase was defined as an OD600 of 1.0 to 1.1, and late stationary phase was defined as an OD600 of 1.2 to 1.3. When examining CA and SOD activities, we prepared test cultures with TSB–2.5% NaCl. This level of NaCl was added for maximum production of enzyme activities (26). These cultures were then harvested by centrifugation (16,300 × g, 10 min, 4°C) and suspended in 100 ml of sterile distilled water (dH2O), 10 mM phosphate-buffered saline (PBS; 130 mM NaCl, pH 7.4), or 50 mM potassium phosphate buffer (PPB; pH 7.4).
Ozone apparatus.
An Infinity Corona Discharge Ozone Generator (CD-7; DEL Industries, San Luis Obispo, Calif.) or a benchtop UV ozone generator (ZO-151; DEL Industries) and an ozone sensor (1054B; Rosemount Analytical, Irvine, Calif.) were used to generate and detect the levels of ozone. Residual ozone was also determined by the indigo colorimetric method (1). Ozone was diffused-bubbled in a 1-liter spinner flask with 900 ml of sterile dH2O, PBS, or PPB to the proper ozone concentration. The suspended culture was then added to this flask, and aliquots were taken at different intervals. These aliquots were diluted in 0.1% peptone and plated onto tryptic soy agar (Fisher Scientific) and incubated at 37°C. Plates were read after 36 to 48 h. Results were reported as CFU per milliliter. Cabbage was cut into 25-g pieces, and each piece was inoculated with 1 ml of early stationary-phase cells (108 cells/ml; OD600, 1.0 to 1.1). This culture was spread aseptically over the cabbage and allowed to dry for at least 1 h. Each sample was then submerged in 1 liter of sterile dH2O and ozonated. Heterotrophic plate counts (HPC) were determined on Plate Count Agar (Fisher Scientific), and L. monocytogenes counts were determined on McBride Listeria Agar (Fisher Scientific) containing 0.2 g of cycloheximide per liter to enhance selectivity. These plates were also incubated at 37°C for 36 to 48 h.
Enzyme activities.
CA and SOD activities were determined prior to exposure to ozone in accordance with the procedure described by Dallmier and Martin (3). One unit of CA decomposed 1 μmol of H2O2 per min at 25°C and pH 7.0, whereas the H2O2 concentration fell from 10.3 to 9.2 μmol/ml of reaction mixture. SOD activity was measured by the cytochrome c reduction method of McCord and Fridovich (24). One unit of SOD activity was defined as the amount required to inhibit the rate of reduction of cytochrome c by 50%.
Statistical analyses.
Statistical analyses were performed using StatView 512+ Version 1.2 (Brain Power Inc., Calabasas, Calif.). A one-way analysis of variance was used to determine any significant differences between the tested strains. The means and standard errors of the means of triplicate experiments are shown in all of the figures and tables.
RESULTS AND DISCUSSION
Ozonation of L. monocytogenes in dH2O and PBS.
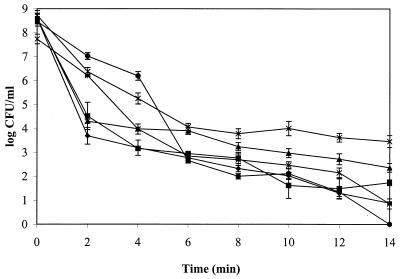
The effects of ozone on six strains of L. monocytogenes were evaluated. Figure 1 shows the inactivation of L. monocytogenes subjected to ozone at 0.25 ppm in dH2O at 24°C. Many studies have shown that death rate kinetics using ozone on a variety of bacteria and viruses exhibit a biphasic curve over an extended period of time (2, 16, 18, 27). This biphasic curve is also evident in Fig. 1.
FIG. 1.
Ozone (0.25 ppm) inactivation of L. monocytogenes 19112 (⧫), Scott A (■), 10403S (▴), SLCC 5764 (×), 7644 (∗), and LO28 (●) in dH2O at 24°C.
