INTRODUCTION
This article discusses the current clinical and research landscape with respect to cardiovascular magnetic resonance (CMR) imaging in several types of cardiotoxicity related to cancer therapy, because cardio-oncology is a heterogeneous indication for CMR (Boxes 1–4). CMR imaging can provide valuable, noninvasive diagnostic assessments of myocardial function and composition as well as vascular assessments (Fig. 1). Because CMR does not use ionizing radiation, its use in oncology patients who receive external radiation therapy is advantageous compared with other imaging modalities that may repetitively expose patients to additional radiation for serial cardio-oncology evaluations. This, combined with the high spatial and temporal resolutions associated with CMR imaging, make it an increasingly valued tool in cardio-oncology assessments.
Box 1. When CMR is useful after echocardiography.
Poor acoustic windows and difficult imaging by echocardiography
Borderline LVEF assessment by echocardiography
Box 4. Contrasted tissue characterization.
Diffuse and focal LGE have been reported in studies related to inflammation, edema, and fibrosis
Extracellular volume (ECV) calculated from native and contrasted T1 mapping may aid in identifying myocardial fibrosis late after cancer-related treatment
Fig. 1.
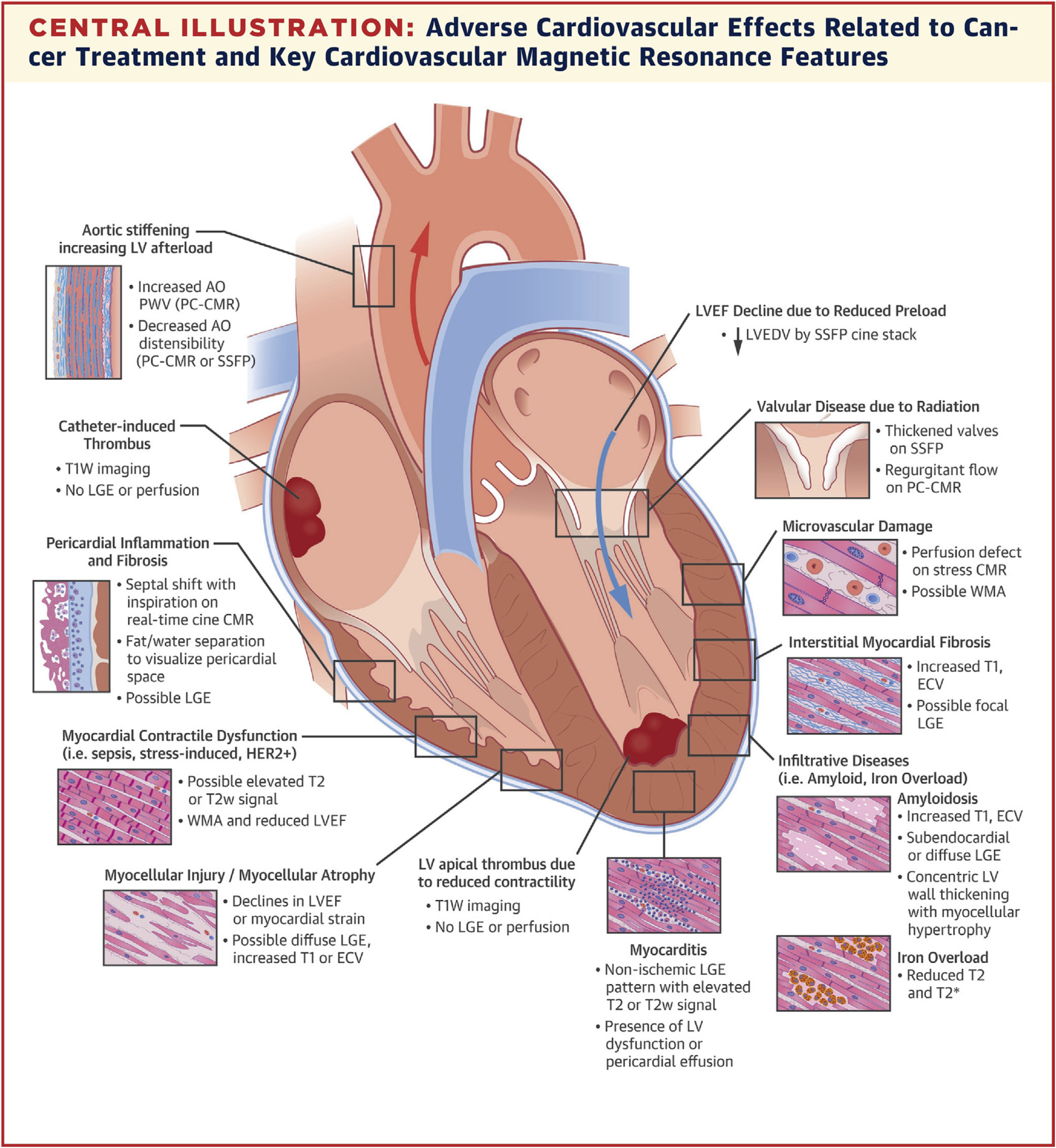
Utility of cardiovascular magnetic resonance imaging (CMR) in the oncology patient. (From Jordan JH, Todd RM, Vasu S, Hundley WG. Cardiovascular magnetic resonance in the oncology patient. JACC Cardiovasc Imaging. 2018;11(8):1150–1172; with permission.)
Although several recent position papers and guidelines have been published in cardio-oncology,1–4 there remains a lack of consensus on the appropriate clinical and research use of CMR in cardio-oncology screening and surveillance. A point of agreement, however, is that CMR examinations should be considered secondary to poor echocardiographic measures where acoustic windows may be poor or that the LVEF may be borderline.2 The use of CMR for screening and surveillance of cardiotoxicity extends beyond looking at systolic function measures, such as LVEF, for identifying the underlying cause of changes in tissue characterization properties, assessments of vasculature and perfusion, and characterization of masses within the left ventricle (LV) (see Fig. 1).5 We refer to other sections in this issue with regard to mechanisms of action for these examples of cardiotoxicity. This article summarizes current knowledge surrounding the use of CMR in cardiotoxicity to evaluate functional capacity, anatomic and structural abnormalities, and both noncontrasted and contrasted tissue characterization techniques. Although imaging of cardiotoxicity related to anthracyclines is presented in most of the literature and in this review, we also discuss CMR imaging of cardiotoxicity related to radiation therapy, trastuzumab, and immune checkpoint inhibitors (ICIs) used in immunotherapy.
