Abstract
Pericycle cells possess proliferative activity long after leaving the root apical meristem. Depending on the developmental stage and external stimuli, pericycle cell division leads to the production of lateral roots, vascular cambium and periderm, and callus. Therefore, pericycle cell division competence underlies root branching and secondary growth, as well as plant regeneration capacity. In this review, we first briefly present an overview of the molecular pathways of the four developmental programs originated, exclusively or partly, from pericycle cells. Then, we provide a review of up-to-date knowledge in the mechanisms determining pericycle cells’ competence to undergo cell division. Furthermore, we discuss directions of future research to further our understanding of the pericycle’s characteristics and functions.
Keywords: cell division competence, lateral root development, pericycle, plant regeneration, secondary growth
Introduction
The pericycle is a primary root tissue that forms the cell layer surrounding xylem, phloem, and procambium cells. In Arabidopsis, the pericycle layer can be recognized in the hypocotyl but not in the shoot (Bougourd et al. 2000). Pericycle cells possess continued cell division activity for a long time after they leave the root meristem (Dubrovsky et al. 2000). According to their position relative to the xylem and phloem, pericycle cells can be categorized into xylem pole pericycle (XPP) and phloem pole pericycle (PPP) cells (Figure 1). XPP and PPP cells are distinctive in their cell morphology, marker gene expression, and especially in their competence for cell division and organogenesis (Parizot et al. 2008). In the Arabidopsis root, XPP cells above the root meristem can be marked by the GFP expression pattern in the GAL4-GFP enhancer-trap line J0121 (Laplaze et al. 2005).
Figure 1. Pericycle in Arabidopsis. Transverse section of an Arabidopsis root approximately 1 mm from the tip. Asterisks: pericycle cells. Xylem cells are highlighted in pink. XPP cells are highlighted in blue. Scale bar, 20 µm.
Depending on the developmental stage and external stimuli, pericycle cell’s proliferative activity has various functional outputs: lateral root (LR) initiation (Motte and Beeckman 2019), vascular cambium initiation (Chaffey et al. 2002), cork cambium (phellogen) initiation (Wunderling et al. 2018), and tissue regeneration (callus formation) (Atta et al. 2009; Sugimoto et al. 2010). LRs are responsible for root branching. Vascular cambium and cork cambium are considered secondary meristems in the root. Vascular cambium cell division produces secondary xylem (wood) inward and secondary phloem outward, resulting in an increase of the root diameter. Cork cambium cell division produces inwardly phelloderm, a living parenchyma-like tissue, and outwardly phellem (or cork), which is non-living and has suberized cell walls. Phelloderm, cork cambium, and phellem are collectively called the periderm and serve as the outermost barrier in aged roots (Campilho et al. 2020). Pericycle or pericycle-like cell represents a primary source tissue for callus formation during in vitro tissue culture and de novo organ regeneration (Atta et al. 2009; Bustillo-Avendaño et al. 2018; Sugimoto et al. 2010).
The plant hormone auxin plays a central role in all four of these developmental programs. Auxin regulates gene expression through the Aux/IAA-ARF signaling cascade. At low auxin levels, AUXIN RESPONSE FACTOR (ARF) transcription factors are bound to Aux/IAA proteins, which recruit the TPL corepressor and other chromatin-modifying proteins to repress auxin-responsive genes. In response to an auxin level increase, SCFTIR1 E3 ligases promote the degradation of Aux/IAA proteins and free the ARFs to activate auxin-responsive gene expression. The large Aux/IAA and ARF gene families and their diversity in expression patterns, interactive activity and auxin sensitivity underlie cell-type-specific auxin responses during plant development (Salehin et al. 2015). Pericycle cells are unique in that they can undergo cell division and initiate various de novo organogenesis programs, even whole plant regeneration. Most of the players involved in the developmental programs of LR development, vascular cambium and cork cambium initiation, and hormone-induced callus formation identified so far are members of auxin signalling modules or their downstream auxin-responsive genes. We are just beginning to understand the competence of pericycle cell’s auxin-responsiveness.
In this review, we first summarize recent research advances in the genetic pathways and hormone regulation during the four developmental processes, focusing on the auxin-regulated pathways at early initiation steps (Figure 2). Then, we review research progress in the molecular mechanisms underlying pericycle cell division competency. Finally, we discuss interesting questions and future challenges in pericycle research.
Figure 2. During root development, pericycle cell division gives rise to LRs, vascular cambium and periderm. LR development starts early and close to the primary root tip and is deemed the primary functional output of pericycle cell division. In the aged part of the root, pericycle cells are activated to divide, giving rise to two types of secondary meristems: vascular cambium and cork cambium. Yellow: PFA-PFB-conferred cell division competence. Blue arrow and inhibitory arrows: promotive or inhibitory effect between the developmental programs.