L. monocytogenes strains SLCC 5764 and 10403S were found to be significantly more resistant after 14 min of exposure to ozone (0.25 ppm) than the other four test strains (P < 0.05). After 14 min, strain SLCC 5764 showed a 5.3-log reduction, strain 10403S showed a 6.2-log reduction, strain Scott A showed a 6.8-log reduction, strain 7644 showed a 6.9-log reduction, and strain 19112 showed a 7.7-log reduction while strain LO28 was completely inactivated. These results suggested that differences in sensitivity to the killing effects of ozone existed among the listerial strains examined. Restaino et al. (27) found differences in sensitivity to ozone between many food-related microorganisms. They showed that gram-negative bacteria and L. monocytogenes (only one strain was examined) were more sensitive to ozone than were other gram-positive bacteria. Results from the present study suggest that there are differences in sensitivity to ozone among all of the L. monocytogenes strains examined.
Differences in sensitivity among listerial strains were also found after exposure to ozone at 0.25 ppm in PBS (data not shown). L. monocytogenes strain SLCC 5764 was again found to be the most resistant of the tested listerial strains after 10 min of ozone exposure (P < 0.05). Ozone inactivation of the listerial strains showed similarities in resistance in both distilled water and PBS.
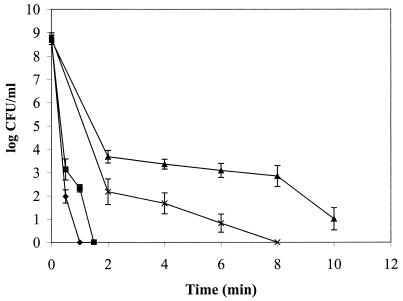
L. monocytogenes strains SLCC 5764 and 10403S, the most resistant to O3 at 0.25 ppm, were then subjected to ozone at 0.40 ppm in dH2O at 24°C (Fig. 2). After 6 min of ozone exposure, L. monocytogenes strain SLCC 5764 demonstrated significantly less inactivation than did strain 10403S (P < 0.05). L. monocytogenes strain SLCC 5764 showed a 5.6-log reduction, while strain 10403S showed an 8.0-log reduction after 6 min of exposure. These results suggest that ozone may need to be used at higher concentrations and for longer times to inactivate less-sensitive strains. The presence of any organic matter will also exert an ozone demand and prevent full utilization of the applied dosage (2, 4, 11, 33).
FIG. 2.
Ozone inactivation of L. monocytogenes 10403S (×, 0.40 ppm; ⧫, 1.00 ppm) and SLCC 5764 (▴, 0.40 ppm; ■, 1.00 ppm) at 24°C.
Higher concentrations of ozone were required to completely inactivate L. monocytogenes. Exposure to ozone at 1.00 ppm in PBS (24°C) eliminated the two strains tested within 1.5 min (Fig. 2). The use of this higher concentration of ozone indicated complete inactivation and suggested that ozone can be an effective disinfectant at this level.
Ozone exposure of different-phase cells at different temperatures.
The log reductions of L. monocytogenes strains Scott A, 10403S, and SLCC 5764 after 2 min of exposure to O3 at 0.25 ppm in PBS (24°C) are shown in Table 1. Mid-exponential-, early stationary-, and late stationary-phase cells were evaluated. Mid-exponential- and late stationary-phase cells were more sensitive to ozone than were early stationary-phase cells. However, strain Scott A showed no significant decreases between phases of growth while 10403S showed a significant decrease (P < 0.05) between the early and late stationary phases. Mid-exponential- and late stationary-phase cells were significantly more sensitive to ozone (P < 0.05) than were early stationary-phase cells of strain SLCC 5764. In general, mid-exponential- and late stationary-phase cells were more sensitive to adverse conditions than were early stationary-phase cells.
TABLE 1.
