Abstract
Unlike animals, terrestrial plants are sessile and able to give rise to new organs throughout their lifetime. In the most extreme cases, they can survive for over a thousand years. With such protracted life cycles, plants have evolved sophisticated strategies to adapt to variable environments by coordinating their morphology as well as their growth, and have consequently acquired a high degree of developmental plasticity, which is supported by small groups of long-lived stem cells found in proliferative centers called meristems. Shoot apical meristems (SAMs) contain multipotent stem cells and provide a microenvironment that ensures both a self-renewable reservoir, to produce primordia and sustain growth, and a differentiating population that develops into all of the above-ground organs of land plants. The homeodomain transcription factor WUSCHEL (WUS) is expressed in the organizing center and acts as a master regulator to govern shoot stem cell homeostasis. In this review, I highlight recent advances in our understanding of the molecular mechanisms and signaling networks that underlie SAM maintenance, and discuss how plants utilize WUS to integrate intrinsic and extrinsic cues.
Keywords: CLAVATA3, environmental stress, homeostasis, shoot apical meristem, WUSCHEL
Introduction
Stem cells residing in plant meristems possess dual functions, controlling both self-renewal and organogenesis by precisely coordinating the balance between proliferation and differentiation. Meristems, whose name derives from the Greek word merizein, which means “to divide”, have been studied for more than a century, and several key components regulating stem cell homeostasis have been identified to date. Meristems are protected within a specialized microenvironment from undergoing differentiation through a variety of regulatory systems, and are located in the apical regions of shoots and roots, and as lateral meristems such as vascular cambium, where they comprise a small number of cells with undifferentiated and dividing states. While common regulators and factors exist in both the shoot and root stem cell niches (Stahl and Simon 2010), the mechanism underlying maintenance of the shoot apical meristem (SAM) is still largely uncertain compared to that of the root apical meristem (RAM). The SAM is composed of three layers in Arabidopsis (L1, epidermal layer; L2, subepidermal layer; and L3, innermost layer) (Figure 1A); cells in the central zone (CZ) rarely divide, whereas the surrounding cells actively divide, yielding a sustainable reservoir that is responsible for the organogenesis of all above-ground tissue. Cell fate in the SAM is not strictly controlled as it is in the RAM, but is determined according to the cell’s position in relation to the organizing center (OC) (Szymkowiak and Sussex 1996).
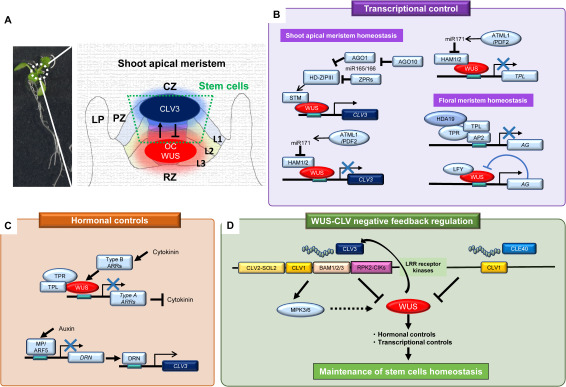
Figure 1. Regulation of Arabidopsis shoot apical meristem homeostasis. A. Whole plant (left) and schematic representation of the shoot apical meristem (right). The SAM is composed of layers L1 (blue), L2 (green), and L3 (red). OC: organizing center. CZ: central zone. PZ: peripheral zone. LP: leaf primordium. RZ: rib zone. Dark blue and red ovals with haloes represent the gradients of CLV3 and WUS expression, respectively. The trapezoid defined by green dots covers the area of stem cells in the SAM. B, C. Transcriptional regulatory networks (B) and hormonal control system (C) through WUS and CLV3 as described in the text. D. Overview of the WUS–CLV negative feedback loop for maintenance of stem cell homeostasis. In B–D, WUS and CLV3 are highlighted by red and dark blue, respectively. Arrows depict positive regulatory relationships and bars and crosses mark negative regulatory relationships. The broken line indicates that direct molecular evidence is not yet available.
In 2000, key factors controlling plant development in the SAM were strikingly elucidated by Schoof et al., who found that the precise program of SAM homeostasis is largely dependent on the activities of the WUSCHEL–CLAVATA3 (WUS–CLV3) negative feedback loop. WUS encodes a plant homeobox protein, and it is becoming clear that it is functionally dispensable for stem cell initiation but plays a central role in maintaining stem cell homeostasis in the SAM (Figure 1A) (Schoof et al. 2000; Zhang et al. 2017). CLV3 encodes a secreted peptide of 13 amino acids at its C-terminal end (the CLE domain), which is post-translationally modified and activated by glycosylation (Ito et al. 2006; Ohyama et al. 2009). The expression level of CLV3 is specifically elevated by WUS in the CZ, and the mature CLV3 peptide diffuses through the apoplast and finally binds to leucine-rich repeat (LRR)-based receptor complexes anchored in the plasma membrane, such as CLAVATA 1 (CLV1), a heterodimer complex of CLV1 and CLAVATA 2 (CLV2), and a combination of CLV1 and BARELY ANY MERISTEMs (BAM1/2/3) (Guo et al. 2010). Genetic and biochemical studies have suggested that the molecular interaction between CLV3 and its receptors can trigger the activation of intercellular signaling cascades toward the L2 and L3 layers of the meristem where, in turn, CLV3 represses WUS expression (Schoof et al. 2000). The CLV3 expression level is tightly coupled with the dosage of WUS protein, and a cis-regulatory module within the CLV3 promoter to which WUS binds is required for fine-tuning of the CLV3 expression level and subsequent stem cell homeostasis (Perales et al. 2016). Loss-of-function mutations in the WUS and CLV3 genes cause developmental defects derived from improper stem cell numbers and irregular division planes, suggesting that the WUS–CLV3 pair is a major signaling module for stem cell functions and dynamically maintains homeostasis through a balance between cell differentiation and self-renewal in stem cells (Waites and Simon 2000). Interestingly, functional paralogs of WUS and CLV3 exist in Arabidopsis stem cell niches that have different developmental origins, specifically in RAMs as well as vascular cambiums; orthologs of this pair also occur in most flowering plants, suggesting that the core module for stem cell maintenance must be broadly conserved across the plant kingdom. The molecular dynamics of WUS and CLV3 proteins have also been studied; WUS itself is also a mobile protein and moves from the OC into the stem cells through plasmodesmata (Figure 1A), and directly binds the CLV3 promoter to activate transcription of CLV3 (its own inhibitor) and negatively regulates the size of the stem cell population in the SAM (Daum et al. 2014; Yadav and Reddy 2011). However, important mechanistic insights—especially into the intercellular signaling pathway that restricts WUS expression, and the key transcriptional pathways or additional unexplained regulatory systems to maintain homeostasis in response to variable external/internal cues—are currently lacking.
