Abstract
In two-step culture systems for efficient shoot regeneration, explants are first cultured on auxin-rich callus-inducing medium (CIM), where cells are activated to proliferate and form calli containing root-apical meristem (RAM)-type stem cells and stem cell niche, and then cultured on cytokinin-rich shoot-inducing medium (SIM), where stem cells and stem cell niche of the shoot apical meristem (SAM) are established eventually leading to shoot regeneration. In the present study, we examined the effects of inhibitors of auxin biosynthesis and polar transport in the two-step shoot regeneration culture of Arabidopsis and found that, when they were applied during CIM culture, although callus growth was repressed, shoot regeneration in the subsequent SIM culture was significantly increased. The regeneration-stimulating effect of the auxin biosynthesis inhibitor was not linked with the reduction in the endogenous indole-3-acetic acid (IAA) level. Expression of the auxin-responsive reporter indicated that auxin response was more uniform and even stronger in the explants cultured on CIM with the inhibitors than in the control explants. These results suggested that the shoot regeneration competence of calli was enhanced somehow by the perturbation of the endogenous auxin dynamics, which we discuss in terms of the transformability between RAM and SAM stem cell niches.
Keywords: Arabidopsis, auxin biosynthesis, auxin transport, shoot regeneration competence
Introduction
Plants possess a small number of stem cells in the meristems at two ends of the body axis, shoot apical meristem (SAM) and root apical meristem (RAM). All cells of the shoot and root organs are derived from stem cells of SAM and RAM, respectively. During post-embryonic development, the meristematic stem cells and their niches are formed from specific tissues at specific positions under the developmental control, contributing to the increase of the body axis and construction of branching architecture (Gaillochet and Lohmann 2015; Yang and Jiao 2016). Additionally, in response to internal or external stimuli, meristematic stem cells/stem cell niches can arise from various tissues that are not the regular sites of meristem formation, which enables plants to plastically alter their development and regenerate the body parts when they are lost by injury or some other reasons (Ikeuchi et al. 2016).
In plant tissue culture, shoot regeneration can be induced from tissue explants by treatment with phytohormones. For efficient shoot regeneration, a two-step culture protocol is widely used, in which explants are primarily cultured on callus-inducing medium (CIM) rich in auxin and then the resultant calli are cultured on shoot-inducing medium (SIM) rich in cytokinin (Ikeuchi et al. 2019; Motte et al. 2014). Molecular biological studies of the two-step shoot regeneration of Arabidopsis have revealed that calli induced by culture on CIM share many characteristics with lateral root primordia (LRPs) and RAM and that their formation follows the pathway of lateral root development (Atta et al. 2009; Sugimoto et al. 2010). These calli contain RAM-type stem cells/stem cell niches, which are considered to confer pluripotency including shoot regeneration competence and to give birth to SAM-type stem cells/stem cell niches after transfer onto SIM, leading to the establishment of adventitious bud SAMs (Ikeuchi et al. 2019).
Auxins to be added to CIM have been extensively examined to optimize callus formation and shoot regeneration. In many cases, the synthetic auxin 2,4-dichlorophenoxyacetic acid (2,4-D) has been shown to be most effective (e.g., Lai and Liu 1982; Maheshwari et al. 2011; Motte et al. 2011). Compared to the exogenously applied auxins, the roles of the endogenously produced natural auxin indole-3-acetic acid (IAA) has received less attention in tissue culture experiments. Information about the causal relationships between endogenous IAA and shoot regeneration is rather limited and fragmentary, although there are several reports on endogenous IAA contents and on effects of IAA-related drugs in explants induced to form calli and regenerate shoots (Bhau and Wakhlu 2001; Cassells 1979; Hisano et al. 2016; Huang et al. 2012). Considering that calli are formed according to the lateral root development pathway and that lateral root development involves dynamic changes in the content and distribution of IAA (Du and Scheres 2018), however, it is possible that endogenous IAA has some important role in the regulation of callus growth and properties and thereby shoot regeneration.
In the present study, we investigated effects of inhibitors of auxin biosynthesis and transport in the two-step shoot regeneration culture of Arabidopsis. The results obtained showed that the inhibitors applied during CIM culture enhance shoot regeneration after transfer onto SIM, suggesting a negative impact of endogenous IAA on shoot regeneration competence of calli. Taken together with the data of endogenous IAA levels, spatial patterns of the auxin-responsive reporter expression, and expression levels of lateral root initiation and/or RAM establishment-related genes, we will discuss how endogenous IAA influences the organogenic properties of calli.
Materials and methods
Plant materials and growth conditions
The Landsberg erecta (Ler) strain of Arabidopsis thaliana were used as a plant material unless otherwise indicated. The CYCB1;1p::CYCB1;1db:GUS reporter line was derived from Ler transformed with pCDG (Colón-Carmona et al. 1999; Tamaki et al. 2009). The DR5::GUS reporter line was originally derived from the Columbia strain (Ulmasov et al. 1997) and was crossed with Ler three times before use in this study.
For tissue culture, seedlings were grown aseptically on germination medium under continuous light (10–15 µmol m−2 s−1). The germination medium was a half strength Murashige-Skoog medium supplemented with 1.0% (w/v) sucrose, buffered to pH 5.7 with 0.05% (w/v) 2-(N-morpholino)ethanesulfonic acid (MES), and solidified with 0.8% (w/v) gellan gum.
