Abstract
Background
Respiratory syncytial virus (RSV) causes substantial morbidity and mortality in older adults and adults with comorbidities. An effective vaccine is needed. An investigational bivalent prefusion F vaccine (RSVpreF) was assessed in healthy adults.
Methods
This phase 1/2 study randomized adults 18–85 years old to receive placebo or 60, 120, or 240 µg RSVpreF (with or without aluminum hydroxide) alone or concomitantly with seasonal inactivated influenza vaccine (SIIV). Safety and immunogenicity were assessed.
Results
In older adults, reactogenicity events were predominantly mild or moderate among RSVpreF recipients; adverse events through 1 month postvaccination were similar across formulations. Coadministration with SIIV did not appear to affect safety among younger or older adults. All RSVpreF formulations with or without concomitant SIIV elicited robust RSV serum-neutralizing responses in adults aged 50–85 years 1 month postvaccination. Neutralizing titers 1 and 12 months postvaccination were 6.9–14.9 and 2.9–4.5 times, respectively, those before vaccination. SIIV immune responses trended lower when coadministered with RSVpreF.
Conclusions
RSVpreF formulations administered alone or with SIIV were well tolerated and highly immunogenic in older adults, supporting the potential for RSVpreF to protect older adults from RSV disease.
Clinical Trials Registration
Keywords: respiratory syncytial virus, F protein, vaccine, safety, immunogenicity, older adults
Respiratory syncytial virus stabilized prefusion F subunit vaccine (RSVpreF) formulations administered alone or with seasonal inactivated influenza vaccine were well tolerated and highly immunogenic in older adults, suggesting that RSVpreF may protect older adults from respiratory syncytial virus disease.
(See the Editorial Commentary by Phijffer and Bont on pages 2053–5.)
Respiratory syncytial virus (RSV) is a major cause of severe lower respiratory disease in infants and young children [1] and an important respiratory pathogen in older adults [2]. In adults older than 65 years in the United States, attack rates are 3%–7%, and RSV is associated with approximately 177 000 hospitalizations and 14 000 deaths annually [3]. However, disease burden in older adults is likely underestimated due to lack of standard RSV testing practices and lower viral shedding compared with infant RSV disease [2, 4, 5].
Immunity to RSV is incomplete, with reinfection throughout life despite the presence of relatively high serum RSV neutralization titers [2]. Several risk factors for serious disease have been identified, including older age [6] and comorbidities such as chronic obstructive pulmonary disease, congestive heart failure, stroke, chronic kidney disease, and immunosuppression [7, 8]. Poor protection against reinfection and risk of severe disease in older adults are likely associated with age-related changes in T-cell responses and effector memory to the virus [9]. Despite no known correlate of immunity among older adults, limited data associate higher serum RSV neutralization titers with less severe disease, and low serum and nasal RSV antibodies are associated with increased risk [10]. Currently, RSV disease management in adults is limited to supportive measures [8]. Antiviral approaches have proven unsuccessful in natural infection [11]. Vaccination remains a high priority.
Despite a large medical need and years of development, no RSV vaccine is licensed for use in any population [12]. Vaccines using live-attenuated viruses, recombinant vectors, messenger RNA, and recombinant protein subunits are in clinical trials [13, 14]. The fusion (F) protein, an RSV envelope glycoprotein, is a prominent vaccine target due to its conserved neutralizing epitopes [15, 16]. The protein has distinct conformational states: a metastable prefusion state on intact virions that is primed to mediate cell entry, and a stable postfusion state that forms after entry-associated rearrangement [16, 17]. Antibodies specific to the prefusion F conformation account for most of the serum-neutralizing activity against RSV [18], explaining the relatively low neutralizing titers elicited by postfusion F vaccines [19–21].
A bivalent prefusion F vaccine (RSVpreF) containing trimeric F glycoproteins from both major RSV subgroups (A and B) engineered for stability in the prefusion conformation is in clinical development in adults. The vaccine is intended to protect older adults directly and protect infants via maternal immunization during pregnancy. This dose-ranging, hypothesis-generating, first-in-human, phase 1/2 study evaluated RSVpreF safety and immunogenicity in healthy adults 18–85 years of age. In the first phase of the study, the sentinel cohort comprised younger participants aged 18–49 years and older participants aged 50–85 years. A larger second phase of the study included expanded cohorts of younger (aged 18–49 years) and older (65–85 years) participants. Reported here are safety and immunogenicity results for older participants 50–85 years of age, with a focus on the expanded cohort (ie, participants 65–85 years of age). We also report safety and immunogenicity data for all participants 18–49 and 65–85 years of age who concomitantly received RSVpreF and seasonal inactivated influenza vaccine (SIIV) during the trial, as RSVpreF may be coadministered with a seasonal influenza vaccine.
METHODS
Study Design
This was a phase 1/2 randomized, placebo-controlled, observer-blinded, dose-finding, first-in-human study (NCT03529773) conducted at 36 sites in the United States between 18 April 2018 and 28 December 2020. Safety, tolerability, and immunogenicity of 3 RSVpreF doses (60 µg, 120 µg, and 240 µg) formulated with or without aluminum hydroxide (Al[OH]3) were evaluated in 2 phases. First, a sentinel cohort of participants 18–49 or 50–85 years of age was randomized (1:3:3 ratio) to receive a single dose of placebo, 1 of the 3 antigen doses without Al(OH)3, or 1 of the 3 antigen doses with Al(OH)3. Randomization began at the 60-µg dose and escalated to 120- and 240-µg doses following review of 14-day safety and tolerability data by an internal review committee. In the second phase, an expanded cohort of participants 18–49 or 65–85 years of age was randomized within age groups to receive placebo or 1 of the 3 dose levels of RSVpreF ± Al(OH)3, with or without SIIV. These participants received 2 vaccinations approximately 1 month apart: either concomitant SIIV with RSVpreF at vaccination 1 then placebo alone for vaccination 2, or concomitant placebo and RSVpreF at vaccination 1 then SIIV alone at vaccination 2. Placebo recipients received 2 doses of placebo at vaccination 1 then SIIV alone for vaccination 2. In the expanded cohort, participants were at least 65 years of age, allowing administration of a commercially available high-dose SIIV licensed for adults ≥65 years of age. Additional details are in the Supplementary Appendix, and the study protocol is available at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/study/NCT03529773).