Log reduction of L. monocytogenes after 2 min of exposure to ozone at 0.25 ppm in PBS at 24°C
| Growth phase | Mean Log reduction (CFU/ml) ± SEM
|
||
|---|---|---|---|
| Scott A | 10403S | SLCC 5764 | |
| Mid-exponential | 5.4 ± 0.4 | 5.0 ± 0.1 | 5.2 ± 0.2 |
| Early stationary | 5.2 ± 0.2 | 4.4 ± 0.1 | 4.0 ± 0.1 |
| Late stationary | 5.3 ± 0.1 | 5.3 ± 0.1 | 5.1 ± 0.1 |
The log reductions of L. monocytogenes strains 10403S and SLCC 5764 after 2 min of exposure to ozone at 0.25 ppm and 4, 24, and 37°C in PBS are shown in Table 2. Results indicated that ozonation at 4°C was more effective at killing L. monocytogenes strains 10403S and SLCC 5764 than was ozonation at 24 and 37°C (P < 0.05). Leiguarda et al. (22) and Ewell (7) found no difference in the bactericidal power of ozone in the temperature range of 10 to 24°C. However, Ingram and Haines (16) observed that lower concentrations of ozone were more inhibitory near 0°C than at 20°C. Kaesz (17) and Kefford (19) also found significantly greater effects at lower temperatures. Rice et al. (29) suggested that below 10°C, the metabolism of the microorganisms was slower, allowing ozone to be more effective, suggesting that at lower temperatures, the treatment time may be shorter. Ozone increases in solubility as the temperature of the water decreases (9).
TABLE 2.
Log reduction of L. monocytogenes after 2 min of exposure to ozone at 0.25 ppm in 4, 24, and 37°C PBS
| Temp (°C) | Mean log reduction (CFU/ml) ± SEM
|
|
|---|---|---|
| 10403S | SLCC 5764 | |
| 4 | 6.1 ± 0.1 | 5.8 ± 0.2 |
| 24 | 5.0 ± 0.1 | 4.0 ± 0.1 |
| 37 | 3.3 ± 0.1 | 3.4 ± 0.1 |
Ozone inactivation of L. monocytogenes on cabbage.
The ability of ozone to kill L. monocytogenes inoculated onto cabbage is shown in Table 3. Cabbage (25-g pieces) was inoculated with 1 ml of an overnight early stationary-phase culture (OD600, 1.0 to 1.1). L. monocytogenes counts and plate counts were examined after 2 and 5 min of exposure to ozone at 1.00 ppm in dH2O (24°C). After 2 min, L. monocytogenes strains Scott A, 10403S, and SLCC 5764 showed 100, 75, and 70% inactivation (McBride Listeria Agar), respectively, while HPC were reduced by 18, 23, and 32%. After 5 min, all L. monocytogenes strains were inactivated while HPC were reduced by 42, 38, and 41%. Cabbage was also ozonated without the inoculation with L. monocytogenes. There were 69 and 79% decreases in HPC after 2 and 5 min of exposure, respectively. These results suggest that ozone at 1.00 ppm may be effective at inactivating all L. monocytogenes cells and in reducing the HPC.
TABLE 3.
Inactivation by ozone at 1.00 ppm of L. monocytogenes on cabbage in dH2O at 24°C
| Strain used to inoculate cabbage | % of microorganisms killed after exposure for:
|
|||
|---|---|---|---|---|
| 2 min
|
5 min
|
|||
| HPC | McBride Listeria Agar | HPC | McBride Listeria Agar | |
| Scott A | 18 | 100 | 42 | 100 |
| 10403S | 23 | 75 | 38 | 100 |
| SLCC 5764 | 32 | 70 | 41 | 100 |
| Nonea | 69 | NCb | 79 | NC |
Uninoculated-cabbage control.
NC, no initial counts.
Survival of wild-type and mutant strains.
The residual ozone concentrations in sterile PPB at 4, 24, and 37°C determined prior to the addition of listerial cells were 0.30, 0.25, and 0.19 mg/liter, respectively, after 40, 60, and 90 min of exposure using the benchtop ozone generator. The time required to reach saturation was dependent on the temperature solubility of ozone (A. D. Venosa and E. J. Opatken, 52nd Annu. Pollut. Contr. Fed. Conf., Houston, Texas, 1979). The ability of ozone to kill each of the three listerial strains during the mid-exponential, early stationary, and late stationary phases when the bacteria were exposed to ozone-saturated PPB at 4, 24, and 37°C was examined. Results for all of the strains grown at the three temperatures were similar: rapid death in the first 2 to 4 min of ozone exposure, followed by a more gradual decrease in cell number. Complete inactivation was found in 6 to 20 min, depending on the strain and growth phase. All of the listerial inactivation experiments demonstrated similar biphasic death curves.