Tissue culture
Tissue-culture experiments were performed at 22°C under continuous light (20–30 µmol m−2 s−1) as previously described (Tamaki et al. 2009) with slight modifications. Hypocotyl segments of 5 mm in length were excised from 7-day-old seedlings and cultured on CIM for the induction of callus followed by culture on SIM for the induction of adventitious bud formation. CIM and SIM were Gamborg’s B5-based media that were supplemented with 2.0% (w/v) glucose and phytohormones, buffered to pH 5.7 with 0.05% (w/v) MES, and solidified with 0.25% (w/v) gellan gum. As phytohormones, 0.5 mg l−1 2,4-D and 0.1 mg l−1 kinetin were added to CIM, whereas 0.04 mg l−1 IAA and 1.5 mg l−1 N6-Δ2-isopentenyladenine (2iP) were added to SIM. Chemicals to be tested for their effects in tissue culture, such as 4-phenoxyphenylboronic acid (PPBo), L-kynurenine (Kyn), 1-naphthylphthalamic acid (NPA), 2,3,5-triiodobenzoic acid (TIBA), IAA, and indole-3-pyruvic acid (IPA), were dissolved in dimethyl sulfoxide (DMSO) and added to CIM or SIM at the indicated concentrations. The final concentration of DMSO did not exceed 0.25% (v/v) in any case and it was the same between conditions in each experiment. Reagents used for tissue culture were mostly the products from Sigma-Aldrich (for glucose, IAA, 2iP, Kyn, IPA, and some others), TCI (for glucose, kinetin, PPBo, NPA, and some others), Wako (for kinetin, PPBo, NPA, and some others), Alfa Aesar (for 2,4-D and IAA), Duchefa Biochemie (for 2iP), Dojindo (for MES), or Kanto Chemical (for gellan gum).
Quantification of IAA
IAA was extracted from hypocotyl explants of approximately 30 mg in fresh weight and analyzed by liquid chromatography-tandem mass spectrometry using 13C6-labeled IAA (Cambridge Isotope Laboratories) as an internal standard as previously described (Mashiguchi et al. 2019).
Histological and histochemical analyses
Explants were fixed overnight at 4°C in a 9 : 1 (v/v) mixture of ethanol and acetic acid, and then rehydrated through a graded series of aqueous ethanol solutions. Samples were mounted with a few drops of a clearing solution (8 : 1 : 2 (w/v/v) mixture of 2,2,2-trichloroethane-1,1-diol, glycerol, and water). Differential interference contrast images were taken under a microscope equipped with Nomarski optics (BX50-DIC; Olympus).
Histochemical detection of the GUS activity signal was performed as previously described (Ohtani and Sugiyama 2005) with slight modifications. Briefly, explants of the reporter lines were fixed at –20°C in 90% (v/v) acetone, rinsed with 100 mM sodium phosphate buffer (pH 7.2), and then incubated in X-gluc solution (0.5 mg ml−1 5-bromo-4-chloro-3-indolyl-β-D-glucuronide cyclohexylammonium salt, 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 100 mM sodium phosphate (pH 7.4)) for GUS reaction at 37°C for 2 h (CYCB1;1p::CYCB1;1db:GUS) reporter or for 30 min (DR5::GUS). After the GUS reaction, samples were decolored with 70% (v/v) ethanol, cleared with the clearing solution, and examined under a microscope with Nomarski optics (BX50-DIC; Olympus).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA samples were prepared from explants using Direct-zol™ RNA Miniprep (ZYMO RESEARCH) and reverse transcribed into cDNAs using PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa). qPCR was performed for LATERAL ORGAN BOUNDARIES DOMAIN 16 (LBD16), LBD18, PLETHORA3 (PLT3), PLT5, PLT7, and WUSCHEL-RELATED HOMEOBOX 5 (WOX5) with α-TUBULIN 4 (TUA4) as an internal standard using TB Green™ Premix ExTaq™ II (Tli RNaseH Plus) (TaKaRa) on StepOne Real-Time PCR System (Applied Biosystems). Primers used for qPCR are listed in Supplementary Table S1.
Results
Effects of auxin biosynthesis inhibitors on organogenic competence
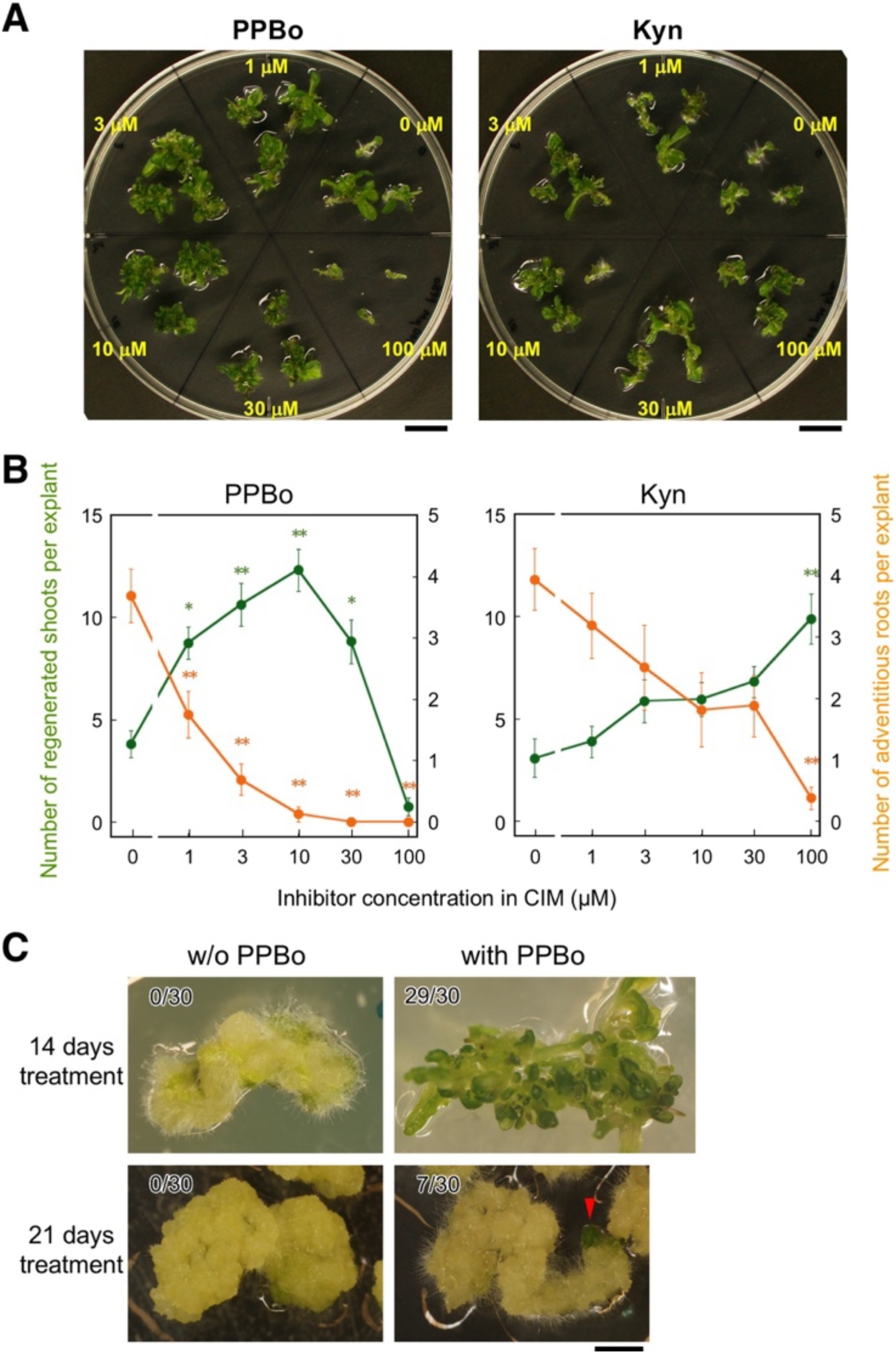