Participants
Participants were required to use effective contraception for 28 days after the last dose or otherwise be unable to bear or father children. Individuals with preexisting, stable disease not requiring any significant change in therapy or hospitalization in the past 6 weeks were eligible for enrollment. Full inclusion and exclusion criteria are provided in the Supplementary Appendix.
Randomization and Masking
Participants were randomized using an interactive response technology system. Participants, study and sponsor team members, and laboratory personnel were blinded as appropriate; site dispensers and administrators were unblinded because the vaccines’ physical appearances differed.
Investigational Product
RSVpreF formulations contained equal amounts of 2 stabilized prefusion F antigens, 1 from the subgroup A/Ontario strain and 1 from the subgroup B/Buenos Aires strain, totaling 60 μg, 120 μg, or 240 μg, with or without 0.2 mg Al(OH)3. Commercially available quadrivalent SIIV (Fluzone Quadrivalent, Sanofi Pasteur, Swiftwater, Pennsylvania) was administered to participants 18–49 years of age, and high-dose trivalent SIIV (Fluzone High-Dose, Sanofi Pasteur) was administered to participants 65–85 years of age [22, 23]. For the 2018–2019 influenza season, high-dose trivalent SIIV contained strains A/Michigan/45/2015 (H1N1)pdm09-like, A/Singapore/INFIMH-16-0019/2016 A(H3N2)-like, and B/Colorado/06/2017-like (Victoria lineage), and the quadrivalent SIIV additionally contained a B/Phuket/3073/2013-like (Yamagata lineage) strain. Placebo was a sterile 0.9% sodium chloride solution.
Outcomes
The primary study objective evaluated the safety and tolerability of RSVpreF administered alone or concomitantly with SIIV. Safety endpoints included local reactions (redness, swelling, and pain at the injection site) and systemic events (fatigue, headache, vomiting, nausea, diarrhea, muscle pain, joint pain, and fever) within 14 days postvaccination, as recorded by electronic diary; adverse events (AEs) within 1 month post–vaccination 1 and 1 month post–vaccination 2 (expanded cohort only); and medically attended AEs (MAEs) and serious AEs (SAEs) within 12 months postvaccination.
Immunogenicity objectives described immune responses elicited by RSVpreF administered alone or concomitantly with SIIV. Corresponding endpoints included serum-neutralizing titers to RSV A and RSV B up to 12 months post–vaccination 1, serum hemagglutination inhibition (HAI) titers for the applicable 3 or 4 influenza strains in the administered SIIV and H3N2-neutralizing titers up to 1 month after SIIV (expanded cohort), and levels of RSV A and RSV B prefusion F–binding immunoglobulin G (IgG) through 12 months post–vaccination 1, as measured by Luminex immunoassay. Details regarding the RSV neutralization assay, Luminex immunoassays, and HAI assays, as well as the evaluable safety and immunogenicity populations, are available in the Supplementary Appendix.
This report presents RSVpreF and SIIV safety and immunogenicity data from sentinel cohort participants 50–85 years of age and expanded cohort participants 65–85 years of age. RSVpreF and SIIV immunogenicity data from expanded cohort participants 18–49 years of age are also presented. Immunogenicity data from expanded cohort participants 18–49 years of age who received placebo are reported here and in the companion report. Safety data and RSVpreF (without concomitant SIIV) immunogenicity data from combined sentinel and expanded cohort participants 18–49 years of age are reported separately.
Statistical Analyses
This was a dose-ranging, hypothesis-generating study with safety as a primary objective and was not designed or powered for formal hypothesis testing regarding dose or formulation comparisons. All data were analyzed descriptively. Safety endpoints were reported using counts and percentages with 2-sided 95% confidence intervals (CIs). Geometric mean titers (GMTs) at each time point and geometric mean fold rises (GMFRs) from prevaccination to each postvaccination time point were calculated and presented with 95% CIs based on a Student t distribution of log-transformed data followed by back-transformation to the original scale. Geometric mean ratios (GMRs) were calculated for HAI of strains in SIIV by comparing sera obtained 1 month postvaccination with SIIV from participants vaccinated with SIIV and RSVpreF concomitantly (vaccination 1) or with SIIV alone (vaccination 2). Two-sided CIs for the GMRs were obtained by calculating CIs using 2-sample Student t distribution for the mean difference of the logarithmically transformed assay results and exponentiating the confidence limits.
