ABSTRACT
We report a patient who presented with a rapidly expanding symptomatic tuberculous aortitis and mycotic pseudo-aneurysm of the infra-renal aorta, after intra-vesical BCG chemotherapy for bladder cancer, treated by required emergency open aneurysm repair. His case highlights this rare complication of intravesical BCG treatment, haematological seeding causing tuberculous aortitis and mycotic pseudo-aneurysm formation of the infra-renal aorta. It also illustrates successful treatment with emergency open surgery, local debridement of mycotic pseudoaneurysm, in-situ surgical reconstruction using a custom bovine-wrap interposition graft to create a neo-aorta and multi-agent anti-tuberculous chemotherapy.
CASE REPORT
A 65-year-old man presented to the Emergency Department with a six-week history of generalised lower abdominal pain radiating posteriorly to his sacroiliac joint. He had past history of bladder cancer (grade 2 pT1 transitional cell carcinoma) and had undergone planned curative Trans-Urethral Resection Bladder Tumour (TURBT) surgery with adjuvant intravesical BCG treatment, receiving 2 cycles of treatment over the last year. A recognised but rare complication of intravesical BCG treatment is the development of Iatrogenic Tuberculosis (TB).
CT Abdomen and Pelvis with contrast was performed in the Emergency Department on the day of presentation which showed a large thick-walled saccular pseudo-aneurysm of the distal infra-renal abdominal aorta with the radiological appearances consistent with a mycotic pseudo-aneurysm with signs of impending rupture. The clinical working diagnosis was of tuberculous aortitis and mycotic pseudo-aneurysm formation of the infra-renal aorta. Within 18 hours, the patient underwent emergency open surgery, with local debridement of mycotic pseudoaneurysm and para-aortic tissue and in-situ surgical reconstruction using a custom bovine-pericardial-wrap interposition tube-graft to create a neo-aorta (Figure 2.), with no intra-operative complications. Aortic tissue and fluid samples were taken intra-operatively and sent for microbiological investigations. The patient was treated in conjunction with an infectious diseases specialist with initially empirical antibiotic therapy.
Figure 2.

Intra-operative view of retroperitoneum, after local debridement of mycotic pseudoaneurysm and para-aortic tissue, showing in-situ surgical reconstruction using a custom bovine-pericardial-wrap interposition tube-graft to create a neo-aorta.
Figure 1.
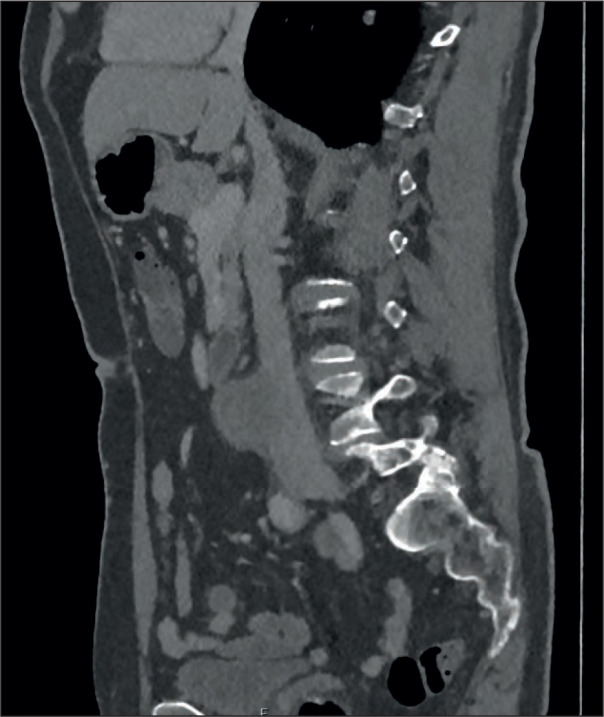
CT Abdomen and Pelvis with contrast was performed in the Emergency department which showed a large thick-walled saccular pseudo-aneurysm of the distal infra-renal abdominal aorta with the radiological appearances consistent with a mycotic pseudo-aneurysm. Signs of impending rupture were evident from a concerning defect in the anterior wall of the aorta, alongside localised reactive changes in peri-aortic fat stranding and para-aortic lymphadenopathy.
Microbiology results from both blood culture samples, and intra-operative samples confirmed Tuberculosis (TB) with acid-fast bacilli seen on tissue sample processing. The Infectious Diseases specialist treated the patient by commencing the combination chemotherapy regime; Voractiv (Rifampicin, Isoniazid, Pyrazinamide and Ethambutol) and the patient made an uncomplicated recovery from surgery and was discharged home following a 17 day stay in hospital.
DISCUSSION
Currently, for high risk, non-muscle-invasive bladder cancers (NMIBC), defined by NICE Guidance1 following a transurethral resection of a bladder tumour (TURBT), patients are offered the choice of intra-vesicular BCG therapy2 or a radical cystectomy. In this case, the patient opted for intra-vesical BCG therapy. In addition to the BCG therapy, the patient received a single dose of mitomycin C, given within 24 hours of resection, which is standard practice of care and reduces recurrence risk by 40-50% with a very low incidence of adverse events3. BCG therapy reduces the likelihood of recurrence and progression of bladder tumours that have been managed with transurethral resection4. It is thought the anti-tumour effect of BCG therapy results from a T-cell mediated inflammatory reaction. Commonly, following this therapy, patients can experience symptoms such as dysuria, increased urinary frequency and fevers.