FUNCTIONAL MEASURES
The LVEF is the primary imaging marker of cardiotoxicity and may be measured noninvasively using modalities such as 2D or 3D echocardiography, radionuclide ventriculography or multigated acquisition scans, or CMR imaging. CMR imaging is advantageous for measuring LVEF because of its high temporal and spatial resolutions and the use of Simpson’s rule to quantify LV volumes that does not require geometric assumptions. Furthermore, CMR does not use ionizing radiation and, thus, may be beneficial for monitoring LVEF in serial settings that may be necessitated by the longterm care of patients with cancer in survivorship. The LVEF is quantified from a short-axis cine stack of steady-state free-precession (SSFP) images in which the left ventricular end-diastolic (LVEDV) and left ventricular end-systolic (LVESV) volumes are identified, and the endocardial surface is contoured in postprocessing software. The discs of volumes are summed by Simpson’s rule and the LVEF is then calculated as the difference in LVEDV and LVESV and divided by the LVEDV. Baseline and serial screening of cardiotoxicity with riskbased algorithms have been proposed by Panjrath and Jain6 for monitoring trastuzumab cardiotoxicity, and by Hall and colleagues7 for monitoring cardiotoxicity from targeted therapies; in general, echocardiographic or radionuclide measures of LVEF are proposed. Although the American Society of Echocardiography and the European Association of Cardiovascular Imaging consensus statement recommends screening of the LVEF with echocardiography, CMR is recommended in circumstances where poor acoustic windows or body habitus may limit the quality of the study, or if a borderline LVEF is measured and CMR may provide additional clarity.2
When the LVEF drops to a value below normal (50%–53%) or changes by more than 10 absolute points without other intervening factors (such as sepsis or myocardial infarction), cancer therapyrelated cardiotoxicity should be considered when chemotherapy that has been associated with myocellular injury has been administered. Before confirming this diagnosis, however, the cause for a change in LVEF should be investigated. In a cohort of 112 patients with cancer receiving potentially cardiotoxic chemotherapy regimens (72% anthracycline-based), Melendez and colleagues8 demonstrated that nearly 20% of patients experienced a cardiotoxic LVEF drop (>10 absolute points or to a value <50%) owing to a large decline in LVEDV. These findings may suggest potential intravascular volume depletion 3 months after initiating treatment.8 Additional unanswered questions regarding imaging LVEF with CMR after anthracyclines include whether acute changes recover after cessation of treatment and if early subclinical changes (changes of <10 points or to a value >50%) portend worse outcomes later in survivorship.
Deteriorations in LV myocardial strain after anthracyclines have been observed with CMR imaging in several studies.9–14 Several myocardial strain techniques exist for CMR, including spatially modulated magnetization with line or grid tags, feature tracking from SSFP cine images, displacement encoding with stimulated echoes, and strainencoded imaging. Values of CMR strain are oriented to planes of the LV and include global radial strain (GRS), global circumferential strain (GCS), and global longitudinal strain (GLS) (Fig. 2).15 The GCS and GLS are most widely used and reported in CMR because of the technical limitations in measuring GRS.15 In a prospective assessment of GCS in 53 patients receiving low to moderate doses of anthracyclines (50–375 mg/m2 of doxorubicin equivalent), early subclinical deteriorations in GCS tracked concurrently with subclinical declines in LVEF (Fig. 3).9
Fig. 2.
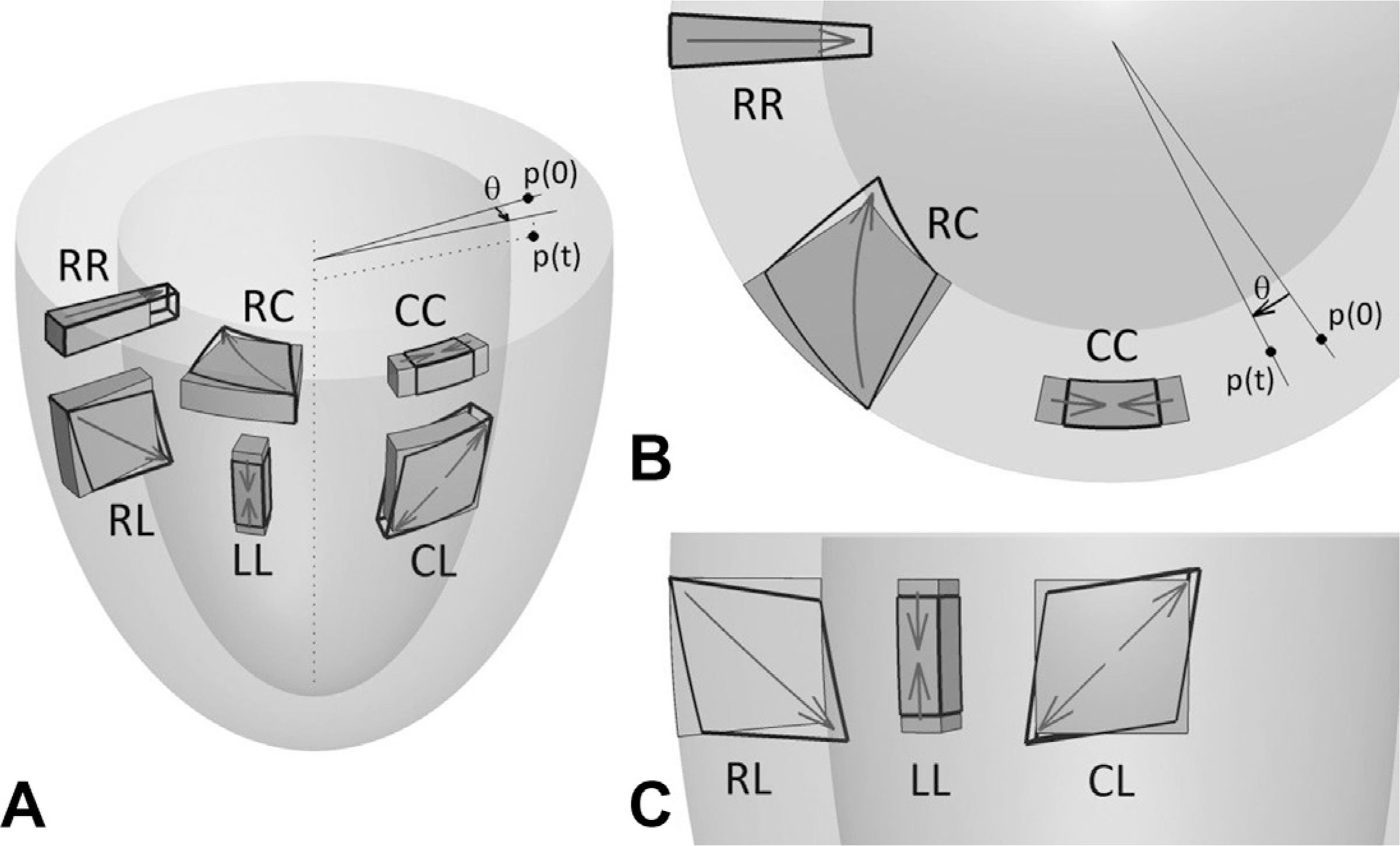
Cardiovascular magnetic strain directions shown (A) in 3D, (B) short axis, and (C) long axis. Global circumferential strain (GCS) is calculated from circumferential changes (CC) and deformation in the long-axis planes (LL), and is used to calculate global longitudinal strain (GLS). (From Zhong X, Gibberman LB, Spottiswoode BS, et al. Comprehensive cardiovascular magnetic resonance of myocardial mechanics in mice using three-dimensional cine DENSE. J Cardiovasc Magn Reson. 2011;13:83; with permission.)
Fig. 3.