RESULTS
Participants
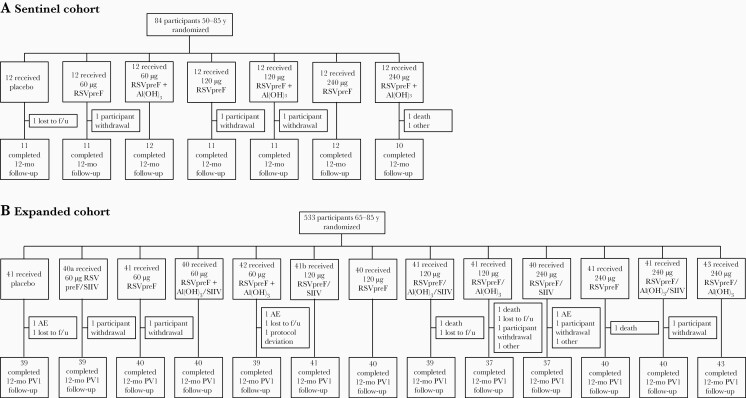
The study randomized 617 older adult participants 50–85 years of age in the sentinel (n = 84; 50–85 years) and expanded (n = 533; 65–85 years) cohorts (Figure 1). In the sentinel cohort, all participants were vaccinated and 92.9% (78/84) completed the 12-month follow-up visit. In the expanded cohort, all but 1 participant were vaccinated; 96.4% (514/533) completed the 12-month follow-up visit. Demographic characteristics were broadly similar across vaccine groups and study cohorts (Supplementary Table 1). The disposition of participants 18–49 years of age is shown in Supplementary Figure 1; demographic characteristics are reported separately.
Figure 1.
Disposition of participants 50–85 years of age in the sentinel (A) and expanded (B) cohorts. Participants who did not receive seasonal inactivated influenza vaccine (SIIV) concomitantly with the respiratory syncytial virus (RSV) vaccine received it 1 month later. aOne participant randomized to the 60-µg bivalent RSV prefusion F vaccine (RSVpreF) + SIIV group did not receive vaccination. bOne participant randomized to the 120-µg RSVpreF + SIIV group was instead administered RSVpreF 240 µg + aluminum hydroxide + SIIV; this participant is included in the population to which the participant was randomized. Abbreviations: AE, adverse event; Al(OH)3, aluminum hydroxide; f/u, follow-up; PV1, post–vaccination 1; RSV, respiratory syncytial virus; RSVpreF, bivalent respiratory syncytial virus prefusion F vaccine; SIIV, seasonal inactivated influenza vaccine.
Safety
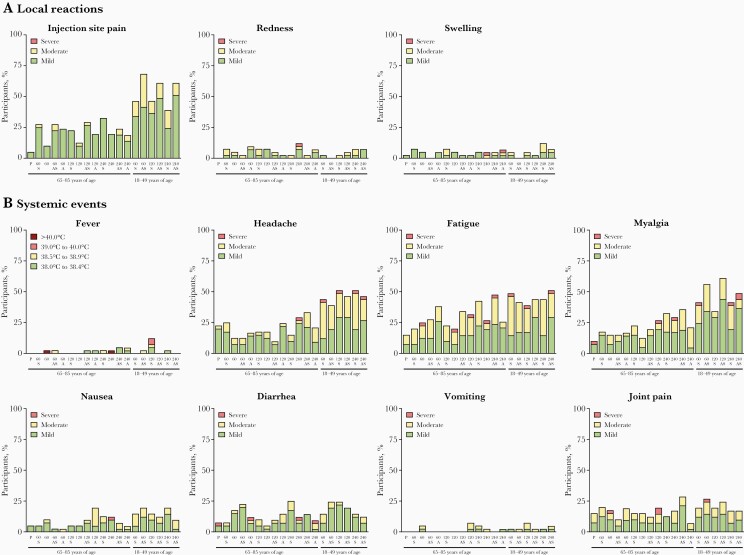
In the expanded cohort of older adults, 25.3% (124/490) of participants in the RSVpreF groups had local reactions within 14 days post–vaccination 1 (Figure 2). The majority (82.2% [102/124]) of participants who reported local reactions rated them mild in severity; pain at the injection site was the most common (22.2% [109/490]). Incidence and severity of local reactions were similar whether RSVpreF was administered with or without SIIV or Al(OH)3. One participant reported severe swelling (RSVpreF 240 µg + Al[OH]3), and 1 participant (RSVpreF 240 µg) reported severe swelling and redness. The median duration of local reactions was 1.0–6.5 days. In the expanded cohort of younger adults, incidence and severity of local reactions was similar across groups and comparable with older adults, except for a higher incidence of injection site pain in younger adults. In the sentinel cohort of older adults (without concomitant SIIV), 8.3%–41.7% of participants reported local reactions across RSVpreF groups, with frequency and severity trending higher in groups receiving Al(OH)3-containing formulations (Supplementary Figure 2).
Figure 2.
Percentages of expanded cohort participants reporting individual local reactions (A) or systemic events (B) by severity within 14 days postvaccination. Total number of participants = 40–43 per group. 60 = 60 µg bivalent respiratory syncytial virus prefusion F vaccine (RSVpreF); 120 = 120 µg RSVpreF; 240 = 240 µg RSVpreF. Abbreviations: A, aluminum hydroxide; P, placebo; RSVpreF, bivalent respiratory syncytial virus prefusion F vaccine; S, seasonal inactivated influenza vaccine.
A systemic event was reported within 14 days post–vaccination 1 by 48.6% (238/490) of RSVpreF recipients in the older adult expanded cohort (Figure 2). Most (95.0% [226/238]) who reported systemic events rated them as mild or moderate in severity, with incidence and severity generally similar across RSVpreF groups. Fatigue (30.2% [148/490]) was the most commonly reported systemic event. Median duration was typically 1.0–6.5 days, with the exceptions of diarrhea (10 days in RSVpreF 60-μg + Al[OH]3 recipients, n = 5) and joint pain (8 days in RSVpreF 120-μg recipients, n = 6; 8.5 days in RSVpreF 120-μg + Al[OH]3 recipients, n = 8). Severe systemic events were reported by 0–7.3% and 5.0% of RSVpreF and placebo recipients, respectively. Two participants were reported to experience fever >40.0°C (1 each in the RSVpreF 60-μg and 240-μg groups). One of these grade 4 fevers was an entry error and the other was associated with an influenza illness reported as an AE the following day. Younger adults in the expanded cohort who received RSVpreF and SIIV reported similar proportions of systemic events compared with older adults, but had slightly higher rates of headache, fatigue, and myalgia. Similar proportions of RSVpreF recipients reported systemic events in the older adult sentinel cohort groups (Supplementary Figure 2). Systemic events were reported by 40.0% (16/40) and 33.3% (4/12) of placebo recipients in the older adult expanded and sentinel cohorts, respectively.
