Abstract
Background
PC786 is a nebulized nonnucleoside respiratory syncytial virus (RSV) polymerase inhibitor designed to treat RSV, which replicates in the superficial layer of epithelial cells lining the airways.
Methods
Fifty-six healthy volunteers inoculated with RSV-A (Memphis 37b) were randomly dosed with either nebulized PC786 (5 mg) or placebo, twice daily for 5 days, from either 12 hours after confirmation of RSV infection or 6 days after virus inoculation. Viral load (VL), disease severity, pharmacokinetics, and safety were assessed until discharge. RSV infection was confirmed by reverse-transcription quantitative polymerase chain reaction with any positive value (intention-to-treat infected [ITT-I] population) or RSV RNA ≥1 log10 plaque-forming unit equivalents (PFUe)/mL (specific intention-to-treat infection [ITT-IS] population) in nasal wash samples.
Results
In the ITT-I population, the mean VL area under the curve (AUC) was lower in the PC786 group than the placebo group (274.1 vs 406.6 log10 PFUe/mL × hour; P = .0359). PC786 showed a trend toward reduction of symptom score and mucous weight. In ITT-IS (post hoc analysis), the latter was statistically significant as well as VL AUC (P = .0126). PC786 showed an early time to maximum plasma concentration, limited systemic exposure, and long half-life and consequently a 2-fold accumulation over the 5-day dosing period. PC786 was well tolerated.
Conclusions
Nebulized PC786 demonstrated a significant antiviral effect against RSV, warranting further clinical study.
Clinical Trials Registration
ClinicalTrials.gov: NCT03382431; EudraCT: 2017-002563-18.
Keywords: challenge, respiratory syncytial virus, nonnucleoside polymerase inhibitor, nasal wash, pharmacokinetics, healthy volunteers
This manuscript reports the first human therapeutic proof of principle for a novel nonnucleoside respiratory syncytial virus (RSV) polymerase inhibitor, PC786. Nebulized PC786 exhibited significant antiviral effects and was well tolerated in healthy volunteers experimentally infected with RSV.
Human respiratory syncytial virus (RSV) is the most common cause of childhood acute lower respiratory infection [1–3] and can produce severe disease in patients with underlying medical conditions [4–8]. Despite significant efforts to develop safe and effective ways to prevent or treat RSV, there is no effective vaccine, and therapeutic options are limited [9]. In recent years, new compounds intended for the treatment of RSV have been evaluated, including fusion protein inhibitors and a nucleoside polymerase inhibitor [10–16].
The initial portal of entry by RSV is through the nose or eye [17]. Once established in the upper respiratory tract, the infection may progress to the lungs. Viral load appears to drive the clinical manifestations of RSV disease [18–22], although there is the conflicting evidence that infants with severe RSV bronchiolitis have lower nasal viral load [23]. The pathophysiology of RSV infection in lung tissues obtained postmortem from children who died within their first week of infection [24, 25] or later [26] showed the presence of RSV to be restricted to epithelial cells. Due to this specific localization of RSV infection, drug concentrations need to be maintained at higher levels within epithelial cells to be efficacious. This poses a therapeutic challenge for oral therapies, as the high drug concentrations required increase the risk of systemic side effects. Inhaled/nebulized therapy where high drug concentrations can be achieved in the respiratory epithelium while minimizing systemic exposure is, therefore, an attractive approach for preventing or treating RSV infection.
PC786 (N-(2-fluoro-6-methylphenyl)-6-(4-(5-methyl-2-(7-oxa-2-azaspiro [3.5]nonan-2-yl)nicotinamido)benzoyl)-5,6-dihydro-4H-benzo[b]thieno[2,3-d]azepine-2-carboxamide) [27, 28] is a nonnucleoside inhibitor of the RSV RNA-dependent RNA polymerase (L-protein), which results in inhibition of viral replication as well as viral messenger RNA synthesis. PC786 is active against RSV-A and RSV-B clinical isolates [27]. PC786 has properties favoring delivery by inhalation. It is sparingly water soluble, highly plasma protein bound, and has prolonged tissue retention in the lung and low systemic exposure. There was no evidence of local irritancy in the lung in preclinical inhaled toxicology studies (Supplementary Document 1).
A first-in-human study (ClinicalTrials.gov identifier NCT03236233) of nebulized PC786 identified no safety signals in either healthy volunteers or people with mild asthma [29, 30]. Furthermore, there was no evidence of hyperreactivity or bronchospasm. The current experimental viral challenge study was conducted to evaluate proof of principle for the antiviral activity of PC786 in healthy adults infected with a clinical strain of RSV [31].