This review provides an overview of recent advances in elucidating the role of known regulatory networks in SAM homeostasis, introduces the role of signaling modules in maintaining stem cell homeostasis, and finally discusses the future direction of plant stem cell research.
Controlling homeostasis of the shoot meristem by transcriptional regulation
SAMs are strictly established at specific positions and at distinct times during early embryogenesis, and post-embryonically they give rise to axillary shoot and floral meristems depending on positional cues triggered by fluctuations in their surrounding environmental conditions. Several experimental observations imply that this conventional model is not sufficient and that additional levels of control must be required for the maintenance of SAM homeostasis.
WUS belongs to the WUSCHEL-LIKE HOMEOBOX (WOX) transcription factor gene family and mainly represses a set of downstream genes that integrate hormone signaling pathways and cell differentiation. For instance, WUS can directly bind to the promoter region of TOPLESS (TPL), which is involved in auxin signaling, to repress its expression (Figure 1B) (Dinesh et al. 2016; Szemenyei et al. 2008). In addition, WUS negatively regulates the A-type ARABIDOPSIS RESPONSE REGULATOR 7 (ARR7) by interacting with its promoter region and thus represses ARR7 function in cytokinin signaling in the center of the SAM (L1–L3 layers) (Figure 1C) (Leibfried et al. 2005). In contrast, WUS interacts with LEAFY and directly activates AGAMOUS (AG), and in turn AG represses WUS expression (Figure 1B) (Lohmann et al. 2001) during early flower development, suggesting that WUS is a multifunctional transcriptional factor whose functions are tightly regulated by its coregulators. To comprehensively identify the target genes of WUS protein, its chromatin binding preferences have been investigated, revealing that WUS potentially binds more than 100 target genes (Busch et al. 2010). However, the other post-translational regulatory factors of WUS have not yet been clarified. Recently, Su et al. (2020) showed that WUS interacts directly with SHOOT MERISTEMLESS (STM), which encodes a class I KNOTTED 1-related homeodomain transcription factor. STM is a local specifier for stem cell characteristics whose loss-of-function mutant exhibits a disrupted SAM, and its strong allele terminates SAM activity at the cotyledon stage, whereas its weaker allele develops only a few leaves and then terminates prematurely due to a disturbance of SAM homeostasis (Clark et al. 1996). WUS and STM proteins can directly bind to distinct sequences in the promoter region of CLV3: WUS to a TAAT motif at −1,082 to −1,079 bp, and STM at −797 to −793 bp (Su et al. 2020). Loss-of-function mutants of the STM gene have obvious pleiotropic phenotypes relating to stem cell establishment such as leaf initiation, loss of stem cells, and abnormal axillary branches (Balkunde et al. 2017; Hake et al. 2004; Hay and Tsiantis 2010; Scofield and Murray 2006). STM acts as a cofactor to guide WUS to access the WUS binding site (TAAT motif) in the CLV3 promoter. Thus, the binding sites of these two factors are coordinately essential for CLV3 expression and subsequently for increasing shoot meristem size. Because STM is broadly expressed in the SAM to prevent differentiation whereas WUS expression is restricted to the OC and confers stem cell activation, their expression domains overlap only in the OC. WUS and STM are thought to constitute a bona fide co-regulatory complex for their relevant functions specifically in stem cell regulation.
Other regulatory factors of WUS include two HAIRY MERISTEMs (HAMs), HAM1 and HAM2; their expression gradually increases from the epidermis to the inner stem cell niche and confines common downstream target gene expression, including CLV3, to the outer apical meristem layers (Figure 1B) (Zhou et al. 2018, 2015). In the rib meristem region, this HAM1/2–WUS complex regulates target genes including TPL. The HAM family gene expression gradient is defined by repression caused by microRNA171 (miR171) family members, which are selectively upregulated by epidermis-specific expression of ARABIDOPSIS THALIANA MERISTEM LAYER 1/PROTODERMAL FACTOR 2 (Han et al. 2020). Interestingly, in a biochemical and in vivo imaging analysis, Perales et al. (2016) found that WUS activity is tightly shifted in a concentration-dependent manner: where WUS level is lower in the meristem (e.g. in the L1 layer), WUS binds the cis-element (TAAT) of the promoter of CLV3, and where WUS level is higher in the meristem (e.g. in the OC), WUS changes its conformation, loses binding affinity with this cis-element, and starts repressing CLV3 expression in the OC, implying that a concentration-dependent transcriptional discrimination system plays an important role in determination of stem cell fate choice between maintenance of progenitors and formation of new organs, as occurs in animal stem cells (Perales et al. 2016; Snipes et al. 2018; Whittington et al. 2015). Therefore, WUS somehow regulates the balance between cell proliferation and maintenance of stem cell activity by changing partners with these various regulatory cofactors and its own protein level in different cell types; however, the mechanism underlying this behavior remains to be solved. The binding of such regulatory factors to WUS may prevent WUS from activating CLV3 in the OC, and may contribute to the establishment of a concentration gradient of WUS activity throughout the SAM.
Class III homeodomain leucine zipper (HD-ZIPIII) transcription factors promote shoot meristem development through STM expression (Shi et al. 2016), and in turn are negatively regulated by LITTLE ZIPPER proteins (ZPRs) through nonfunctional heterodimer formation or by miR165/miR166 (Figure 1B) (Byrne 2006; Kim et al. 2008; Prigge et al. 2005; Wenkel et al. 2007; Yu et al. 2017; Zhu et al. 2011) through ARGONAUTE protein (AGO) RNA-binding protein interaction. The HD-ZIPIII–ZPR complexes confine CLV3 and WUS expression domains to the SAM in Arabidopsis and tomato (Kim et al. 2008; Xu et al. 2019). AGO1 protein can bind to microRNAs (miR165/166) to cleave target HD-ZIPIII mRNAs, and also binds another microRNA (miR168) to cleave its own AGO1 mRNA to maintain SAM homeostasis (Figure 1B) (Baumberger and Baulcombe 2005). AGO1 protein is a component of the RNA-induced silencing chaperone complex, which causes gene silencing and reduced expression of a set of target genes, and an AGO1 loss-of-function mutant displays over-proliferation of the shoot and flower meristems, which can suppress shoot stem cell termination caused by CLV3 overproduction (Du et al. 2020). Among the 10 paralogs of AGO genes in the Arabidopsis genome, AGO10/ZWILLE (ZLL) is indispensable and has an overlapping function with AGO1 for shoot meristem maintenance over different developmental stages (Du et al. 2020; Tucker et al. 2008). Further detailed biochemical analysis has provided evidence that biological functions of AGO1 and AGO10 are different from previous reports, in that AGO10/ZLL protein may act as a lure for other AGO proteins; it preferentially binds to miR165/166 to reduce their levels and protect HD-ZIPIII mRNAs from AGO1-mediated gene silencing (Yu et al. 2017). Thus, while the biochemical functions of AGO1 and AGO10/ZLL seem opposite, they genetically complement each other’s functions to promote stem cell maintenance (Du et al. 2020). Future studies on SAM homeostasis will need to take integrated and comprehensive approaches, especially regarding the molecular nature of the WUS–CLV3 feedback loop and the intersection of the regulatory cofactors within this loop.