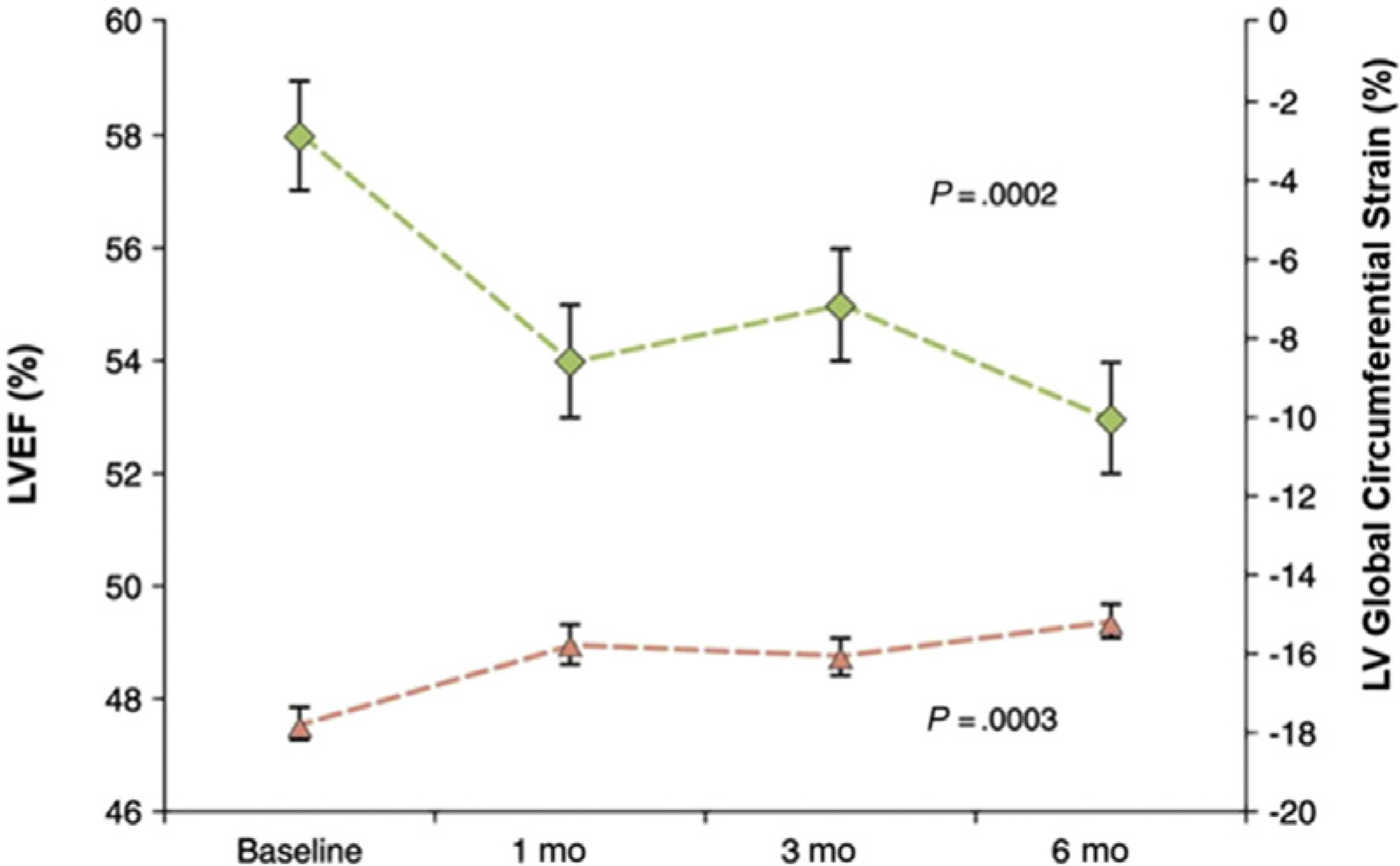
Reduced LV global circumferential strain occurred concurrent with subclinical left ventricular ejection fraction changes early after initiation of chemotherapy. (From Drafts BC, Twomley KM, D’Agostino R, Jr., et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6(8):877–885; with permission.)
Similar to LVEF, the LVEDV and LVESV should be considered when interpreting changes in GCS with the administration of anthracyclines. In a cohort of 101 patients receiving cardiotoxic chemotherapies (71% anthracycline-based regimens), Jordan and colleagues16 found that up to 16% of individuals experienced a deterioration in circumferential myocardial strain mediated by a decline in LVEDV rather than an increase in LVESV. Recent data from Haslbauer and colleagues10 demonstrated that, although GCS changes only trended toward being higher than in controls, GLS did significantly increase (or worsen) early (<3 months) and late (>12 months) after treatment (21% ± 8% and 17% ± 11%, respectively) when compared with controls (24% ± 5%, P<.001) using feature tracking strain analysis.
Although studies have demonstrated the usefulness of CMR to identify subclinical cardiac dysfunction with LVEF and strain,8–11,14,16,17 the usefulness of imaging to intervene and guide therapy remains uncertain. The ongoing SUCCOUR Trial, although echocardiography based, may answer this gap in knowledge because it is evaluating the hypothesis that cardioprotective therapy guided by GLS rather than LVEF would benefit patients at risk of developing future declines in LVEF.18
ANATOMY AND STRUCTURE
CMR imaging is also useful for investigating anatomic and structural features in the heart and surrounding structures that may occur in oncology patients. For instance, CMR images may be useful for identifying the causes of masses in the cardiac field of view or to identify thickening of the pericardial space (see Fig. 1).
An LV mass can be quantified from the SSFP cine stack images (acquired for the assessment of LV volumes) by adding an additional endocardial border to contour the myocardial tissue. Anthracyclines such as doxorubicin may be associated to atrophic remodeling due to the mechanisms of action which include topoisomerase Iiβ-mediated myocellular death, downregulation of myocellular GATA4 expression, and DNA oxidant damage.19–24 Several recent studies have demonstrated early and late decreases in LV mass in response to anthracyclines.1,25–30 However, these findings are not uniform and may be related to the timing of measurement in the cycle of remodeling.
Using a novel method of noninvasively measuring cardiomyocyte size with CMR, called intracellular water lifetime (τic), de Souza and colic leagues31 demonstrated that women with breast cancer treated with anthracyclines had a decrease in LV mass resulting from cardiomyocyte atrophy. Importantly, other studies have demonstrated that declines in the LV mass of anthracycline-treated patients with cancer portended increased risk of future cardiac events27 and were more associated with heart failure symptoms than with changes in LVEF early after treatment.30 Willis and colleagues32 recently suggested that this atrophic mechanism following doxorubicin exposure may be dependent on the striated muscle-specific ubiquitin ligase MuRF1. The compensatory mechanism following atrophic responses remains to be determined and is likely due to multifactorial processes.
TISSUE CHARACTERIZATION: NONCONTRAST TECHNIQUES
One of the major advantages of CMR imaging in cardio-oncology patients is the ability to assess both functional capacity and changes in tissue characteristics in a single, noninvasive examination. Increases in noncontrasted T1, or native T1, are associated with pathology in the myocardium including edema, inflammation, and fibrosis.33 T2 relaxation is a water-sensitive process, and increases above normal myocardial T2 values are therefore strongly associated with acute processes and myocardial edema.33,34
Historical work in nuclear magnetic resonance spectroscopy of rodents exposed to cardiotoxic drugs demonstrated that histologic changes in myocardial tissue were associated with an increase in myocardial T1 and T2 relaxation.35,36 Because T2 relaxation is tightly linked to myocardial water content and edema, the ability to identify changes in myocardial T2 is thus dependent on timing of the CMR examination with respect to the cardiotoxic remodeling process.34
Quantitative parametric mapping of myocardial T1 and T2 relaxation may be accomplished in a single breath hold that characterizes the myocardial relaxation constants in a voxel-by-voxel basis. As MRI scanner vendors provide motion-corrected registration of these maps and sites, and establish normative/abnormal values for their specific scanner, parametric mapping may be used to identify myocardial tissue characteristics associated with cardiotoxicity.10,33,34 For example, using a cut point of T2 greater than 59 ms, Thavendiranathan and colleagues37 demonstrated that quantitative T2 mapping was elevated in HER2-positive patients with breast cancer treated with sequential anthracycline and trastuzumab treatment who were diagnosed with subclinical cardiotoxicity. A recent communication from Lustberg and colleagues38 followed 29 patients with breast cancer with a preserved LVEF and normal baseline T2 (51.8 ± 3.5 ms); after the first anthracycline treatment there was no change in functional measures; however, T2 increased by 3.3 ± 0.8 ms (P<.001) and continued to increase by 5.4 ± 0.8 ms after the fourth anthracycline treatment (Fig. 4). Although LVEF declined in the overall group, early changes in T2 were not associated with LVEF decline, thus the prognostic significance of identifying acute cardiotoxicity with T2 mapping remains unclear.38
Fig. 4.