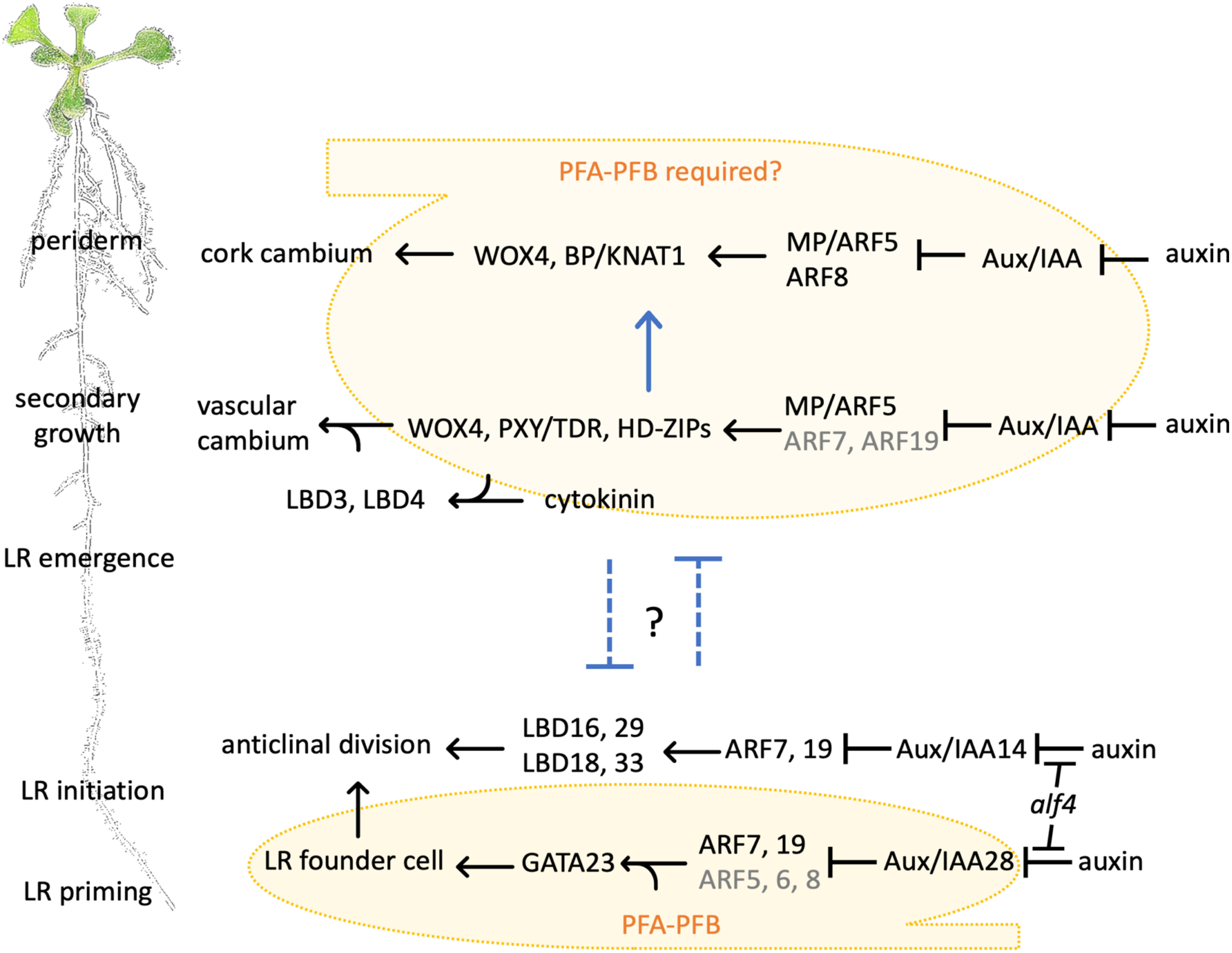
Lateral root initiation
Higher plants continually produce LRs throughout their lifetime, resulting in a branched root architecture. In all seed plants that exhibit monopodial branching, i.e. the parental root is the axis from which LRs emerge, LRs initiate from the pericycle (Motte and Beeckman 2019). In most species including Arabidopsis thaliana, LR initiation occurs post-germination in the elongation zone or the young differentiation zone (Dubrovsky et al. 2000). However, in some species including Cucurbitaceae, LR initiation occurs in the root apical meristem as early as during embryogenesis (Dubrovsky and Laskowski 2017; Ilina et al. 2018; Kiryushkin et al. 2019). Only XPP cells have the competency to become initial LR founder cells in most dicots including Arabidopsis. In monocot cereals (including maize, wheat, and rice), LRs initiate from PPP cells, where local auxin maxima are formed (Jansen et al. 2012).
There is a series of key events during LR development: priming, lateral root founder cell specification, and well-organized cell divisions that eventually give rise to the lateral root primordium (LRP), which consists of a root tip meristem that ensures indeterminate growth (Trinh et al. 2018). Auxin plays a pivotal role throughout LR development. In Arabidopsis, cells in the layers outside the pericycle (endodermis, cortex, and epidermis) never divide, even when treated with auxin (Himanen et al. 2002). However, this lack of mitotic activation in the endodermis and cortex seems to have evolved only in the family Brassicaceae. In many other seed plants, the LR formation process involves cell division in the endodermis and cortex (Xiao et al. 2019).
Priming takes place in the transition zone (a region between the root apical meristem and elongation zone) where an oscillating auxin response maximum (about every 6 hours) in protoxylem cells “primes” the adjacent pericycle cells into an incipient branching site. The periodic auxin response maxima predetermine the interval between future LR emergence (Moreno-Risueno et al. 2010). To date, several factors have been proposed to play roles in generating the endogenous oscillating mechanism: the metabolite retinols (Dickinson et al. 2021), cell wall remodeling and vesicle trafficking (Wachsman et al. 2020), the cyclic programmed cell death in the lateral root cap (Xuan et al. 2016), and the interplay between auxin transport and root tip growth dynamics (van den Berg et al. 2021).