PPBo blocks the conversion of IPA to IAA catalyzed by the flavin monooxygenase YUCCA (Kakei et al. 2015), whereas Kyn blocks the conversion of tryptophan to IPA catalyzed by the TAA1 family aminotransferases (He et al. 2011). To address possible roles of endogenous IAA in shoot regeneration, we first examined effects of PPBo and Kyn by culturing hypocotyl explants on CIM with various concentrations of PPBo or Kyn for 4.5 days and then on SIM without PPBo or Kyn. After 2 weeks on SIM, explants cultured with PPBo or Kyn at appropriate concentrations showed more vigorous shoot regeneration than the control explants and were covered densely by regenerated shoots (Figure 1A). Quantitative evaluation of shoot regeneration at 10 days of SIM culture indicated that PPBo and Kyn given in the CIM culture significantly increased the number of regenerated shoots at the concentrations of 1 to 30 µM and of 100 µM, respectively (Figure 1B). In these explants, we also noticed that the number of adventitious roots, which are often formed after transfer onto SIM in the two-step culture employed here, was decreased seemingly in association with the rise of shoot regeneration by PPBo and Kyn. The promotive effect of PPBo on shoot regeneration was observed even more obviously when CIM culture was prolonged to 2 weeks or 3 weeks (Figure 1C). These results indicated that IAA biosynthesis inhibitors added to the CIM culture affected organogenic competence of calli to enhance shoot regeneration and to repress adventitious root formation in the subsequent culture on SIM.
Figure 1. Effects of inhibitors of auxin biosynthesis in CIM culture on shoot regeneration in hypocotyl explants. (A) Hypocotyl explants cultured on SIM for 14 days after 4.5 days of CIM culture supplemented with the indicated concentrations of PPBo (left) or Kyn (right). Scale bars=1 cm. (B) Numbers of regenerated shoots (green) and adventitious roots (orange) in hypocotyl explants cultured on SIM for 10 days after 4.5 days of CIM culture with the indicated concentrations of PPBo (left) or Kyn (right). Mean values of 10 explants are shown with standard errors. Values with an asterisk(s) are significantly different from the control (0 µM) value at p<0.05 (*) or 0.01 (**) (Tukey’s test). (C) Hypocotyl explants cultured on SIM for 14 days after 14 or 21 days of CIM culture supplemented with and without 25 µM PPBo. The number of explants with regenerated shoots per the total number of explants is shown at the upper left corner of each photograph. The red arrowhead indicates a regenerated shoot bud. Scale bar=2 mm.
Next, we examined organogenic responses of calli cultured with 25 µM PPBo and various concentrations of IAA to test whether exogenous IAA counteracts the effects of PPBo on shoot regeneration and adventitious root formation. Over the concentrations from 0.1 to 10 mg l−1, however, IAA addition did not much affect the numbers of regenerated shoots and adventitious roots (Supplementary Figure S1A). We also tried to test whether IPA counteracts the effects of Kyn but no clear tendency was observed (Supplementary Figure S1B). These results imply that the effects of auxin biosynthesis inhibitors on organogenic competence of calli is not exerted simply through the overall reduction of the IAA level.
Effective timing of PPBo treatment
In consideration of the possibility that auxin biosynthesis/transport inhibitors are not specifically effective during CIM culture but also effective during SIM culture for enhancing shoot regeneration, we examined the influence of PPBo added to SIM by culturing explants on CIM without inhibitors and then on SIM with various concentrations of inhibitors. In contrast to the PPBo treatment during CIM, PPBo added to SIM rather decreased the number of regenerated shoots at as low concentrations as 1 to 10 µM (Supplementary Figure S2). This result showed that shoot regeneration is enhanced by PPBo only when it is given during CIM culture in the two-step culture protocol.
To further dissect the effective timing of PPBo treatment, the period of 4.5 days of CIM culture was divided into three subperiods of 1.5 days each and explants were treated with PPBo under various time schedules, including conditions in which explants were exposed to PPBo during only one or two subperiods (Supplementary Figure S3A). PPBo treatment for 3 days from the first to second subperiods or from the second to third subperiods was fully effective in enhancing shoot regeneration and repressing adventitious root formation (Supplementary Figure S3B). Furthermore, PPBo treatment for only 1.5 days also effectively enhanced shoot regeneration when given in the first subperiod, while it did not when given in the third subperiod (Supplementary Figure S3B). From these effective timings, it was suggested that IAA biosynthesis in the early phase of callus formation is particularly important for the regulation of organogenic competence.