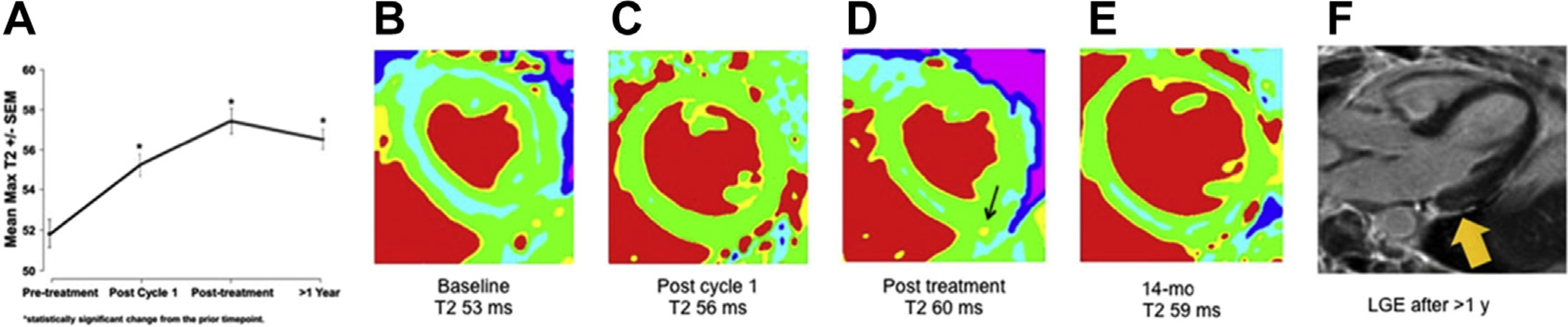
(A) Serial increases in a population of patients with breast cancer during and after treatment (B–E), and representative T2 images from a woman in whom T2 increased significantly (denoted by arrow in D) and who developed subepicardial scarring on late gadolinium enhancement (LGE) images 1 year after treatment (denoted by arrow in F). (From Lustberg MB, Reinbolt R, Addison D, et al. Early detection of anthracycline-induced cardiotoxicity in breast cancer survivors with T2 cardiac magnetic resonance. Circ Cardiovasc Imaging 2019;12(5):e008777; with permission.)
Haslbauer and colleagues10 sought to answer these gaps in knowledge regarding early and late noncontrasted tissue characterization features in 115 patients receiving cancer-related therapy. Early cardiotoxic changes were demonstrated as increased native T1 and T2 in the first 3 months of therapy, whereas late after treatment, the acute processes resolved and increased T1 in absentia of T2 changes indicated myocardial fibrosis (Fig. 5).
Fig. 5.
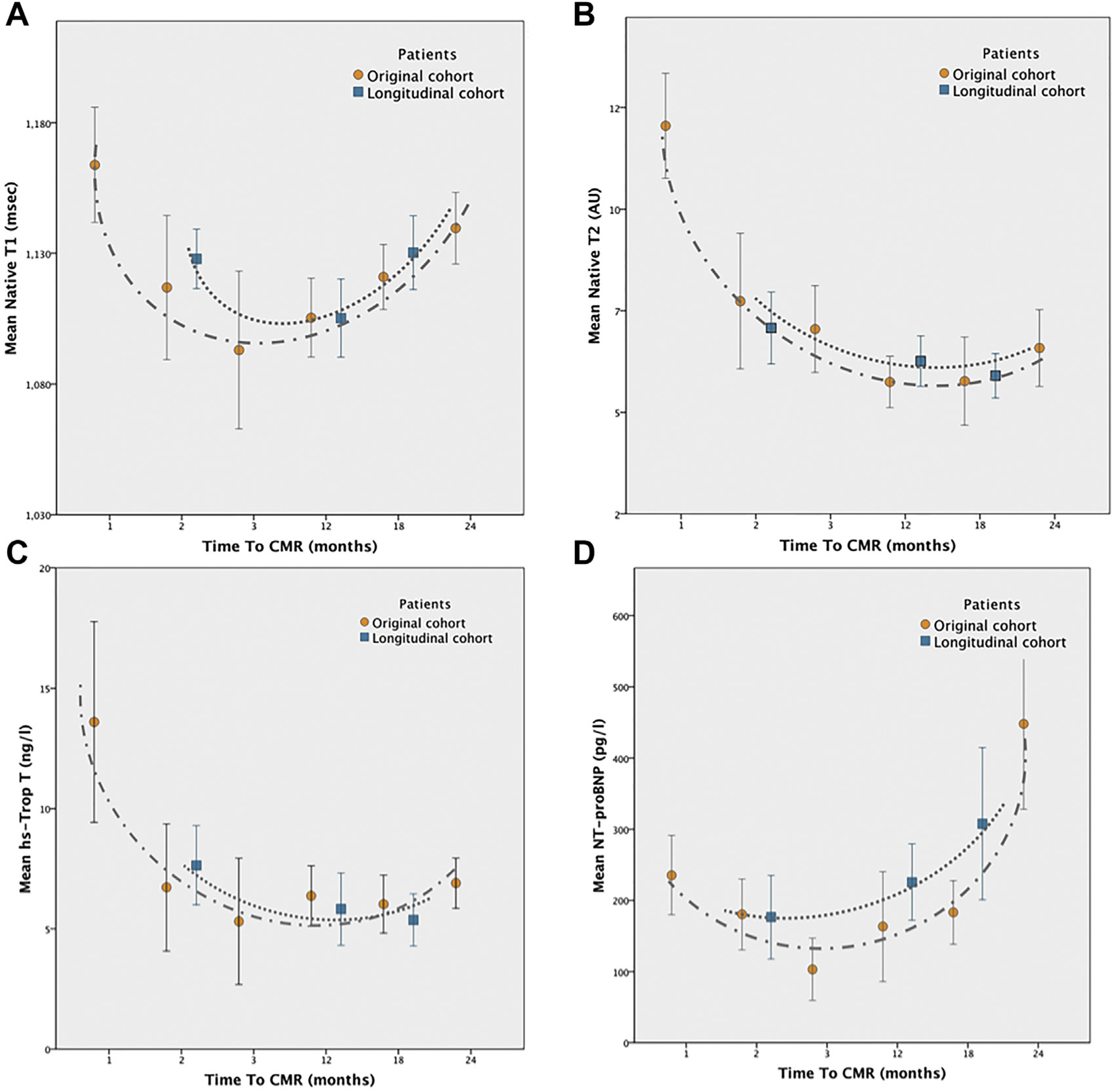
(A) Temporal changes in noncontrast tissue characteristics demonstrate acute processes with elevated native T1 (B) and T2 are then followed by a resolution in T2 values, when late increased native T1 is associated with myocardial fibrosis. Biomarker data (shown in C and D) follow the CMR imaging findings. (From Haslbauer JD, Lindner S, Valbuena-Lopez S, et al. CMR imaging biosignature of cardiac involvement due to cancer-related treatment by T1 and T2 mapping. Int J Cardiol. 2019;275:179–186; with permission.)