Control of homeostasis in the shoot meristem by phytohormones
Because precise spatiotemporal control is essential for stem cell maintenance, communication between cells in the peripheral region and stem cells is another important aspect of homeostasis. Phytohormones and signaling molecules, which can penetrate the cell wall barrier, transmit their signals to neighboring cells, and commit the cells to the perturbation of the WUS–CLV3 regulatory circuit, which is the key role in switching developmental phase and coping with variable environmental stimuli. WUS expression is directly regulated by a family of cytokinin signaling transduction components, B-type ARRs; in turn, WUS enhances cytokinin responses by repressing at least four A-type ARR genes (ARR5, ARR6, ARR7, and ARR15), which negatively regulate cytokinin signaling (Figure 1C) (Gordon et al. 2009; Leibfried et al. 2005).
WUS also modulates auxin responsiveness in the shoot (Ma et al. 2019). Auxin-dependent organ initiation is mediated by AUXIN RESPONSE FACTOR 5/MONOPTEROUS (ARF5/MP) transcription factor, which directly represses the transcription of DORNRÖSCHEN (DRN) in the SAM by binding to a conserved auxin response element in the DRN promoter (Figure 1C). The expression patterns of ARF5/MP and DRN are complementary: ARF5/MP is expressed predominantly in the peripheral zone (PZ) and not in the CZ, whereas DRN is predominantly expressed in the CZ and to a lesser extent in the PZ (Luo et al. 2018). DRN directly binds the promoter region of CLV3 and promotes CLV3 expression, which is reduced in the double knockout mutant of DRN and its closest homolog DORNRÖSCHEN-LIKE genes, resulting in an enlarged SAM (Figure 1C). Taken together, these data indicate that ARF5/MP-mediated auxin signaling negatively regulates CLV3 expression in the shoot stem cell niche and maintains homeostasis by repressing DRN in the PZ of the SAM. They also suggest that auxin does not promote cell differentiation but actively engages in the CZ to maintain stem cell proliferation by repressing CLV3 expression.
Among the direct targets of WUS, phytohormone-related key genes such as ARR7 (cytokinin signaling), CLV1 (meristem-related processes), JAZ5 (jasmonate signaling), KAN1/2, YABBY 3 (YAB3), and AS2 are all included. Auxin is required for entry into mitosis and promotes cell division to cause cell proliferation by interacting with the signaling cascade of another canonical phytohormone, cytokinin (Shimotohno et al. 2021). The stem cell niche is robustly maintained in the undifferentiated state despite exposure to a dynamically changing tissue environment and endogenous hormonal status. Indeed, live-cell imaging for monitoring in situ auxin level using two auxin markers, R2D2 (designated as the auxin input) and DR5v2 (designated as an auxin output reporter), in the SAM revealed that auxin was distributed quite uniformly across the SAM except in young primordium and at the boundaries of developing organs (Ma et al. 2019). The level of the auxin response is inversely correlated with the amount of WUS protein, and subsequent WUS target analysis revealed that WUS acts by regulating the histone acetylation status of many genes that are involved in auxin signaling and responses. The BIG/TRANSPORT INHIBITOR RESPONSE 3 (TIR3) mutant tir3-1 is known to show a reduction in the size of the root meristem (Kanyuka et al. 2003; Ruegger et al. 1997) due to the perturbation of polar auxin transport and multiple hormonal responses (Gil et al. 2001; López-Bucio et al. 2005; Paciorek et al. 2005), although recent fascinating research has reported that tir3-1 also shows a typical phenotype with enlarged SAM size (Zhang et al. 2020). Transcripts of the CLV3, WUS, and STM genes were all significantly increased and overlapped spatially in a T-DNA insertion mutant of the BIG gene, designated the big-shoot meristem (big-m) mutant, which has an expanded expression domain in the SAM compared with that in the wild type (Zhang et al. 2020). clv3-2 showed increased SAM size; however, the double mutant phenotype of the big-m and clv3-2 combination was genetically additive, whereas big-m wus and big-m stm phenotypes were identical to the wus and stm single mutant phenotypes, implying that BIG mediates stem cell homeostasis by affecting expression of WUS and STM, and indirectly affecting the CLV3 pathway. In addition, the PIN1 transcript was significantly reduced, along with lower auxin maxima, in the big-m mutant. Therefore, BIG regulates PIN1 protein distribution to perturb auxin transport, and the tir3-1 pin1 double mutant displayed severe defects in auxin-dependent growth of inflorescence meristems (Gil et al. 2001). Taken together, these observations suggest that BIG acts synergistically and influences auxin transport by controlling PIN1 localization to distribute proper auxin maxima and to maintain SAM homeostasis (Zhang et al. 2020).
More in-depth studies have suggested that WUS directly controls the balance between cytokinin and auxin levels by manipulating the expression levels of their related genes. For instance, Ma et al. (2019) reported that WUS rheostatically controls both the auxin signaling and auxin response pathways in the SAM, and does so sufficiently to inhibit active auxin responses in the center of the SAM, suggesting that WUS protects stem cells from high auxin levels to maintain stemness (Ma et al. 2019). WUS interacts with TPL and TOPLESS RELATED (TPR) (Dolzblasz et al. 2016; Kieffer et al. 2006; Shi et al. 2018) as corepressors to manipulate their downstream target genes. Both TPL and TPR proteins can also associate with HISTONE DEACETYLASE (HDAC) 19 to form a huge transcriptional repression complex (Krogan et al. 2012; Wang et al. 2013). Although there is no direct evidence of a direct interaction between WUS–TPL and HDAC proteins to date, short-term WUS induction is sufficient to change the histone acetylation status of over 40% of genes across the whole genome, whereas histone methylation patterns are largely unaffected. In addition, WUS binding to, and reduced transcription of, a set of genes controlling auxin signaling and response is tightly coupled with HDAC activity, implying that these hormonal regulatory effects, as well as the HDAC-dependent chromatin remodeling mechanism, also contribute to the broadening of WUS activity at the transcriptional level between the CZ and PZ in the SAM (Ma et al. 2019). By increasing the spatiotemporal resolution of experimental systems to the level of the individual cell, a low-auxin spot was detected in the OC, which is a critical location for SAM homeostasis (Snipes et al. 2018). Therefore, WUS probably acts as a hub to perturb cytokinin responses, and restricts auxin signaling and rheostatically maintains stem cells in the SAM.