Specification of LR founder cells is marked by the expression of GATA23, a transcription factor-coding gene inducible by auxin via the Aux/IAA28 signaling cascade, in subsets of XPP cells before the first asymmetric anticlinal cell division (de Rybel et al. 2010). The auxin-signalling module Aux/IAA14-ARF7/ARF19 and its downstream transcription factors LATERAL ORGAN BOUNDARIES DOMAIN16 (LBD16) and LBD29, together with LBD18 and LBD33, are required to activate the first anticlinal division of LR founder cells (Berckmans et al. 2011; Goh et al. 2019; Okushima et al. 2007). The Aux/IAA gene IAA14 gain-of-function mutant solitary-root (slr), the ARF7 and ARF19 double loss-of-function mutant arf7arf19, and the LBD16 dominant negative mutant LBD16-SRDX (in which the LBD16 is converted into a dominant repressor) have no or severely impaired lateral root formation (Fukaki et al. 2002; Goh et al. 2012; Okushima et al. 2005). The LR-deficient mutant aberrant lateral root formation4 (alf4) displays blocked pericycle cell division even in the presence of exogenous auxin (DiDonato et al. 2004). The ALF4 gene encodes a widely expressed regulator protein of SCF E3 ligases. And alf4 mutant displays reduced auxin response partly due to increased levels of Aux/IAA proteins (Bagchi et al. 2018).
Vascular cambium initiation
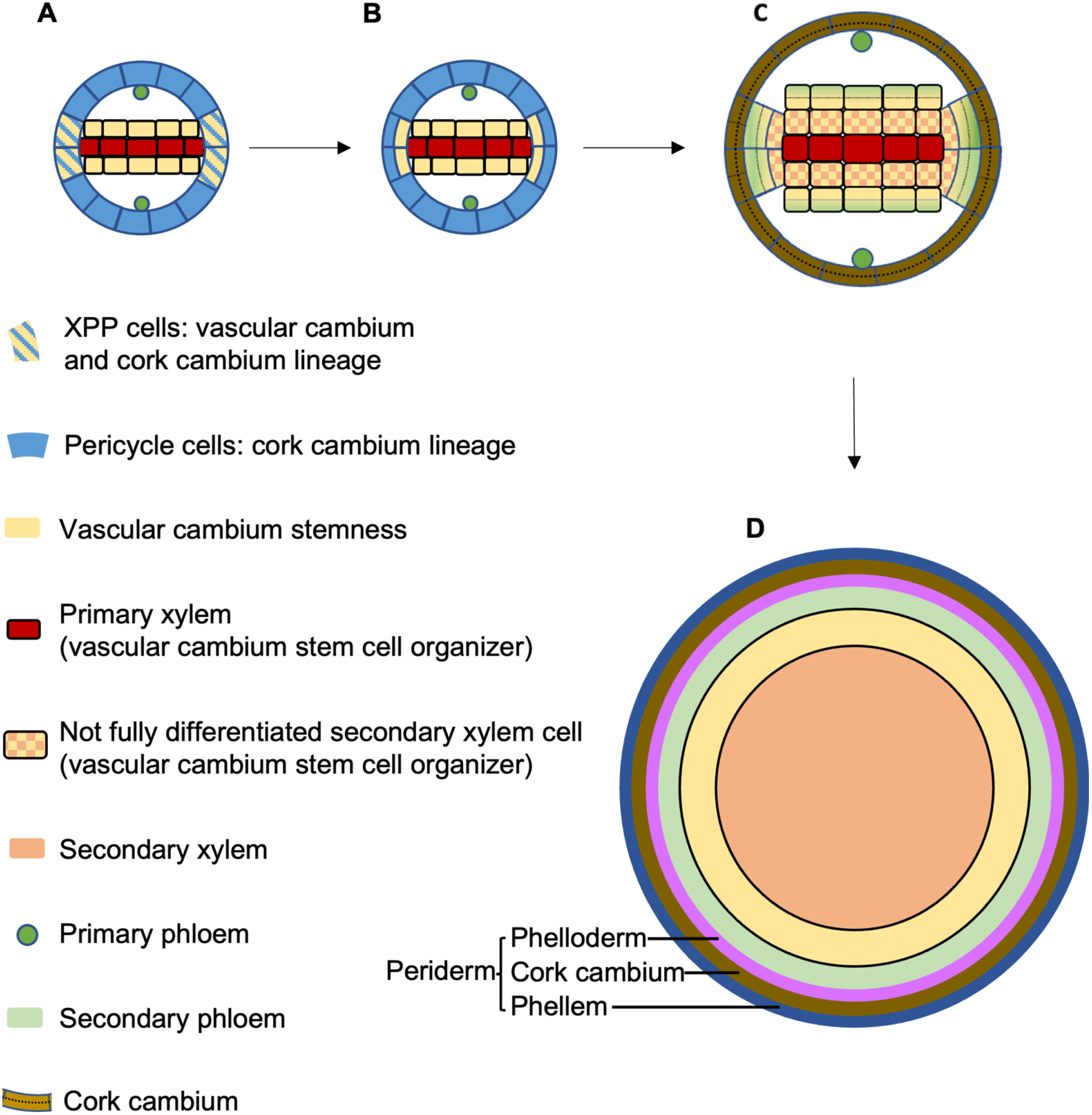
Vascular cambium originates heterogeneously from procambium and pericycle (Figure 3A). The vascular cambium stem cell then divides bifacially to produce secondary xylem and secondary phloem. The first cell divisions that contribute to vascular cambium initiation are observed in the procambium and pericycle of the aged root region (15–18 mm from the root tip) (Ye et al. 2021). XPP cells undergo periclinal cell division, from which the daughter cell adjacent to the xylem acts as a stem cell for the vascular cambium while the other daughter cell retains its pericycle identity (Figure 3B). Similarly, only procambium cells that are in physical contact with xylem gain vascular cambium stem cell identity. It was proposed that cells with xylem identity act as stem cell “organizers” to activate adjacent vascular cambium stem cell division, and to ensure the daughter cell adjacent to the organizer cell maintains stemness while the other daughter cell differentiates into secondary phloem (Smetana et al. 2019). During secondary growth, the vascular cambium stem-cell organizers eventually differentiate into xylem and undergo programmed cell death while their adjacent vascular cambium stem cells become new organizers (Figure 3C). The outcome of this dynamic stem cell specification and division is that, in thicker roots, secondary xylem occupies the centre while secondary phloem is pushed to the periphery, breaking the bilaterally symmetric xylem–phloem pattern seen in young roots (Smetana et al. 2019; Thamm et al. 2019) (Figure 3D). Auxin is proposed to be essential in defining the stem-cell organiser function (Smetana et al. 2019). In vascular cambium stem-cell organizer cells, local auxin response maxima promote expression of WUSCHEL-RELATED HOMEOBOX4 (WOX4), PHLOEM INTERCALATED WITH XYLEM/TDIF RECEPTOR (PXY/TDR) and CLASS-III HOMEODOMAIN-LEUCINE ZIPPERs (HD-ZIPs) transcription factors via MONOPTEROS (MP)/ARF5 (probably also ARF7 and ARF19). This auxin-induced pathway is necessary and sufficient for adjacent vascular cambium stem cell division and differentiation (Smetana et al. 2019). Intriguingly, the molecular regulatory system and cellular organization dynamics of vascular cambium are remarkably similar to those of the root apical meristem and shoot apical meristem (Greb and Lohmann 2016; Smetana et al. 2019).