A summary of the reduction after 2 min of exposure is found in Table 4. These results show that all three strains were rapidly killed following exposure to ozone. The rate of listerial killing was significantly increased (P < 0.05) as the exposure temperature decreased from 37 and 24 to 4°C. No significant differences (P > 0.05) were observed between 24 and 37°C. The increased effectiveness of ozone at the lower temperature was probably due to the greater solubility and stability of ozone at reduced temperatures. Late stationary-phase cells were more sensitive to ozone treatment than were mid-exponential- or early stationary-phase cells (P < 0.05).
TABLE 4.
| Strain and growth phase | Mean no. of CFU/ml ± SEM
|
Mean activity ± SEM
|
|||
|---|---|---|---|---|---|
| 4°C | 24°C | 37°C | CA | SOD | |
| 10403S (wild type) | |||||
| Mid-exponential | 5.3 ± 0.1 | 4.3 ± 0.4 | 3.7 ± 0.3 | 26.4 ± 2.3 | 72.3 ± 4.4 |
| Early stationary | 4.1 ± 0.4 | 3.3 ± 0.5 | 2.6 ± 0.1 | 29.3 ± 1.3 | 83.3 ± 2.9 |
| Late stationary | 5.5 ± 0.3 | 5.2 ± 0.3 | 4.0 ± 0.4 | 18.6 ± 1.7 | 43.7 ± 4.6 |
| 1370 (CA− SOD+) | |||||
| Mid-exponential | 5.7 ± 0.1 | 4.6 ± 0.1 | 4.4 ± 0.3 | NDc | 145.7 ± 4.8 |
| Early stationary | 5.2 ± 0.3 | 4.4 ± 0.3 | 3.8 ± 0.2 | ND | 157.6 ± 4.6 |
| Late stationary | 6.0 ± 0.1 | 5.2 ± 0.2 | 5.1 ± 0.2 | ND | 89.3 ± 7.3 |
| DHL1 (CA+ SOD−) | |||||
| Mid-exponential | 6.5 ± 0.1 | 5.9 ± 0.1 | 5.5 ± 0.1 | 29.6 ± 2.1 | ND |
| Early stationary | 6.2 ± 0.2 | 5.6 ± 0.1 | 5.1 ± 0.4 | 34.4 ± 2.5 | ND |
| Late stationary | 6.7 ± 0.2 | 6.3 ± 0.1 | 5.9 ± 0.1 | 19.5 ± 2.9 | ND |
The ozone concentrations at 4, 24, and 37°C were 0.30, 0.25, and 0.19 mg/liter, respectively.
Cells were grown in TSB–2.5% NaCl at the indicated temperatures, pelleted, and suspended in PPB prior to ozone exposure.
ND, none detected.
Influence of CA and SOD.
Another objective of this study was to examine the effects of in vivo CA and SOD on ozone sensitivity and resistance. A wild-type strain and two mutants were utilized, i.e., L. monocytogenes strains 10403S (wild type), 1370 (CA− SOD+), and DHL1 (CA+ SOD−). The CA and SOD activities of the three strains are presented in Table 4. The highest CA and SOD activities were found in early stationary-phase cells of all three strains (when present). The mid-exponential-phase cells had enzymatic activities similar to those of early stationary-phase cells (P > 0.05), while the activities decreased in cells in the late stationary phase. Strain 1370 produced significantly more SOD than did strain 10403S (P < 0.05). This increased production of SOD may be in compensation for the lack of CA. An approximately twofold increase in SOD production in a CA-negative mutant compared with a CA-positive strain was previously observed when listerial cells were exposed to oxygen (12, 34). No overproduction of CA was observed in the SOD-negative mutant DHL1, and the level was similar to that of strain 10403S. Imlay and Linn (15) obtained similar results with a SOD-negative strain of E. coli compared to a SOD-positive strain.
