Abstract
Background
Prevention of respiratory syncytial virus (RSV) disease in infants is an unmet vaccine need, and maternal immunization is a potential strategy to address this need. This study evaluated concomitant administration of RSV stabilized prefusion F subunit vaccine (RSVpreF) and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed (Tdap) in healthy, nonpregnant women 18‒49 years of age.
Methods
In this phase 2b, multicenter, placebo-controlled, observer-blind, noninferiority study, participants were randomized to receive RSVpreF in a range of doses and formulations with Tdap or alone, or Tdap alone. Safety and immunogenicity were assessed.
Results
Local reactions and systemic events were generally similar across vaccine groups. Noninferiority of anti-RSV-A and anti-RSV-B immune responses induced by RSVpreF with Tdap was demonstrated compared to RSVpreF alone. Noninferiority of anti-diphtheria toxoid and anti-tetanus toxoid immune responses after administration of RSVpreF with Tdap was demonstrated compared to Tdap alone; noninferiority was not met for anti-pertussis component responses.
Conclusions
RSVpreF was safe and well tolerated when administered with Tdap or alone in nonpregnant women 18‒49 years of age. Immune responses induced by Tdap administered with RSVpreF were noninferior for the tetanus and diphtheria components of Tdap, but not for pertussis.
Clinical Trials Registration
Keywords: respiratory syncytial virus, RSV vaccine, Tdap vaccine, safety, immunogenicity, maternal immunization
Respiratory syncytial virus stabilized prefusion F subunit vaccine (RSVpreF) was safe and well tolerated when administered with Tdap or alone in nonpregnant women. Tdap-induced immune responses with concomitant RSVpreF were noninferior for tetanus and diphtheria, but not the pertussis component.
Human respiratory syncytial virus (RSV) is a leading cause of acute lower respiratory tract disease in infants and children, causing considerable morbidity and mortality [1–5]. There is no specific treatment for RSV and no vaccine to protect against RSV disease. Prophylactic antibody use is currently limited to high-risk populations [6, 7].
Maternal immunization is a promising strategy to reduce the risk of RSV disease in very young infants, providing neonatal protection via transplacental transfer of maternally derived circulating antibodies [8–10]. The bivalent RSV stabilized prefusion F subunit vaccine (RSVpreF), which covers RSV subgroups A and B, is being developed to prevent medically attended RSV-associated lower respiratory tract illness in infants by active immunization of pregnant women in the late second or third trimester. If demonstrated to be safe and effective, this could be the first vaccine licensed specifically for use in pregnancy [11]. In the first-in-human study of RSVpreF with or without aluminum hydroxide (Al[OH]3), all formulations and dose levels were safe, well tolerated, and highly immunogenic in adults 18‒49 years of age [12].
Pertussis can cause significant morbidity and mortality in young infants before they are fully vaccinated [13, 14]. Because of this and the failure of measures such as cocooning to adequately protect newborns, the tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) is recommended during each pregnancy as standard of care in the United States (US) and several other high-income countries around the world, preferably in the second or third trimester to maximize passive antibody transfer [13–20]. In lower-income countries, tetanus toxoid–containing vaccine or tetanus and diphtheria toxoid–containing vaccine is typically prioritized for pregnant women [21, 22]. This variation in immunization recommendations during pregnancy supports alternative concomitant use, tetanus, diphtheria, and pertussis vaccine coadministration strategies with a potential maternal RSV vaccine based on country immunization activities and schedules.
If RSVpreF is licensed for maternal immunization, it is likely that pregnant women will receive both the Tdap and RSVpreF vaccines during the late second or third trimester; therefore, it is important to assess the safety of concomitant administration and the potential for immune interference. Influenza vaccine is also recommended during any trimester for women who are pregnant or are planning to become pregnant during influenza season but can be given at any time [23, 24]. The RSVpreF maternal immunization program includes a phase 2b study that is evaluating the safety, tolerability, and immunogenicity of RSVpreF at 2 dose levels formulated with or without Al(OH)3 in pregnant women and the safety and transplacental transfer of immunity to their infants (ClinicalTrials.gov identifier NCT04032093). A pivotal phase 3 study is evaluating efficacy, safety, and RSV serum neutralizing titers in infants born to mothers vaccinated with RSVpreF during pregnancy and safety and immunogenicity in the mothers (NCT04424316).
This phase 2b study evaluated the safety and immunogenicity of concomitant administration of RSVpreF and US-licensed Tdap in healthy, nonpregnant women 18‒49 years of age. The study was conducted in nonpregnant women to prepare for concomitantly immunizing pregnant women with vaccine regimens that minimize disruption to the prenatal care schedule and maximize RSV neutralizing titers in newborns.
METHODS
Study Design
This phase 2b randomized, multicenter, placebo-controlled, observer-blind, noninferiority study (NCT04071158) was conducted at 16 sites in the US from October through December, 2019. A total of 713 participants were randomized through an interactive response technology system in a 1:1:1:1:1 ratio to receive 1 of 5 schedules: 120 μg RSV vaccine antigen (60 μg A and 60 μg B) with sterile water for injection (sWFI) and Tdap; 120 μg RSV vaccine antigen with sWFI and placebo; 240 μg RSV vaccine antigen (120 μg A and 120 μg B) with Al(OH)3 and Tdap; 240 μg RSV vaccine antigen with Al(OH)3 and placebo; or placebo and Tdap. Administering the lower dose level of RSVpreF without Al(OH)3 increased the likelihood of observing interference of Tdap with RSVpreF; administering the higher dose level of RSVpreF with Al(OH)3 increased the likelihood of observing interference of RSVpreF with Tdap. RSVpreF was injected in the left deltoid muscle, Tdap in the right, and placebo on the opposite side to treatment. Blood was collected for serology before vaccination and approximately 1 month (28–35 days) after, and safety was assessed through 1 month after vaccination.