Figure 3. Secondary growth of the Arabidopsis root. XPP cells can give rise to both vascular cambium and cork cambium (A). Upon secondary growth activation, XPP cells undergo periclinal cell division. The XPP-daughter cell and procambium cells adjacent to the primary xylem act as vascular cambium stem cells. The XPP-daughter cell not in contact with the primary xylem, as well as other pericycle cells, give rise to cork cambium (B). Cells with xylem identity (primary xylem cells or, during subsequent cell division, not yet terminally differentiated secondary xylem cells) act as adjacent vascular cambium stem cell organizers. The organizer activates cell division of the vascular cambium stem cell and ensures the organizer-adjacent daughter cell maintains vascular cambium stemness while the other daughter cell differentiates into protophloem (C). The organizer eventually fully differentiates and undergoes programed cell death. The organizer-adjacent vascular stem cell then acquires xylem identity and acts as a new organizer. After vascular cambium formation starts, cork cambium formation initiates from tangential divisions of XPP pericycle cells, followed by periclinal cell divisions of pericycle cells that give rise to cork cambium cells (C). Divisions of cork cambium cells eventually give rise to periderm surrounding the concentric structure of the secondary xylem, vascular cambium and secondary phloem (D).
Cytokinins are rate-limiting factors in the activation of secondary growth (Matsumoto-Kitano et al. 2008). Elevated cytokinin responses in the upper part of the root induce expression of LBD3 and LBD4 in procambial and pericycle cells to activate the transition from primary growth to secondary growth (Ye et al. 2021). LBD3 and LBD4 have also been shown to be regulated by auxin and TDR-mediated signaling (Smit et al. 2020), suggesting complex crosstalk between auxin- and cytokinin-mediated pathways.
Cork cambium initiation
During secondary growth, periderm replaces the epidermis to become the outermost defensive barrier of the aged root (Campilho et al. 2020). Periderm development has been intensively studied in trees and crops such as potatoes for its physiological importance (Neubauer et al. 2013). Recently, the Arabidopsis root and hypocotyl have been adopted as a model system to study periderm development (Wunderling et al. 2018).
Periderm is initiated from the pericycle only in the aged part of the root (the upper 20% of the root of 6-day-old Arabidopsis seedlings). The onset of periderm development follows the first cell division in vascular cambium formation. The loss-of-function mutant of the master regulator of cambial activity PXY/TDR displays reduced periderm formation, indicating that vascular cambium establishment acts as a developmental switch to trigger periderm formation (Xiao et al. 2020). The very first cell divisions observed in the periderm developmental program are longitudinal anticlinal divisions of XPP cells that increase the cell number of the pericycle layer in the transverse plane, followed by periclinal cell divisions of the pericycle that give rise to a two-layer structure called cork cambium (Figure 3C). Cork cambial cells then further divide and differentiate outwardly into phellem and inwardly into phelloderm. The mature periderm is a four- to five-cell-layer structure comprising the phellem (cork), cork cambium, and phelloderm (Figure 3D). At the same time, cells at the outer surface (endodermis, cortex, and epidermis) undergo programmed cell death and are shed off, leaving the periderm as the outermost structure of aged roots. The cell walls of the phellem, the outermost layer in the aged root, contain suberin, lignin and waxes, and thus have protective barrier properties (Wunderling et al. 2018). Auxin promotes phellogen formation via MP/ARF5 and ARF8 (and probably other ARFs) and through the activation of WOX4 and BREVIPEDICELLUS/KNAT1 (BP/KNAT1) (Xiao et al. 2020), both of which are positive regulators of meristem and cambial activity (Zhang et al. 2019).
