Abstract
Background
Porcine circovirus type 1 (PCV-1) material was detected in the human rotavirus vaccine (HRV) in 2010. In this study we compared immunogenicity and safety of the PCV-free HRV vaccine (PCV-free HRV) with HRV. PCV-free HRV is an HRV with no detectable PCV-1 and PCV-2 according to the limit of detection of the tests used.
Methods
Healthy infants 6–12 weeks of age were randomized (1:1:1:1) to receive 2 doses of 1 of the 3 lots of PCV-free HRV or HRV. The study objectives were to demonstrate lot-to-lot consistency of the PCV-free HRV and noninferiority of PCV-free HRV as compared to HRV in terms of immunogenicity, 1–2 months post dose 2. Reactogenicity and safety were also assessed.
Results
Overall, 1612 infants were enrolled and 1545 completed the study. Study objectives were demonstrated because the predefined criteria were met. Among participants receiving PCV-free HRV and HRV, 79.27% and 81.76% seroconverted and geometric mean concentrations were 159.5 and 152.8 U/mL, respectively. The incidences of adverse events and serious adverse events were similar between the pooled PCV-free HRV and HRV groups.
Conclusions
The 3 PCV-free HRV lots demonstrated consistency and PCV-free HRV was noninferior compared to HRV in terms of immunogenicity.
Clinical trials registration
Keywords: rotavirus vaccine, porcine circovirus type 1, porcine circovirus-free, immunogenicity, safety
The immunogenicity of PCV-free HRV was noninferior to that of HRV when administered to infants according to a 2-dose series. Consistency across different PCV-free HRV lots was demonstrated. Adverse events incidence was similar following vaccination with PCV-free HRV or HRV.
Rotavirus (RV) is the leading cause of severe dehydrating gastroenteritis (GE) in children younger than 5 years and accounts for substantial morbidity globally and mortality in resource-limited countries [1]. Although effective RV vaccines have been available for more than a decade and have led to a substantial decrease in disease burden and mortality rates caused by severe RV GE, in 2016 RV infection caused 128 530 deaths and 258 173 278 episodes of diarrhea among children younger than 5 years, with the majority of these deaths occurring in developing countries [2].
The World Health Organization first recommended RV vaccination in 2006 and afterwards, in 2009, issued a reinforcement, mentioning that RV vaccination should be offered to infants in all regions worldwide, especially in countries with high diarrhea-related mortality rates [3]. More than 95 countries globally have implemented RV vaccination in their national immunization programs [4]. The live-attenuated human RV vaccine licensed at the time of study conduct (HRV, Rotarix; GSK) has proved to be efficacious, immunogenic, and well tolerated in various clinical trials conducted worldwide [5–9]. In addition, postmarketing studies have also demonstrated indirect benefits from RV vaccination such as herd effect and reduction of nosocomial infections along with significant cost savings for the societal and health care systems [10–12].
In 2010, porcine circovirus 1 (PCV-1) material was unexpectedly discovered in Rotarix and PCV-1 and PCV-2 material was identified in human-bovine reassortant vaccine (HBRV, RotaTeq; Merck) [13, 14]. PCV-1 and PCV-2 are not known to cause disease in humans. Subsequent independent and internal investigations indicated that human infection with PCV-1 did not occur after vaccination [13, 15–17]. In addition, the postmarketing surveillance data (with more than 69 million doses distributed at the time of the finding) had not detected any signal or a safety risk attributable to the presence of PCV-1 in the vaccine [13].
GSK informed health authorities worldwide (including the European Medicines Agency [18], Food and Drug Administration [16], and World Health Organization [19]) about the presence of PCV-1 DNA in Rotarix and committed to develop a PCV-free vaccine. The newly developed PCV-free vaccine is an HRV vaccine with no detection of PCV-1 and PCV-2 according to the limit of detection of the tests used. As part of this development, a phase 3 clinical study was conducted to compare the PCV-free product to the HRV after a sufficient level of comparability was reached for technical and production data. Here we present the immunogenicity and safety outcomes of this first trial with the PCV-free HRV, which will eventually replace HRV after approval by regulatory authorities.
METHODS
Study Design and Participants
This was a phase 3a, randomized, observer-blind study conducted at 66 centers in 8 countries (Costa Rica, Finland, Germany, Japan, Republic of Korea, Spain, Taiwan, and the United States) between October 2016 and November 2018.
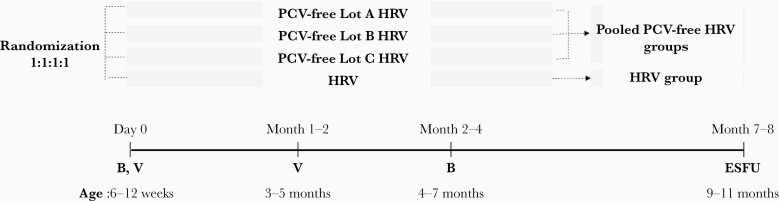
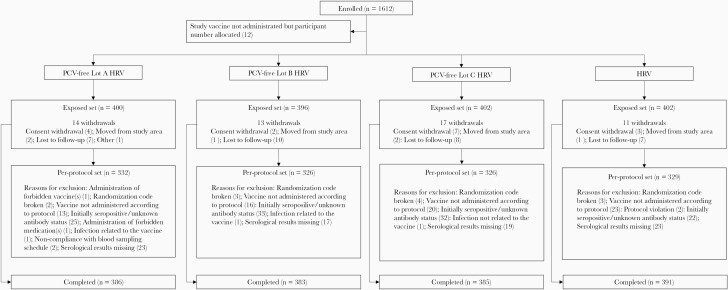
Healthy infants, 6–12 weeks of age were eligible for enrollment if their parents/legally acceptable representatives (LARs) provided written informed consent and were able and willing to comply with the study procedures. Infants who previously received any vaccination against RV, had a confirmed case of RV GE, or had a history of intussusception were not eligible for enrollment. A full list of inclusion and exclusion criteria is provided in Supplementary Text 1. Infants were randomized (1:1:1:1) in 4 groups. Three groups received PCV-free HRV, each group a different lot (lots A, B, and C), and 1 group received HRV, at 6–12 weeks and 3–5 months of age (Figure 1). Concomitant administration of routine pediatric vaccines was allowed, according to the immunization practices in each country. The study enrollment was staggered, with approximately 10% of the participants initially enrolled. An independent data monitoring committee (IDMC) assessed the reactogenicity and safety data of these infants before enrollment of the remaining approximately 90% (Figure 2).