The observation that mid-exponential- and early stationary-phase cells were more resistant to ozone treatment than late stationary-phase cells correlates with both the CA and SOD activities. Lower enzyme activities were found in late stationary-phase cells.
Killing curves demonstrated that the wild-type strain was more resistant to ozone exposure than were strains 1370 and DHL1 (P < 0.05). Strain 1370 demonstrated increased sensitivity to ozone in spite of the substantial increase in SOD activity compared to strain 10403S. This result suggests that CA is necessary for protection against ozone exposure. Strain 1370 had the ability to detoxify superoxide radicals but was unable to eliminate hydrogen peroxide, thus accounting for the increase in sensitivity observed. The strain most sensitive to ozone exposure was the mutant DHL1. Strain DHL1 was rapidly inactivated by 2 min of exposure to ozone (Table 4). There were no significant differences in CA activity between this mutant and strain 10403S. This result suggests that SOD is critical to cell survival following ozone exposure. Ozone dissociates into various free radicals through autodecomposition. SOD acts by eliminating the superoxide radical, thereby preventing the formation of hydroxyl radicals.
ACKNOWLEDGMENT
This work was supported by USDA award 98-35201-6217.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 17th ed. Washington, D.C.: American Public Health Association; 1989. [Google Scholar]
- 2.Broadwater W T, Hoehn R R, King F H. Sensitivity of three selected bacterial species to ozone. Appl Microbiol. 1973;26:391–393. doi: 10.1128/am.26.3.391-393.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dallmier A W, Martin S E. Catalase and superoxide dismutase activities after heat injury of Listeria monocytogenes. Appl Environ Microbiol. 1988;54:581–582. doi: 10.1128/aem.54.2.581-582.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickerman J M, Castraberti A O, Fuller J E. Action of ozone on water-borne bacteria. J N Engl Water Works Assoc. 1954;68:11. [Google Scholar]
- 5.Dominquez L, Garayazabal J F F, Ferri E R, Vazquez J A, Gomez-Lucia E, Ambrosis C, Suarez G. Viability of Listeria monocytogenes in milk treated with hydrogen peroxide. J Food Prot. 1987;50:636–639. doi: 10.4315/0362-028X-50.8.636. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly C W. Listeria monocytogenes. In: Hui Y H, Gorham J R, Murrell K D, Cliver D O, editors. Foodborne disease handbook. Vol. 1. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 215–252. [Google Scholar]
- 7.Ewell A W. Researches on ultra-violet and ozone. Refrig Eng. 1941;41:331. [Google Scholar]
- 8.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham D M. Use of ozone for food processing. Food Technol. 1997;51:72–75. [Google Scholar]
- 10.Gray M L, Killinger A H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966;30:309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guinvarch P. Three years of ozone sterilization of water in Paris. Adv Chem Serol. 1959;21:416. [Google Scholar]
- 12.Haas A, Brehm K, Kreft J, Goebel W. Cloning, characterization, and expression in Escherichia coli of a gene encoding Listeria seeligeri catalase, a bacterial enzyme highly homologous to mammalian catalase. J Bacteriol. 1991;173:5159–5167. doi: 10.1128/jb.173.16.5159-5167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamelin C, Chung Y S. Repair of ozone-induced DNA lesions in Escherichia coli B cells. Mutat Res. 1989;214:253–255. doi: 10.1016/0027-5107(89)90169-3. [DOI] [PubMed] [Google Scholar]