The results of that work produced an algorithm of phenotypical signatures for cardiac involvement after cancer treatment in which early involvement, defined as native T1 ≥ 2 SD and native T2 ≥ 2 SD, and late involvement, defined as native T1 ≥ 2 SD, and normal T2 and/or GLS ≤ 17%, led to a detection rate of 84% in their cohort. Importantly, both native T1 and T2 outperformed functional measures such as LVEF and GLS in identifying patients with cardiotoxicity (Fig. 6).
Fig. 6.
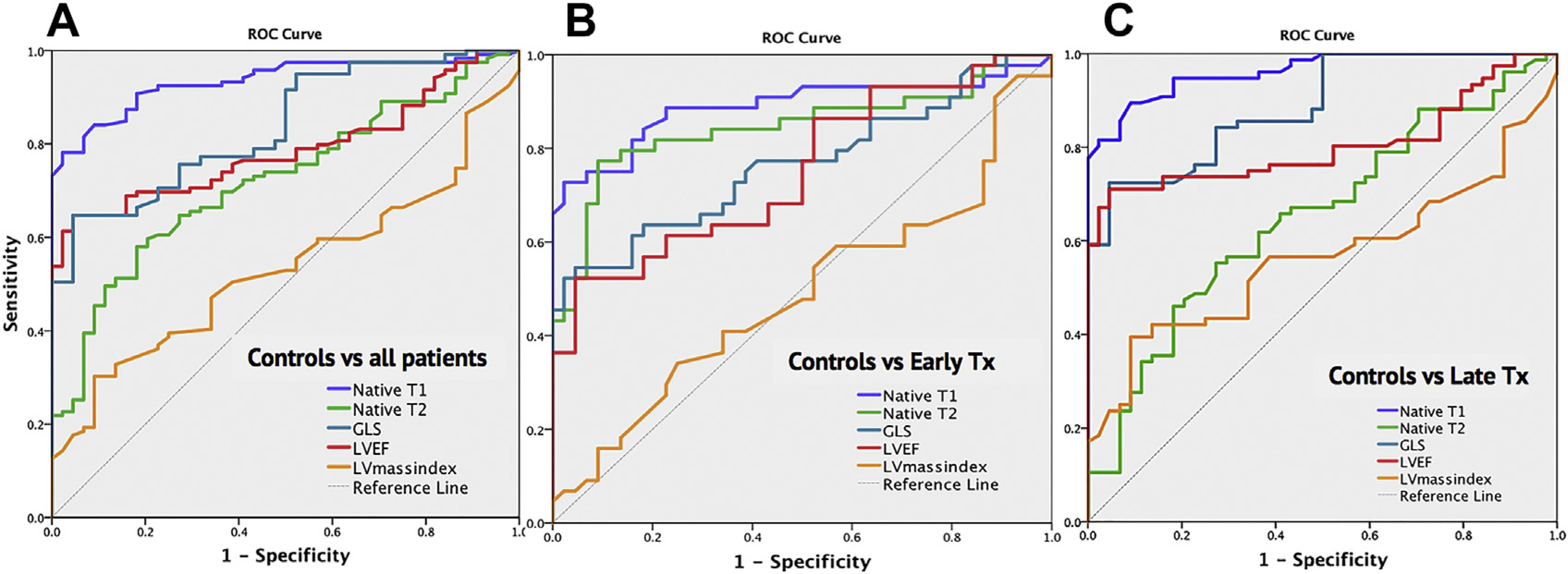
ROC curve from all patients (A) demonstrating noncontrasted CMR tissue characterization (native T1 and T2) outperform functional measures such as left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) in identifying cardiotoxicity following cancer-related therapy. Native T1 outperformed other CMR measures whether early after treatment (B) or late after treatment (C). (From Haslbauer JD, Lindner S, Valbuena-Lopez S, et al. CMR imaging biosignature of cardiac involvement due to cancer-related treatment by T1 and T2 mapping. Int J Cardiol. 2019;275:179–186; with permission.)
TISSUE CHARACTERIZATION: CONTRASTED TECHNIQUES
Myocardial tissue characterization with CMR imaging may also involve one of several contrasted techniques with an extracellular gadolinium contrast agent. Of note, contrasted tissue characterization has limited use in patients with contraindications to gadolinium contrast or with renal insufficiency, and noncontrasted techniques must be used. Qualitative contrasted imaging using T1-weighted sequences assesses the infiltration of contrast to identify areas with early and late signal enhancement, generally highlighting areas in which underlying pathologic conditions such as inflammation, edema, and fibrosis may exist.39,40 Wassmuth and colleagues39 were able to show that an increase of more than 5 times in the relative early enhancement on T1-weighted images identified future LVEF declines after the first month of an anthracycline-based regimen. Anthracyclines have also been associated with acute, diffuse late gadolinium enhancement (LGE) in several studies. In an animal model of anthracycline cardiotoxicity, increased diffuse myocardial signal intensity in LGE images was associated with future LV systolic dysfunction and evidence of vacuolization and extracellular volume (ECV) on histopathologic examination (Fig. 7).40 Similar imaging results were confirmed in a clinical study of patients receiving anthracyclines and other cardiotoxic chemotherapy regimens.41
Fig. 7.