MATERIALS AND METHODS
Study Participants and Design
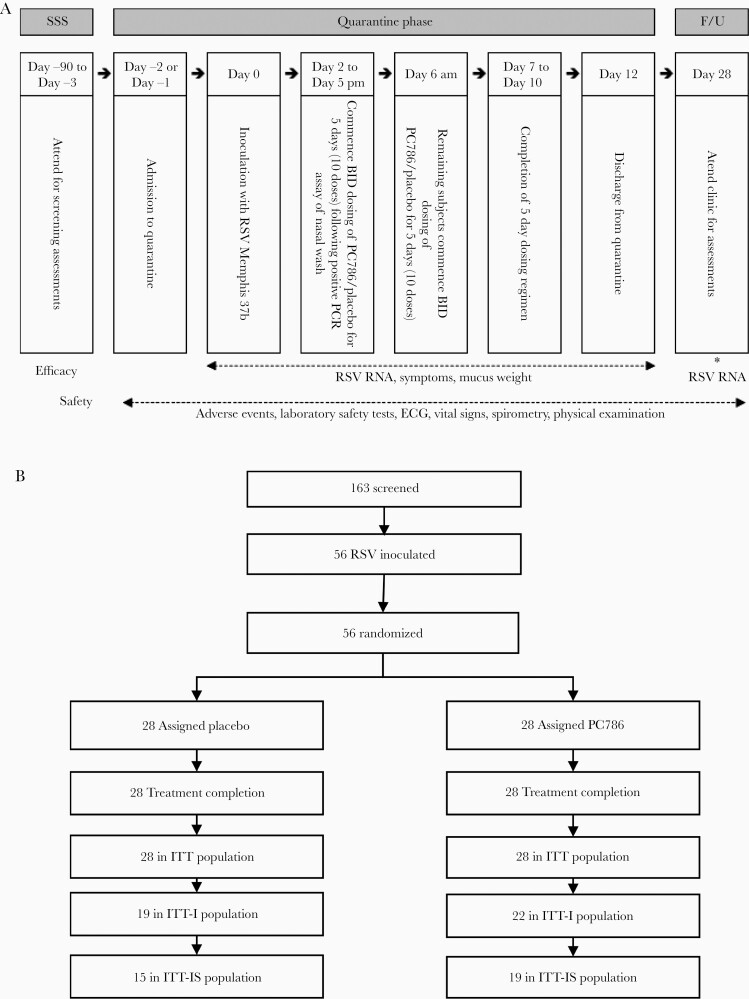
Healthy male and nonpregnant female subjects aged 18–55 years, with low serum neutralizing antibody titers against the challenge virus were enrolled. This randomized, single-blind, placebo-controlled, repeat-dose study was conducted over 7 quarantine sessions. Because of the difference in appearance between the placebo and PC786, the study was categorized as single blind. The following blinding methodologies were used (Supplementary Document 2, page 37): All study drug dosing procedures were performed by a separate unblinded team that was not involved in any postdose assessments. The subjects, investigator, investigator’s study assessment team, and the sponsor were all blinded with respect to treatment assignment. Viral load determinations were carried out in a physically separate laboratory (all separate personnel) that was blinded as to treatment assignment. Fifty-six eligible participants were confined to a specialized quarantine unit for 15 days. A further outpatient assessment occurred on study day 28 (Figure 1A; Supplementary Document 2). On study day 0, participants were inoculated intranasally with 4 log10 plaque-forming units (PFUs) of RSV-A Memphis 37b challenge virus [31]. From study day 2 until randomization, RSV infection was monitored twice daily in nasal washings using a Simplexa reverse-transcription quantitative polymerase chain reaction (RT-qPCR) assay. Participants received the first dose of either PC786 (5 mg) or placebo delivered by nebulizer (PARI LC SPRINT device) via facemask approximately 12 hours after detection of RSV or on the morning of day 6 (regardless of RSV infection status), whichever occurred first. PC786 or placebo was also given every 12 hours over 5 days (a total of 10 doses).
Figure 1.
Study design and participant disposition. A, Schematic overview of the study. B, Subject disposition flowchart. Of the 56 participants (intention-to-treat [ITT] population), 41 (73%) met the criteria to be included in the ITT infected population (respiratory syncytial virus [RSV] RNA cutoff >0 log10 plaque-forming unit equivalents (PFUe)/mL, determined by a Simplexa reverse-transcription quantitative polymerase chain reaction assay), and 34 (61%) were included in the specific ITT infected population (RSV RNA cutoff ≥1 log10 PFUe/mL). *, Quantitative RSV RT-PCR assay was conducted in nasal wash samples. Abbreviations: BID, twice daily; ECG, electrocardiogram; F/U, follow-up; ITT, intention-to-treat; ITT-I, intention-to-treat infected; ITT-IS, specific intention-to-treat infected; PCR, polymerase chain reaction; RSV, respiratory syncytial virus; SSS, study-specific screening.
The study was conducted in accordance with the Declaration of Helsinki (1996 version), the International Conference on Harmonisation Good Clinical Practice guidelines, applicable local regulations, and the study protocol (Supplementary Document 2). The protocol was approved by the Medicines and Healthcare Products Regulatory Agency (EudraCT identifier 2017-002563-18) and the Ethics Committee of the National Research Ethics Service in the United Kingdom (North West-Liverpool East Ethics Committee, Manchester, REC number 17/NW/0488). All participants provided written informed consent.
Study Population
All participants who were inoculated with the challenge virus and received at least 1 dose of study drug were included in the intention-to-treat (ITT) population (Figure 1B). Participants who were RSV PCR positive immediately before dosing (baseline), or who subsequently became positive on at least 2 occasions after baseline, were defined as infected and were included in the efficacy analysis population (ITT infected [ITT-I]) (Figure 1; Supplementary Table 1). In addition, in a post hoc analysis, an RSV viral load cutoff value of 1.0 log10 PFU equivalents (PFUe)/mL was used to score a measurement as positive. This analysis defines a new population, the specific ITT-I (ITT-IS) population. This cutoff avoided PCR detection of the inoculum itself in the absence of infection and improved the specificity of the assay to identify subjects with true RSV infection.
Endpoints and Assessments
The prespecified primary endpoint was the area under the curve (AUC) for RT-qPCR RSV viral load in nasal washes [10, 13, 16, 32, 33]. Secondary virology endpoints included time to nondetectability of the virus from commencement of study medication, viral load clearance rate during the first 24 and 48 hours after the start of dosing, peak viral load and time to peak viral load from just before starting study medication, and the proportion of subjects with detectable virus by time following commencement of study medication. The scores for symptoms related to RSV infection [32] and the AUC for mucous weight [10, 13, 16, 21] were also measured. L-gene analysis and a quantitative plaque culture assay were also conducted in aliquots of nasal washes.
Safety data were collected through study day 28 (±3 days) (Supplementary Document 2). Serial plasma samples, nasosorption-collected mucosal lining fluid (MLF), and cell pellets prepared from nasal washes were collected for determination of PC786 concentrations by liquid chromatography–tandem mass spectrometry with liquid–liquid extraction (LGC, Fordham, Cambridgeshire, United Kingdom). The lower limit of quantification was 0.1 ng/mL and the validated assay range was 0.1–100 ng/mL. The assay internal standard was deuterated (D4) PC786.