The participant, investigator, study coordinator, site staff, sponsor study team, and laboratory personnel were blinded. Study site dispensers and administrators were unblinded because the physical appearance of vaccines differed. The protocol, amendments, and informed consent form were reviewed and approved for all sites by a central institutional review board (Advarra, Columbia, Maryland). This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Council for Harmonisation Good Clinical Practice Guidelines. All local regulatory requirements were followed. All participants provided written informed consent before any study-specific activities were performed.
Study Participants
Participants were healthy, nonpregnant women 18‒49 years of age regardless of childbearing potential. Key exclusion criteria included vaccination within 5 years with diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed or tetanus and diphtheria toxoids adsorbed vaccine before investigational product administration.
Investigational Product
Each RSVpreF formulation was provided as a lyophilized vial containing a mixture of equal amounts of 2 stabilized prefusion F antigens from RSV subgroups A and B at 2 dose levels (120 μg and 240 μg). Before administration, the RSVpreF lyophilized cake was reconstituted with diluent; the 120-μg dose was reconstituted with sWFI, and the 240-μg dose was reconstituted with sterile Al(OH)3 suspension (0.4mg/mL). The placebo was sterile normal saline (0.9% NaCl). US-licensed Tdap (Boostrix; GlaxoSmithKline, Brentford, United Kingdom) contained 5 limits of flocculation (Lf) of tetanus toxoid (TTd), 2.5 Lf of diphtheria toxoid (DTd), and acellular pertussis antigens (8 μg of inactivated pertussis toxin [PT], 8 μg of filamentous hemagglutinin [FHA], and 2.5 μg of pertactin [PRN]) per dose. Participants received 2 intramuscular injections of a 0.5-mL dose in accordance with the randomization schedule.
Safety Assessments
The primary safety objective was to evaluate safety and tolerability of RSVpreF administered concomitantly with Tdap and RSVpreF administered alone. Safety endpoints included local reactions (redness, swelling, and pain at the injection site) at the left arm injection site (RSVpreF in the RSVpreF groups or placebo in the placebo/Tdap group) and systemic events (fatigue, headache, vomiting, nausea, diarrhea, muscle pain, joint pain, and fever) collected for 7 days following vaccination and recorded in an electronic diary. Local reactions and systemic events were categorized during analysis as mild, moderate, or severe based on a predefined grading scale following the US Food and Drug Administration Center for Biologics Evaluation and Research guidance [25]. Participant-reported local reactions and systemic events could only be classified as grade 4 after evaluation by the site investigator. Adverse events (AEs) within 1 month after vaccination and medically attended AEs and serious AEs (SAEs) throughout the study were recorded. Safety analyses used the safety population, which included all randomized participants who received the investigational product.
Immunogenicity Assessments
A primary immunogenicity objective was to demonstrate that immune responses induced by Tdap when administered concomitantly with RSVpreF (RSVpreF/Tdap) were noninferior to those induced by Tdap alone (placebo/Tdap). Endpoints included anti-TTd, anti-DTd, and anti-pertussis component antibodies (anti-PT, anti-FHA, and anti-PRN) measured 1 month after vaccination. The criteria for noninferiority were a geometric mean titer ratio (GMR) (coadministration/Tdap alone) >0.67 for pertussis components and a response difference (coadministration – Tdap alone) of > –10% for diphtheria and tetanus components. A co-primary immunogenicity objective was to demonstrate that the RSV-A– and RSV-B– neutralizing titers elicited 1 month after immunization with RSVpreF administered concomitantly with Tdap (RSVpreF/Tdap) were noninferior to the neutralizing titers elicited 1 month after immunization with RSVpreF alone (RSVpreF/placebo). The criterion for noninferiority was a GMR (coadministration/RSVpreF alone) >0.5. Achieving the primary objective of noninferiority required meeting statistical criteria for all Tdap antibody endpoints and the RSV-A– and RSV-B–neutralizing titer endpoints. Noninferiority margins were chosen based on precedents used for licensure of Tdap and other vaccines. The secondary immunogenicity objective was to assess noninferiority of RSV-A and RSV-B neutralizing titers at the more stringent margin of 0.67.
Immunogenicity analyses used the evaluable immunogenicity population, which included all participants who were eligible, received all doses of investigational products to which they were randomized, had blood drawn for assay testing 1 month after vaccination, ≥1 valid and determinate assay result at the 1 month postvaccination visit, and no major protocol deviations.
Pfizer Inc (New York, New York) participated in the interlaboratory studies that assessed the first World Health Organization (WHO) International Standard for Antiserum to RSV (16/284; National Institute for Biological Standards and Control, Hertfordshire, United Kingdom) in our neutralization assays for RSV-A [26] and RSV-B [27]. We report a neutralization geometric mean titer for the first WHO International Standard of 5243 for RSV-A and 5619 for RSV-B. To approximate WHO International Units (IU)/mL, Pfizer neutralization titers may be multiplied by the conversion factor of 0.379 for RSV-A and 0.356 for RSV-B. Additional details for the detection of RSV-A, RSV-B, and Tdap antigens are provided in the Supplementary Methods.
Statistical Analyses
The study sample size was based on the evaluation of noninferiority of RSV vaccine, Tdap coadministration to Tdap alone or to RSV vaccine alone on all co-primary immunogenicity endpoints (Supplementary Table 1); the overall power to demonstrate noninferiority on all co-primary immunogenicity endpoints was 92.1%. Safety endpoints were evaluated as the percentage of participants reporting each event for each vaccine group; exact 2-sided 95% confidence intervals (CIs) for percentages were calculated using the Clopper–Pearson method. The primary objective required success for all co-primary endpoints; therefore, no adjustment for multiplicity was necessary.