Changes in the IAA level during CIM culture
To gain information about changes in the IAA level during CIM culture and to examine the influence of PPBo on the IAA level, explants cultured on CIM with or without PPBo for 4 days and 14 days were subjected to IAA quantification. During 4 days of CIM culture, regardless of the addition or not of PPBo, the IAA content dropped to less than one third of the initial level (Table 1). At this time, the decrease of the IAA level was not much different between PPBo-treated and control cultures in the presence of 2,4-D but rather smaller in the absence of 2,4-D, suggesting 2,4-D-dependent reduction of endogenous IAA in a short term of CIM culture. After 14 days, the IAA content was increased to two times higher than the initial level in the CIM culture without PPBo, while it remained at a low level in the culture with PPBo in accordance with the inhibition of IAA biosynthesis by PPBo (Table 1).
Table 1. IAA content in hypocotyl explants cultured on CIM.
| Culture duration | Culture medium (phytohormones) | Inhibitor | IAA content (pmol/g fresh weight) |
|---|---|---|---|
| 0 day | — | — | 39.8±13.9a |
| 4 days | CIM (2,4-D and kinetin) | — | 9.8±2.5a |
| 4 days | CIM (2,4-D and kinetin) | 25 µM PPBo | 12.5±4.7a |
| 4 days | 2,4-D-depleted CIM (kinetin) | — | 22.3±6.4a |
| 14 days | CIM (2,4-D and kinetin) | — | 88.3±19.1b |
| 14 days | CIM (2,4-D and kinetin) | 25 µM PPBo | 17.5±4.8a |
Data are means±SD of 4 biological replicates. Values with different letters are significantly different from each other at p<0.01 (Tukey’s test).
Effects of auxin transport inhibitors on organogenic competence
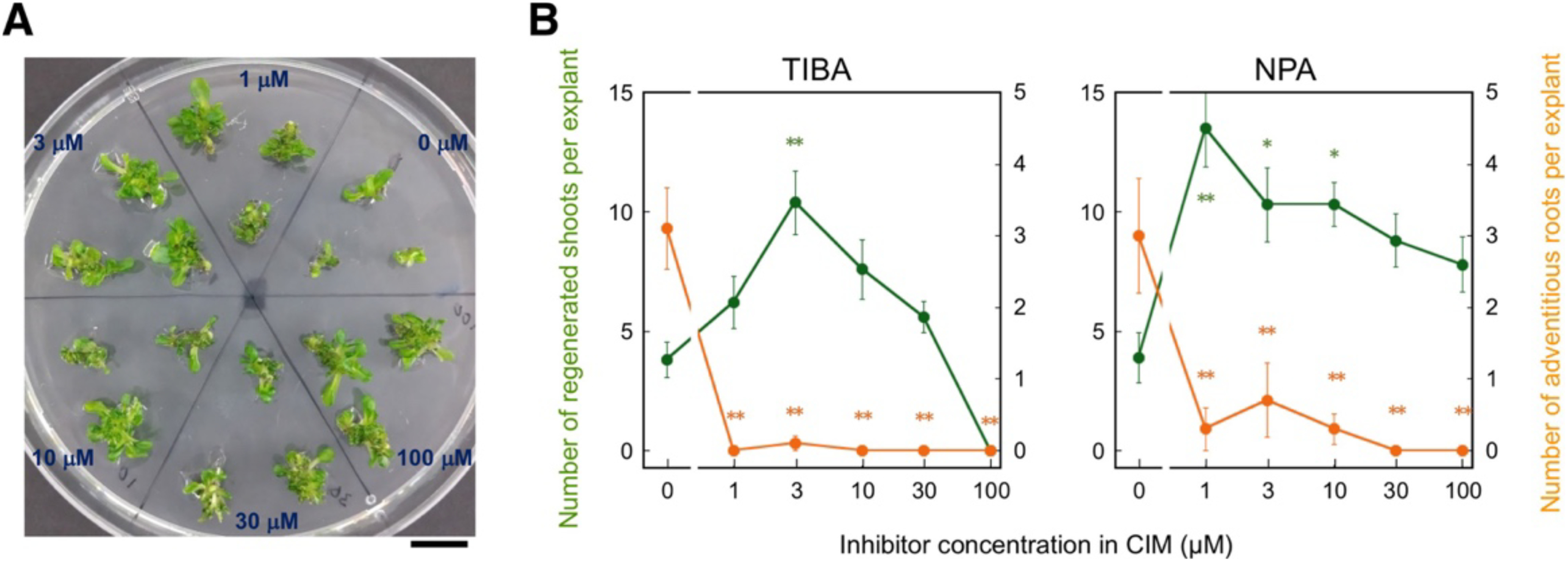
In addition to auxin biosynthesis, auxin polar transport mainly driven by the auxin efflux carriers PIN-FORMED (PIN) proteins also constitutes the dynamics of endogenous auxin, which generally plays a critical role in plant organ development (Benková et al. 2003). As the auxin exogenously supplied in CIM is 2,4-D, which is not transported by PIN proteins (Delbarre et al. 1996), inhibition of PIN-dependent polar transport of auxin is expected to perturb endogenous IAA without direct influence on the actions of exogenous 2,4-D. In the light of this difference between IAA and 2,4-D, we examined effects of treatment with inhibitors of auxin polar transport, NPA and TIBA, during CIM culture, to obtain further insights into the relationship between endogenous IAA and organogenic competence. When hypocotyl explants were cultured on CIM that contained various concentrations of NPA or TIBA and then on inhibitor-free SIM, the number of regenerated shoots was increased over a broad range of concentrations with a peak at 3 µM or less (Figure 2). As was the case for the treatment with auxin biosynthesis inhibitors, a significant decrease of adventitious roots was also observed in calli induced in the presence of NPA or TIBA (Figure 2). These results suggest that the disturbance of the endogenous auxin dynamics due to the inhibition of auxin transport facilitates the competence of calli to regenerate shoots on SIM concomitantly with diminishing the root-forming competence.