In the expanded cohort, percentages of participants who reported AEs through 1 month postvaccination were 5.0%–26.2% across RSVpreF groups and 12.2% among placebo recipients (Table 1). The most common system organ class of reported AEs was infections and infestations. Six RSVpreF recipients reported AEs that were considered related (injection site pruritus [n = 1]; injection site swelling [n = 1]; injection site pain [n = 1]; sensation of foreign body [n = 1]; skin exfoliation [n = 1]; upper abdominal pain, chills, decreased appetite, myalgia, and hyperhidrosis [n = 1]). Within 1 month after vaccination 2 (SIIV or placebo), percentages of participants reporting any AE were 7.3%–32.5% across RSVpreF groups and 17.1% among placebo recipients (corresponding percentages in younger adults were 7.3%–26.8% and 9.8%, respectively). Percentages of participants reporting SAEs through 12 months postvaccination were 4.9%–19.0% and 7.3% across the RSVpreF and placebo groups, respectively. No SAEs were considered vaccine related. MAEs through 12 months after vaccination were reported by 15.0%–47.5% and 14.6% of participants in the RSVpreF and placebo groups, respectively. One MAE reported by a participant in the RSVpreF 240-µg + SIIV group (upper abdominal pain) was considered vaccine related. In the sentinel cohort of older adults, percentages of participants reporting AEs, related AEs, SAEs, and MAEs were comparable across RSVpreF groups (Supplementary Table 2). No reported SAEs or MAEs in the sentinel cohort were considered related to the vaccine. One sentinel recipient of 240 µg + Al(OH)3 reported an AE leading to withdrawal (myocardial infarction) that was not considered vaccine related. Four participants 50–85 years of age and 1 participant 18–49 years of age died during the study; no deaths were considered vaccine related.
Table 1.
Percentages of Participants in the Expanded Cohort Reporting Adverse Events After Vaccination a
| Group (No.b) | Any AE, No.c (%) | Related AE, No.c (%) | SAE, No.c (%) | MAE, No.c (%) |
|---|---|---|---|---|
| 65–85 years of age | ||||
| Placebo (41) | 5 (12.2) | 0 | 3 (7.3) | 6 (14.6) |
| 60 µg RSVpreF + SIIV (40) | 4 (10.0) | 0 | 2 (5.0) | 6 (15.0) |
| 60 µg RSVpreF (41) | 6 (14.6) | 0 | 7 (17.1) | 10 (24.4) |
| 60 µg RSVpreF + Al(OH)3 + SIIV (40) | 2 (5.0) | 0 | 4 (10.0) | 12 (30.0) |
| 60 µg RSVpreF + Al(OH)3 (42) | 11 (26.2) | 1 (2.4) | 8 (19.0) | 13 (31.0) |
| 120 µg RSVpreF + SIIV (40) | 9 (22.5) | 1 (2.5) | 4 (10.0) | 9 (22.5) |
| 120 µg RSVpreF (40) | 8 (20.0) | 1 (2.5) | 2 (5.0) | 9 (22.5) |
| 120 µg RSVpreF + Al(OH)3 + SIIV (41) | 6 (14.6) | 0 | 7 (17.1) | 7 (17.1) |
| 120 µg RSVpreF + Al(OH)3 (41) | 6 (14.6) | 0 | 2 (4.9) | 14 (34.1) |
| 240 µg RSVpreF + SIIV (40) | 6 (15.0) | 3 (7.5) | 4 (10.0) | 19 (47.5) |
| 240 µg RSVpreF (41) | 4 (9.8) | 0 | 3 (7.3) | 18 (43.9) |
| 240 µg RSVpreF + Al(OH)3+ SIIV (42) | 5 (11.9) | 0 | 3 (7.1) | 12 (28.6) |
| 240 µg RSVpreF + Al(OH)3(43) | 7 (16.3) | 0 | 3 (7.0) | 11 (25.6) |
| 18–49 years of age | ||||
| 60 µg RSVpreF + SIIV (41) | 7 (17.1) | 1 (2.4) | 1 (2.4) | 9 (22.0) |
| 60 µg RSVpreF + Al(OH)3 + SIIV (41) | 6 (14.6) | 2 (4.9) | 0 | 4 (9.8) |
| 120 µg RSVpreF + SIIV (41) | 10 (24.4) | 1 (2.4) | 1 (2.4) | 9 (22.0) |
| 120 µg RSVpreF + Al(OH)3 + SIIV (41) | 3 (7.3) | 0 | 0 | 5 (12.2) |
| 240 µg RSVpreF + SIIV (41) | 4 (9.8) | 0 | 2 (4.9) | 10 (24.4) |
| 240 µg RSVpreF + Al(OH)3 + SIIV (41) | 5 (12.2) | 0 | 1 (2.4) | 10 (24.4) |
Abbreviations: AE, adverse event; Al(OH)3, aluminum hydroxide; MAE, medically attended adverse event; RSVpreF, bivalent respiratory syncytial virus prefusion F vaccine; SAE, serious adverse event; SIIV, seasonal inactivated influenza vaccine.
AEs and related AEs were assessed through 1 month post–vaccination 1; SAEs and MAEs were assessed through 12 months post–vaccination 1.
Total number of participants in the specified vaccine group.