For immunogenicity endpoints, 95% CIs for the differences in the percentages of participants with anti-TTd and anti-DTd concentrations ≥0.1 IU/mL were calculated using the Miettinen and Nurminen method. GMRs for anti-PT, anti-FHA, and anti-PRN antibody concentrations and RSV-A– and RSV-B–neutralizing titers, with 2-sided 95% CIs, were obtained by calculating CIs using Student t distribution for the mean difference of logarithmically transformed assay results and transforming confidence limits back to original units. Noninferiority was declared when the lower bound of the 95% CI exceeded the noninferiority margin for a given component.
RESULTS
Study Participants
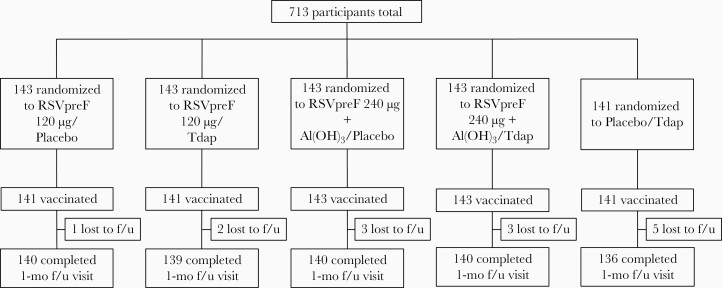
Of 713 randomized female participants aged 18‒49 years, 709 were vaccinated across all groups (Figure 1). Overall, 695 participants completed the visit 1 month after vaccination, and 14 withdrew before the visit; all withdrawals were lost to follow-up. Demographic characteristics were similar across vaccine groups; 70.9% were White, 21.0% Black or African American, 13.7% Hispanic or Latino, and 5.8% Asian. Mean age was 35.6 (standard deviation, 8.9) years (Table 1).
Figure 1.
Participant disposition. Abbreviations: Al(OH)3,aluminum hydroxide; f/u,follow-up; RSVpreF,respiratory syncytial virus stabilized prefusion F subunit vaccine; Tdap,tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed.
Table 1.
Participant Demographics of the Safety Population
| Characteristic | Vaccine Group, as Administered | ||||
|---|---|---|---|---|---|
| RSVpreF 120 μg/Placebo (na=141) | RSVpreF 120 μg/Tdap (na=141) | RSVpreF 240 μg + Al(OH)3/Placebo (na=142) | RSVpreF 240 μg + Al(OH)3/Tdap (na=144) | Placebo/Tdap (na=141) | |
| Sex, nb (%) | |||||
| Female | 141 (100.0) | 141 (100.0) | 142 (100.0) | 144 (100.0) | 141 (100.0) |
| Race, nb (%) | |||||
| White | 100 (70.9) | 98 (69.5) | 101 (71.1) | 107 (74.3) | 97 (68.8) |
| Black or African American | 29 (20.6) | 28 (19.9) | 25 (17.6) | 29 (20.1) | 38 (27.0) |
| Asian | 12 (8.5) | 9 (6.4) | 10 (7.0) | 5 (3.5) | 5 (3.5) |
| American Indian or Alaska Native | 0 | 2 (1.4) | 1 (0.7) | 0 | 1 (0.7) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (0.7) | 2 (1.4) | 1 (0.7) | 0 |
| Multiracial | 0 | 2 (1.4) | 3 (2.1) | 1 (0.7) | 0 |
| Not reported | 0 | 1 (0.7) | 0 | 1 (0.7) | 0 |
| Ethnicity, nb (%) | |||||
| Hispanic or Latino | 23 (16.3) | 16 (11.3) | 23 (16.2) | 19 (13.2) | 16 (11.3) |
| Not Hispanic or Latino | 116 (82.3) | 125 (88.7) | 119 (83.8) | 124 (86.1) | 125 (88.7) |
| Not reported | 2 (1.4) | 0 | 0 | 1 (0.7) | 0 |
| Age at vaccination, y | |||||
| Mean (SD) | 35.6 (9.2) | 35.7 (8.7) | 36.0 (8.3) | 36.1 (9.1) | 34.4 (9.2) |
| Median | 39.0 | 38.0 | 37.0 | 38.0 | 35.0 |
| Min, max | 19, 49 | 18, 49 | 18, 49 | 18, 49 | 18, 49 |
Abbreviations: Al(OH)3,aluminum hydroxide; RSVpreF,respiratory syncytial virus stabilized prefusion F subunit vaccine; SD, standard deviation; Tdap,tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed.
No. of participants in the specified vaccine group. These values were used as the denominators for the percentage calculations.
No. of participants in the specified category.
Safety
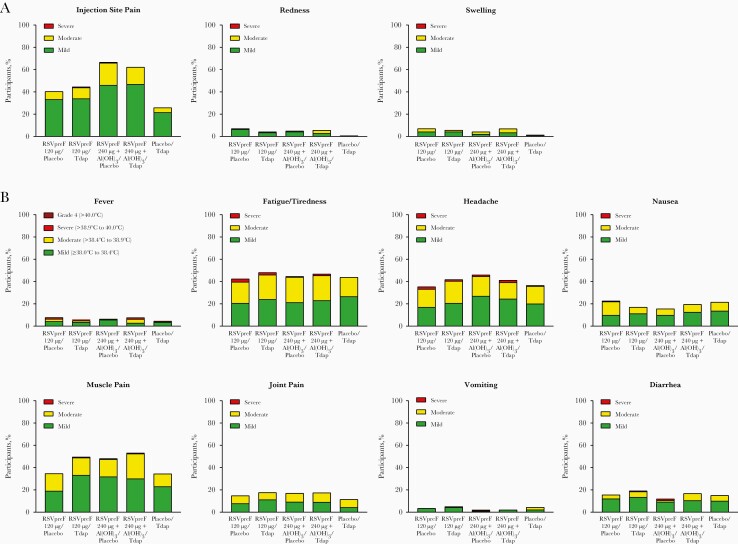
The observed incidence of reported local reactions in the left arm (RSVpreF in the RSVpreF groups or placebo in the placebo/Tdap group) and systemic events was generally similar across vaccine groups with the exception of injection site pain, which was more common in groups that received 240 μg antigen with Al(OH)3 (Figure 2). Two participants reported severe pain at the injection site (1 participant each in the RSVpreF 120 μg/Tdap and RSVpreF 240 μg + Al[OH]3/placebo groups). Three participants reported a fever of grade ≥4 (2 in the RSVpreF 120 μg/Tdap group and 1 in the placebo/Tdap group). Most other local reactions and systemic events were mild or moderate in severity. Median duration of local reactions after vaccination was similar across vaccine groups and ranged from 1.0 to 2.0 days for injection site pain and redness and 1.5 to 2.5 days for swelling. Median duration of systemic events was also similar across vaccine groups and ranged from 1.0 to 2.0 days for nausea, vomiting, diarrhea, headache, muscle pain, and joint pain and 2.0 to 3.0 days for fatigue/tiredness. Median fever duration was 1.0 day.