Small-peptide modules contribute to SAM homeostasis
As described above, the WUS–CLV negative feedback loop regulating SAM homeostasis was identified at the beginning of this century (Figure 1D) (Schoof et al. 2000). Among many small peptides and their receptors encoded in the Arabidopsis genome, CLV3 is perceived by at least four different types of receptor-like kinases (RLKs): CLV1, the homologs BAM1/2/3, RPK2, and CLV2/CORYNE (CRN). These constitute functional modules that are complementarily integrated into SAM homeostasis, and each of their loss-of-function mutants shows broadly expanded WUS expression with a dramatically enlarged and disorganized SAM, as does the clv3 mutant. In addition, CLV3 INSENSITIVE KINASEs 1–4 (CIK1–4) were originally isolated as coreceptors of four RLKs (CLV1, BAMs, RPK2, and CRN), and perturb CLV3 signaling to maintain SAM homeostasis (Hu et al. 2018). Insensitivity to CLV3 peptide application in the quadruple mutant of ciks (cik1 cik2 cik3 cik4) as well as the phosphorylation level of endogenous CIKs was not elevated in the clv1 bam1 bam2 triple mutant, implying that CIKs function as shared cofactors in a CLV-related signaling pathway. However, other reports showed that the phenotype of the cik1 cik2 cik3 cik4 quadruple mutant was much weaker than that of a receptor mutant (clv1 bam1 bam2 triple mutant), implying that additional unknown factors may contribute to the regulation of SAM homeostasis (Deyoung and Clark 2008; Hu et al. 2018).
Recently, Zhu et al. (2021) have reported that two additional CIK paralogs, CIK5 and CIK6, also contribute to SAM homeostasis; their sextuple mutant (cik1 cik2 cik3 cik4 cik5 cik6) displays an extremely enlarged SAM and a high number of rosette leaves, reminiscent of the phenotypes of the clv1 bam1 bam2 bam3 mutant, suggesting that CIK genes are functionally highly redundant in regulating SAM homeostasis (Zhu et al. 2021). Interestingly, although the mechanisms and functional diversity have not yet been clarified, CIK3, CIK4, and CIK5 are expressed in the distal root meristem in wild type and the expression of CIK2 and CIK6 is induced in the cik1 cik3 cik4 cik5 and cik2 cik3 cik4 cik5 mutant, respectively, perhaps to compensate these functions (Hu et al. 2022; Zhu et al. 2021). CIK kinases directly interact as coreceptors with ARABIDOPSIS CRINKLY 4 (ACR4), and they can be phosphorylated by ACR4 to suppress ectopic WOX5 expression in distal root apical meristem homeostasis. Therefore, CIK kinases make unequal contributions but function as coreceptors of different receptors to maintain stemness in shoot and root apical meristems.
Functional modules consisting of a small peptide and its ligand have also been identified in other species. For instance, weak mutants of THICK TASSEL DWARF1 or FASCIATED EAR2 (FEA2), encoding maize (Zea mays) orthologs of CLV1 or CLV2, respectively, show an enlarged ear inflorescence meristem and dramatically increased crop yields (Je et al. 2018; Lunde and Hake 2009). In maize, independent from the CLV2 regulatory pathway, a novel LRR-RLP receptor with 12 LRRs plus a short cytoplasmic tail, FEA3, perceives FON2 CLE PEPTIDE-RELATED1 and restricts WUS expression at the base of the OC, and its loss-of-function mutant displays significantly higher yield (Je et al. 2016). In tomato, two fasciation weak allele mutants have been isolated, fasciated and branched (fab) and fasciated inflorescence (fin), which respectively encode a tomato CLV1 homolog and the HYDROXYPROLINE O-ARABINOSYLTRANSFERASE gene that commits the plant to full activation of CLE peptides (Wang et al. 2020). Both of these weak allele mutants display reduced expression of the tomato CLV3 ortholog (SlCLV3) and/or lower activity of SlCLV3, and both mutants eventually show increased meristem size, higher carpel numbers, and enlarged fruit size with greater locule numbers; conversely, however, null CLV3-related signaling modules are characterized by smaller meristem size and poorer yield traits than those of weaker alleles.
Mitogen-activated protein kinase (MAPK) cascades act as central hubs in physiological and developmental pathways across kingdoms. Among over 100 MAPK cascade-related proteins in plants, the two best characterized MAPKs, MPK3 and MPK6, are tightly coupled with abiotic/biotic stresses as well as development pathways, including stomatal/ovule and lateral root development. Genetic analysis of clv1, clv2, and receptor-like protein kinase 2 (rpk2) loss-of-function mutants revealed that CLV3 peptide triggers a rapid MAPK signaling cascade, which is compositively or additively regulated by changing the composition of receptors (CLV1, CLV2–SOL2, and RPK2) (Figure 1D) (Betsuyaku et al. 2011; Kinoshita et al. 2010). Exogenously applied CLV3 peptide can directly enhance the activities of both MPK3 and MPK6 kinases in vitro, and such activations are diminished in the clv1 bam1 double mutant in Arabidopsis, indicating that the CLV1 and BAM1 receptors perceived CLV3 peptide signaling and phosphorylate MPK3 and MPK6 to activate MPK3/MPK6 cascades (Figure 1D) (Lee et al. 2019). In support of this interpretation, expression of WUS and WUS target genes (ARR7, ARR15, Growth-regulating factor 6 (GRF6), and YAB3) is drastically impaired in a loss-of-function mpk3 mpk6 double mutant, and the mpk3 mpk6 double mutation suppressed the SAM termination phenotype caused by exogenous application of CLV3 peptide, implying that MPK3 and MPK6 signaling cascades play important roles in the regulation of SAM homeostasis by manipulation of the WUS–CLV3 negative feedback loop.
Taken together, these findings indicate that functional CLE signaling modules as well as the regulatory system of WUS–CLV3-mediated SAM homeostasis are highly conserved among plant species and have the potential to optimize crop yields, and extending these studies to other species will illuminate the modules’ utility for increasing food production. CLV3 encodes a 96-amino acid (aa) protein that can be processed to two mature forms of CLV3 peptides in vivo, the 12-aa MCLV3 or the tri-arabinosylated 13-aa [Ara3]CLV3, and they act as ligands to regulate stem cell homeostasis in the SAM (Ito et al. 2006; Ohyama et al. 2009; Shinohara and Matsubayashi 2015). Due to the technical difficulties of analyzing these peptides, Kim et al. (2017) have carefully demonstrated their bioactive properties for SAM homeostasis and root growth inhibition by chemically synthesizing CLV3 peptides. Most strikingly, the phenotype of a clv3 null mutant (clv3-2) with a slightly enlarged SAM was fully restored by physiological amounts of peptides: treatment with 100 nM synthetic peptides of MCLV3 and [Ara3]CLV3 was sufficient to complement the clv3-2 enlarged SAM phenotype to wild type. Application of both synthetic peptides resulted in suppression of a pair of marker genes (SULFOTRANSFERASE 17 and ADENOSINE-5′-PHOSPHOSULFATE KINASE 2) that are involved in stem cell proliferation and are co-expressed with WUS in clv3-2 mutants, implying that these two synthetic forms of CLV3 peptides retain authentic bioactive properties, at least in SAM maintenance.