Number of participants reporting ≥1 specified event.
Immunogenicity
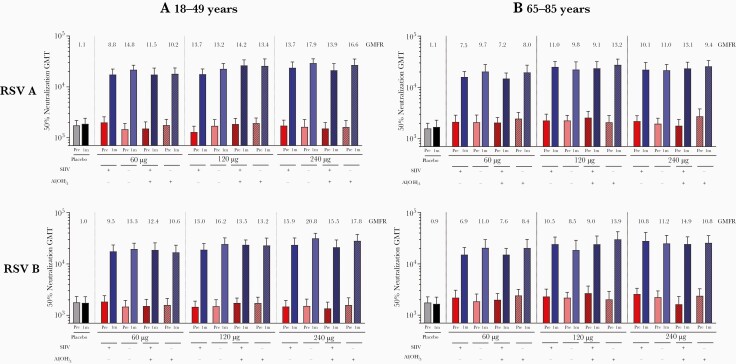
Among older adults 65–85 years in the expanded cohort, RSV A 50% neutralizing GMTs in RSVpreF recipients increased from 1793–2734 before vaccination to 14 905–27 600 at 1 month postvaccination (Figure 3). RSV B neutralizing titers increased from 1635–2685 before vaccination to 15 169–30 071 at 1 month postvaccination. Postimmunization neutralizing titers were similarly high across RSVpreF dose levels and formulations; corresponding GMFRs were 7.2–13.2 for RSV A and 6.9–14.9 for RSV B across RSVpreF groups, and 1.1 for RSV A and 0.9 for RSV B for placebo recipients (findings were similar for older adults in the sentinel cohort; Supplementary Figure 3). Similar high RSV A and RSV B titers 1 month postvaccination were observed for expanded cohort participants 18–49 years of age (Figure 3A). Coadministration of RSVpreF with SIIV did not affect RSV neutralizing GMTs and GMFRs in either age group (Figure 3A and 3B). Older adult RSVpreF recipients in the combined sentinel and expanded cohorts (restricted to participants without concomitant SIIV) with lower baseline titers showed greater fold rise at 1 month postvaccination, with a steep inverse dependence between GMFRs and baseline titers (correlation, –0.53; Supplementary Figure 4).
Figure 3.
Respiratory syncytial virus (RSV) neutralizing geometric mean titers before and 1 month post–vaccination 1 for RSV subgroups A and B among expanded cohort participants aged 18–49 years (A) and 65–85 years (B). Neutralizing titer geometric mean fold rises from prevaccination to 1 month postvaccination are indicated above each pair of bars. Total number of participants: 38–41 per group (18–49 years) and 38–43 per group (65–85 years). Abbreviations: Al(OH)3, aluminum hydroxide; GMFR, geometric mean fold rise; GMT, geometric mean titer; m, month; RSV, respiratory syncytial virus; SIIV, seasonal inactivated influenza vaccine.
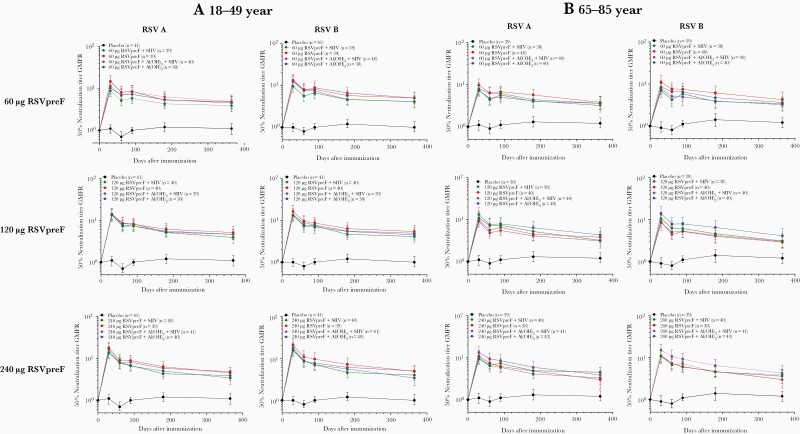
Serum RSV neutralizing titers in the older adult expanded cohort remained above baseline through 12 months postvaccination (Figure 4), with GMFRs of 3.0–4.5 for RSV A and 2.9–4.3 for RSV B; corresponding GMFRs for placebo recipients were 1.2 for each RSV subgroup (findings were similar for older adults in the sentinel cohort; Supplementary Figure 5). GMFRs were also above baseline through 12 months postvaccination in younger adults in the expanded cohort (Figure 4). A similar trend was observed for prefusion F–binding IgG (Supplementary Figures 6 and 7). Neither dose nor formulation had a major impact on durability of antibody responses.
Figure 4.
Respiratory syncytial virus (RSV) neutralizing titer geometric mean fold rises through 12 months post–vaccination 1 compared with prevaccination for RSV subgroups A and B in participants aged 18–49 years (A) and 65–85 years (B) in the expanded cohort. Abbreviations: Al(OH)3, aluminum hydroxide; GMFR, geometric mean fold rise; RSV, respiratory syncytial virus; RSVpreF, bivalent respiratory syncytial virus prefusion F vaccine; SIIV, seasonal inactivated influenza vaccine.
A high GMR was observed for the combined RSV A and B neutralizing titer fold rise to prefusion F binding IgG fold rise among 50- to 85-year-old RSVpreF recipients (without concomitant SIIV) 1 month postvaccination, regardless of RSVpreF formulation (Supplementary Figure 8).