Auxin plays a pivotal role in the three developmental processes that give rise to LRs, vascular cambium, and periderm. The molecular pathways revealed to date in periderm and vascular cambium development overlap but are distinct from those of LR development pathways (Figure 2).
Hormone-induced callus in the root is initiated from pericycle cells in Arabidopsis
The remarkable developmental plasticity of plants is best manifested by their regeneration capacity. Mechanical wounding triggers callus formation to repair lost tissues. During wounding-triggered callus formation without exogenous application of hormones, cell division initiates from pericycle and vascular cells of the wounded hypocotyl (Ikeuchi et al. 2017). One example of organ regeneration is adventitious rooting (commonly referred to as de novo root organogenesis), a process through which a detached leaf blade or leaf petiole regenerates adventitious roots without exogenous hormone treatment (Bustillo-Avendaño et al. 2018; Chen et al. 2014). A wound-induced auxin maximum upregulates WUSCHEL-RELATED HOMEOBOX11 (WOX11) and WOX12 expression, which in turn activates LBD16 and LBD29, resulting in the first step of cell fate transition from a leaf procambium or nearby parenchyma cell to a root founder cell (Liu et al. 2014). Interestingly, in intact plants, WOX11 is usually expressed in root protoxylem but can be rapidly induced in XPP by exogenous auxin (Liu et al. 2014), implying a possible requirement for pericycle cell characteristics during the auxin-dependent cell fate transition from leaf procambium to root founder cell.
The callus formation process has been exploited to facilitate de novo organogenesis and asexual propagation (see a historical review by Sugiyama, 2015). In a stepwise in vitro regeneration system, explants are first incubated on an auxin/cytokinin-containing callus-induction medium (CIM) to induce callus formation, and then root or shoot regeneration from the callus is induced by auxin or cytokinin, respectively (Skoog and Miller 1957). Hormone-induced calli arise from the pericycle cells of root explants via the LR development pathway (Atta et al. 2009; Sugimoto et al. 2010). Very long chain fatty acids, which are synthesised by the enzyme ketoacyl-CoA synthase (KCS) in cells surrounding pericycle, restrict pericycle cell proliferation during hormone-induced callus formation possibly through suppressing the transcription of ALF4 (Shang et al. 2016). Hormone-induced callus formation from cuttings of aerial organs initiates in procambium cells, procambium-derived cells, and xylem parenchyma cells (Yu et al. 2010). These callus-precursor cells are referred to as “pericycle-like cells” because they express the XPP marker GFP J0121 during callus induction (Sugimoto et al. 2010).
While the pericycle cell identity provides the cellular basis for hormone-induced callus in most cases, it is also possible to reprogram fully differentiated cells in exceptional cases such as mesophyll protoplasts (Chupeau et al. 2013; Takebe et al. 1971).
Pericycle cell division competence
The Arabidopsis GAL4-GFP enhancer trap line J0121 expresses GFP in XPP cells above the meristem and has been widely used to study LR initiation (Dubrovsky et al. 2006; Parizot et al. 2008) and callus formation (Sugimoto et al. 2010). The enhancer element directly responsible for GAL-4 expression in J0121 is not clear (Laplaze et al. 2005). Importantly, J0121 marker expression appears to be independent from auxin-mediated pathways, because the J0121 GFP expression pattern is not altered by exogenous auxin 1-naphtyl acetic acid (NAA) treatment, by blocking auxin polar transportation with 1-naphthylphthalamic acid (NPA) (Casimiro et al. 2001), or in the auxin signaling mutant slr (Vanneste et al. 2005). Wild-type roots have two protoxylem poles. In the lonesome highway (lhw) mutant, which has a single protoxylem pole, expression of J0121-marker GFP and lateral root formation occur on the side associated with the single-pole xylem (Ohashi-Ito and Bergmann 2007; Parizot et al. 2008). Therefore, J0121 GFP is closely associated with cell division competence and participation in LR formation. Using J0121 GFP as an indicator for such competence, a regulatory network consisting of two groups of basic helix-loop-helix (bHLH) transcription factors that govern pericycle cell competence to undergo auxin-induced cell division have been identified (Zhang et al. 2021). The two groups of bHLH transcription factors, namely PERICYCLE FACTOR TYPE-A (PFA) and PERICYCLE FACTOR TYPE-B (PFB) proteins, interact and possibly form PFA-PFB dimers to activate target gene expression. Overexpression of PFA genes induced ectopic expression of J0121 GFP, while repression of PFA-PFB target genes by expressing PFB1-SRDX or PFB2-SRDX caused complete loss of J0121 expression from the root and blocked LR initiation. PFA-overexpression also confers pericycle characteristics including competence to undergo auxin-induced cell division and auxin-induced expression of the lateral root founder cell marker GATA23. Knocking-out all six PFA genes causes reduced LR initiation. Moreover, when treated with auxin, the sextuple mutant pfa123456 shows greatly reduced pericycle cell division. Therefore, PFAs are required for pericycle cell division competency and participation in LR initiation in Arabidopsis.
Ectopic cell divisions are induced by auxin treatment in cell types other than pericycle in roots overexpressing PFA1 or PFA2 genes. Without exogenous auxin treatment, ectopic cell divisions outside the pericycle are rare, and the LR initiation frequency remains unaltered in PFA1- or PFA2-overexpressing roots. Therefore, activation of the PFA-PFB network confers competence to undergo auxin-induced cell division, but not cell division per se. Both PFA-PFB controlled gene expression and auxin signalling pathways are required to induce pericycle cell division. An important question is where the two pathways converge to activate cell division. Functional analysis of downstream target genes of the PFA-PFB network may serve to pinpoint the intersection point of the PFA-PFB and auxin-mediated pathways.