The incidence of mycotic aortic aneurysms is rare, with 0.9%-1.3% of aneurysms resulting from an infective cause5. The most commonly associated pathogens are Staphylococcus Aureus (most common), Streptococcus spp., Salmonella and Escherichia coli. The incidence of mycotic aneurysms as a result of intra-vesicular BCG therapy is therefore very rare indeed, upon review of the published literature, we found a total of only 35 cases dating back to 1988, including this patient. The interval between final instillation and first presentation of infection in our patient was just over a year which compared to similar cases is a relatively short duration – the median of these cases being 17 months6.
Our patient’s only presenting symptom was abdominal discomfort for a period of 6 weeks, they did report weight loss but stated this was intentional. The most common presenting symptoms for mycotic aneurysms are pain, 79%, and fever, 48%, and whilst pain was a major feature in our case, fever was not reported, and the patient was apyrexic on admission.
It is thought that there are a number of ways that the causative Tuberculous Bacilli can reach the aortic wall in order to cause the initial infection and aortitis. A risk factor for complications of BCG therapy is commencing the treatment temporally close to the surgical bladder wall trauma after TURBT. It is widely recommended that BCG therapy should not start for several weeks following procedures that could damage the bladder and grant bacteria access to the blood stream7. Once bacilli are in the blood stream, and are haematologically spread, they may infect the aorta by entering through damage to the vessel wall secondary to atherosclerosis, or through the vasa vasorum and invading the adventitia or media. Alternatively, the bacilli can infect the vessel by means of a contiguous focus such as a lymph node or paraspinal abscess8.
Other than positive blood cultures and aortic tissue taken intra-operatively which grew acid-fast bacilli, there was no evidence of systemic TB in this case. Treatment by open surgery remains the gold-standard for mycotic pseudoaneurysm of the infra-renal aorta, as it offers an opportunity for surgical debridement of infected tissue and tissue sampling for microbiology assessment with confirmation of the diagnosis. Where possible anatomical in-situ reconstruction of the aorta is preferred, using autologous vein or as in this case, a custom bovine-pericardium-wrap fashioned into a tube-graft, which is remarkably versatile and resistant to infection. When in-situ reconstruction is not feasible, an alternative approach is aortic ligation and extra-anatomic bypass by axillo-bi-femoral bypass grafting. Endovascular Repair with an Endograft Covered-Stent can offer a temporary solution, particularly in extremis or ruptured aneurysms, but infection typically persists and despite long-term antimicrobial therapy, the majority suffer late infective complications and death.
This case highlights a rare complication of intravesical BCG therapy for Bladder Cancer with the development of a life-threatening Tuberculous aortitis and rapidly expanding pseudoaneurysm of the infra-renal abdominal aorta. Untreated rupture and death would have resulted, but we are pleased to report that after open surgical repair and adjuvant combination anti-tuberculous chemotherapy this man made an uncomplicated recovery. It is important for clinicians to consider the possibility of a mycotic aneurysm in patients with a history of bladder cancer managed with BCG therapy. This allows the conduction of relevant microbiological investigations so that positive diagnoses can facilitate emergency surgical treatment to be complimented by targeted anti-microbial therapy for a successful outcome.
Footnotes
UMJ is an open access publication of the Ulster Medical Society (http://www.ums.ac.uk).
REFERENCES
- 1.NICE Guideline. London: National Institute for Health and Care Excellence; 2015. 2. Bladder cancer: diagnosis and management. Available from: https://www.nice.org.uk/guidance/NG2. [PubMed] [Google Scholar]
- 2.Porten SP, Leapman MS, Greene KL. Intravesical chemotherapy in non-muscle-invasive bladder cancer. Indian J Urol. 2015;31(4):297–303. doi: 10.4103/0970-1591.166446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostafid H. London: NICE Shared Learning Database; 2015. [cited 2021 Jan 7]. NICE Guidance: Conditions and diseases: cancer: bladder cancer. Immediate instillation of intravesical chemotherapy (Mitomycin-C) following transurethral resection for bladder tumour. [View the supporting material.] [monograph on the Internet] Available from: https://www.nice.org.uk/sharedlearning/immediate-instillation-of-intravesical-chemotherapy-mitomycin-c-following-transurethral-resection-for-bladder-tumour. [Google Scholar]
- 4.Shelley M, Court JB, Kynaston H, Wilt TJ, Fish R, Mason M. Cochrane Database Systematic Reviews; 2000. Intravesical Bacillus Calmette-Guérin in Ta and T1 bladder cancer. Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001986/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller BT, Wegener OR, Grabitz K, Pillny M, Thomas L, Sandmann W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001;33(1):106–13. doi: 10.1067/mva.2001.110356. [DOI] [PubMed] [Google Scholar]
- 6.Higashi Y, Nakamura S, Kidani K, et al. Mycobacterium bovis-induced Aneurysm after Intravesical Bacillus Calmette-Guérin therapy: a case study and literature review. Intern Med. 2018;57(3):429–35. doi: 10.2169/internalmedicine.9102-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steg A, Adjiman S, Debre B. BCG therapy in superficial bladder tumours – complications and precautions. Eur Urol. 1992;21(Suppl 2):35–40. doi: 10.1159/000474920. [DOI] [PubMed] [Google Scholar]
- 8.Long R, Guzman R, Greenberg H, Safneck J, Hershfield E. Tuberculous mycotic aneurysm of the aorta: review of published medical and surgical experience. Chest. 1999;115(2):522–31. doi: 10.1378/chest.115.2.522. [DOI] [PubMed] [Google Scholar]