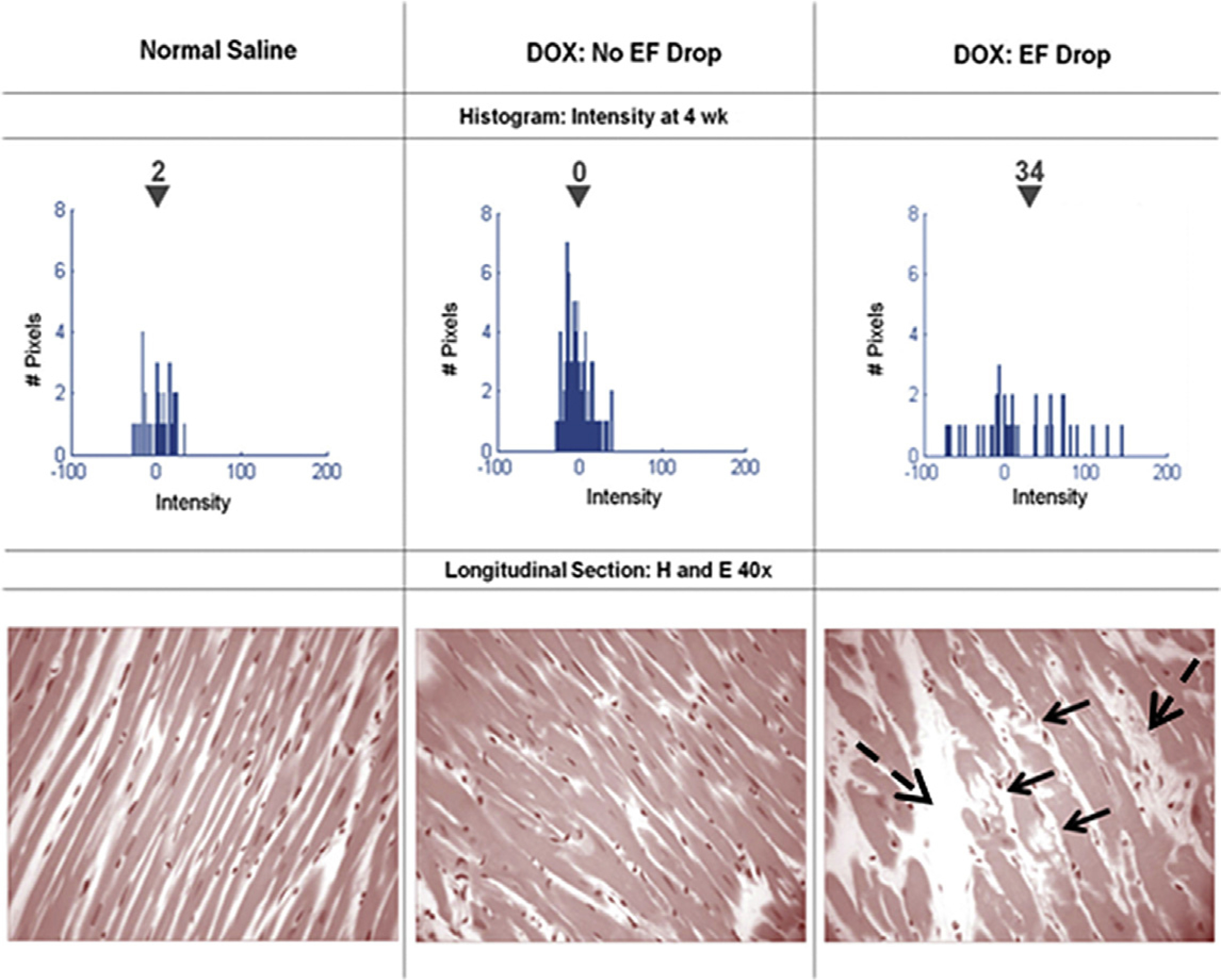
Serial histograms of myocardial LGE signal intensity (top, mean intensity shown above the inverted black triangles) and corresponding histopathology (bottom) of individual animals 4 weeks after receipt of normal saline (left), doxorubicin without an LVEF drop (middle), and doxorubicin with an LVEF drop (right). Vacuolization (arrows) and increased extracellular space (dashed arrows) were observed in animals with doxorubicin cardiotoxicity. (From Lightfoot JC, D’Agostino RB, Jr., Hamilton CA, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. 2010;3(5):550–558; with permission.)
Limited reports of focal LGE have been observed following anthracyclines (see Fig. 4)38,42 and trastuzumab therapy, predominantly in a subepicardial linear pattern.43,44 Of interest to many may be the that the increasing use of ICIs in the treatment of cancer45–47 has resulted in an increase in case reports of fulminant ICI-associated myocarditis.27,48,49 Zhang and colleagues50 recently reported initial findings from the International ICI Myocarditis Study involving a registry from 19 sites and a total of 102 ICI-associated cases of myocarditis. Only half of the myocarditis patients in the registry had LGE noted on CMR imaging, and LGE was not associated with outcomes, emphasizing that an LGE-only approach would not be sufficient in ICI-associated myocarditis patients with preserved LVEF (Fig. 8).50
Fig. 8.
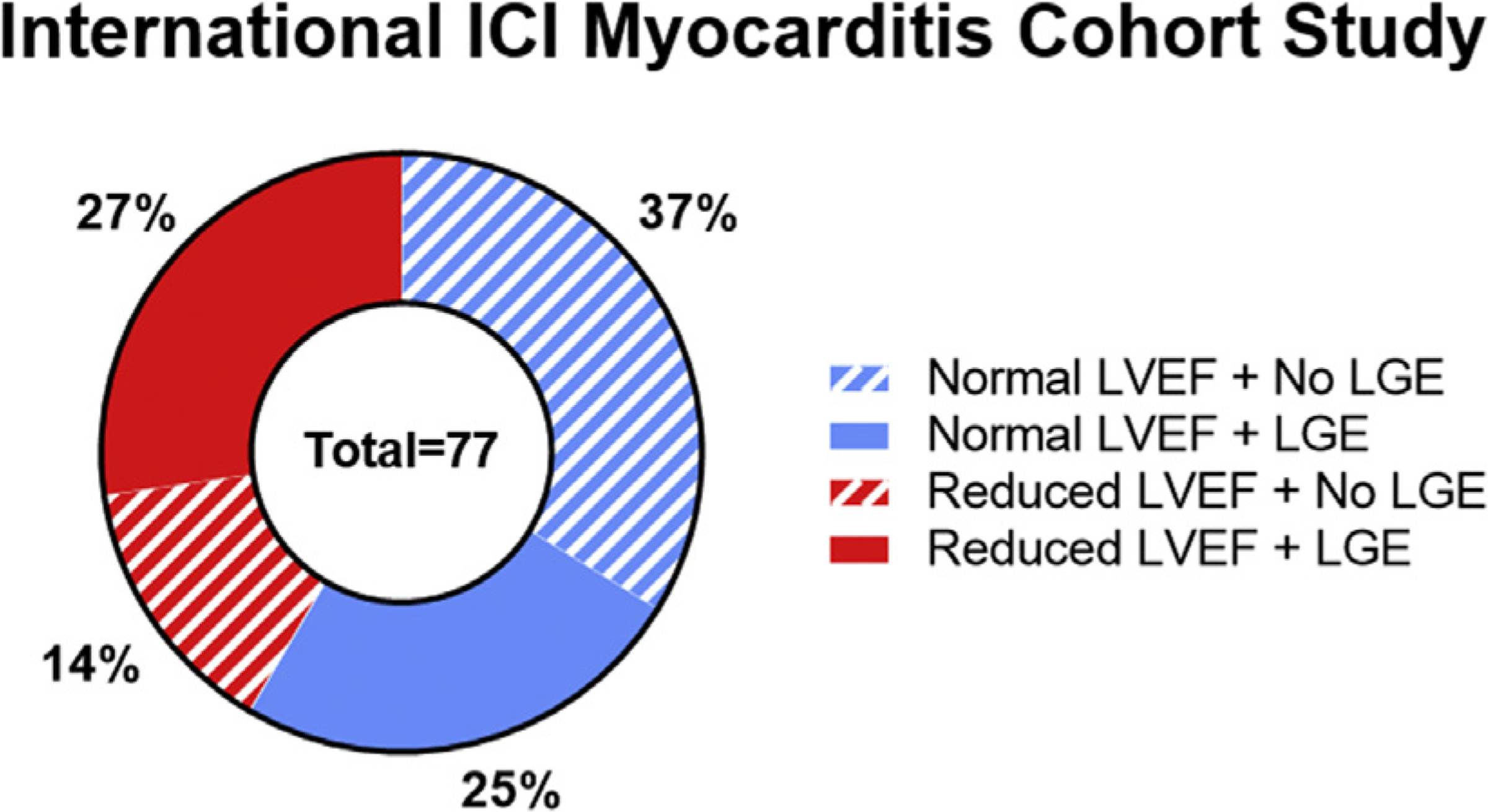
Data recently presented at the 2019 American College of Cardiology on behalf of the International ICI Myocarditis Cohort Study demonstrating that roughly only half of ICI-associated myocarditis patients have LGE and more than half have a preserved LVEF. (Data from Zhang L, Awadalla M, Mahmood SS, et al. Late gadolinium enhancement in patients with myocarditis from immune checkpoint inhibitors. J Am Coll Cardiol. 2019;73(9 Supplement 1):675.)