Figure 2. Effects of inhibitors of polar auxin transport in CIM culture on shoot regeneration in hypocotyl explants. (A) Hypocotyl explants cultured on SIM for 14 days after 4.5 days of CIM culture supplemented with the indicated concentration of NPA. Scale bar=1 cm. (B) Numbers of regenerated shoots (green) and adventitious roots (orange) in hypocotyl explants cultured on SIM for 10 days after 4.5 days of CIM culture with the indicated concentrations of TIBA (left) and NPA (right), respectively. Mean values of 16 explants (PPBo) or 10 explants (Kyn) are shown with standard errors. Values with an asterisk(s) are significantly different from the control (0 µM) value at p<0.05 (*) or 0.01 (**) (Tukey’s test).
Characteristics of calli as influenced by auxin biosynthesis/transport inhibitors
The above-mentioned experiments showed that calli formed in the presence of inhibitors of auxin biosynthesis or polar transport have a higher competence for shoot regeneration. To characterize such calli with the enhanced regeneration competence, we compared the characteristics of calli cultured on CIM with and without inhibitors for cell proliferation and auxin response. In the standard CIM culture, calli were initiated from the stele tissue within 2 days and grew up to large mounds of actively proliferating cells (Figure 3A, B). When cultured on CIM with PPBo, callus growth was slower due to less active cell division resulting in the formation of relatively compact and flat calli (Figure 3A, B). These differences in the appearance of calli between CIM cultures with and without PPBo became much more obvious after prolonged culture (Figure 3C). Similar effects on the growth and shape of calli were observed in the treatment with NPA (Figure 3D).
Figure 3. Effects of PPBo and NPA on callus formation of hypocotyl explants. (A) Differential interference contrast images of hypocotyl explants cultured on CIM with and without 25 µM PPBo for the indicated days. Yellow dotted lines show the outlines of calli proliferated from the stele tissues. Scale bar=100 µm. (B) Expression patterns of CYCB1;1p::CYCB1;1:GUS in hypocotyl explants cultured on CIM with or without 25 µM PPBo for the indicated days. Scale bar=100 µm. (C) Callus formation induced from hypocotyl explants by culture on CIM with or without 25 µM PPBo for 21 days. Scale bar=1 cm. (D) Differential interference contrast images (upper micrographs) and CYCB1;1p::CYCB1;1:GUS expression patterns (lower micrographs) of hypocotyl explants cultured on CIM with or without 3 µM NPA for 8 days. Yellow dotted lines show the outlines of calli proliferated from the stele tissues. Scale bar=100 µm. (E) Expression patterns of DR5::GUS in hypocotyl explants cultured on CIM with or without 25 µM PPBo for. 4.5 days. Scale bar=2 mm. (F) Expression patterns of DR5::GUS in hypocotyl explants cultured on CIM with or without 2.5 µM NPA for 4.5 days. Scale bar=2 mm.

Inhibition of auxin biosynthesis and transport was expected to alter the endogenous auxin distribution in calli, which might be reflected in the alteration of the spatial pattern of auxin response. This possibility was examined using DR5::GUS as an auxin-responsive reporter. In the explants cultured on CIM for 4.5 days without the addition of inhibitors, the GUS activity was quite unevenly distributed: a strong GUS activity was observed in limited regions and there were large areas of a relatively weak GUS activity (Figure 3E, F). In contrast, explants cultured with PPBo showed a strong GUS activity almost throughout them (Figure 3E). The effect of NPA on DR5::GUS expression was not so striking, but the strong GUS activity regions were expanded in the NPA-treated explants compared to the control CIM cultures (Figure 3F). These results indicated that the addition of inhibitors of auxin biosynthesis and transport may make auxin response stronger and more uniform over the explants while they suppress cell proliferation to alter the callus shape flatter and more compact.
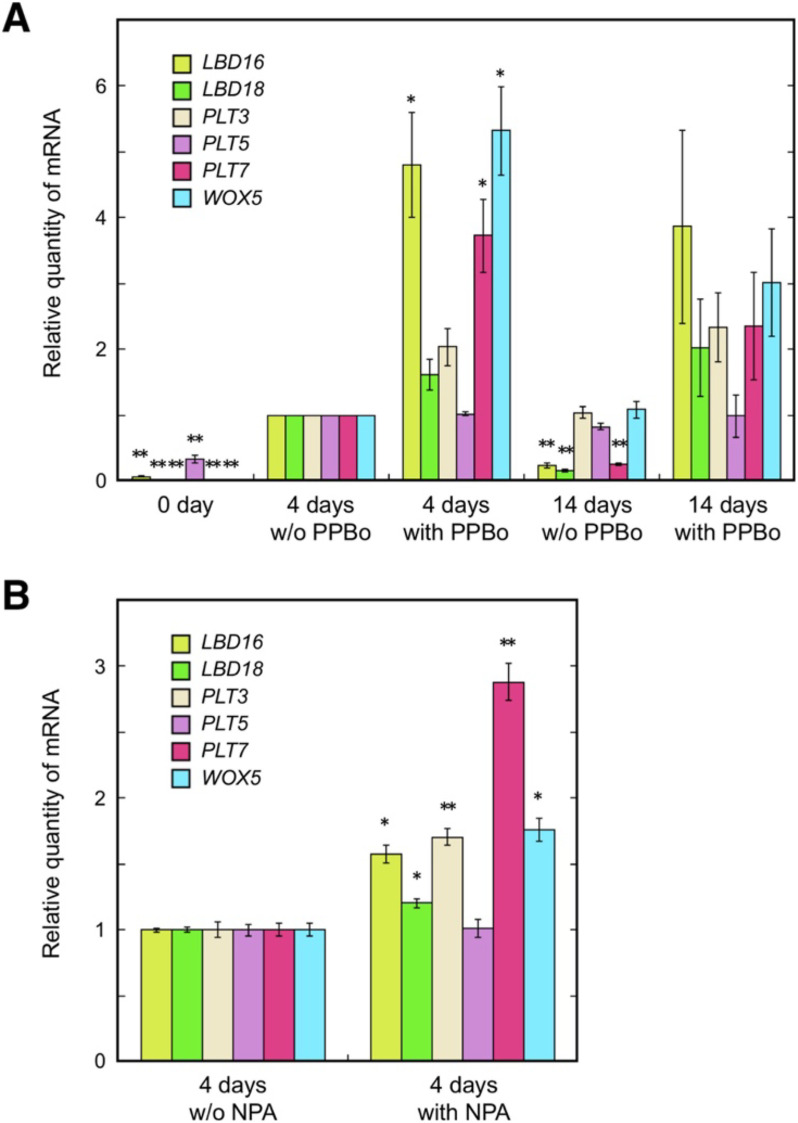
Auxin-induced formation of shoot regeneration-competent calli has recently been revealed to involve auxin-responsive expression of lateral root initiation and/or RAM stem cell niche establishment-related genes such as LBDs, PLTs, and WOXs (Fan et al. 2012; Kareem et al. 2015; Kim et al. 2018; Liu et al. 2018). We assessed expression levels of these genes in calli cultured with PPBo or NPA by RT-qPCR analysis. All genes examined, except for PLT5, were found to be up-regulated by both PPBo and NPA treatments (Figure 4), which seems to agree with the stronger auxin response in PPBo or NPA-treated calli suggested by DR5::GUS expression (Figure 3E, F). LBD16, LBD18, and PLT7 not only showed increased expression in cultures with PPBo or NPA, where shoot regeneration competence was enhanced, but also showed decreased expression in a long-term culture (Figure 4A), where regeneration competence declined (Figure 1C). Out of these three genes, only PLT7 was affected in its expression level to a comparable extent by both PPBo and NPA treatments (Figure 4), which enhanced shoot regeneration competence to a comparable extent (Figures 1B, 2B). These results indicated that shoot regeneration competence of calli under various culture conditions is more closely correlated to the expression level of PLT7 than to the expression levels of the other lateral root/RAM-related genes tested.