Statistical Analysis
Details of the sample size calculations for this study are described in Supplementary Document 2. The primary and secondary antiviral endpoints assessed the effects of PC786 vs placebo using a mixed-effects model (details shown in Supplementary Document 2, pages 60–61). A t test was used to compare the means of AUC of RSV viral load and other parameters between PC786 and placebo.
RESULTS
Study Population
The pretreatment characteristics of the participants were similar across treatment regimens in the ITT, ITT-I, and ITT-IS populations (Table 1). The viral load in the placebo group remained lower in ITT-I than that in the ITT-IS population (Supplementary Figure 1). The viral load in ITT-IS was also less variable and more closely resembled that observed in previously published studies with this model [13, 15, 16]. Thus, ITT-IS results (as post hoc analysis) are also presented in this manuscript.
Table 1.
Baseline Characteristics of the Intention-to-Treat (ITT), ITT Infected, and Specific ITT Infected Populations
| Characteristic | ITT | ITT-I | ITT-IS | |||
|---|---|---|---|---|---|---|
| Placebo (BID) | PC786 (5 mg BID) | Placebo (BID) | PC786 (5 mg BID) | Placebo (BID) | PC786 (5 mg BID) | |
| (n = 28) | (n = 28) | (n = 19) | (n = 22) | (n = 15) | (n = 19) | |
| Age, y, mean (SD) | 26.3 (5.89) | 25.4 (5.63) | 26.7 (6.60) | 25.1 (5.48) | 27.3 (6.98) | 23.8 (3.24) |
| BMI, kg/m2, mean (SD) | 23.88 (2.548) | 23.65 (2.650) | 24.06 (2.624) | 23.63 (2.584) | 24.21 (2.612) | 23.53 (2.097) |
| Sex, male, No. (%) | 19 (67.9) | 18 (64.3) | 14 (73.7) | 14 (63.6) | 13 (86.7) | 11 (57.9) |
| Race, No. (%) | ||||||
| White | 22 (78.6) | 20 (71.4) | 16 (84.2) | 17 (77.3) | 13 (86.7) | 15 (78.9) |
| Asian | 3 (10.7) | 4 (14.3) | 2 (10.5) | 2 (9.1) | 2 (13.3) | 2 (10.5) |
| Black/African American | 2 (7.1) | 1 (3.6) | 1 (5.3) | 0 | 0 | 0 |
| Other | 1 (3.6) | 3 (10.7) | 0 | 3 (13.6) | 0 | 2 (10.5) |
| Baseline viral load, log10 PFUe/mLa, mean (SD) | 1.053 (1.304) | 1.244 (1.476) | 1.552 (1.317) | 1.583 (1.497) | 1.966 (1.165) | 1.833 (1.459) |
| Time from inoculation to the first dose, h, mean (SD) | 119 (29) | 114 (30) | 107 (28) | 106 (29) | 97 (22) | 100 (27) |
Abbreviations: BID, twice daily; BMI, body mass index; ITT, intention-to-treat; ITT-I, intention-to-treat infected; ITT-IS, specific intention-to-treat infected; PFUe, plaque-forming unit equivalents; SD, standard deviation.
aBaseline samples were obtained from the last nasal wash collected before treatment was initiated.
Efficacy
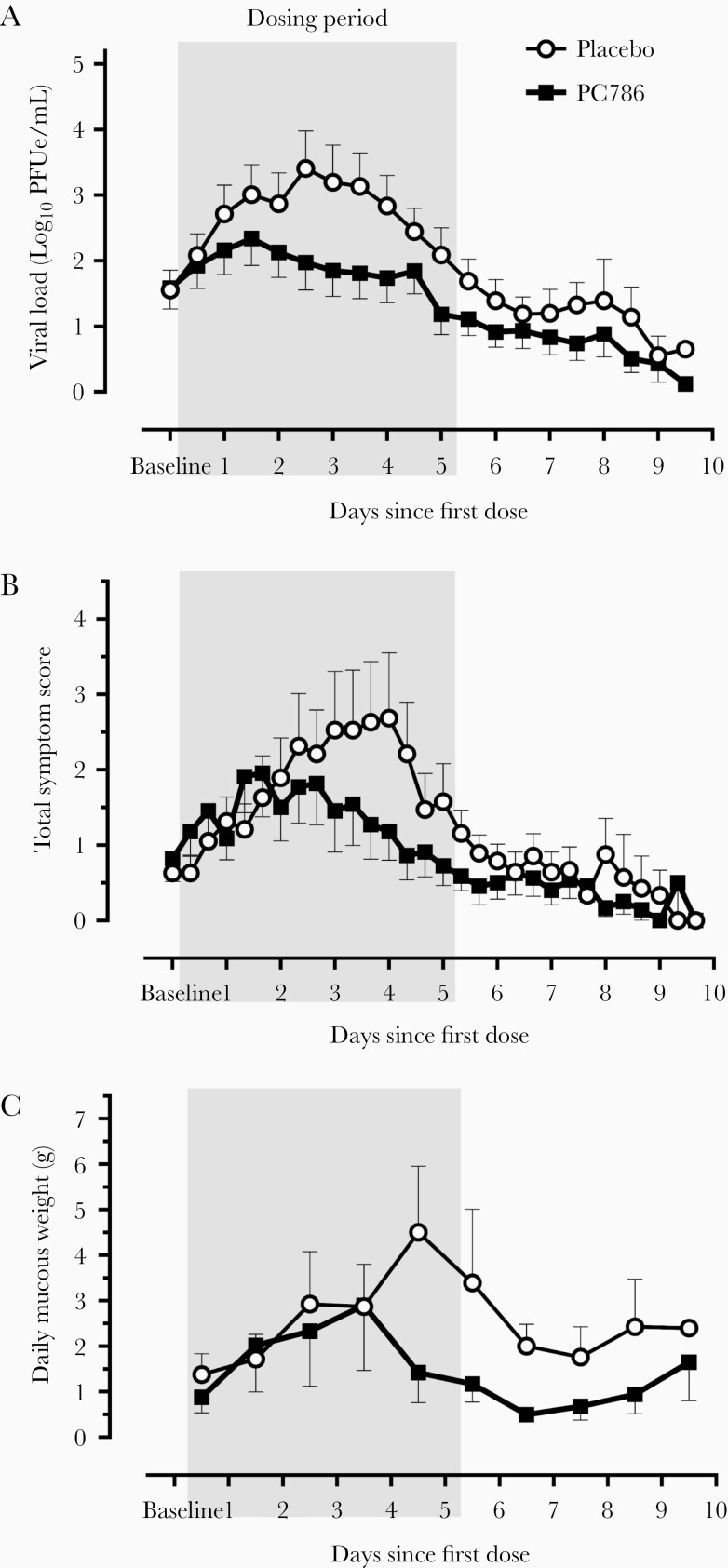
In the ITT-I population, viral load at baseline was similar between the placebo and PC786 groups. The viral load in the placebo group increased logarithmically, peaking approximately 2.5 days after randomization, and declined thereafter (Figure 2A). The mean viral load in the PC786 group remained lower than the placebo group during and after treatment, and a statistically significant reduction (32.57%; P = .0359) in the AUC for viral load was observed for PC786 compared with placebo (Table 2). The slope of the viral load curve and mean peak viral load for PC786 tended to be lower compared to placebo (P = .0571 and P = .0747, respectively). The mean viral load at the peak time in placebo (2.5 days after the first dose) was significantly lower on PC786 (P = .0064) (Table 2; Figure 2A). There was no evidence of rebound of viral load after the completion of dosing. RSV disease severity peaked 1 day after the peak of viral load and was less severe on PC786 than on placebo (Figure 2B). The AUC for the mean total subject self-assessed score for RSV symptoms was 29% lower in the PC786, although this was not statistically significant (Table 2). Mean AUC of mucous weight tended to be lower (38%; P = .1095) on PC786 (Figure 2C; Table 2).