Figure 2.
Percentages of participants reporting local reactions (A) or systemic events (B) by severity within 7 days after vaccination. Number of participants, 141–143 per vaccine group. Abbreviations: Al(OH)3,aluminum hydroxide; RSVpreF,respiratory syncytial virus stabilized prefusion F subunit vaccine; Tdap,tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed.
The frequency of reported AEs within 1 month after vaccination was highest in the RSVpreF 240 μg + Al(OH)3/Tdap and placebo/Tdap groups (Table 2). Of 6 AEs considered related to vaccine, 2 were severe and included constipation in the RSVpreF 120 μg/placebo group and lymphadenopathy in the RSVpreF 120 μg/Tdap group. None of 7 reported medically attended AEs were considered serious, immediate, or related to vaccine. There were no serious, immediate, or life-threatening AEs reported within 1 month of vaccination and no AEs led to withdrawal; no deaths occurred during the study. One SAE of spontaneous abortion was reported in the RSVpreF 240 μg + Al(OH)3/placebo group after visit 2 and was considered unrelated to the vaccine.
Table 2.
Percentages of Participants Reporting Adverse Events Within 1 Month After Vaccination in the Safety Population
| Vaccine Group, as Administered (na) | Adverse Events, nb (%) [95% CIc] | |||
|---|---|---|---|---|
| Any | Severe | Related | Medically Attended | |
| RSVpreF 120 μg/Placebo (141) | 8 (5.7) [2.5–10.9] | 2 (1.4) [0.2–5.0] | 1 (0.7) [0.0–3.9] | 2 (1.4) [0.2–5.0] |
| RSVpreF 120 μg/Tdap (141) | 11 (7.8) [4.0–13.5] | 1 (0.7) [0.0–3.9] | 1 (0.7) [0.0–3.9] | 0 [0.0–2.6] |
| RSVpreF 240 μg + Al(OH)3/Placebo (142) | 8 (5.6) [2.5–10.8] | 1 (0.7) [0.0–3.9] | 1 (0.7) [0.0–3.9] | 0 [0.0–2.6] |
| RSVpreF 240 μg + Al(OH)3/Tdap (144) | 15 (10.4) [5.9–16.6] | 0 [0.0–2.5] | 3 (2.1) [0.4–6.0] | 2 (1.4) [0.2–4.9] |
| Placebo/Tdap (141) | 13 (9.2) [5.0–15.3] | 0 [0.0–2.6] | 0 [0.0–2.6] | 3 (2.1) [0.4–6.1] |
Abbreviations: Al(OH)3,aluminum hydroxide; CI, confidence interval; RSVpreF,respiratory syncytial virus stabilized prefusion F subunit vaccine; Tdap,tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed.
No. of participants in the vaccine group. These values were used as the denominators for the percentage calculations.
No. of participants reporting ≥1 event of the type specified.
Exact 2-sided CI calculated using the Clopper–Pearson method.
Immunogenicity
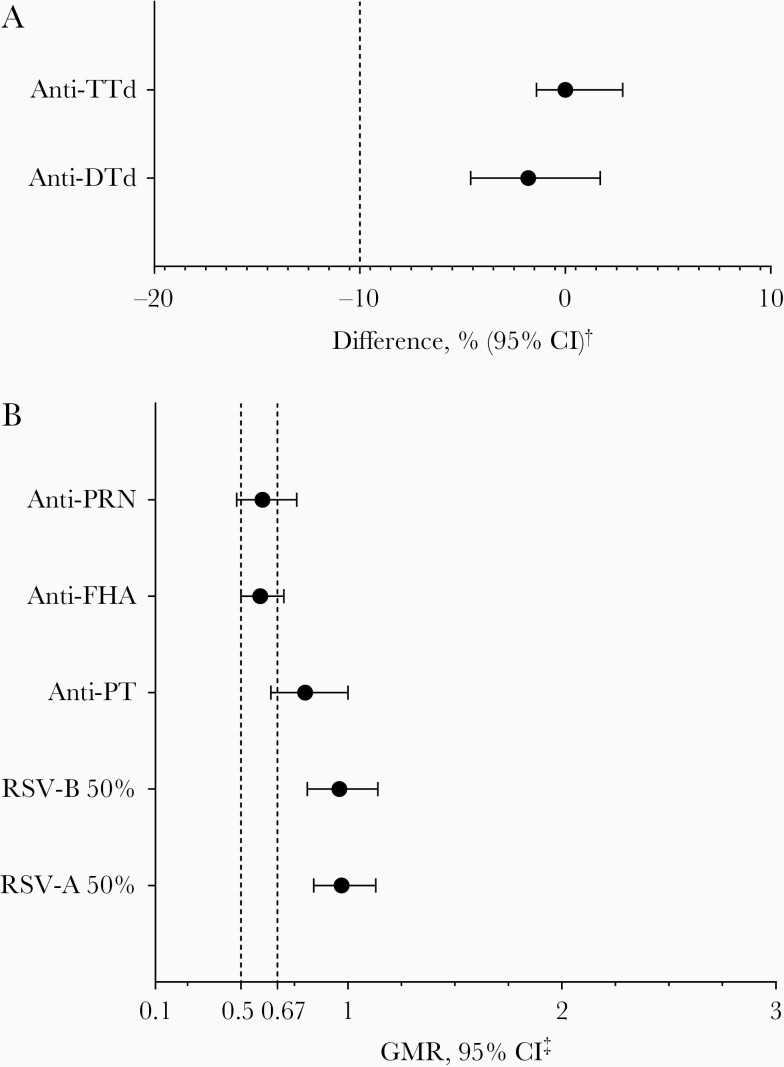
At 1 month after vaccination, the difference in the percentage of participants with anti-DTd antibody concentrations ≥0.1 IU/mL between the combined RSVpreF/Tdap groups and placebo/Tdap group was –1.8% (95% CI, −4.6 to 1.7). At 1 month after vaccination, all participants in the combined RSVpreF/Tdap groups and placebo/Tdap group achieved anti-TTd antibody concentrations ≥0.1 IU/mL, resulting in a difference of 0.0% (95% CI, −1.4 to 2.8). Thus, compared with the criterion of –10%, noninferiority was established for these components (Figure 3).