Figure 4. Effects of PPBo and NPA on the expression of lateral root/RAM-related genes in calli. (A) mRNA levels of LBD16, LBD18, PLT3, PLT5, PLT7, and WOX5 determined by RT-qPCR analysis for hypocotyl explants cultured on CIM with or without 25 µM PPBo for the indicated days. Data obtained from three independent experiments are presented as means±standard errors of relative quantities compared to the quantities of the 4-day culture without PPBo. Asterisks indicate significant differences from the value of the 4-day culture without PPBo for each gene at p<0.05 (*) or 0.01 (**) (Tukey’s test). (B) mRNA levels of LBD16, LBD18, PLT3, PLT5, PLT7, and WOX5 determined by RT-qPCR analysis for hypocotyl explants cultured on CIM with or without 2.5 µM NPA for 4 days. Data obtained from three biological replicates are presented as means±standard errors of relative quantities compared to the quantities of the culture without NPA. Asterisks indicate significant differences from the value of the culture without NPA for each gene at p<0.05 (*) or 0.01 (**) (Student’s t test).
Discussion
Our results obtained with the two-step culture of Arabidopsis demonstrated that treatment with inhibitors of auxin biosynthesis or polar transport during CIM culture suppresses callus growth but increases shoots regenerated in the subsequent SIM culture, suggesting the enhancement of the shoot regeneration competence of calli by the perturbation of the endogenous auxin dynamics. The effects of auxin biosynthesis and transport inhibitors on shoot regeneration have been examined in various tissue culture systems. These inhibitors were shown to affect shoot regeneration negatively in some experimental conditions (Cassells 1979; Christianson and Warnick 1984; Dhaliwal et al. 2004; Kim and Ernst 1994; Koike et al. 2020; Liu et al. 2017) and positively in others (Belaizi et al. 1991; Bhau and Wakhlu 2001; Cambecèdes et al. 1991; Pal et al. 2012; Shukla et al. 2014; van Aartrijk and Blom-Barnhoorn 1984; Zhang et al. 2008). Most of the positive effects were observed in one-step cultures, in which shoot regeneration is induced directly on a cytokinin-rich medium without a preliminary callus-inducing culture (Belaizi et al. 1991; Cambecèdes et al. 1991; Pal et al. 2012; Shukla et al. 2014; van Aartrijk and Blom-Barnhoorn 1984), whereas, in a few cases, auxin transport inhibitors could increase the number of regenerated shoots when added to SIM in the two-step culture (Bhau and Wakhlu 2001; Zhang et al. 2008). Such improvement of shoot regeneration has been interpreted to be attributable to a shift of the hormonal balance in favor of shoot regeneration at the regeneration sites due to the prevention of auxin transport across the explant (Belaizi et al. 1991; Cambecèdes et al. 1991). Compared to these previously reported positive effects, the shoot regeneration-stimulating effects of the auxin biosynthesis and transport inhibitors described here is clearly distinct in that they were exerted during CIM culture in the presence of a high concentration of the non-transportable auxin 2,4-D and in that they seem to reflect the enhancement of the shoot regeneration competence resulting from the impairment of the auxin dynamics in developing calli.
Treatment with PPBo only for the first 1.5 or 3 days of CIM culture was effective in stimulating shoot regeneration. As the PPBo-treated explants contained almost the same amount of IAA as the control explants even after 4 days of CIM culture, the shoot regeneration-stimulating effect of PPBo cannot be simply attributed to the reduction in the IAA level but is likely caused by the disruption of finely controlled spatiotemporal patterns of auxin distribution. This speculation is supported by the result that inhibitors of auxin transport applied during CIM culture showed similar effects on shoot regeneration to the auxin biosynthesis inhibitors, and also appears to be consistent with the result that the exogenous addition of IAA and IPA did not cancel the effects of PPBo and Kyn. Moreover, of great interest, auxin response visualized by the DR5::GUS reporter expression appeared to be more uniform and even stronger in the explants cultured on CIM with PPBo or NPA than the control explants. In agreement with the stronger DR5::GUS expression, PPBo and NPA treatments tended to increase expression of auxin-responsive lateral root/RAM-related genes. It is possible that these changes in the pattern of auxin response and the relative expression levels of the lateral root/RAM-related genes, perhaps induced by the disturbance of the local dynamics of endogenous IAA, are associated with some alteration of the callus cell status leading to the enhancement of the shoot regeneration competence.