The quantitative contrasted characterization equivalent to qualitative LGE imaging is considered to be ECV mapping, in which a native T1 map and a postcontrast T1 map are acquired and the relaxivities of the tissue and LV blood pool are compared and adjusted to the hematocrit.33,51,52 Each voxel in an ECV map corresponds to the percentage of extracellular space in the imaged tissue. Extracellular space may increase acutely because of interstitial edema and inflammation; however, increases in ECV late after injury (or cancer treatment) are considered to be due to myocardial fibrosis.33,34,52 A major advantage of ECV is the ability to readily identify diffuse myocardial fibrosis that is not easily identified visually with LGE imaging, a claim that has been histologically validated by several groups.53–56
Interestingly, an increased myocardial ECV has been observed both in survivors diagnosed with anthracycline cardiomyopathy57 and in asymptomatic cancer survivors years after treatment with anthracycline-based chemotherapies compared with healthy individuals and untreated patients with cancer.58 Although those late changes are attributable to myocardial fibrosis, prospective measurements of acute increases in ECV have been observed after initiation of anthracyclines, which are likely more associated with edema and acute injury processes.59
SUMMARY AND FUTURE DIRECTIONS
CMR imaging is useful for identifying systolic dysfunction, particularly in patients in whom echocardiographic imaging is not acceptable because of poor acoustic windows or that the LVEF is inconclusive by other modalities and an accurate LVEF or strain measurement is needed. Of particular advantage is capability of CMR to perform tissue characterization (noncontrasted or contrasted techniques) to noninvasively identify changes in pathologic conditions related to cancer therapy or to discriminate causes of disease that may confound presentation in cardio-oncology patients (ie, regional wall abnormalities from ischemia or downregulation of contractility secondary to trastuzumab administration).
CMR imaging does not use ionizing radiation and is the gold standard for many cardiovascular measurements, making it a great serial imaging option if equipment and expertise are available. As cardio-oncology grows, more research with regard to screening and surveillance guidelines specific to CMR is needed, because most data are produced by other imaging modalities. The scope of cardio-oncology is wide and includes many types of diseases and therapies, and an everchanging landscape of emerging therapies that need to be considered when developing these guidelines. Given the advantages of CMR imaging in screening and surveillance, CMR certainly has a role to play in the future of cardio-oncology.
KEY POINTS.
Cardiovascular magnetic resonance imaging is a safe, nonionizing imaging modality to noninvasively and comprehensively assess myocardial structure, function, and tissue changes in cardio-oncology patients.
Accurate quantification of LVEF and LV strain may be performed with CMR and should be considered in cases in whom values from other modalities (echo, multigated acquisition scan) are borderline or of poor imaging quality.
Acute cardiotoxic changes in the myocardium involving edema and inflammation may be identified using T2-based tissue characterization; in the absence of T2-based changes, late cardiotoxic changes may be identified using T1-based methods such as LGE and ECV.
Noncontrasted and contrasted tissue characterization can identify underlying myopathic processes such as inflammation, edema, and fibrosis in cardio-oncology patients and may precede LV systolic dysfunction.
Box 2. Functional assessment pearls.
Assess for large changes in LVEF (>10% decline) and/or to a value <50%
Assess for relative strain changes >15%
Evaluate both LVEDV and LVESV in the context of LVEF and strain changes
Box 3. Noncontrast tissue characterization.
Increased native T1 indicates many pathologic changes, whereas increased T2 indicates an acute process with myocardial edema
Establish local normative native T1 and T2 values
Normative values are effected by field strength, version of acquisition sequence, and local field inhomogeneities
ACKNOWLEDGMENTS
The authors wish to acknowledge the following National Institutes of Health grants for their efforts: R01CA199167, R21CA226960, and R01HL118740.
Footnotes
Disclosure: The authors have nothing to disclose.
REFERENCES
- 1.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol 2012; 30(23):2876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014; 15(10):1063–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2017;35(8):893–911. [DOI] [PubMed] [Google Scholar]
- 4.Eschenhagen T, Force T, Ewer MS, et al. Cardiovascular side effects of cancer therapies: a position statement from the heart failure association of the European Society of Cardiology. Eur J Heart Fail 2011;13(1):1–10. [DOI] [PubMed] [Google Scholar]
- 5.Jordan JH, Todd RM, Vasu S, et al. Cardiovascular magnetic resonance in the oncology patient. JACC Cardiovasc Imaging 2018;11(8):1150–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panjrath GS, Jain D. Trastuzumab-induced cardiac dysfunction. Nucl Med Commun 2007;28(2): 69–73. [DOI] [PubMed] [Google Scholar]
- 7.Hall PS, Harshman LC, Srinivas S, et al. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail 2013;1(1):72–8. [DOI] [PubMed] [Google Scholar]
- 8.Melendez GC, Sukpraphrute B, D’Agostino RB Jr, et al. Frequency of left ventricular end-diastolic volume-mediated declines in ejection fraction in patients receiving potentially cardiotoxic cancer treatment. Am J Cardiol 2017;119(10):1637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drafts BC, Twomley KM, D’Agostino R Jr, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 2013;6(8):877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haslbauer JD, Lindner S, Valbuena-Lopez S, et al. CMR imaging biosignature of cardiac involvement due to cancer-related treatment by T1 and T2 mapping. Int J Cardiol 2019;275:179–86. [DOI] [PubMed] [Google Scholar]
- 11.Thavendiranathan P, Wintersperger BJ, Flamm SD, et al. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging 2013;6(6): 1080–91. [DOI] [PubMed] [Google Scholar]
- 12.Jolly MP, Jordan JH, Melendez GC, et al. Automated assessments of circumferential strain from cine CMR correlate with LVEF declines in cancer patients early after receipt of cardio-toxic chemotherapy. J Cardiovasc Magn Reson 2017;19(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano S, Takahashi M, Kimura F, et al. Cardiac magnetic resonance imaging-based myocardial strain study for evaluation of cardiotoxicity in breast cancer patients treated with trastuzumab: a pilot study to evaluate the feasibility of the method. Cardiol J 2016;23(3):270–80. [DOI] [PubMed] [Google Scholar]
- 14.Ong G, Brezden-Masley C, Dhir V, et al. Myocardial strain imaging by cardiac magnetic resonance for detection of subclinical myocardial dysfunction in breast cancer patients receiving trastuzumab and chemotherapy. Int J Cardiol 2018;261:228–33. [DOI] [PubMed] [Google Scholar]
- 15.Zhong X, Gibberman LB, Spottiswoode BS, et al. Comprehensive cardiovascular magnetic resonance of myocardial mechanics in mice using three-dimensional cine DENSE. J Cardiovasc Magn Reson 2011;13:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan JH, Sukpraphrute B, Melendez GC, et al. Early myocardial strain changes during potentially cardiotoxic chemotherapy may occur as a result of reductions in left ventricular end-diastolic volume: the need to interpret left ventricular strain with volumes. Circulation 2017;135(25):2575–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II–positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol 2011; 57(22):2263–70. [DOI] [PubMed] [Google Scholar]