Open questions and future challenges
In Arabidopsis, PPP cells were thought to be less mitotically active and never participate in LR formation. However, a recent study revealed that PPP cells were also recruited as founder cells at a later step of LR initiation and were induced to divide (Torres-martínez et al. 2020). Therefore, it is possible that, during LR initiation, all pericycle cells possess competence to undergo auxin-induced cell division, but pericycle cells positioned at the xylem pole may respond to auxin more rapidly to become pioneer founder cells. This possible difference in auxin responsiveness may be regulated by a protoxylem-derived signal. The identification of this protoxylem-derived signal will be an important future study topic.
The functions of the PFA-PFB network have been examined only in the context of LR initiation (Zhang et al. 2021). Whether PFA/PFB genes play roles in pericycles’ competence for secondary meristem (vascular cambium and cork cambium) initiation and regeneration processes needs investigation. LR initiation is thought to be the primary functional output of pericycle cell division because LR initiation takes place very close to the root tip early on after germination. The LR-deficient mutant LBD16-SRDX displays an early onset of periderm formation accompanied with an increased vascular cell number (Xiao et al. 2020), suggesting that LR development exerts an inhibitory effect on secondary growth, and in turn inhibits periderm development. Normally, LRs are not initiated from aged roots where secondary growth has already been activated. However, under certain circumstances such as root tip excision, LRs do initiate from the root region undergoing secondary growth (Chiatante et al. 2018). It needs to be clearly demonstrated whether and how secondary growth exerts inhibitory effects on LR development. Notably, the spatiotemporal patterns of cell division differ across different pericycle cell-originated developmental programs; LR founder cells divide anticlinally for several rounds before periclinal divisions take place (Malamy and Benfey 1997), while secondary meristem is formed mainly through periclinal divisions (Smetana et al. 2019; Wunderling et al. 2018). Cytokinins activate the transition from primary to secondary growth (Matsumoto-Kitano et al. 2008) and act in the XPP to inhibit LR initiation (Bielach et al. 2012; Laplaze et al. 2007). Therefore, it is tempting to speculate that cytokinins may switch pericycle cells’ developmental potential from LR initiation to secondary meristem initiation by controlling cell division orientations.
Pericycle cells divide and, depending on the developmental context, give rise to various tissues and organs. Dissecting the common and distinct pathways underlying pericycle cells’ various developmental potentials may further our understanding of the plasticity of plant development.
Acknowledgments
This work was supported by MEXT KAKENHI grant number 20H04886, JSPS KAKENHI grant numbers 19H03246 and 21K19264 to T.K., and MEXT KAKENHI grant numbers 17H06470, 17H06477 and 21H04715 to M. U. We thank Dr. Pingping Qian for discussion. We thank Robbie Lewis, MSc, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. We apologise to colleagues whose work could not be cited due to space limitation.
Abbreviations
- ALF4
ABERRANT LATERAL ROOT FORMATION4
- ARF
AUXIN RESPONSE FACTOR
- bHLH
basic helix-loop-helix
- BP/KNAT1
BREVIPEDICELLUS/KNAT1
- CIM
callus-induction medium
- KCS
ketoacyl-CoA synthase
- LBD
LATERAL ORGAN BOUNDARIES DOMAIN
- LR
lateral root
- LRP
lateral root primordium
- MP
MONOPTEROS
- NAA
1-naphtyl acetic acid
- NPA
1-naphthylphthalamic acid
- PFA
PERICYCLE FACTOR TYPE-A
- PFB
PERICYCLE FACTOR TYPE-B
- PPP
phloem pole pericycle
- PXY/TDR
PHLOEM INTERCALATED WITH XYLEM/TDIF RECEPTOR
- WOX
WUSCHEL-RELATED HOMEOBOX
- XPP
xylem pole pericycle
References
- Atta R, Laurens L, Boucheron-Dubuisson E, Guivarc’h A, Carnero E, Giraudat-Pautot V, Rech P, Chriqui D (2009) Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J 57: 626–644 [DOI] [PubMed] [Google Scholar]
- Bagchi R, Melnyk CW, Christ G, Winkler M, Kirchsteiner K, Salehin M, Mergner J, Niemeyer M, Schwechheimer C, Calderón Villalobos LIA, et al. (2018) The Arabidopsis ALF 4 protein is a regulator of SCF E3 ligases. EMBO J 37: 255–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berckmans B, Vassileva V, Schmid SP, Maes S, Parizot B, Naramoto S, Magyar Z, Kamei CLA, Koncz C, Bögre L, et al. (2011) Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by lateral organ boundary proteins. Plant Cell 23: 3671–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielach A, Podlešáková K, Marhavý P, Duclercq J, Cuesta C, Müller B, Grunewald W, Tarkowski P, Benková E (2012) Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 24: 3967–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougourd S, Marrison J, Haseloff J (2000) An aniline blue staining procedure for confocal microscopy and 3D imaging of normal and perturbed cellular phenotypes in mature Arabidopsis embryos. Plant J 24: 543–550 [DOI] [PubMed] [Google Scholar]