Figure 2.
Viral load, total symptom score, and daily mucous weight over time in the intention-to-treat infected (ITT-I) population. Mean viral loads (A), total symptom scores (B), and daily mucous weights (C) in the ITT-I population are shown from the time immediately before administration of the first dose up to 10 days afterward. Although participants were inoculated with respiratory syncytial virus on the same day, they became infected on different days and consequently, began treatment at different timepoints. Viral loads were determined with the use of a reverse-transcription quantitative polymerase chain reaction assay of nasal-wash samples, which were collected twice daily (A). Participants recorded symptoms in diaries 3 times daily (B). Mucous weights were measured daily and plotted at the time corresponding to the midpoint of the mucous collection period each day (ie, weights plotted midway between the first and second days of treatment are from facial tissues collected between the morning of the first day of study treatment and the morning of the second day) (C). Mean + standard error (SE) for placebo and mean - SE for PC786 are shown. PFUe denotes plaque-forming unit equivalents.
Table 2.
Summary of Analysis of Derived Respiratory Syncytial Viral Load Parameters
| Parameter | ITT-I Population | ITT-IS Population | ||||
|---|---|---|---|---|---|---|
| Placebo (BID) (n = 19) | PC786 (5 mg BID) (n = 22) | P Value | Placebo (BID) (n = 15) | PC786 (5 mg BID) (n = 19) | P Value | |
| LS Mean (SD) | LS Mean (SD) | LS Mean (SD) | LS Mean (SD) | |||
| Primary (virology) | ||||||
| AUC VL from baseline through day 12, log10 PFUe/mL × h | 406.6 (231.86) | 274.1 (234.42) [32.57% reduction] | .0359 a | 495.5 (199.22) | 325.8 (199.19) [34.25% reduction] | .0209 a |
| Secondary (virology) | ||||||
| Time, d, to nondetectability (or <1 log) of RSV RNA | 5.4 (3.24) | 4.6 (3.75) | .3211a | 5.7 (4.13) | 4.9 (4.13) | .5573a |
| VL slope: baseline to 24 h after first dose, log10 PFUe/mL/24 h | 1.3 (1.84) | 0.8 (1.89) | .1801a | 1.5 (1.49) | 0.7 (1.50) | .0988a |
| VL slope: baseline to 48 h after first dose, log10 PFUe/mL/48 h | 1.5 (1.50) | 0.6 (1.52) | .0571a | 1.7 (1.45) | 0.6 (1.45) | .0330 a |
| Peak VL, log10 PFUe/mL | 4.2 (1.73) | 3.4 (1.76) | .0747a | 5.0 (1.30) | 3.7 (1.30) | .0107 a |
| VL at 2.5 d (peak at placebo) after first dose, log10 PFUe/mLb | 3.5 (1.81) | 2.0 (1.82) | .0064 a | 4.2 (1.66) | 2.3 (1.66) | .0025 a |
| Other parameters | ||||||
| AUC of symptom score from baseline through day 12, score × h | 269.4 (322.08) | 192.3 (244.97) | .5123c | 340.6 (327.91) | 221.2 (252.05) | .2182c |
| AUC of mucous weight from baseline through day 12, g × No. of days | 18.9 (21.98) | 11.8 (22.99) | .1095c | 23.4 (22.71) | 12.7 (24.69) | .0126 c |
Baseline values were obtained immediately before the first dose of investigational product. The boldface text indicates statistically significant.
Abbreviations: AUC, area under the curve; BID, twice daily; ITT-I, intention-to-treat infected; ITT-IS, specific intention-to-treat infected; LS, least squares; PFUe, plaque-forming unit equivalents; RSV, respiratory syncytial virus; SD, standard deviation; VL, viral load.
aMixed-effects model with treatment group as main effect, baseline VL as a covariate, and quarantine period as a random effect.
bTime at which peak mean VL occurred in the placebo group.
cFrom a nonparametric Kruskal-Wallis test due to the nonnormality of the data for these 2 endpoints
In the ITT-IS population, as a post hoc analysis, statistically significant reductions in the PC786 group compared to the placebo group were observed in the viral load AUC (34% reduction; P = .0209), the viral load slope during the first 48 hours (P = .0330), the mean peak viral load (P = .0107), and the mean viral load at the peak time in the placebo group (P = .0025), as well as mean AUC of mucous weight (46% reduction; P = .0126) (Table 2; Supplementary Figure 2). PC786 treatment produced a significant inhibition of infectious RSV viral load determined with plaque assay (as an exploratory read-out), and the AUC for viable infectious viral load was lower on PC786 than on placebo in the ITT-I (P = .0601) and the ITT-IS (P = .0405) populations (Supplementary Figure 3; Supplementary Document 3).