In the light of our knowledge that auxin-induced callus formation on CIM resembles lateral root formation in many aspects (Atta et al. 2009; Sugimoto et al. 2010), the functions of IAA in the development of lateral roots may provide us with a clue to understand how endogenous IAA regulates the callus cell status and the competence for shoot regeneration. In the process of lateral root formation, biosynthesis and polar transport of IAA determine the pattern of IAA distribution in each LRP, which plays a pivotal role in proper LRP development (Du and Scheres 2018). The recent study by Rosspopoff et al. (2017) demonstrated that the RAM stem cell niche in the LRP only within a narrow window of developmental stages (Stage VI or VII) can be transformed by high concentrations of cytokinin into the SAM stem cell niche, leading to shoot regeneration. Assuming callus status transition corresponding to the IAA-dependent progression of LRP development, it can be reasonably speculated that the perturbation of endogenous auxin dynamics in calli may interfere with the status transition to make them stay longer at the status with the RAM stem cell niche transformable to the SAM stem cell niche and thus enhance their competence for shoot regeneration. Spatiotemporal expression analysis of the stem cell-related genes between calli induced with and without the auxin inhibitors and in comparison with LRPs at different stages would be helpful for testing this hypothesis. In our RT-qPCR experiment, PLT7 expression showed the closest correlation to shoot regeneration competence under different culture conditions among genes examined. Therefore, PLT7 may be a promising candidate for the target of future research in this line.
Acknowledgments
We thank the Salk Institute and Dr. Peter Doerner (Edinburgh University) for providing pCDG and Dr. Tom J. Guilfoyle (University of Missouri) for providing the DR5::GUS line. This work was supported by the grants from National Cheng-Kung University to I.O. and by Japan Society for the Promotion of Science KAKENHI (JP18H04830 and JP20H04881 to M.S.).
Abbreviations
- CIM
callus-inducing medium
- DMSO
dimethyl sulfoxide
- IAA
indole-3-acetic acid
- IPA
indole-3-pyruvic acid
- Kyn
L-kynurenine
- LBD
LATERAL ORGAN BOUNDARIES DOMAIN
- Ler
Landsberg erecta
- LRP
lateral root primordium
- MES
2-(N-morpholino)ethanesulfonic acid
- NPA
1-naphthylphthalamic acid
- PLT
PLETHORA
- PPBo
4-phenoxyphenylboronic acid
- RAM
root apical meristem
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- SAM
shoot apical meristem
- SIM
shoot-inducing medium
- TIBA
2,3,5-triiodobenzoic acid
- TUA4
α-TUBULIN 4
- 2,4-D
2,4-dichlorophenoxyacetic acid
- 2iP
N6-Δ2-isopentenyladenine
- WOX
WUSCHEL-RELATED HOMEOBOX
Author contributions
Tissue culture experiments were performed by IO, YS, and MS. IAA quantification was carried out by HKu and HKa. IO and MS wrote the manuscript.
Supplementary Data
References
- Atta R, Laurens L, Boucheron-Dubuisson E, Guivarc’h A, Carnero E, Giraudat-Pautot V, Rech P, Chriqui D (2009) Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J 57: 626–644 [DOI] [PubMed] [Google Scholar]
- Belaizi M, Paul H, Sangwan RS, Sangwan-Norreel BS (1991) Direct organogenesis from internodal segments of in vitro grown shoots of apple cv. Golden delicious. Plant Cell Rep 9: 471–474 [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bhau BS, Wakhlu AK (2001) Effect of genotype, explant type and growth regulators on organogenesis in Morus alba. Plant Cell Tissue Organ Cult 66: 25–29 [Google Scholar]
- Cambecèdes J, Duron M, Decourtye L (1991) Adventitous bud regeneration from leaf explants of the shrubby ornamental honeysuckle, Lonicera nitida Wils. cv. ‘Maigrün’: Effects of thidiazuron and 2,3,5-triiodobenzoic acid. Plant Cell Rep 10: 471–474 [DOI] [PubMed] [Google Scholar]
- Cassells AC (1979) The effect of 2,3,5-triiosobenzoic acid on caulogenesis in callus cultures of tomato and Pelargonium. Physiol Plant 46: 159–164 [Google Scholar]
- Christianson ML, Warnick DA (1984) Phenocritical times in the process of in vitro shoot organogenesis. Dev Biol 101: 382–390 [DOI] [PubMed] [Google Scholar]
- Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P (1999) Spatio-temporal analysis of mitotic activity with labile cyclin-GUS fusion protein. Plant J 20: 503–508 [DOI] [PubMed] [Google Scholar]
- Delbarre A, Muller P, Inhoff V, Guern VJ (1996) Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198: 532–541 [DOI] [PubMed] [Google Scholar]
- Dhaliwal H, Yeung EC, Thorpe TA (2004) TIBA inhibition of in vitro organogenesis in excised tobacco leaf explants. In Vitro Cell Dev Biol—Plant 40: 235–238 [Google Scholar]
- Du Y, Scheres B (2018) Lateral root formation and the multiple roles pf auxin. J Exp Bot 69: 155–167 [DOI] [PubMed] [Google Scholar]
- Fan M, Xu C, Xu K, Hu Y (2012) LATERAL ORGAN BOUNDAIRES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res 22: 1169–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillochet C, Lohmann JU (2015) The never-ending story: From pluripotency to plant developmental plasticity. Development 142: 2237–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F, et al. (2011) A small-molecule screen identifies L-kynurenin as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisano H, Matsuura T, Mori IC, Yamane M, Sato K (2016) Endogenous hormone levels affect the regeneration ability of callus derived from different organs in barley. Plant Physiol Biochem 99: 66–72 [DOI] [PubMed] [Google Scholar]