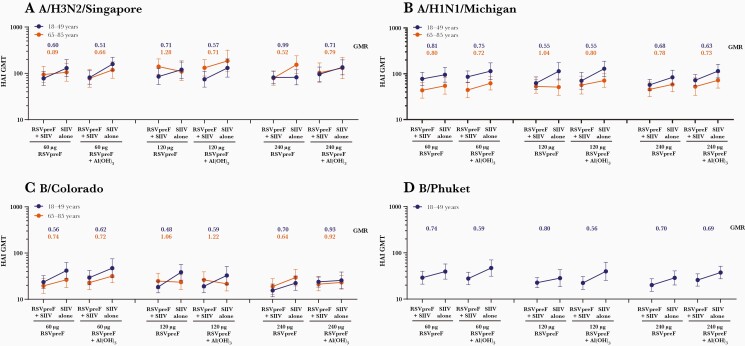
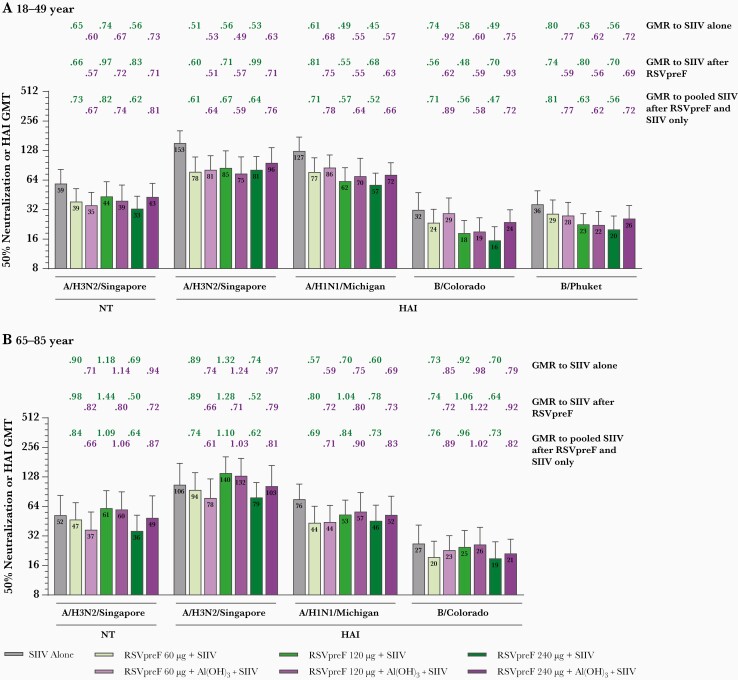
Before vaccination, HAI and neutralizing GMTs were generally comparable between groups except for titers against A/H1N1/Michigan, which were lower in expanded cohort participants 65–85 years of age than in participants 18–49 years of age. One month postvaccination, immune responses to SIIV as measured by HAI GMTs were generally lower for those expanded cohort participants 18–49 and 65–85 years of age coadministered RSVpreF with SIIV vs those receiving SIIV separately (Figure 5). In general, the reduction in SIIV immune responses with RSVpreF coadministration was more pronounced among younger participants, who received standard dose SIIV, than among older participants, who received high-dose SIIV (Figure 6).
Figure 5.
Hemagglutination inhibition (HAI) geometric mean titers to A/H3N2/Singapore (A), A/H1N1/Michigan (B), B/Colorado (C), and B/Phuket (D) at 1 month after seasonal inactivated influenza vaccine (SIIV) in participants aged 18–49 and 65–85 years receiving either SIIV concomitantly with bivalent respiratory syncytial virus prefusion F vaccine (RSVpreF) (RSVpreF + SIIV) or SIIV 1 month after RSVpreF (SIIV alone) in the expanded cohort (evaluable influenza immunogenicity population). Total number of participants = 31–41 per group. Geometric mean ratios at 1 month after SIIV for concomitant (RSVpreF + SIIV) to sequential (SIIV alone) administration are shown above each bar. Participants 18–49 years of age received quadrivalent SIIV containing A/H3N2/Singapore, A/H1N1/Michigan, B/Colorado, and B/Phuket; participants 65–85 years of age received high-dose trivalent SIIV containing A/H3N2/Singapore, A/H1N1/Michigan, and B/Colorado. Abbreviations: Al(OH)3, aluminum hydroxide; GMR, geometric mean ratio; GMT, geometric mean titer; HAI, hemagglutination inhibition; RSVpreF, bivalent respiratory syncytial virus prefusion F vaccine; SIIV, seasonal inactivated influenza vaccine.
Figure 6.
Seasonal inactivated influenza vaccine (SIIV) neutralizing geometric mean titers (GMTs) (A/H3N2/Singapore) and hemagglutination inhibition (HAI) GMTs (A/H3N2/Singapore, A/H1N1/Michigan, B/Colorado, B/Phuket) at 1 month after SIIV in participants aged 18–49 years (A) and 65–85 years (B) in the expanded cohort (evaluable influenza immunogenicity population). Total number of participants = 34–41 per group. Geometric mean ratios at 1 month after SIIV for bivalent respiratory syncytial virus prefusion F vaccine (RSVpreF) + SIIV to SIIV alone (ie, 1 month after placebo), to SIIV after RSVpreF, and to pooled SIIV after RSVpreF and SIIV alone are shown above each bar. Participants 18–49 years of age received quadrivalent SIIV containing A/H3N2/Singapore, A/H1N1/Michigan, B/Colorado, and B/Phuket; participants 65–85 years of age received high-dose trivalent SIIV containing A/H3N2/Singapore, A/H1N1/Michigan, and B/Colorado. Abbreviations: Al(OH)3, aluminum hydroxide; GMR, geometric mean ratio; GMT, geometric mean titer; HAI, hemagglutination inhibition; NT, neutralization titer; RSVpreF, bivalent respiratory syncytial virus prefusion F vaccine; SIIV, seasonal inactivated influenza vaccine.