No Y1631H substitutions in the RSV L region, which was previously found to be associated with in vitro resistance to PC786, were detected after 5 days of treatment in a blinded single-nucleotide polymorphism (SNP) analysis performed on all nasal wash samples (Supplementary Document 4). The RSV L gene region was also sequenced from selected nasal wash samples, and Y1631H substitutions were observed in only 2 samples from different subjects treated with PC786. The substitutions were found in low allele frequency (<5%) of the viral population sequenced and failed to be detected during retesting of those 2 samples, suggesting a low substitution frequency at most, around the limit of assay detection. Additionally, the mutation did not persist, and the viral load declined after the timepoints when the substitutions were detected.
Safety
PC786 was well tolerated and no serious adverse events (AEs), deaths, or other significant AEs were reported, and no subject interrupted dosing or withdrew from the study because of an AE. Treatment-emergent AEs (TEAEs) were reported between the first dose of either PC786 or placebo as investigational products and the day 28 follow-up visit in 14 subjects (50.0%) in the placebo group and 12 subjects (42.9%) in the PC786 group, and those reported by >1 subject are summarized in Table 3.
Table 3.
Treatment-Emergent Adverse Events Reported in 2 or More Subjects
| Event Abnormality | Placebo (BID) (n = 28) | PC786 (5 mg BID) (n = 28) |
|---|---|---|
| No. (%); No. of Events | No. (%); No. of Events | |
| No. of subjects with AEs | 14 (50.0) | 12 (42.9) |
| ALT increased | 4 (14.3); 4 | 4 (14.3); 4 |
| AST increased | 4 (14.3); 4 | 4 (14.3); 5 |
| Dizziness | 3 (10.7); 3 | 0 |
| FEV1 decreaseda | 2 (7.1); 2 | 2 (7.1); 3 |
| Epistaxis | 2 (7.1); 2 | 1 (3.6); 1 |
| Blood creatine phosphokinase increased | 1 (3.6); 1 | 1 (3.6); 1 |
| Headache | 1 (3.6); 1 | 1 (3.6); 1 |
| Nausea | 1 (3.6); 1 | 0 |
| Ocular hyperemia | 0 | 2 (7.1); 2 |
| No. of subjects with AEs attributed to study drug | 2 (7.1) | 1 (3.6) |
| Conjunctivitis | 1 (3.6); 1 | 0 |
| FEV1 decreaseda | 1 (3.6); 1 | 0 |
| Headache | 0 | 1 (3.6); 1 |
| Photophobia | 1 (3.6); 1 | 0 |
Numbers represent the number of subjects reporting AEs. Safety analyses were based on the primary safety analysis (intention-to-treat) population, which was defined as those participants who were inoculated with the challenge virus and received at least 1 dose of the study drug. An AE was listed in this table if it occurred in 2 or more participants receiving a treatment regimen and occurred between the start of the study medication and day 28 (±3 days). The blood creatine phosphokinase increases occurred during the follow-up period with a well-documented history of strenuous exercise and training with heavy weights in these subjects.
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BID, twice daily; FEV1, forced expiratory volume in 1 second.
aThe FEV1 decrease from baseline was 16%, approximately 30 minutes after the final dose of placebo.
The majority of TEAEs were of mild or moderate severity. Three severe TEAEs, all following strenuous exercise, and none attributed to study treatment, were reported in 2 subjects: increased blood creatine phosphokinase in a subject in the placebo group and increased aspartate aminotransferase and blood creatine phosphokinase in a subject in the PC786 group. There were no notable differences between the placebo and PC786 groups for mean clinical laboratory values, vital signs, electrocardiography, or spirometry values. Additional safety data are provided in Supplementary Document 5.
Pharmacokinetics
Mean plasma concentrations of PC786 in the ITT population on dosing schedule A (first dose in the morning) and schedule B (first dose in the evening) were plotted on linear scales in Supplementary Figure 4. Following repeated twice-daily dosing of PC786, the time to maximum plasma concentration (Tmax) was 0.5 hours after end of inhalation (at the first specimen collection time point). This rapid plasma Tmax suggests rapid exposure of the respiratory epithelium. Observed geometric mean maximum concentration (Cmax) for the combined groups (A and B) was 1490 pg/mL with an AUC0-τ of 11 700 pg × hour/mL (Table 4).
Table 4.
Pharmacokinetic Measures of PC786 in Plasma After Repeated Twice-Daily Dosing of 5 mg
| Measure (Plasma) | PC786 (5 mg BID) | |
|---|---|---|
| Geometric Mean (CV%) | No. | |
| Cmax, pg/mL | 1490 (38.7) | 28 |
| Tmax, h, median (range) | 0.542 (0.383–2.08) | 28 |
| AUC0-τ, h × pg/mL | 11 700 (39.2) | 28 |
| t½, h | 12.1 (35.1) | 28 |
| RO, AUC0-τ | 2.14 (38.3) | 28 |
All data are presented as geometric mean of the 2 groups unless otherwise indicated.
Abbreviations: AUC0-τ, area under the concentration vs time curve within a dosing interval; BID, twice daily; Cmax, maximum observed concentration; CV%, coefficient of variation; RO, observed extent of accumulation in plasma; t½, apparent terminal half-life; tmax, time at which maximum observed concentration was apparent.
An approximate 2-fold accumulation of PC786 was observed in plasma, and steady state was reached by day 4. The effective plasma elimination half-life, consistent with the extent of accumulation in plasma and the time to reach steady state, was 12–24 hours. Thus, the pharmacokinetic (systemic and predicted lung) data were similar to those observed in the first-in-human study [30]. Nasal concentrations of PC786 (in nasal MLF and cell pellets) were measurable in most subjects up to 144 hours after the end of first inhalation, confirming the persistence of PC786 in the nasal cavity after dosing was complete (Supplementary Table 2). The pharmacokinetic profile of PC786 demonstrated the properties desirable for an inhaled molecule as previously described [34].