Figure 3.
Noninferiority of respiratory syncytial virus stabilized prefusion F subunit vaccine (RSVpreF) and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed (Tdap) coadministration (combination of RSVpreF 120 μg/Tdap and RSVpreF 240 μg + Al(OH)3/Tdap groups) compared with Tdap alone for anti-tetanus toxoid (TTd) and anti-diphtheria toxoid (DTd) antibodies (A) and anti-pertussis components (B). †Difference in the percentage of participants achieving anti-TTd or anti-DTd antibody concentrations ≥0.1 IU/mL between the combined RSVpreF/Tdap groups and placebo/Tdap group (RSVpreF/Tdap – placebo/Tdap). ‡Geometric mean ratios (GMRs) were calculated as the group mean differences of logarithmically transformed antibody levels and back-transformed to the original units. Anti-pertussis component antibody GMRs were calculated using combined RSVpreF/Tdap GMCs as numerators and placebo/Tdap GMCs as denominators, and RSV neutralizing titer GMRs were calculated using combined RSVpreF/Tdap geometric mean titers (GMTs) as numerators and combined RSVpreF/placebo GMTs as denominators. Noninferiority for anti-pertussis toxin, anti-pertactin, and anti-filamentous hemagglutinin required the lower 95% confidence limit to be >0.67. Noninferiority for RSV-A– and RSV-B– neutralizing titers required the lower 95% confidence limit to be >0.5 for the primary objective and >0.67 for the secondary objective. Abbreviations: CI, confidence interval; DTd,diphtheria toxoid; FHA,filamentous hemagglutinin; GMR,geometric mean ratio; PRN,pertactin; PT,pertussis toxin; TTd,tetanus toxoid; RSV,respiratory syncytial virus.
At 1 month after vaccination, the ratios of anti-PT, anti-FHA, and anti-PRN antibody geometric mean concentrations (GMCs) for the combined RSVpreF/Tdap groups relative to the corresponding GMCs for the placebo/Tdap group were 0.80 (95% CI, 0.64–1.00), 0.59 (95% CI, 0.50–0.70), and 0.60 (95% CI, 0.48–0.76), respectively. Thus, compared with the criterion of 0.67, noninferiority was not established for these components (Figure 3; Supplementary Table 2).
At 1 month after vaccination, the GMRs of RSV-A and RSV-B 50% neutralizing titers for the combined RSVpreF/Tdap groups divided by the corresponding neutralizing titers for the combined RSVpreF/placebo groups were 0.97 (95% CI, 0.84–1.13) and 0.96 (95% CI, 0.81–1.08), respectively. Thus, compared with both the criterion of 0.5 for the primary objective and 0.67 for the secondary objective, noninferiority was established for these components (Figure 3; Supplementary Table 3).
The main immunogenicity analyses predefined combining the RSVpreF vaccine groups; immunogenicity results from exploratory analyses of each individual RSVpreF dose and formulation (data not shown) were consistent with combined results. Post hoc reverse cumulative distribution curves (RCDCs) showed an effect of coadministration with RSVpreF on responses to all Tdap components (Supplementary Figures 1 and 2). RCDCs for RSV-A– and RSV-B–neutralizing titers are also provided (Supplementary Figure 3).
DISCUSSION
The safety and immunogenicity of RSVpreF and Tdap administered concomitantly can inform the design of potential future maternal immunization schedules that include a dose of RSVpreF to prevent infant RSV disease. Both formulations of RSVpreF were safe and well tolerated when administered alone or concomitantly with Tdap. Most reported reactogenicity events were mild or moderate in intensity, and there were no SAEs, immediate AEs, or life-threatening AEs reported within 1 month of vaccination. The study demonstrated noninferiority of antibody responses to the tetanus and diphtheria components of Tdap when administered concomitantly with RSVpreF compared with antibody responses elicited by Tdap alone, as well as noninferiority of RSV-A– and RSV-B–neutralizing responses elicited by RSVpreF administered concomitantly with Tdap compared with neutralizing responses elicited by RSVpreF alone (Supplementary Figure 3). In all vaccine groups, there was a substantial increase in antibody responses against pertussis antigen after vaccination; however, responses to the pertussis components of Tdap did not meet the predefined noninferiority threshold for concomitant administration of Tdap with RSVpreF compared with administration of Tdap alone. This effect of concomitant RSVpreF immunization on pertussis responses in the combined group was also observed in exploratory analyses of each RSVpreF formulation separately (data not shown). Anti-DTd and anti-TTd titers were reduced in the RSVpreF coadministration groups compared with Tdap alone 1 month after vaccination (Supplementary Figure 1); however, noninferiority was met for these components with defined correlates of protection. A similar effect was seen with the pertussis antigens (Supplementary Figure 2); however, noninferiority criteria were not met. Because mean age was slightly lower in the Tdap alone group compared with the RSVpreF and Tdap coadministration groups, and differences in baseline titers of antibodies against pertussis antigens could affect GMRs, additional analyses were performed to adjust GMRs for age and baseline titers or age only. GMRs were not different for pertussis components when adjusted for age and baseline titers or age only. Additionally, noninferiority criteria would have been met for some components in the unadjusted and adjusted analyses if a 2-fold margin were used.