DISCUSSION
Historically, RSV has primarily been considered a pediatric pathogen, but during the past 30 years it has been increasingly recognized as an important cause of respiratory disease among older adults. Despite 50 years of vaccine-development efforts, no vaccine to protect against RSV disease in any population has been licensed [12]. The current study evaluated the safety and immunogenicity of a novel RSV vaccine (RSVpreF) in healthy older adults with the overall aim to boost immune responses in this population sufficiently to protect against RSV disease.
In healthy adults 50–85 years of age, RSVpreF was safe and well tolerated. The majority of local reactions and systemic events were mild or moderate in severity across RSVpreF doses and formulations, with a potential trend toward higher rates of local reactions for RSVpreF formulated with Al(OH)3. Coadministration with SIIV did not have a substantive impact on reactogenicity or safety. Proportions of participants reporting AEs were similar across RSVpreF groups, and no SAEs were considered related to the study vaccine. These safety findings are consistent with phase 1/2 study results in adults 18–49 years of age (reported separately).
At 1 month postvaccination, RSVpreF elicited vigorous serum-neutralizing responses in older adults that were consistent with responses observed in younger adults. From baseline to 1 month after vaccination, serum-neutralizing GMFRs ranged from 7 to 15 in participants 65–85 years of age, 9 to 21 among those aged 18–49 years, and 11 to 27 among those aged 50–85 years in the sentinel cohort. Observed GMFRs were substantially higher than those previously shown in clinical studies of RSV vaccine candidates in which F was not stabilized in the prefusion conformation [19, 20]. Additionally, robust neutralizing responses were observed against RSV subgroups A and B, both of which cause substantial disease, varying temporally and geographically [24]. Furthermore, much of the vaccine-elicited antibody effectively neutralizes RSV, as the GMR of the combined RSV A and B neutralizing titer fold rise to prefusion F–binding IgG fold rise among 50- to 85-year-old RSVpreF recipients was 0.78–0.87 across dose levels and formulations. Durability of responses were demonstrated with neutralizing titer GMFRs remaining 3- to 4-fold above baseline levels in adults aged 65–85 years and 3- to 5-fold higher in adults aged 18–49 years through 12 months of study. These findings are comparable to those for another investigational RSV prefusion F subunit vaccine, which in adults aged 18–50 years elicited robust RSV neutralizing activity at week 4 postvaccination that remained ≥2.8-fold higher than baseline at week 44 [25]. Adsorption to Al(OH)3 did not augment neutralizing responses to RSVpreF, similar to other investigational RSV F vaccines [25, 26]. Robust immunogenicity in older adults is particularly encouraging given the age-related reductions in antibody responses observed for other vaccines in this population, including influenza vaccines [27]. Taken together, RSVpreF induces robust immune responses in healthy adults 18–85 years of age, with an observed persistence through 12 months, suggesting potential benefit beyond a single RSV season.
For all ages, coadministration of RSVpreF with SIIV did not affect RSV immunogenicity, regardless of dose level or formulation. However, immune responses to SIIV trended lower when RSVpreF was coadministered with SIIV compared with SIIV administered alone. This reduction in responses to SIIV was more pronounced in younger adults, who received standard dose SIIV (15 µg hemagglutinin per strain), than in older adults, who received high-dose SIIV (60 µg hemagglutinin per strain) [22, 23]. The underlying reason for the interference is unclear, but it is notable that RSV prefusion F is a relatively immunodominant antigen, as shown by (1) the high RSV neutralizing responses to RSVpreF without adjuvant, and (2) the lack of effect of SIIV on RSVpreF immunogenicity. However, as this was a dose-ranging, hypothesis-generating study designed primarily to evaluate safety, these observations require further study to determine clinical significance.
The strengths of this study include its large size, randomized controlled design, and evaluation of multiple vaccine formulations administered alone or concomitantly with SIIV. Limitations are a lack of statistical power to analyze comparisons across groups and uncertain generalizability of these findings to adults at greatest risk of severe disease (ie, elderly with cardiopulmonary disease or immunosuppression). Additional safety and immunogenicity evaluations of RSVpreF in adult populations will further inform use for this population, including a phase 1/2 study in older adults (NCT03572062) assessing additional adjuvanted formulations of RSVpreF and a phase 2 study in adults (NCT04785612) assessing RSVpreF safety, immunogenicity, and efficacy against experimental challenge with RSV.
This phase 1/2 study showed that different formulations and dose levels of RSVpreF administered alone or with SIIV were safe, well tolerated, and highly immunogenic in healthy older adults. A 120-µg RSVpreF dose formulated without Al(OH)3 has been selected for further evaluation, as this dose formulation elicited the strongest immune response in the presence of the most favorable overall safety profile across multiple studies. In addition, future evaluation of RSVpreF/SIIV coadministration in adults will use high-dose or adjuvanted SIIV formulations. This study supports continued development of RSVpreF to protect vulnerable adult populations from RSV disease, with further study regarding potential annual concomitant immunization with SIIV.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank those who participated in the study and those who conducted the study, including nurses, physicians, coordinators, clinical scientists, laboratory personnel, and other critical colleagues. We also thank the following individuals at Pfizer Inc: Katie McDonald, Christine Juergens, David Radley, Mary Beth Turner, Han-Qing Jiang, Luke Cunliffe, Naren Surampalli, Michelle McLean, and Keri Clarke at SRG; and the following individuals at the University of Rochester: Angela R. Branche, Emily Pierce, Mary Criddle, Sally Thomas, and Doreen Francis. This study was sponsored by Pfizer Inc. Editorial/medical writing support was provided by Emily Stackpole, PhD, Allison Gillies, PhD, and Philippa Jack, PhD, of ICON (Blue Bell, PA, USA), and was funded by Pfizer Inc.