- 14.Herbold K, Flehmig B, Botzenhart K. Comparison of ozone inactivation in flowing water of hepatitis A virus, poliovirus 1, and indicator organisms. Appl Environ Microbiol. 1989;55:2949–2953. doi: 10.1128/aem.55.11.2949-2953.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imlay J, Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol. 1987;169:2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingram M, Haines R B. Inhibition of bacterial growth by pure ozone in the presence of nutrients. J Hyg. 1949;47:146–158. doi: 10.1017/s0022172400014406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaesz G. Der Einfluss von Ozon auf die Haltbarkeit von gekühltem Fleisch. Z Ges Kalteind. 1936;7:152. [Google Scholar]
- 18.Katzenelson E, Klettel B, Shuval H I. Inactivation kinetics of viruses and bacteria in water by use of ozone. J Am Water Works Assoc. 1974;66:725–729. [Google Scholar]
- 19.Kefford J. Effect of ozone on microbial growth on beef muscle, and on the flavour of beef fat. J Counc Sci Ind Res Aust. 1948;21:116. [Google Scholar]
- 20.Komanapalli I R, Lau B H S. Ozone-induced damage of Escherichia coli K-12. Appl Environ Microbiol. 1996;46:610–614. doi: 10.1007/s002530050869. [DOI] [PubMed] [Google Scholar]
- 21.Komanapalli I R, Mudd J B, Lau B H S. The effects of ozone on the metabolic activities of Escherichia coli K-12. Toxicol Lett. 1997;90:61–66. doi: 10.1016/s0378-4274(96)03830-1. [DOI] [PubMed] [Google Scholar]
- 22.Leiguarda R H, Peso O A, Palazzolo A Z. Bacterial action of ozone. Ann Soc Quim Argent. 1949;37:165–176. [Google Scholar]
- 23.Linnan M J, Mascola L, Lou X D, Goulet V, May S, Salminen C, Hird D W, Yonekura M L, Hayes P, Weaver R, Audurier A, Plikaytis B D, Fannin S, Kleks A, Broome C V. Epidemic listeriosis associated with Mexican-style cheese. N Eng J Med. 1988;319:823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- 24.McCord J M, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein. J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 25.Morris C E. Microbe tracking: the new dangers. Food Eng. 1986;June:64. [Google Scholar]
- 26.Myers E R, Dallmier A W, Martin S E. Sodium chloride, potassium chloride, and virulence in Listeria monocytogenes. Appl Environ Microbiol. 1993;59:2082–2086. doi: 10.1128/aem.59.7.2082-2086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Restaino L, Frampton E W, Hemphill J B, Palnikar P. Efficacy of ozonated water against various food-related microorganisms. Appl Environ Microbiol. 1995;61:3471–3475. doi: 10.1128/aem.61.9.3471-3475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice R G, Robson C M, Miller G W, Hill A G. Uses of ozone in drinking water treatment. J Am Water Works Assoc. 1981;73:44–57. [Google Scholar]
- 29.Rice R G, Farquhar J W, Bollyky L J. Review of the applications of ozone for increasing storage times of perishable foods. Ozone Sci Eng. 1982;4:147–163. [Google Scholar]
- 30.Southwick F S, Purich D L. Intracellular pathogenesis of listeriosis. N Eng J Med. 1996;334:770–776. doi: 10.1056/NEJM199603213341206. [DOI] [PubMed] [Google Scholar]
- 31.Sugita H, Asai T, Hayashi K, Mitsuya T, Amanuma K, Maruyama C, Deguchi Y. Application of ozone disinfection to remove Enterococcus seriolicida, Pasteurella piscicida, and Vibrio anguillarum from seawater. Appl Environ Microbiol. 1993;58:4072–4075. doi: 10.1128/aem.58.12.4072-4075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenney R L. Ozone, the add-nothing sterilant. Tech Q. 1972;10:35–41. [Google Scholar]
- 33.Torricelli A. Drinking water purification. Adv Chem Serol. 1959;21:453. [Google Scholar]
- 34.Welch D F, Sword C P, Brehn S, Dusanic D. Relationship between superoxide dismutase and pathogenic mechanisms of Listeria monocytogenes. Infect Immun. 1979;23:863–872. doi: 10.1128/iai.23.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whiteside C, Hassan H M. Induction and inactivation of catalase and superoxide dismutase of Escherichia coli by ozone. Arch Biochem Biophys. 1987;257:467–471. doi: 10.1016/0003-9861(87)90591-1. [DOI] [PubMed] [Google Scholar]