Post Hoc Assessment of Relationship of Pharmacokinetics and Pharmacodynamics
The PC786 treatment group was dichotomized using the median value for the viral load AUC (called poor responders and responders) in the ITT-IS population, and the relationship between PC786 exposure and each parameter was analyzed. Viral loads before the treatment commenced were approximately 1 log higher in poor responders than those in responders (Supplementary Figure 5A and 5B), and there was a strong negative correlation between initial viral load and effects of PC786 (P = .0079, r = –0.5896; Supplementary Figure 6). The responder group showed remarkable reduction of viral load and mucous weight over time (Supplementary Figure 5B and 5D). There was no significant difference in the PC786 concentration in MLF between responders and poor responders at earlier timepoints, but the levels of PC786 remained higher at later timepoints in the responder group (Supplementary Figure 5E and 5F). In the cell pellets, the PC786 concentrations in responders were higher than those in poor responders (Supplementary Figure 5G and 5H). There was no difference in plasma concentrations between responders and poor responders (Supplementary Figure 5I and 5J).
Discussion
In this study, PC786, a nebulized nonnucleoside small molecule RSV polymerase inhibitor, was shown to be well tolerated and have significant antiviral effects in healthy subjects infected with the RSV Memphis 37 strain. This was demonstrated by a reduction in the AUC for viral load, confirming that PC786 met the primary endpoint for this clinical study. PC786 also showed a trend toward reduced total daily mucous production, the most objective available measure of disease severity [21]. As predicted, systemic PC786 exposure was limited (mean plasma Cmax 1.5 ng/mL), while local concentrations in the respiratory tract exceeded the 90% effective concentration on an in vitro RSV cytopathic effect inhibition assay [27] by several orders of magnitude throughout the dosing period. PC786 nebulization was well tolerated, and safety assessments were similar between groups. The study therefore provides the first human therapeutic proof for the principle that a nebulized antiviral can inhibit RSV replication without causing local irritation, while limiting the potential for systemic side effects.
The sample size calculations for this study assumed that 70% of subjects recruited would become infected, so the 56 subjects recruited were expected to ensure 40 infected subjects with available primary endpoint data [10, 15]. In fact, the ITT-I population comprised 41 subjects (73% of randomized subjects) as expected, but 7 subjects in quarantine 7 (4 placebo and 3 PC786 subjects) had intermittent, sparse, low viral loads that were not representative of true RSV infection in this challenge model and were therefore excluded from the ITT-I population (Supplementary Table 1). The resulting ITT-IS population identified for a post hoc analysis therefore included only 34 subjects (61% of randomized subjects). The mean peak viral load in placebo-treated subjects in the ITT-I population (4.2 log10 PFUe/mL; Table 2) was much lower than that in previous reports, whereas the mean of peak viral load in the ITT-IS population (5.0 log10 PFUe/mL) was still slightly lower but more consistent with that seen in previously published studies (5.3–6.0 log10 PFUe/mL) [10, 15, 32].
Although PC786 showed significant antiviral effects, the activity was modest and more variable than anticipated from preclinical PC786 studies [27, 28] and that observed in previous challenge studies with other antivirals [10, 15]. The antiviral effect of PC786 was more similar to that observed in an RSV challenge study with JNJ-53718678 (41%–53% AUC reduction) [13], where the mean AUC for the viral load in the placebo group (432.8 log10 PFUe/mL × hour) was similar to that in the current study (495.5 log10 PFUe/mL × hour) and lower than that in other studies [10, 15, 16]. As PC786 is a polymerase inhibitor and inhibits RSV replication within cells [27, 28, 35], intracellular PC786 exposure of cells is key for this mode of action, whereas fusion-protein inhibitors may work extracellularly. As shown in Supplementary Figure 5G and 5H, cellular exposures of PC786 were found to be higher and more persistent in the responders than in the poor responders. The initial viral load before treatment was also significantly higher in the poor responders than responders. This higher baseline viral load in the poor responder group may have been associated with more extensive inflammation, nasal congestion, or nasal secretions, which could have provided a “barrier” and prevented effective delivery of the drug to the site of virus replication. Alternatively, cells already infected by RSV may take up PC786 at lower concentrations than uninfected cells. The PC786 local exposure might also be affected by the volunteer’s breathing technique, such as oral breathing or nasal dominant breathing, even though subjects were instructed to inhale through their nose. In addition, in cell-free, intact cell, and in vivo preclinical studies [27, 28], PC786 consistently showed a very steep dose-response curve, with 3- to 5-fold changes in concentration able to result in profound changes in activity. Therefore, variability in facemask delivery may have resulted in interindividual variability in concentrations of PC786 at the site of viral replication in the nose, resulting in some subjects being lower on the dose response curve than others.
In parallel with virus replication, several proinflammatory biomarkers are known to be increased during natural or experimental RSV infection [21, 36]. In the current study, levels of RANTES, CXCL10, interleukin 15 (IL-15), CXCL8, and mucin in nasal washings and those of fibrinogen, C-reactive protein, and CXCL8 in plasma were increased by RSV infection in the placebo group (Supplementary Document 6). Although the study was not powered to assess biomarkers, a statistically significant reduction or trends toward reduction by PC786 treatment was indicated in CXCL10, IL-15, and CXCL8 in nasal washings and fibrinogen and CXCL8 in plasma in the ITT-I and/or ITT-IS populations.