Data sharing. Upon request and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Disclaimer. The funder was involved with study concept and design; collection, analysis, and interpretation of the data; drafting of the manuscript; and the decision to submit the manuscript for publication.
Financial support. This work was supported by Pfizer Inc.
Potential conflicts of interest. A. R. F. reports grants from Merck, Pfizer, BioFire Diagnostics, and Janssen and serves on a data and safety monitoring board for Novavax. E. E. W. reports grants from Merck and Janssen and has also served as an unpaid consultant to Novavax, Merck, GlaxoSmithKline, and Janssen. All other authors are employees of Pfizer Inc and may hold stock and/or stock options.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek, Washington, District of Columbia, USA, 2–6 October 2019; and Fifth ReSViNET conference (RSVVW), Accra, Ghana, 12–14 November 2019.
Contributor Information
Ann R Falsey, Department of Medicine, Infectious Diseases Division, Rochester General Hospital and University of Rochester Medical Center, Rochester, New York, USA.
Edward E Walsh, Department of Medicine, Infectious Diseases Division, Rochester General Hospital and University of Rochester Medical Center, Rochester, New York, USA.
Daniel A Scott, Vaccine Research and Development, Pfizer Inc, Collegeville, Pennsylvania, USA.
Alejandra Gurtman, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Agnieszka Zareba, Vaccine Research and Development, Pfizer Inc, Collegeville, Pennsylvania, USA.
Kathrin U Jansen, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
William C Gruber, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Philip R Dormitzer, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Kena A Swanson, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Qin Jiang, Vaccine Research and Development, Pfizer Inc, Collegeville, Pennsylvania, USA.
Emily Gomme, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
David Cooper, Vaccine Research and Development, Pfizer Inc, Pearl River, New York, USA.
Beate Schmoele-Thoma, Vaccine Research and Development, Pfizer Pharma GMbH, Berlin, Germany.
References
- 1. Li Y, Johnson EK, Shi T, et al. National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med 2021; 9:175–85. [DOI] [PubMed] [Google Scholar]
- 2. Walsh EE. Respiratory syncytial virus infection: an illness for all ages. Clin Chest Med 2017; 38:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE.. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 4. Falsey AR, McElhaney JE, Beran J, et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis 2014; 209:1873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, Falsey AR.. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis 2013; 207:1424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sundaram ME, Meece JK, Sifakis F, Gasser RA Jr, Belongia EA.. Medically attended respiratory syncytial virus infections in adults aged ≥50 years: clinical characteristics and outcomes. Clin Infect Dis 2014; 58:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoon JG, Noh JY, Choi WS, et al. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci Rep 2020; 10:12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wyffels V, Kariburyo F, Gavart S, Fleischhackl R, Yuce H.. A real-world analysis of patient characteristics and predictors of hospitalization among US Medicare beneficiaries with respiratory syncytial virus infection. Adv Ther 2020; 37:1203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cusi MG, Martorelli B, Di Genova G, Terrosi C, Campoccia G, Correale P.. Age related changes in T cell mediated immune response and effector memory to respiratory syncytial virus (RSV) in healthy subjects. Immun Ageing 2010; 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walsh EE, Falsey AR.. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis 2004; 190:373–8. [DOI] [PubMed] [Google Scholar]
- 11. Domachowske JB, Anderson EJ, Goldstein M.. The future of respiratory syncytial virus disease prevention and treatment. Infect Dis Ther 2021; 10:47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyoglu-Barnum S, Chirkova T, Anderson LJ.. Biology of infection and disease pathogenesis to guide RSV vaccine development. Front Immunol 2019; 10:1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aranda SS, Polack FP.. Prevention of pediatric respiratory syncytial virus lower respiratory tract illness: perspectives for the next decade. Front Immunol 2019; 10:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aliprantis AO, Shaw CA, Griffin P, et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum Vaccin Immunother 2021; 17:1248–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson PR, Collins PL.. The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J Gen Virol 1988; 69:2623–8. [DOI] [PubMed] [Google Scholar]
- 16. McLellan JS. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr Opin Virol 2015; 11:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calder LJ, Gonzalez-Reyes L, Garcia-Barreno B, et al. Electron microscopy of the human respiratory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology 2000; 271:122–31. [DOI] [PubMed] [Google Scholar]
- 18. Ngwuta JO, Chen M, Modjarrad K, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 2015; 7:309ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Falloon J, Yu J, Esser MT, et al. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017; 216:1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med 2020; 383:426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crank MC, Ruckwardt TJ, Chen M, et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019; 365:505–9. [DOI] [PubMed] [Google Scholar]
- 22. Sanofi Pasteur Inc. FLUZONE quadrivalent full prescribing information. Swiftwater, PA: Sanofi Pasteur Inc, 2018. [Google Scholar]
- 23. Sanofi Pasteur Inc. FLUZONE high-dose full prescribing information. Swiftwater, PA: Sanofi Pasteur Inc, 2018. [Google Scholar]
- 24. Hall CB, Walsh EE, Schnabel KC, et al. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis 1990; 162:1283–90. [DOI] [PubMed] [Google Scholar]
- 25. Ruckwardt TJ, Morabito KM, Phung E, et al. Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir Med 2021; 9:1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beran J, Lickliter JD, Schwarz TF, et al. Safety and immunogenicity of 3 formulations of an investigational respiratory syncytial virus vaccine in nonpregnant women: results from 2 phase 2 trials. J Infect Dis 2018; 217:1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gustafson CE, Kim C, Weyand CM, Goronzy JJ.. Influence of immune aging on vaccine responses. J Allergy Clin Immunol 2020; 145:1309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.