- Bustillo-Avendaño E, Ibáñez S, Sanz O, Sousa Barros JA, Gude I, Perianez-Rodriguez J, Micol JL, Del Pozo JC, Moreno-Risueno MA, Pérez-Pérez JM (2018) Regulation of hormonal control, cell reprogramming, and patterning during de novo root organogenesis. Plant Physiol 176: 1709–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campilho A, Nieminen K, Ragni L (2020) The development of the periderm: The final frontier between a plant and its environment. Curr Opin Plant Biol 53: 10–14 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. (2001) Auxin transport promotes arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffey N, Cholewa E, Regan S, Sundberg B (2002) Secondary xylem development in Arabidopsis: A model for wood formation. Physiol Plant 114: 594–600 [DOI] [PubMed] [Google Scholar]
- Chen X, Qu Y, Sheng L, Liu J, Huang H, Xu L (2014) A simple method suitable to study de novo root organogenesis. Front Plant Sci 5: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiatante D, Rost T, Bryant J, Scippa GS (2018) Regulatory networks controlling the development of the root system and the formation of lateral roots: A comparative analysis of the roles of pericycle and vascular cambium. Ann Bot 122: 697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chupeau MC, Granier F, Pichon O, Renou JP, Gaudin V, Chupeau Y (2013) Characterization of the early events leading to totipotency in an arabidopsis protoplast liquid culture by temporal transcript profiling. Plant Cell 25: 2444–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B, Demeulenaere M, Grunewald W, Audenaert D, Van Campenhout J, Overvoorde P, Jansen L, Vanneste S, et al. (2010) A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol 20: 1697–1706 [DOI] [PubMed] [Google Scholar]
- Dickinson AJ, Zhang J, Luciano M, Wachsman G, Sandoval E, Schnermann M, Dinneny JR, Benfey PN (2021) A plant lipocalin promotes retinal-mediated oscillatory lateral root initiation. Science 373: 1532–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato RJ, Arbuckle E, Buker S, Sheets J, Tobar J, Totong R, Grisafi P, Fink GR, Celenza JL (2004) Arabidopsis ALF4 encodes a nuclear-localized protein required for lateral root formation. Plant J 37: 340–353 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol 124: 1648–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Gambetta GA, Hernández-Barrera A, Shishkova S, González I (2006) Lateral root initiation in Arabidopsis: Developmental window, spatial patterning, density and predictability. Ann Bot 97: 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Laskowski M (2017) Lateral root initiation. In: Thomas B, Murray BG, Murphy DJ (eds) Encyclopedia of Applied Plant Sciences, 2nd edition. Academic Press, Oxford, pp 256–264
- Fukaki H, Tameda S, Masuda H, Tasaka M (2002) Lateral root formation is blocked by a gain-of-function mutation in the solitary-root/IAA14 gene of Arabidopsis. Plant J 29: 153–168 [DOI] [PubMed] [Google Scholar]
- Goh T, Joi S, Mimura T, Fukaki H (2012) The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139: 883–893 [DOI] [PubMed] [Google Scholar]
- Goh T, Toyokura K, Yamaguchi N, Okamoto Y, Uehara T, Kaneko S, Takebayashi Y, Kasahara H, Ikeyama Y, Okushima Y, et al. (2019) Lateral root initiation requires the sequential induction of transcription factors LBD16 and PUCHI in Arabidopsis thaliana. New Phytol 224: 749–760 [DOI] [PubMed] [Google Scholar]
- Greb T, Lohmann JU (2016) Plant stem cells. Curr Biol 26: R816–R821 [DOI] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Iwase A, Rymen B, Lambolez A, Kojima M, Takebayashi Y, Heyman J, Watanabe S, Seo M, De Veylder L, et al. (2017) Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol 175: 1158–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilina EL, Kiryushkin AS, Semenova VA, Demchenko NP, Pawlowski K, Demchenko KN (2018) Lateral root initiation and formation within the parental root meristem of Cucurbita pepo: Is auxin a key player? Ann Bot 122: 873–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen L, Roberts I, de Rycke R, Beeckman T (2012) Phloem-associated auxin response maxima determine radial positioning of lateral roots in maize. Philos Trans R Soc Lond B Biol Sci 367: 1525–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryushkin AS, Ilina EL, Puchkova VA, Guseva ED, Pawlowski K, Demchenko KN (2019) Lateral root initiation in the parental root meristem of Cucurbits: Old players in a new position. Front Plant Sci 10: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, et al. (2007) Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19: 3889–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Parizot B, Baker A, Ricaud L, Martinière A, Auguy F, Franche C, Nussaume L, Bogusz D, Haseloff J (2005) GAL4-GFP enhancer trap lines for genetic manipulation of lateral root development in Arabidopsis thaliana. J Exp Bot 56: 2433–2442 [DOI] [PubMed] [Google Scholar]
- Liu J, Sheng L, Xu Y, Li J, Yang Z, Huang H, Xu L (2014) WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 26: 1081–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Václavíková K, Miyawaki K, Kakimoto T (2008) Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA 105: 20027–20031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Risueno MA, van Norman JM, Moreno A, Zhang J, Ahnert SE, Benfey PN (2010) Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329: 1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte H, Beeckman T (2019) The evolution of root branching: Increasing the level of plasticity. J Exp Bot 70: 785–793 [DOI] [PubMed] [Google Scholar]