- Huang W-L, Lee C-H, Chen Y-R (2012) Levels of endogenous abscisic acid and indole-3-acetic acid influence shoot organogenesis in callus cultures of rice subjected to osmotic stress. Plant Cell Tissue Organ Cult 108: 257–263 [Google Scholar]
- Ikeuchi M, Favero DS, Sakamoto Y, Iwase A, Coleman D, Rymen B, Sugimoto K (2019) Molecular mechanisms of plant regeneration. Annu Rev Plant Biol 70: 377–406 [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K (2016) Plant regeneration; cellular origins and molecular mechanisms. Development 143: 1442–1451 [DOI] [PubMed] [Google Scholar]
- Kakei Y, Yamazaki C, Suzuki M, Nakamura A, Sato A, Ishida Y, Kikuchi R, Higashi S, Kokudo Y, Ishii T, et al. (2015) Small-molecule auxin inhibitors that target YUCCA are powerful tools for studying auxin function. Plant J 84: 827–837 [DOI] [PubMed] [Google Scholar]
- Kareem A, Durgaprasad K, Sugimoto K, Du Y, Pulianmackal AJ, Trivedi ZB, Abhayadev PV, Pinon V, Meyerowitz EM, Scheres B, et al. (2015) PLETHORA genes control regeneration by a two-step mechanism. Curr Biol 25: 1017–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-Y, Yang W, Forner J, Lohmann JU, Noh B, Noh Y-S (2018) Epigenetic reprogramming by histone acetyltransferase HAG1/AtGCN5 is required for pluripotency acquisition in Arabidopsis. EMBO J 37: e98726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-N, Ernst SG (1994) Effects of inhibitors on phenocritical events of in vitro shoot organogenesis in tobacco thin cell layers. Plant Sci 103: 59–66 [Google Scholar]
- Koike I, Watanabe S, Okazaki K, Hayashi K, Kasahara H, Shimomura K, Umehara M (2020) Endogenous auxin determines the pattern of adventitious shoot formation on internodal segments of ipecac. Planta 251: 73. [DOI] [PubMed] [Google Scholar]
- Lai K-L, Liu L-F (1982) Induction and plant regeneration of callus from immature embryos of rice plants (Oryza sativa L.). Jpn J Crop Sci 51: 70–74 [Google Scholar]
- Liu J, Hu X, Qin P, Prasad K, Hu Y, Xu L (2018) The WOX11-LBD16 pathway promotes pluripotency acquisition in callus cells during de novo shoot regeneration in tissue culture. Plant Cell Physiol 59: 739–748 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang H-L, Guo H-R, Xie L, Zeng R-Z, Zhang X-Q, Zhang Z-S (2017) Transcriptomic and hormonal analyses reveal that YUC-mediated auxin biogenesis is involved in shoot regeneration from rhizome in Cymbidium. Front Plant Sci 8: 1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari P, Selvaraji G, Kovalchuk I (2011) Optimization of Brassica napus (canola) explant regeneration for genetic transformation. N Biotechnol 29: 144–155 [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Hisano H, Takeda-Kamiya N, Takebayashi Y, Ariizumi T, Gao Y, Ezura H, Sato K, Zhao Y, Hayashi K, et al. (2019) Agrobacterium tumefaciens enhances biosynthesis of two distinct auxins in the formation of crown galls. Plant Cell Physiol 60: 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte H, Vereecke D, Geelen D, Werbrouck S (2014) The molecular path to in vitro regeneration. Biotechnol Adv 32: 107–121 [DOI] [PubMed] [Google Scholar]
- Motte H, Verstraeten I, Werbrouck S, Geelen D (2011) CUC2 as an early marker for regeneration competence in Arabidopsis root explants. J Plant Physiol 168: 1598–1601 [DOI] [PubMed] [Google Scholar]
- Ohtani M, Sugiyama M (2005) Involvement of SRD2-mediated activation of snRNA transcription in the control of cell proliferation competence in Arabidopsis. Plant J 43: 479–490 [DOI] [PubMed] [Google Scholar]
- Pal AK, Acharya K, Ahuja PS (2012) Endogenous auxin level is a critical determinant for in vitro adventitious shoot regeneration in potato (Solanum tuberosum L.). J Plant Biochem Biotechnol 21: 205–212 [Google Scholar]
- Rosspopoff O, Chelysheva L, Saffar J, Lecorgne L, Gey D, Caillieux E, Colot V, Roudier F, Hilson P, Berthomé R, et al. (2017) Direct conversion of root primordium into shoot meristem relies on timing of stem cell niche development. Development 144: 1187–1200 [DOI] [PubMed] [Google Scholar]
- Shukla PS, Das AK, Jha B, Agarwal PK (2014) High-frequency in vitro shoot regeneration in Cucumis sativus by inhibition of endogenous auxin. In Vitro Cell Dev Biol Plant 50: 729–737 [Google Scholar]
- Sugimoto K, Jiao Y, Meyerowitz EM (2010) Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell 18: 463–471 [DOI] [PubMed] [Google Scholar]
- Tamaki H, Konishi M, Daimon Y, Aida M, Tasaka M, Sugiyama M (2009) Identification of novel meristem factors involved in shoot regeneration through the analysis of temperature-sensitive mutants of Arabidopsis. Plant J 57: 1027–1039 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/lAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Aartrijk J, Blom-Barnhoorn GJ (1984) Adventitious bud formation from bulb-scale explants of Lilium speciosum Thunb. in vitro. Interacting effects of NAA, TIBA, wounding, and temperature. J Plant Physiol 116: 409–416 [DOI] [PubMed] [Google Scholar]
- Yang M, Jiao Y (2016) Regulation of axillary meristem initiation by transcription factors and plant hormones. Front Plant Sci 7: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu Z, Huang C (2008) Effects of gibberellin mutations on in vitro shoot bud regeneration of Arabidopsis. Afr J Biotechnol 7: 4159–4163 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.