A multidimensional regulatory circuit enables the meristem to fine-tune stem cell activities
Although the key dialogue between the shoot stem cells and their underlying niche (i.e. the OC) is carried out by the WUS–CLV feedback regulatory circuit, recent reports imply the existence of an alternative WUS-independent pathway for the control of stem cell proliferation during plant development (Huang et al. 2015; Kimura et al. 2018; Lee and Clark 2015). ERECTA (ER), ER-LIKE 1 (ERL1), and ER-LIKE 2 (ERL2) encode receptor-like kinases that are expressed throughout the SAM (Chen et al. 2013; Uchida et al. 2013). It has been shown that the er erl1 erl2 triple mutant exhibits an enlarged SAM (Uchida et al. 2013), but the molecular mechanism of how the ER pathway functions in SAM homeostasis remains uncertain. Interestingly, the wus loss-of-function mutant exhibits a smaller SAM, whereas the addition of the er erl1 erl2 triple mutation complemented the defects of the vegetative and inflorescence SAMs in the wus mutant. In this quadruple mutant, CLV3 and STM gene expression in the L3, as well as the cytokinin response in the OC, was specifically maintained, unlike in the wus mutant, implying that ER-mediated signaling in the epidermis is independent of the canonical WUS–CLV negative feedback regulation, but is indispensable for the maintenance of SAM homeostasis by coordination of CLV3 signaling and cytokinin responses in a tissue layer-specific manner (Kimura et al. 2018; Uchida et al. 2013). Overall meristem size is determined by this ER family signaling pathway, which is activated by EPIDERMAL PATTERNING FACTOR (EPF)-LIKE (EPFL) ligands secreted from the meristem peripheral region, and properly restricts the expression of both CLV3 and WUS. EPFL1 and EPFL2 are also expressed in the shoot apex, but it is uncertain whether ER receptors directly perceive EPFL signals to maintain SAM homeostasis. In addition, EPFLs repress WUS expression, but it is still unclear whether EPF/EPFL signaling controls WUS transcription itself or WUS mRNA stability (Zhang et al. 2021). Thus, signaling molecules that can act as triggers to drive the ER pathway, which is involved in SAM function, remain to be identified.
As described above, WUS function in the OC is regulated multidimensionally. However, how stem cell activity is coordinated, in terms of the balance between stem cell maintenance and differentiation, and how zonation from the PZ is determined, are still largely unknown. Recently, Schlegel et al. (2021) reported that the PZ of the SAM produces a secreted peptide called CLE40, a paralog of CLV3, which is distributed antagonistically to CLV3 in the SAM. Although the molecular mechanism still needs to be addressed, in contrast to CLV3, CLE40 signaling enables WUS expression to be boosted, in a BAM1-dependent manner, predominantly in peripheral areas of the meristem such as the inflorescence meristem, flower meristem, and floral organs. Contrarily, CLV1 represses BAM1 expression, so that regions not expressing the BAM1 gene include the OC or deep inner central cell layers, with expression restricted to the PZ of the SAM. WUS promotes CLV3 expression in the CZ in the SAM; in contrast, CLE40 expression is specifically repressed in a WUS-dependent manner. Therefore, CLE40 is not expressed in the CZ of the SAM, but is preferentially expressed in the PZ of the SAM. BAM1 expression is upregulated in regions where the CLV3–CLV1 signaling module is not available, implying that the antagonistic functions of the CLV3–CLV1–WUS and CLE40–BAM1–WUS negative feedback pathways are reflected in their cross-regulated expression patterns. The bam1 bam2 bam3 triple mutant enhances the clv1 loss-of-function phenotype (Nimchuk et al. 2015), implying that some transcriptional/translational cross-regulatory networks exist among receptor complexes and that some peptide signaling can compensate functions of CLV3 and/or CLE40 signals, at least to a certain extent, to maintain stem cell fate.
Furthermore, the fact that CLV3 promoter-fused CLE40 can fully complement clv3-1 shoot and flower meristem defects (Hobe et al. 2001) implies that CLV3 and CLE40 are functional homologs, at least in their ability to maintain SAM homeostasis. This raises the question: how are CLV3 and CLE40 specifically controlled by WUS? Further investigations, especially on the downstream target events of each signaling module at the cellular level, are required to evaluate how the CLE40–BAM1 signaling module enables the enhancement of incongruous WUS expression in the SAM zone. CLV3 and CLE40 belong to the same evolutionary clade as the CLV3 orthologs in rice, maize, barley, and tomato, implying that these combined regulatory circuits arose from two CLE peptides; the regulatory circuit comprising the CLV3 and CLE40 signaling modules may thus be a common system to maintain SAM homeostasis among plant species (Goad et al. 2017; Suzuki et al. 2019).
Conclusions and future perspectives
Sessile plants have evolved unique mechanisms to support long-lived stem cell activities so that they can adapt their morphogenesis in response to variable environmental stimuli. Such stem cell activities and maintenance are secured by a microenvironment called the stem cell niche. An intercellular signaling module known as the WUS–CLV pathway is highly conserved in flowering plants (Fletcher 2018). Important findings concerning new regulatory factors for WUS and CLVs and their orthologs in different plant species have been reported to date (Lopes et al. 2021; Zhou et al. 2018). For instance, orthologs of the fundamental WUS protein co-factors, HAMs and STM, have been identified in tomato (SlHAM1 and SlHAM2), petunia, and pepper and are known to be involved in meristem maintenance and leaf morphogenesis (David-Schwartz et al. 2013; Hendelman et al. 2016; Stuurman et al. 2002). The CLE peptide signaling module is also highly conserved in flowering plants (Rodriguez-Leal et al. 2019). For instance, the rice CLV3 homologs, FON2 and FON2 SPARE1 (FOS1) genes, are redundantly involved in stem cell activities in the floral meristem: FON2 affects the maintenance of the inflorescence meristem, whereas FOS1 contributes to vegetative SAM homeostasis (Suzaki et al. 2009).