A higher barrier to resistance has been observed with PC786 in vitro compared with RSV fusion inhibitors [27] (Supplementary Document 7) and mutants to GS-5806 frequently occurred in the corresponding challenge study [37]. In the current study, we found no emergence of confirmed resistant virus (Y1631H substitutions) at any timepoints based on SNP PCR analysis. Only transient and low-frequency appearance of Y1631H mutation was detected in 2 samples from 2 different subjects treated with PC786 by RSV L protein gene sequencing. The mutation never represented >5% allele frequency of the viral population sequenced and disappeared at all subsequent timepoints. More definitive conclusions regarding viral resistance to PC786 should be drawn from patients with naturally acquired disease, particularly in immunocompromised patients, who are known to have prolonged and elevated levels of RSV replication.
This proof-of-principle study had several limitations. The nebulized formulation tested was not optimized for nasal delivery nor for a nasal viral challenge model. As PC786 is designed to be delivered to the lung, natural upper lung or lower respiratory tract infection would provide a more appropriate setting in which to establish clinical proof of concept for this drug. In addition, because the nebulized dose was determined from preclinical and first-in-human data for lung delivery and not optimized for nasal delivery, a higher dose via facemask with more exposure to the nose may have improved the outcome. This model is unlike natural infection in at-risk populations in that infections in this model are concentrated in the upper respiratory tract. Patients who are infected naturally are likely to be admitted to hospital later in the course of disease, with greater disease severity and more limited preexisting immunity. Therefore, it may be difficult to directly extrapolate the results of this study to a clinical setting. The ability of PC-786 to reduce viral load when applied relatively late in an RSV infection has been evaluated in vitro compared with fusion inhibitors [27, 28]. Timing windows of clinical efficacy will have to be determined in various naturally infected populations. Furthermore, PC786 showed a trend toward reduction of symptom scores, but this was not statistically significant. This result was not surprising as the study was underpowered to detect an effect on symptom scores and, indeed, the self-reported data were highly variable. Future clinical studies in patients naturally infected with RSV will be designed and powered to assess the impact of treatment on relevant clinical outcomes. Given the long residence time in the respiratory tract and duration of action of PC786, prophylactic treatment is a promising option for future consideration.
Overall, PC786 was well tolerated by healthy subjects inoculated with RSV and demonstrated antiviral effects. This represents the first-in-human therapeutic effect of a nonnucleoside class. These data support further studies in naturally infected patients to evaluate the use of PC786 to treat or prevent RSV infections in at-risk human populations.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. L. C., K. W., V. M., J. M., P. S., G. R., and J. D. were involved in preparation and participated in the design of the original clinical trial. J. D. and E. M. conducted respiratory syncytial virus (RSV) polymerase chain reaction analysis and contributed to the data analysis and the manuscript preparation. M. C., L. D., and K. I. contributed to RSV sequence analysis, single-nucleotide polymorphism (SNP) assays, and biomarker analysis. A. M. and B. M. managed subject safety. C. B. and M. M. contributed to data statistical analysis. L. C., A. M., A. D., and G. R. contributed to data interpretation and manuscript preparation. K. I. and P. S. conducted the data analysis and drafted the manuscript.
Acknowledgments. We acknowledge the contribution of the volunteers who participated in this study, and HMR Ltd (London, United Kingdom) for pharmacy services, data management, and statistical support. We also thank Mr Jed Ashman of Pulmocide Ltd for assistance in SNP and biomarker analysis, and Dr John Ayrton of Pulmocide Ltd for providing details of the PC786 measurement method.
Financial support. This work was funded by Pulmocide Ltd, United Kingdom.
Potential conflicts of interest. K. I., P. S., and G. R. are employees and (co-)founders of Pulmocide Ltd and shareholders. L. C., A. M., M. C., and A. D. are employees of Pulmocide Ltd and shareholders. K. W. is a consultant of Pulmocide Ltd. V. W., J. M., and B. M. work at hVIVO Services Ltd, where this clinical study was conducted. J. D. serves on the scientific advisory board of Pulmocide Ltd and is a consultant for other RSV prevention and treatment programs including Pfizer, MedImmune/AstraZeneca, ADMA Biologics, Janssen, Ark Bio, and GSK. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
John DeVincenzo, Department of Pediatrics, University of Tennessee Health Science Center, Memphis, Tennessee, USA; Children’s Foundation Research Institute, Le Bonheur Children’s Hospital, Memphis, Tennessee, USA; Department of Microbiology, Immunology and Biochemistry, University of Tennessee Health Science Center, Memphis, Tennessee, USA.
Lindsey Cass, Pulmocide Ltd, London, United Kingdom.
Alison Murray, Pulmocide Ltd, London, United Kingdom.
Kathy Woodward, Pulmocide Ltd, London, United Kingdom.
Elizabeth Meals, Department of Pediatrics, University of Tennessee Health Science Center, Memphis, Tennessee, USA; Children’s Foundation Research Institute, Le Bonheur Children’s Hospital, Memphis, Tennessee, USA.
Matthew Coates, Pulmocide Ltd, London, United Kingdom.
Leah Daly, Pulmocide Ltd, London, United Kingdom.
Vicky Wheeler, hVIVO Services Ltd, London, United Kingdom.
Julie Mori, hVIVO Services Ltd, London, United Kingdom.
Charlie Brindley, KinetAssist Ltd, Quothquan, United Kingdom.
Amanda Davis, Pulmocide Ltd, London, United Kingdom.
Meabh McCurdy, Exploristics Ltd, Belfast, United Kingdom.
Kazuhiro Ito, Pulmocide Ltd, London, United Kingdom.
Bryan Murray, hVIVO Services Ltd, London, United Kingdom.
Pete Strong, Pulmocide Ltd, London, United Kingdom.
Garth Rapeport, Pulmocide Ltd, London, United Kingdom.