The reasons for the reduced pertussis component antibody responses observed in this study are not known, and the clinical significance is unclear. There are no established serologic correlates of protection for pertussis, and the protective levels of antibodies in pregnant women are unknown [14, 28]. Pertussis antigen–containing vaccines, such as Boostrix or Adacel (Sanofi Pasteur Inc, Bridgewater, New Jersey), often fail to meet noninferiority for ≥1 pertussis antigen when coadministered with other vaccines, including influenza, meningococcal, and human papillomavirus vaccines [29–34]. Nevertheless, coadministration of these vaccines is not excluded owing to overall high responses, additional ad hoc analyses justifying use, or comparability of antibody levels achieved in noninferiority studies with pediatric effective levels [29–34].
In general, the number of recommended antenatal care visits across low-, middle-, and high-income countries provides flexibility in the immunization schedule, with multiple opportunities for vaccination with RSVpreF, Tdap, and influenza vaccines. A 2020 analysis of prenatal care consensus guidelines for low-risk women in 9 high-income countries reported that the median number of recommended visits ranged from 7.5 (France and Netherlands) to 15 (Japan) [35]. In contrast, the 2015 South African maternity care guidelines recommend a basic antenatal care schedule of 5 visits for pregnant women without risk factors [36], and Nepalese guidelines recommend 4 visits [37]. The 2016 WHO guidelines on routine antenatal care generally recommend a minimum of 8 antenatal care contacts [38], an update from the 4 visits recommended in the early 2000s [38, 39], as fewer visits may be associated with unfavorable pregnancy outcomes [40, 41]. Although the percentage of pregnant women attending ≥4 antenatal care visits varies widely by geographic region, a considerable number achieve this target. In the US, the typical intervals for prenatal visits for nulliparous women with uncomplicated pregnancies are every 4 weeks until 28 weeks of gestation, every 2 weeks from 28 to 36 weeks, then weekly until delivery [42]. According to this schedule, a woman with an uncomplicated pregnancy with a first visit at 6 weeks’ gestation and a last visit at 41 weeks will have 16 prenatal visits at which influenza vaccine could be administered [23, 24], 5 opportunities for Tdap administration (from 27 to 36 weeks’ gestation) [13, 14], and 6 opportunities for immunization with RSVpreF (from 24 to 36 weeks’ gestation).
This study evaluated coadministration of RSVpreF with Tdap; however, Tdap is not routinely used worldwide. Tdap is typically recommended in higher-income countries with an elevated infant pertussis risk, including, but not limited to, the US [13, 14], the United Kingdom [15], Australia [16], Canada [17], New Zealand [18], and countries in the European Union [19]. Routine use of Tdap is less common in low- and middle-income countries owing to prohibitive cost and limited data on infant pertussis disease burden [43], and tetanus toxoid or tetanus-diphtheria vaccines are recommended during pregnancy in these countries [21, 22, 44]. Tetanus-diphtheria vaccines are preferred by WHO and the United Nations Children’s Fund because they are cost-effective and address the more prominent risks [21, 44]. The observed tetanus and diphtheria antibody responses were noninferior when coadministered with RSVpreF, supporting concomitant RSV and tetanus toxoid or tetanus-diphtheria immunization during pregnancy in regions that use vaccines without pertussis antigens.
Strengths of this study include the randomized controlled design and the power to demonstrate noninferiority of combined RSVpreF formulations with Tdap to Tdap alone or RSVpreF alone for the immunogenicity endpoints. A potential limitation is that this study was conducted in nonpregnant women and, therefore, transplacental transfer of antibodies against pertussis antigens to infants could not be established. The study was conducted in the US alone, limiting generalizability to other countries.
This phase 2b study provided key data supporting the decision to administer RSVpreF without concomitant Tdap to pregnant women in the pivotal, ongoing phase 3 trial that is designed to evaluate the efficacy and safety of RSVpreF maternal immunization at 24 to 36 weeks of gestation against medically attended lower respiratory tract illness in infants (NCT04424316). Additionally, an ongoing phase 2b proof-of-concept study has provided data on the safety, tolerability, and immunogenicity of 2 dose levels of RSVpreF formulated with and without Al(OH)3 (NCT04032093) and informed the dose and formulation selection for the phase 3 study.
In conclusion, the results of this study confirm that 120 μg RSVpreF antigen without Al(OH)3 and 240 μg RSVpreF antigen with Al(OH)3 were safe and well tolerated when administered alone or concomitantly with Tdap in healthy nonpregnant women 18‒49 years of age. Noninferiority of anti-RSV-A and anti-RSV-B immune responses induced by RSVpreF with Tdap was demonstrated compared with RSVpreF alone. Noninferiority of anti-DTd and anti-TTd immune responses after concomitant administration of RSVpreF with Tdap was demonstrated compared with Tdap alone. Noninferiority of immune response was not met for the anti-pertussis components (PT, FHA, and PRN); further studies are needed to evaluate the clinical significance of these results. These results support the continued clinical development of RSVpreF for maternal immunization to reduce the burden of infant RSV disease.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. Editorial/medical writing support was provided by Kate Russin, PhD, of ICON (North Wales, Pennsylvania) and was funded by Pfizer Inc. We thank Wayde Weston for advice.
Financial support. This study was sponsored by Pfizer Inc.