In the past few years, other internal/external signals that are transmitted in response to nutrient availability and stresses have also been found to affect the WUS–CLV signaling network and to be involved in homeostasis of SAM activities. The CLE gene family has 32 members in the Arabidopsis genome, most of whose functions have yet to be revealed due to their redundancy under normal conditions. However, we are beginning to understand that the program to maintain homeostasis through peptide signaling under fluctuating environmental conditions is more complex and diverse than we previously thought. CLE45 plays a role in pollen tube growth at high temperature, and the CLE3–CLV1 signaling module couples lateral root growth with a nitrogen-poor environment (Araya et al. 2014; Endo et al. 2013). CLE14 is induced by a variety of environmental stresses such as high salinity, abscisic acid, salicylic acid, and jasmonic acid, and it functions in the suppression of leaf senescence by regulating reactive oxygen species (ROS) homeostasis in Arabidopsis (Zhang et al. 2022). In addition, recent reports have demonstrated a tight connection between nitrogen nutrition status, ROS, and hypoxic state in the regulation of stem cell homeostasis (Araya et al. 2014; Kong et al. 2018; Landrein et al. 2018; Shen et al. 2013; Weits et al. 2019; Zeng et al. 2017). Thus, newly uncovered signaling molecules including CLE peptides may have key functions in the response to variable environmental states and may be involved in stem cell maintenance, and further molecular analysis to provide a new framework for understanding what happens after perception by an LRR receptor complex is required. Furthermore, numerous regulatory proteins, microRNAs, and phytohormones have also been found to play roles in operating the maintenance of SAM homeostasis. Therefore, further genetic approaches plus quantitative time-course profiling with the WUS–CLV loop will generate a picture of the predicted mutual transfer of signaling molecules and will improve our understanding of intertwined molecular networks and the robustness of stem cell maintenance in plants.
References
- Araya T, Miyamoto M, Wibowo J, Suzuki A, Kojima S, Tsuchiya YN, Sawa S, Fukuda H, von Wirén N, Takahashi H (2014) CLE-CLAVATA1 peptide-receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proc Natl Acad Sci USA 111: 2029–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkunde R, Kitagawa M, Xu XM, Wang J, Jackson D (2017) SHOOT MERISTEMLESS trafficking controls axillary meristem formation, meristem size and organ boundaries in Arabidopsis. Plant J 90: 435–446 [DOI] [PubMed] [Google Scholar]
- Baumberger N, Baulcombe DC (2005) Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci USA 102: 11928–11933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsuyaku S, Sawa S, Yamada M (2011) The function of the CLE peptides in plant development and plant-microbe interactions. Arabidopsis Book 9: e0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch W, Miotk A, Ariel FD, Zhao Z, Forner J, Daum G, Suzaki T, Schuster C, Schultheiss SJ, Leibfried A, et al. (2010) Transcriptional control of a plant stem cell niche. Dev Cell 18: 849–861 [DOI] [PubMed] [Google Scholar]
- Byrne ME (2006) Shoot meristem function and leaf polarity: The role of class III HD-ZIP genes. PLoS Genet 2: e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Wilson RL, Palme K, Ditengou FA, Shpak ED (2013) ERECTA family genes regulate auxin transport in the shoot apical meristem and forming leaf primordia. Plant Physiol 162: 1978–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Jacobsen SE, Levin JZ, Meyerowitz EM (1996) The clavata and shoot meristemless loci competitively regulate meristem activity in Arabidopsis. Development 122: 1567–1575 [DOI] [PubMed] [Google Scholar]
- Daum G, Medzihradszky A, Suzaki T, Lohmann JU (2014) A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proc Natl Acad Sci USA 111: 14619–14624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Schwartz R, Borovsky Y, Zemach H, Paran I (2013) CaHAM is autoregulated and regulates CaSTM expression and is required for shoot apical meristem organization in pepper. Plant Sci 203-204: 8–16 [DOI] [PubMed] [Google Scholar]
- Deyoung BJ, Clark SE (2008) BAM receptors regulate stem cell specification and organ development through complex interactions with CLAVATA signaling. Genetics 180: 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh DC, Villalobos LIAC, Abel S (2016) Structural biology of nuclear auxin action. Trends Plant Sci 21: 302–316 [DOI] [PubMed] [Google Scholar]
- Dolzblasz A, Nardmann J, Clerici E, Causier B, van der Graaff E, Chen J, Davies B, Werr W, Laux T (2016) Stem cell regulation by Arabidopsis WOX genes. Mol Plant 9: 1028–1039 [DOI] [PubMed] [Google Scholar]
- Du F, Gong W, Boscá S, Tucker M, Vaucheret H, Laux T (2020) Dose-dependent AGO1-mediated inhibition of the miRNA165/166 pathway modulates stem cell maintenance in Arabidopsis shoot apical meristem. Plant Commun 1: 100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo S, Shinohara H, Matsubayashi Y, Fukuda H (2013) A novel pollen-pistil interaction conferring high-temperature tolerance during reproduction via CLE45 signaling. Curr Biol 23: 1670–1676 [DOI] [PubMed] [Google Scholar]
- Fletcher JC (2018) The CLV-WUS stem cell signaling pathway: A roadmap to crop yield optimization. Plants 7: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J (2001) BIG: A calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulini A, Wang J, Jackson D (2004) Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature 430: 1031–1034 [DOI] [PubMed] [Google Scholar]
- Goad DM, Zhu C, Kellogg EA (2017) Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytol 216: 605–616 [DOI] [PubMed] [Google Scholar]
- Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM (2009) Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci USA 106: 16529–16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Han L, Hymes M, Denver R, Clark SE (2010) CLAVATA2 forms a distinct CLE-binding receptor complex regulating Arabidopsis stem cell specification. Plant J 63: 889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake S, Smith HM, Holtan H, Magnani E, Mele G, Ramirez J (2004) The role of knox genes in plant development. Annu Rev Cell Dev Biol 20: 125–151 [DOI] [PubMed] [Google Scholar]
- Han H, Yan A, Li L, Zhu Y, Feng B, Liu X, Zhou Y (2020) A signal cascade originated from epidermis defines apical-basal patterning of Arabidopsis shoot apical meristems. Nat Commun 11: 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A, Tsiantis M (2010) KNOX genes: Versatile regulators of plant development and diversity. Development 137: 3153–3165 [DOI] [PubMed] [Google Scholar]
- Hendelman A, Kravchik M, Stav R, Frank W, Arazi T (2016) Tomato HAIRY MERISTEM genes are involved in meristem maintenance and compound leaf morphogenesis. J Exp Bot 67: 6187–6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobe M, Brand U, Waites R, Simon R (2001) Control of cell fate in plant meristems. Novartis Found Symp 237: 235–243, discussion 243–247 [DOI] [PubMed] [Google Scholar]