- Neubauer JD, Lulai EC, Thompson AL, Suttle JC, Bolton MD, Campbell LG (2013) Molecular and cytological aspects of native periderm maturation in potato tubers. J Plant Physiol 170: 413–423 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC (2007) Regulation of the Arabidopsis root vascular initial population by Lonesome highway. Development 134: 2959–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19: 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al. (2005) Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: Unique and overlapping functions of ARF7 and ARF19. Plant Cell 17: 444–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizot B, Laplaze L, Ricaud L, Boucheron-Dubuisson E, Bayle V, Bonke M, De Smet I, Poethig SR, Helariutta Y, Haseloff J, et al. (2008) Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol 146: 140–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehin M, Bagchi R, Estelle M (2015) SCF TIR1/AFB-based auxin perception: Mechanism and role in plant growth and development. Plant Cell 27: 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang B, Xu C, Zhang X, Cao H, Xin W, Hu Y (2016) Very-long-chain fatty acids restrict regeneration capacity by confining pericycle competence for callus formation in arabidopsis. Proc Natl Acad Sci USA 113: 5101–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11: 118–130 [PubMed] [Google Scholar]
- Smetana O, Mäkilä R, Lyu M, Amiryousefi A, Sánchez Rodríguez F, Wu MF, Solé-Gil A, Leal Gavarrón M, Siligato R, Miyashima S, et al. (2019) High levels of auxin signalling define the stem-cell organizer of the vascular cambium. Nature 565: 485–489 [DOI] [PubMed] [Google Scholar]
- Smit ME, McGregor SR, Sun H, Gough C, Bågman AM, Soyars CL, Kroon JT, Gaudinier A, Williams CJ, Yang X, et al. (2020) A PXY-mediated transcriptional network integrates signaling mechanisms to control vascular development in Arabidopsis. Plant Cell 32: 319–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Jiao Y, Meyerowitz EM (2010) Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell 18: 463–471 [DOI] [PubMed] [Google Scholar]
- Sugiyama M (2015) Historical review of research on plant cell dedifferentiation. J Plant Res 128: 349–359 [DOI] [PubMed] [Google Scholar]
- Takebe I, Labib G, Melchers G (1971) Regeneration of whole plants from isolated mesophyll protoplasts of tobacco. Naturwissenschaften 58: 318–320 [Google Scholar]
- Thamm A, Sanegre-sans S, Paisley J, Meader S, Milhinhos A, Contera S, Agusti J (2019) A simple mathematical model of allometric exponential growth describes the early three-dimensional growth dynamics of secondary xylem in Arabidopsis roots. R Soc Open Sci 6: 190126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Martínez HH, Hernández-Herrera P, Corkidi G, Dubrovsky JG (2020) From one cell to many: Morphogenetic field of lateral root founder cells in Arabidopsis thaliana is built by gradual recruitment. Proc Natl Acad Sci USA 117: 20943–20949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh CD, Laplaze L, Guyomarc’h S (2018) Lateral root formation: Building a meristem de novo. Annu Plant Rev Online 1: 847–890 [Google Scholar]
- van den Berg T, Yalamanchili K, de Gernier H, Santos Teixeira J, Beeckman T, Scheres B, Willemsen V, ten Tusscher K (2021) A reflux-and-growth mechanism explains oscillatory patterning of lateral root branching sites. Dev Cell 56: 2176–2191 [DOI] [PubMed] [Google Scholar]
- Vanneste S, de Rybel B, Beemster GTS, Ljung K, de Smet I, Van Isterdael G (2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17: 3035–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman G, Zhang J, Moreno-Risueno MA, Anderson CT, Benfey PN (2020) Cell wall remodeling and vesicle trafficking mediate the root clock in Arabidopsis. Science 370: 819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderling A, Ripper D, Barra-Jimenez A, Mahn S, Sajak K, Targem MB, Ragni L (2018) A molecular framework to study periderm formation in Arabidopsis. New Phytol 219: 216–229 [DOI] [PubMed] [Google Scholar]
- Xiao TT, van Velzen R, Kulikova O, Franken C, Bisseling T (2019) Lateral root formation involving cell division in both pericycle, cortex and endodermis is a common and ancestral trait in seed plants. Development 146: dev.182592. [DOI] [PubMed] [Google Scholar]
- Xiao W, Molina D, Wunderling A, Ripper D, Vermeer JEM, Ragni L (2020) Pluripotent pericycle cells trigger different growth outputs by integrating developmental cues into distinct regulatory modules. Curr Biol 30: 4384–4398 [DOI] [PubMed] [Google Scholar]
- Xuan W, Band LR, Kumpf RP, Van Damme D, Parizot B, De Rop G, Opdenacker D, Möller BK, Skorzinski N, Njo MF, et al. (2016) Cyclic programmed cell death stimulates hormone signaling and root development in Arabidopsis. Science 351: 384–387 [DOI] [PubMed] [Google Scholar]
- Ye L, Wang X, Lyu M, Siligato R, Eswaran G, Vainio L, Blomster T, Zhang J, Mähönen AP (2021) Cytokinins initiate secondary growth in the Arabidopsis root through a set of LBD genes. Curr Biol 31: 3365–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Feng Z, Wang G, Li F, Du X, Zhu J (2010) Initiation of dedifferentiation and structural changes in in vitro cultured petiole of Arabidopsis thaliana. Protoplasma 241: 75–81 [DOI] [PubMed] [Google Scholar]
- Zhang J, Eswaran G, Alonso-Serra J, Kucukoglu M, Xiang J, Yang W, Elo A, Nieminen K, Damén T, Joung JG, et al. (2019) Transcriptional regulatory framework for vascular cambium development in Arabidopsis roots. Nat Plants 5: 1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mitsuda N, Yoshizumi T, Horii Y, Oshima Y, Ohme-Takagi M, Matsui M, Kakimoto T (2021) Two types of bHLH transcription factor determine the competence of the pericycle for lateral root initiation. Nat Plants 7: 633–643 [DOI] [PubMed] [Google Scholar]