- 18.Negishi T, Thavendiranathan P, Negishi K, et al. Rationale and design of the strain surveillance of chemotherapy for improving cardiovascular outcomes: the SUCCOUR trial. JACC Cardiovasc Imaging 2018; 11(8):1098–105. [DOI] [PubMed] [Google Scholar]
- 19.Childs AC, Phaneuf SL, Dirks AJ, et al. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res 2002;62(16): 4592–8. [PubMed] [Google Scholar]
- 20.An J, Li P, Li J, et al. ARC is a critical cardiomyocyte survival switch in doxorubicin cardiotoxicity. J Mol Med (Berl) 2009;87(4):401–10. [DOI] [PubMed] [Google Scholar]
- 21.Sorensen BS, Sinding J, Andersen AH, et al. Mode of action of topoisomerase II-targeting agents at a specific DNA sequence: uncoupling the DNA binding, cleavage and religation events. J Mol Biol 1992;228(3):778–86. [DOI] [PubMed] [Google Scholar]
- 22.Zhu S-G, Kukreja RC, Das A, et al. Dietary nitrate supplementation protects against doxorubicininduced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol 2011;57(21): 2181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Ma AG, Kitta K, et al. Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol Pharmacol 2003;63(2):368–77. [DOI] [PubMed] [Google Scholar]
- 24.Menna P, Salvatorelli E, Minotti G. Anthracycline degradation in cardiomyocytes: a journey to oxidative survival. Chem Res Toxicol 2010;23(1): 6–10. [DOI] [PubMed] [Google Scholar]
- 25.Ganame J, Claus P, Eyskens B, et al. Acute cardiac functional and morphological changes after anthracycline infusions in children. Am J Cardiol 2007; 99(7):974–7. [DOI] [PubMed] [Google Scholar]
- 26.Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson 2013;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neilan TG, Coelho-Filho OR, Pena-Herrera D, et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol 2012;110(11):1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Wolf D, Suys B, Maurus R, et al. Dobutamine stress echocardiography in the evaluation of late anthracycline cardiotoxicity in childhood cancer survivors. Pediatr Res 1996;39(3):504–12. [DOI] [PubMed] [Google Scholar]
- 29.Iarussi D, Galderisi M, Ratti G, et al. Left ventricular systolic and diastolic function after anthracycline chemotherapy in childhood. Clin Cardiol 2001; 24(10):663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan JH, Castellino SM, Melendez GC, et al. Left ventricular mass change after anthracycline chemotherapy. Circ Heart Fail 2018;11(7):e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Souza TF, Silva TQA, Costa FO, et al. Anthracycline therapy is associated with cardiomyocyte atrophy and preclinical manifestations of heart disease. JACC Cardiovasc Imaging 2018;11(8): 1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willis MS, Parry TL, Brown DI, et al. Doxorubicin exposure causes subacute cardiac atrophy dependent on the striated muscle-specific ubiquitin ligase MuRF1. Circ Heart Fail 2019;12(3):e005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72(24):3158–76. [DOI] [PubMed] [Google Scholar]
- 35.Thompson RC, Canby RC, Lojeski EW, et al. Adriamycin cardiotoxicity and proton nuclear-magneticresonance relaxation properties. Am Heart J 1987; 113(6):1444–9. [DOI] [PubMed] [Google Scholar]
- 36.Cottin Y, Ribuot C, Maupoil V, et al. Early incidence of adriamycin treatment on cardiac parameters in the rat. Can J Physiol Pharmacol 1994;72(2):140–5. [DOI] [PubMed] [Google Scholar]
- 37.Thavendiranathan P, Amir E, Bedard P, et al. Regional myocardial edema detected by T2 mapping is a feature of cardiotoxicity in breast cancer patients receiving sequential therapy with anthracyclines and trastuzumab. J Cardiovasc Magn Reson 2014;16(Suppl 1):P273. [Google Scholar]
- 38.Lustberg MB, Reinbolt R, Addison D, et al. Early detection of anthracycline-induced cardiotoxicity in breast cancer survivors with T2 cardiac magnetic resonance. Circ Cardiovasc Imaging 2019;12(5): e008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassmuth R, Lentzsch S, Erdbruegger U, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging - a pilot study. Am Heart J 2001;141(6):1007–13. [DOI] [PubMed] [Google Scholar]
- 40.Lightfoot JC, D’Agostino RB Jr, Hamilton CA, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging 2010;3(5): 550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan JH, D’Agostino RB Jr, Hamilton CA, et al. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2014; 7(6):872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lunning MA, Kutty S, Rome ET, et al. Cardiac magnetic resonance imaging for the assessment of the myocardium after doxorubicin-based chemotherapy. Am J Clin Oncol 2013;21(12):1283–9. [DOI] [PubMed] [Google Scholar]
- 43.Fallah-Rad N, Lytwyn M, Fang T, et al. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson 2008;10(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawley C, Wainwright C, Segelov E, et al. Pilot study evaluating the role of cardiac magnetic resonance imaging in monitoring adjuvant trastuzumab therapy for breast cancer. Asia Pac J Clin Oncol 2012;8(1): 95–100. [DOI] [PubMed] [Google Scholar]
- 45.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12(4): 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27(4):450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161(2):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moslehi JJ, Salem J-E, Sosman JA, et al. Increased reporting of fatal immune checkpoint inhibitorassociated myocarditis. Lancet 2018;391(10124): 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71(16): 1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Awadalla M, Mahmood SS, et al. Late gadolinium enhancement in patients with myocarditis from immune checkpoint inhibitors. J Am Coll Cardiol 2019;73(9 Supplement 1):675. [Google Scholar]
- 51.Ugander M, Oki AJ, Kellman P, et al. Myocardial extracellular volume imaging allows quantitative assessment of atypical late gadolinium enhancement. J Cardiovasc Magn Reson 2010;12(1):100. [Google Scholar]
- 52.Ugander M, Oki AJ, L H, et al. Myocardial extracellular volume imaging by CMR quantitatively characterizes myocardial infarction and subclinical myocardial fibrosis. J Cardiovasc Magn Reson 2011;13(1):148. [Google Scholar]
- 53.Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation 2010; 122(2):138–44. [DOI] [PubMed] [Google Scholar]
- 54.Bandula S, White SK, Flett AS, et al. Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: validation against histologic findings. Radiology 2013;269(2): 396–403. [DOI] [PubMed] [Google Scholar]
- 55.Fontana M, White SK, Banypersad SM, et al. Comparison of T1 mapping techniques for ECV quantification. Histological validation and reproducibility of ShMOLLI versus multibreath-hold T1 quantification equilibrium contrast CMR. J Cardiovasc Magn Reson 2012;14(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broberg CS, Chugh SS, Conklin C, et al. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging 2010; 3(6):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neilan TG, Coelho-Filho OR, Shah RV, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol 2013;111(5):717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jordan JH, Vasu S, Morgan TM, et al. Anthracyclineassociated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging 2016;9(8) [pii:e004325]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melendez GC, Jordan JH, D’Agostino RB Jr, et al. Progressive 3-month increase in LV myocardial ECV after anthracycline-based chemotherapy. JACC Cardiovasc Imaging 2017;10(6): 708–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