- Hu C, Zhu Y, Cui Y, Cheng K, Liang W, Wei Z, Zhu M, Yin H, Zeng L, Xiao Y, et al. (2018) A group of receptor kinases are essential for CLAVATA signalling to maintain stem cell homeostasis. Nat Plants 4: 205–211 [DOI] [PubMed] [Google Scholar]
- Hu C, Zhu Y, Cui Y, Zeng L, Li S, Meng F, Huang S, Wang W, Kui H, Yi J, et al. (2022) A CLE-BAM-CIK signalling module controls root protophloem differentiation in Arabidopsis. New Phytol 233: 282–296 [DOI] [PubMed] [Google Scholar]
- Huang W, Pitorre D, Poretska O, Marizzi C, Winter N, Poppenberger B, Sieberer T (2015) ALTERED MERISTEM PROGRAM1 suppresses ectopic stem cell niche formation in the shoot apical meristem in a largely cytokinin-independent manner. Plant Physiol 167: 1471–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H (2006) Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313: 842–845 [DOI] [PubMed] [Google Scholar]
- Je BI, Gruel J, Lee YK, Bommert P, Arevalo ED, Eveland AL, Wu Q, Goldshmidt A, Meeley R, Bartlett M, et al. (2016) Signaling from maize organ primordia via FASCIATED EAR3 regulates stem cell proliferation and yield traits. Nat Genet 48: 785–791 [DOI] [PubMed] [Google Scholar]
- Je BI, Xu F, Wu Q, Liu L, Meeley R, Gallagher JP, Corcilius L, Payne RJ, Bartlett ME, Jackson D (2018) The CLAVATA receptor FASCIATED EAR2 responds to distinct CLE peptides by signaling through two downstream effectors. eLife 7: e35673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyuka K, Praekelt U, Franklin KA, Billingham OE, Hooley R, Whitelam GC, Halliday KJ (2003) Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J 35: 57–70 [DOI] [PubMed] [Google Scholar]
- Kieffer M, Stern Y, Cook H, Clerici E, Maulbetsch C, Laux T, Davies B (2006) Analysis of the transcription factor WUSCHEL and its functional homologue in Antirrhinum reveals a potential mechanism for their roles in meristem maintenance. Plant Cell 18: 560–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Wu CY, Yu HM, Sheen J, Lee H (2017) Dual CLAVATA3 peptides in Arabidopsis shoot stem cell signaling. J Plant Biol 60: 506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Kim SG, Lee M, Lee I, Park HY, Seo PJ, Jung JH, Kwon EJ, Suh SW, Paek KH, et al. (2008) HD-ZIP III activity is modulated by competitive inhibitors via a feedback loop in Arabidopsis shoot apical meristem development. Plant Cell 20: 920–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Tasaka M, Torii KU, Uchida N (2018) ERECTA-family genes coordinate stem cell functions between the epidermal and internal layers of the shoot apical meristem. Development 145: dev156380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Betsuyaku S, Osakabe Y, Mizuno S, Nagawa S, Stahl Y, Simon R, Yamaguchi-Shinozaki K, Fukuda H, Sawa S (2010) RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development 137: 3911–3920 [DOI] [PubMed] [Google Scholar]
- Kong X, Tian H, Yu Q, Zhang F, Wang R, Gao S, Xu W, Liu J, Shani E, Fu C, et al. (2018) PHB3 maintains root stem cell niche identity through ROS-responsive AP2/ERF transcription factors in Arabidopsis. Cell Rep 22: 1350–1363 [DOI] [PubMed] [Google Scholar]
- Krogan NT, Hogan K, Long JA (2012) APETALA2 negatively regulates multiple floral organ identity genes in Arabidopsis by recruiting the co-repressor TOPLESS and the histone deacetylase HDA19. Development 139: 4180–4190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrein B, Formosa-Jordan P, Malivert A, Schuster C, Melnyk CW, Yang W, Turnbull C, Meyerowitz EM, Locke JCW, Jönsson H (2018) Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc Natl Acad Sci USA 115: 1382–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Clark SE (2015) A WUSCHEL-independent stem cell specification pathway is repressed by PHB, PHV and CNA in Arabidopsis. PLoS One 10: e0126006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Jun YS, Cha OK, Sheen J (2019) Mitogen-activated protein kinases MPK3 and MPK6 are required for stem cell maintenance in the Arabidopsis shoot apical meristem. Plant Cell Rep 38: 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU (2005) WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438: 1172–1175 [DOI] [PubMed] [Google Scholar]
- Lohmann JU, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D (2001) A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105: 793–803 [DOI] [PubMed] [Google Scholar]
- Lopes FL, Galvan-Ampudia C, Landrein B (2021) WUSCHEL in the shoot apical meristem: Old player, new tricks. J Exp Bot 72: 1527–1535 [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Pérez-Torres A, Rampey RA, Bartel B, Herrera-Estrella L (2005) An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol 137: 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde C, Hake S (2009) The interaction of knotted1 and thick tassel dwarf1 in vegetative and reproductive meristems of maize. Genetics 181: 1693–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Zeng J, Wu H, Tian Z, Zhao Z (2018) A molecular framework for auxin-controlled homeostasis of shoot stem cells in Arabidopsis. Mol Plant 11: 899–913 [DOI] [PubMed] [Google Scholar]
- Ma Y, Miotk A, Šutiković Z, Ermakova O, Wenzl C, Medzihradszky A, Gaillochet C, Forner J, Utan G, Brackmann K, et al. (2019) WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nat Commun 10: 5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchuk ZL, Zhou Y, Tarr PT, Peterson BA, Meyerowitz EM (2015) Plant stem cell maintenance by transcriptional cross-regulation of related receptor kinases. Development 142: 1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Shinohara H, Ogawa-Ohnishi M, Matsubayashi Y (2009) A glycopeptide regulating stem cell fate in Arabidopsis thaliana. Nat Chem Biol 5: 578–580 [DOI] [PubMed] [Google Scholar]
- Paciorek T, Zazímalová E, Ruthardt N, Petrásek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, et al. (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Perales M, Rodriguez K, Snipes S, Yadav RK, Diaz-Mendoza M, Reddy GV (2016) Threshold-dependent transcriptional discrimination underlies stem cell homeostasis. Proc Natl Acad Sci USA 113: E6298–E6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE (2005) Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17: 61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Leal D, Xu C, Kwon CT, Soyars C, Demesa-Arevalo E, Man J, Liu L, Lemmon ZH, Jones DS, van Eck J, et al. (2019) Evolution of buffering in a genetic circuit controlling plant stem cell proliferation. Nat Genet 51: 786–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M (1997) Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel J, Denay G, Wink R, Pinto KG, Stahl Y, Schmid J, Blümke P, Simon RG (2021) Control of Arabidopsis shoot stem cell homeostasis by two antagonistic CLE peptide signalling pathways. eLife 10: e70934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644 [DOI] [PubMed] [Google Scholar]
- Scofield S, Murray JA (2006) KNOX gene function in plant stem cell niches. Plant Mol Biol 60: 929–946 [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang YT, Tian H, Guo FQ (2013) Nitric oxide mediates cytokinin functions in cell proliferation and meristem maintenance in Arabidopsis. Mol Plant 6: 1214–1225 [DOI] [PubMed] [Google Scholar]