References
- 1. Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J 2002; 21:629–32. [DOI] [PubMed] [Google Scholar]
- 2. Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nair H, Nokes DJ, Gessner BD, et al. . Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walsh EE, Falsey AR. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets 2012; 12:98–102. [DOI] [PubMed] [Google Scholar]
- 5. Mehta J, Walsh EE, Mahadevia PJ, Falsey AR. Risk factors for respiratory syncytial virus illness among patients with chronic obstructive pulmonary disease. COPD 2013; 10:293–9. [DOI] [PubMed] [Google Scholar]
- 6. Darveaux JI, Lemanske RF Jr. Infection-related asthma. J Allergy Clin Immunol Pract 2014; 2:658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abman SH, Ogle JW, Butler-Simon N, Rumack CM, Accurso FJ. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J Pediatr 1988; 113:826–30. [DOI] [PubMed] [Google Scholar]
- 8. Abdallah A, Rowland KE, Schepetiuk SK, To LB, Bardy P. An outbreak of respiratory syncytial virus infection in a bone marrow transplant unit: effect on engraftment and outcome of pneumonia without specific antiviral treatment. Bone Marrow Transplant 2003; 32:195–203. [DOI] [PubMed] [Google Scholar]
- 9. American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134:415–20. [DOI] [PubMed] [Google Scholar]
- 10. DeVincenzo JP, Whitley RJ, Mackman RL, et al. . Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med 2014; 371:711–22. [DOI] [PubMed] [Google Scholar]
- 11. Blair W, Cox C. Current landscape of antiviral drug discovery. F1000Res 2016; 5:1000. doi:10.12688/f1000research.7665.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ClinicalTrials.gov. Safety, efficacy and pharmacokinetics of BTA-C585 in a RSV viral challenge study (NCT02718937). 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT02718937. Accessed 30 November 2020. [Google Scholar]
- 13. Stevens M, Rusch S, DeVincenzo J, et al. . Antiviral activity of oral JNJ-53718678 in healthy adult volunteers challenged with respiratory syncytial virus: a placebo-controlled study. J Infect Dis 2018; 218:748–56. [DOI] [PubMed] [Google Scholar]
- 14. Detalle L, Stohr T, Palomo C, et al. . Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob Agents Chemother 2016; 60:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeVincenzo JP, McClure MW, Symons JA, et al. . Activity of oral ALS-008176 in a respiratory syncytial virus challenge study. N Engl J Med 2015; 373:2048–58. [DOI] [PubMed] [Google Scholar]
- 16. DeVincenzo J, Tait D, Efthimiou J, et al. . A randomized, placebo-controlled, respiratory syncytial virus human challenge study of the antiviral efficacy, safety, and pharmacokinetics of RV521, an inhibitor of the RSV-F protein. Antimicrob Agents Chemother 2020; 64:e01884-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hall CB, Douglas RG Jr, Schnabel KC, Geiman JM. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun 1981; 33:779–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buckingham SC, Bush AJ, Devincenzo JP. Nasal quantity of respiratory syncytical virus correlates with disease severity in hospitalized infants. Pediatr Infect Dis J 2000; 19:113–7. [DOI] [PubMed] [Google Scholar]
- 19. DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis 2005; 191:1861–8. [DOI] [PubMed] [Google Scholar]
- 20. El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis 2011; 204:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeVincenzo JP, Wilkinson T, Vaishnaw A, et al. . Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 2010; 182:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uusitupa E, Waris M, Heikkinen T. Association of viral load with disease severity in outpatient children with respiratory syncytial virus infection. J Infect Dis 2020; 222:298–304. [DOI] [PubMed] [Google Scholar]
- 23. Thwaites RS, Coates M, Ito K, et al. . Reduced nasal viral load and IFN responses in infants with respiratory syncytial virus bronchiolitis and respiratory failure. Am J Respir Crit Care Med 2018; 198:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welliver TP, Garofalo RP, Hosakote Y, et al. . Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis 2007; 195:1126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeVincenzo JP. A new direction in understanding the pathogenesis of respiratory syncytial virus bronchiolitis: how real infants suffer. J Infect Dis 2007; 195:1084–6. [DOI] [PubMed] [Google Scholar]
- 26. Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 2007; 20:108–19. [DOI] [PubMed] [Google Scholar]
- 27. Coates M, Brookes D, Kim YI, et al. . Preclinical characterization of the inhaled small molecule respiratory syncytial virus L-protein polymerase inhibitor, PC786. Antimicrob Agents Chemother 2017; 61:e00737-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brookes DW, Coates M, Allen H, et al. . Late therapeutic intervention with a respiratory syncytial virus L-protein polymerase inhibitor, PC786, on respiratory syncytial virus infection in human airway epithelium. Br J Pharmacol 2018; 175:2520–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. ClinicalTrials.gov. A study to investigate the safety and tolerability of single and repeat doses of PC786 (NCT03236233). 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT03236233. Accessed 30 November 2020. [Google Scholar]
- 30. Cass L, Davis A, Murray A, et al. . Safety and pharmacokinetic profile of PC786, a novel inhibitor of respiratory syncytial virus L-protein polymerase, in a single and multiple-ascending dose study in healthy volunteer and mild asthmatics. 2018; 5:S407–S8. [Google Scholar]
- 31. Kim YI, DeVincenzo JP, Jones BG, et al. . Respiratory syncytial virus human experimental infection model: provenance, production, and sequence of low-passaged memphis-37 challenge virus. PLoS One 2014; 9:e113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeVincenzo J, Lambkin-Williams R, Wilkinson T, et al. . A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A 2010; 107:8800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perkins SM, Webb DL, Torrance SA, et al. . Comparison of a real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J Clin Microbiol 2005; 43:2356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strong P, Ito K, Murray J, Rapeport G. Current approaches to the discovery of novel inhaled medicines. Drug Discov Today 2018; 23:1705–17. [DOI] [PubMed] [Google Scholar]
- 35. Mirabelli C, Jaspers M, Boon M, et al. . Differential antiviral activities of respiratory syncytial virus (RSV) inhibitors in human airway epithelium. J Antimicrob Chemother 2018; 73:1823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thompson TM, Roddam PL, Harrison LM, Aitken JA, DeVincenzo JP. Viral specific factors contribute to clinical respiratory syncytial virus disease severity differences in infants. Clin Microbiol 2015; 4:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stray K, Perron M, Porter DP, et al. . Drug resistance assessment following administration of respiratory syncytial virus (RSV) fusion inhibitor presatovir to participants experimentally infected with RSV. J Infect Dis 2020; 222:1468–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.