Data sharing statement. Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Potential conflicts of interest. J. T. P. received payment from Pfizer as a study investigator. B. J. E. reports no conflicts of interest. D. F.-P. reports research funding from Pfizer, Merck, AstraZeneca, Johnson & Johnson, Novartis, and Sanofi. All other authors are employees of Pfizer Inc and may hold stock and/or stock options.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
James T Peterson, J. Lewis Research, Inc, and Foothill Family Clinic, Salt Lake City, Utah, USA.
Agnieszka M Zareba, Pfizer Vaccine Research and Development, Collegeville, Pennsylvania, USA.
David Fitz-Patrick, East-West Medical Research Institute, Honolulu, Hawaii, USA.
Brandon J Essink, Meridian Clinical Research, Omaha, Nebraska, USA.
Daniel A Scott, Pfizer Vaccine Research and Development, Collegeville, Pennsylvania, USA.
Kena A Swanson, Pfizer Vaccine Research and Development, Pearl River, New York, USA.
Dhawal Chelani, Pfizer Vaccine Research and Development, Hurley, United Kingdom.
David Radley, Pfizer Vaccine Research and Development, Pearl River, New York, USA.
David Cooper, Pfizer Vaccine Research and Development, Pearl River, New York, USA.
Kathrin U Jansen, Pfizer Vaccine Research and Development, Pearl River, New York, USA.
Philip R Dormitzer, Pfizer Vaccine Research and Development, Pearl River, New York, USA.
William C Gruber, Pfizer Vaccine Research and Development, Pearl River, New York, USA.
Alejandra Gurtman, Pfizer Vaccine Research and Development, Pearl River, New York, USA.
References
- 1. Hall CB, Simoes EA, Anderson LJ.. Clinical and epidemiologic features of respiratory syncytial virus. In: Anderson LJ, Graham BS, eds. Challenges and opportunities for respiratory syncytial virus vaccines. Berlin/Heidelberg, Germany: Springer, 2013. [Google Scholar]
- 2. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rha B, Curns AT, Lively JY, et al. . Respiratory syncytial virus-associated hospitalizations among young children: 2015-2016. Pediatrics 2020; 146:e20193611. [DOI] [PubMed] [Google Scholar]
- 4. Geoghegan S, Erviti A, Caballero MT, et al. . Mortality due to respiratory syncytial virus. Burden and risk factors. Am J Respir Crit Care Med 2017; 195:96–103. [DOI] [PubMed] [Google Scholar]
- 5. Laudanno SL, Sánchez Yanotti CI, Polack FP.. RSV lower respiratory tract illness in infants of low- and middle-income countries. Acta Med Acad 2020; 49:191–7. [DOI] [PubMed] [Google Scholar]
- 6. MedImmune, LLC. Synagis (palivizumab). Full prescribing information. Gaithersburg, MD: MedImmune, LLC, 2014. [Google Scholar]
- 7. Griffin MP, Yuan Y, Takas T, et al. . Nirsevimab Study Group. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020; 383:415–25. [DOI] [PubMed] [Google Scholar]
- 8. Muňoz FM, Jamieson DJ.. Maternal immunization. Obstet Gynecol 2019; 133:739–53. [DOI] [PubMed] [Google Scholar]
- 9. Muňoz FM, Swamy GK, Hickman SP, et al. . Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. J Infect Dis 2019; 220:1802–15. [DOI] [PubMed] [Google Scholar]
- 10. Madhi SA, Polack FP, Piedra PA, et al. . Prepare Study Group. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med 2020; 383:426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaccines and Related Biological Products Advisory Committee. Clinical development and requirements for licensure of vaccines intended for use during pregnancy to prevent disease in the infant. Silver Springs, MD: US Food and Drug Administration, 2015. [Google Scholar]
- 12. Schmoele-Thoma B, Falsey A, Walsh E, et al. . Phase 1/2, first-in-human study of the safety, tolerability, and immunogenicity of an RSV prefusion F-based subunit vaccine candidate. Open Forum Infect Dis 2019; 6:S970. [Google Scholar]
- 13. Committee on Obstetric Practice and the Immunization and Emerging Infections Expert Work Group. ACOG Committee opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2017/09/update-on-immunization-and-pregnancy-tetanus-diphtheria-and-pertussis-vaccination.pdf. Accessed 2 March 2021. [Google Scholar]
- 14. Liang JL, Tiwari T, Moro P, et al. . Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018; 67:1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Health Service, United Kingdom. Whooping cough vaccination in pregnancy. https://www.nhs.uk/conditions/pregnancy-and-baby/whooping-cough-vaccination-pregnant/. Accessed 12 November 2020. [Google Scholar]
- 16. Australian Government Department of Health. Australian immunisation handbook. https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/pertussis-whooping-cough#expand-collapse-all-top. Accessed 12 November 2020. [Google Scholar]
- 17. Public Health Agency of Canada. An Advisory Committee Statement (ACS), National Advisory Committee on Immunization (NACI): update on immunization in pregnancy with tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis (Tdap) vaccine. https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/update-immunization-pregnancy-tdap-vaccine/update-immunization-pregnancy-tdap-vaccine-eng.pdf. Accessed 2 March 2021. [Google Scholar]
- 18. Immunisation Advisory Centre. Recommended and funded vaccines during pregnancy. https://www.immune.org.nz/sites/default/files/resources/Written%20Resources/ProgrammePregnancyImac20210813%20V01Final.pdf. Accessed 27 October 2021. [Google Scholar]
- 19. European Centre for Disease Prevention and Control. Vaccine scheduler: pertussis: recommended vaccinations. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=3&SelectedCountryIdByDisease=-1#:~:text=Tdap%20vaccination%20recommended%20during%20pregnancy,the%2024th%20week%20of%20pregnancy. Accessed 22 February 2021. [Google Scholar]
- 20. State of Israel Ministry of Health. Whooping cough vaccination in pregnant women. https://www.health.gov.il/English/Topics/Pregnancy/during/Pages/Vaccination-Whooping_cough.aspx. Accessed 22 February 2021. [Google Scholar]