- Shi B, Guo X, Wang Y, Xiong Y, Wang J, Hayashi KI, Lei J, Zhang L, Jiao Y (2018) Feedback from lateral organs controls shoot apical meristem growth by modulating auxin transport. Dev Cell 44: 204–216.e6 [DOI] [PubMed] [Google Scholar]
- Shi B, Zhang C, Tian C, Wang J, Wang Q, Xu T, Xu Y, Ohno C, Sablowski R, Heisler MG, et al. (2016) Two-step regulation of a meristematic cell population acting in shoot branching in Arabidopsis. PLoS Genet 12: e1006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno A, Aki SS, Takahashi N, Umeda M (2021) Regulation of the plant cell cycle in response to hormones and the environment. Annu Rev Plant Biol 72: 273–296 [DOI] [PubMed] [Google Scholar]
- Shinohara H, Matsubayashi Y (2015) Reevaluation of the CLV3-receptor interaction in the shoot apical meristem: dissection of the CLV3 signaling pathway from a direct ligand-binding point of view. Plant J 82: 328–336 [DOI] [PubMed] [Google Scholar]
- Snipes SA, Rodriguez K, DeVries AE, Miyawaki KN, Perales M, Xie M, Reddy GV (2018) Cytokinin stabilizes WUSCHEL by acting on the protein domains required for nuclear enrichment and transcription. PLoS Genet 14: e1007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl Y, Simon R (2010) Plant primary meristems: Shared functions and regulatory mechanisms. Curr Opin Plant Biol 13: 53–58 [DOI] [PubMed] [Google Scholar]
- Stuurman J, Jäggi F, Kuhlemeier C (2002) Shoot meristem maintenance is controlled by a GRAS-gene mediated signal from differentiating cells. Genes Dev 16: 2213–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YH, Zhou C, Li YJ, Yu Y, Tang LP, Zhang WJ, Yao WJ, Huang R, Laux T, Zhang XS (2020) Integration of pluripotency pathways regulates stem cell maintenance in the Arabidopsis shoot meristem. Proc Natl Acad Sci USA 117: 22561–22571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Ohneda M, Toriba T, Yoshida A, Hirano HY (2009) FON2 SPARE1 redundantly regulates floral meristem maintenance with FLORAL ORGAN NUMBER2 in rice. PLoS Genet 5: e1000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki C, Tanaka W, Hirano HY (2019) Transcriptional corepressor ASP1 and CLV-like signaling regulate meristem maintenance in rice. Plant Physiol 180: 1520–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szemenyei H, Hannon M, Long JA (2008) TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386 [DOI] [PubMed] [Google Scholar]
- Szymkowiak EJ, Sussex IM (1996) What chimeras can tell us about plant development. Annu Rev Plant Physiol Plant Mol Biol 47: 351–376 [DOI] [PubMed] [Google Scholar]
- Tucker MR, Hinze A, Tucker EJ, Takada S, Jürgens G, Laux T (2008) Vascular signalling mediated by ZWILLE potentiates WUSCHEL function during shoot meristem stem cell development in the Arabidopsis embryo. Development 135: 2839–2843 [DOI] [PubMed] [Google Scholar]
- Uchida N, Shimada M, Tasaka M (2013) ERECTA-family receptor kinases regulate stem cell homeostasis via buffering its cytokinin responsiveness in the shoot apical meristem. Plant Cell Physiol 54: 343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites R, Simon R (2000) Signaling cell fate in plant meristems. Three clubs on one tousle. Cell 103: 835–838 [DOI] [PubMed] [Google Scholar]
- Wang C, Reid JB, Foo E (2020) The role of CLV1, CLV2 and HPAT homologues in the nitrogen-regulation of root development. Physiol Plant 170: 607–621 [DOI] [PubMed] [Google Scholar]
- Wang L, Kim J, Somers DE (2013) Transcriptional corepressor TOPLESS complexes with pseudoresponse regulator proteins and histone deacetylases to regulate circadian transcription. Proc Natl Acad Sci USA 110: 761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weits DA, Kunkowska AB, Kamps NCW, Portz KMS, Packbier NK, Nemec Venza Z, Gaillochet C, Lohmann JU, Pedersen O, van Dongen JT, et al. (2019) An apical hypoxic niche sets the pace of shoot meristem activity. Nature 569: 714–717 [DOI] [PubMed] [Google Scholar]
- Wenkel S, Emery J, Hou BH, Evans MM, Barton MK (2007) A feedback regulatory module formed by LITTLE ZIPPER and HD-ZIPIII genes. Plant Cell 19: 3379–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington N, Cunningham D, Le TK, De Maria D, Silva EM (2015) Sox21 regulates the progression of neuronal differentiation in a dose-dependent manner. Dev Biol 397: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Li R, Weng L, Sun Y, Li M, Xiao H (2019) Domain-specific expression of meristematic genes is defined by the LITTLE ZIPPER protein DTM in tomato. Commun Biol 2: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Reddy GV (2011) WUSCHEL-mediated cellular feedback network imparts robustness to stem cell homeostasis. Plant Signal Behav 6: 544–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Ji L, Le BH, Zhai J, Chen J, Luscher E, Gao L, Liu C, Cao X, Mo B, et al. (2017) ARGONAUTE10 promotes the degradation of miR165/6 through the SDN1 and SDN2 exonucleases in Arabidopsis. PLoS Biol 15: e2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Dong Z, Wu H, Tian Z, Zhao Z (2017) Redox regulation of plant stem cell fate. EMBO J 36: 2844–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, DeGennaro D, Lin G, Chai J, Shpak ED (2021) ERECTA family signaling constrains CLAVATA3 and WUSCHEL to the center of the shoot apical meristem. Development 148: dev189753. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Zhai LM, Yu HX, Peng J, Wang SS, Zhang XS, Su YH, Tang LP (2020) The BIG gene controls size of shoot apical meristems in Arabidopsis thaliana. Plant Cell Rep 39: 543–552 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu C, Li K, Li X, Xu M, Guo Y (2022) CLE14 functions as a “brake signal” to suppress age-dependent and stress-induced leaf senescence by promoting JUB1-mediated ROS scavenging in Arabidopsis. Mol Plant 15: 179–188 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tucker E, Hermann M, Laux T (2017) A molecular framework for the embryonic initiation of shoot meristem stem cells. Dev Cell 40: 264–277.e4 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liu X, Engstrom EM, Nimchuk ZL, Pruneda-Paz JL, Tarr PT, Yan A, Kay SA, Meyerowitz EM (2015) Control of plant stem cell function by conserved interacting transcriptional regulators. Nature 517: 377–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yan A, Han H, Li T, Geng Y, Liu X, Meyerowitz EM (2018) HAIRY MERISTEM with WUSCHEL confines CLAVATA3 expression to the outer apical meristem layers. Science 361: 502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Hu F, Wang R, Zhou X, Sze SH, Liou LW, Barefoot A, Dickman M, Zhang X (2011) Arabidopsis Argonaute10 specifically sequesters miR166/165 to regulate shoot apical meristem development. Cell 145: 242–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hu C, Cui Y, Zeng L, Li S, Zhu M, Meng F, Huang S, Long L, Yi J, et al. (2021) Conserved and differentiated functions of CIK receptor kinases in modulating stem cell signaling in Arabidopsis. Mol Plant 14: 1119–1134 [DOI] [PubMed] [Google Scholar]