- 21. World Health Organization. WHO/UNICEF guidance note ensuring sustained protection against diphtheria: replacing TT with Td vaccine. https://www.who.int/immunization/diseases/tetanus/Guide_to_TT_to_Td_Replacement_Final_12.09.2018.pdf. Accessed 2 March 2021. [Google Scholar]
- 22. Njuguna HN, Yusuf N, Raza AA, Ahmed B, Tohme RA.. Progress toward maternal and neonatal tetanus elimination—worldwide, 2000-2018. MMWR Morb Mortal Wkly Rep 2020; 69:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ACOG Committee opinion no. 732 summary: influenza vaccination during pregnancy. Obstet Gynecol 2018; 131:752–3. [DOI] [PubMed] [Google Scholar]
- 24. Grohskopf LA, Alyanak E, Broder KR, et al. . Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 influenza season. MMWR Recomm Rep 2020; 69:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Food and Drug Administration. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. https://www.fda.gov/media/73679/download. Accessed 1 April 2020. [Google Scholar]
- 26. McDonald JU, Rigsby P, Dougall T, Engelhardt OG; Study Participants. . Establishment of the first WHO International Standard for antiserum to respiratory syncytial virus: report of an international collaborative study. Vaccine 2018; 36:7641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McDonald JU, Rigsby P, Atkinson E, Engelhardt OG; Study Participants. . Expansion of the 1st WHO International Standard for antiserum to respiratory syncytial virus to include neutralisation titres against RSV subtype B: an international collaborative study. Vaccine 2020; 38:800–7. [DOI] [PubMed] [Google Scholar]
- 28. Healy CM. Pertussis vaccination in pregnancy. Hum Vaccin Immunother 2016; 12:1972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weston WM, Chandrashekar V, Friedland LR, Howe B.. Safety and immunogenicity of a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine when co-administered with influenza vaccine in adults. Hum Vaccin 2009; 5:858–66. [DOI] [PubMed] [Google Scholar]
- 30. Gasparini R, Conversano M, Bona G, et al. . Randomized trial on the safety, tolerability, and immunogenicity of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults. Clin Vaccine Immunol 2010; 17:537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arguedas A, Soley C, Loaiza C, et al. . Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine 2010; 28:3171–9. [DOI] [PubMed] [Google Scholar]
- 32. Weston WM, Friedland LR, Wu X, Howe B.. Immunogenicity and reactogenicity of co-administered tetanus–diphtheria–acellular pertussis (Tdap) and tetravalent meningococcal conjugate (MCV4) vaccines compared to their separate administration. Vaccine 2011; 29:1017–22. [DOI] [PubMed] [Google Scholar]
- 33. Rivera L, Chanthavanich P, Põder A, Suryakiran PV, Jastorff A, Van der Wielen M.. MenACWY-TT is immunogenic when co-administered with Tdap and AS04-HPV16/18 in girls and young women: results from a phase III randomized trial. Vaccine 2018; 36:3967–75. [DOI] [PubMed] [Google Scholar]
- 34. McNeil SA, Noya F, Dionne M, et al. . Comparison of the safety and immunogenicity of concomitant and sequential administration of an adult formulation tetanus and diphtheria toxoids adsorbed combined with acellular pertussis (Tdap) vaccine and trivalent inactivated influenza vaccine in adults. Vaccine 2007; 25:3464–74. [DOI] [PubMed] [Google Scholar]
- 35. Friedman Peahl A, Heisler M, Essenmacher LK, et al. . A comparison of international prenatal care guidelines for low-risk women to inform high-value care. Am J Obstet Gynecol 2020; 222:505–7. [DOI] [PubMed] [Google Scholar]
- 36. National Department of Health, Republic of South Africa. Guidelines for maternity care in South Africa. A manual for clinics, community health centres and district hospitals. 4th ed. https://www.up.ac.za/media/shared/62/ZP_Files/maternal-care-guidelines-2015.zp68511.pdf. Accessed 2 March 2021. [Google Scholar]
- 37. Aryal KK, Sharma SK, Khanal MN, et al. . Maternal health care in Nepal: trends and determinants. DHS further analysis reports No. 118. https://dhsprogram.com/pubs/pdf/FA118/FA118.pdf. Accessed 17 May 2021. [Google Scholar]
- 38. World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. https://www.who.int/publications/i/item/9789241549912. Accessed 2 March 2021. [PubMed] [Google Scholar]
- 39. Carroli G, Villar J, Piaggio G, et al. . WHO Antenatal Care Trial Research Group. WHO systematic review of randomised controlled trials of routine antenatal care. Lancet 2001; 357:1565–70. [DOI] [PubMed] [Google Scholar]
- 40. United Nations Children’s Fund. UNICEF data warehouse. https://data.unicef.org/resources/data_explorer/unicef_f/?ag=UNICEF&df=GLOBAL_DATAFLOW&ver=1.0&dq=.MNCH_ANC4+IMMUNISATION+MNCH..&startPeriod=2016&endPeriod=2021. Accessed 18 February 2021. [Google Scholar]
- 41. Vogel JP, Habib NA, Souza JP, et al. . Antenatal care packages with reduced visits and perinatal mortality: a secondary analysis of the WHO Antenatal Care Trial. Reprod Health 2013; 10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. American Academy of Pediatrics and American College of Obstetricians and Gynecologists. Guidelines for perinatal care. 8th ed. Elk Grove Village, IL/Washington, DC: AAP/ACOG, 2017. [Google Scholar]
- 43. Sobanjo-Ter Meulen A, Duclos P, McIntyre P, et al. . Assessing the evidence for maternal pertussis immunization: a report from the Bill & Melinda Gates Foundation Symposium on Pertussis Infant Disease Burden in Low- and Lower-Middle-Income Countries. Clin Infect Dis 2016; 63:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. World Health Organization. Tetanus vaccines: WHO position paper—February 2017. Wkly Epidemiol Rec 2017; 6:53–76. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.