Figure 1.
Study design. Abbreviations: B, blood sample; ESFU, extended safety follow-up contact; HRV, human rotavirus vaccine licensed at the time of study conduct; PCV-free Lot A HRV, PCV-free Lot B HRV, and PCV-free Lot C HRV, groups receiving the porcine circovirus-free human rotavirus vaccine from lots A, B, or C; Pooled PCV-free HRV, pooled porcine circovirus-free human rotavirus vaccine groups; V, vaccination.
Figure 2.
Flow of participants. Abbreviations: HRV, group receiving human rotavirus vaccine licensed at the time of study conduct; n, number of infants in each group; PCV-free Lot A HRV, PCV-free Lot B HRV, PCV-free Lot C HRV, groups receiving the porcine circovirus-free human rotavirus vaccine from lots A, B, or C.
The study protocol, amendments, informed consent form, and other information that required preapproval were reviewed and approved by a national, regional, or investigational center independent ethics committee or institutional review board. The study was conducted in compliance with the Declaration of Helsinki and International Committee on Harmonization Guidelines for Good Clinical Practice and is registered at ClinicalTrials.gov (NCT02914184) and EudraCT (2016-000598-19). The full protocol is available at https://www.gsk-studyregister.com/study/4517.
Study Objectives
The first coprimary objective assessed the lot-to-lot consistency of the PCV-free HRV in terms of immunogenicity as measured by serum anti-RV immunoglobulin A (IgA) antibody concentrations at 1–2 months after dose 2. Consistency was considered met if the 2-sided 95% confidence intervals (CIs) of the geometric mean concentration (GMC) ratio between all lot pairs were within the .5–2.0 interval (the predefined clinical limit interval for consistency).
The second coprimary objective assessed the immunological noninferiority of PCV-free HRV as compared to HRV in terms of seroconversion rates 1–2 months after dose 2. Noninferiority was demonstrated if the lower limit of the 2-sided 95% CI for the difference in seroconversion rate between the PCV-free HRV group (pooled PCV-free HRV groups) and HRV group was −10% or higher (the predefined clinical limit for noninferiority).
The third coprimary objective assessed the immunological noninferiority of PCV-free HRV as compared to HRV in terms of serum anti-RV IgA antibody GMCs 1–2 months after dose 2. Noninferiority was demonstrated if the lower limit of the 2-sided 95% CI for the GMC ratio (PCV-free HRV [pooled groups]/HRV group) was ≥0.67 (the predefined clinical limit for noninferiority).
Vaccines
The PCV-free HRV is a liquid vaccine presented in a prefilled oral applicator [20]. One dose has a volume of 1.5 mL and contains ≥106.0 median cell culture infective dose 50% (CCID50) of HRV strain RIX4414. PCV-free was defined as a negative result of quantitative polymerase chain reaction (qPCR) for PCV-1 and PCV-2 DNA (limit of detection [LOD] of 15 copies/million cells) and by the absence of PCV-1 infective particle through the PCV-1 infectivity assay (LOD of 10 CCID50 per assay corresponding to 1 CCID50/mL).
The HRV used in this study was the lyophilized formulation licensed at the time of study conduct [20]. One dose had a volume of 1 mL and contained ≥106.0 (CCID50) HRV strain RIX 4414 mixed with a CaCO3-based diluent.
The 2 oral doses of the HRV were administered at 1-month or 2-months interval, as per the RV vaccination schedule in participating countries.
Immunogenicity Assessment
Blood samples were collected before the first dose and 1–2 months after the second dose from each infant to measure serum anti-RV IgA antibody concentrations using a modified enzyme linked immunosorbent assay (ELISA; GSK laboratory, Rixensart, Belgium and Wavre, Belgium), which was described previously [21, 22]. The seroconversion rate was defined as the percentage of infants who were initially seronegative (ie, with anti-RV IgA antibody concentration <20 U/mL prior to the first dose) and developed anti-RV IgA antibody concentration ≥20 U/mL after the second dose.
Stool samples were collected from infants who experienced GE episodes from administration of the first dose up to 2 months post dose 2. A stool sample had to be collected as soon as possible after illness began and preferably not later than 7 days after the start of GE symptoms. Two occurrences of diarrhea were classified as separate episodes if there were 5 or more diarrhea-free days between episodes.
Stool samples were tested for RV antigen by ELISA (GSK laboratory, Rixensart, Belgium). Positive samples were tested by reverse transcription PCR (RT-PCR) followed by sequencing to determine the G and P genotype (DDL Diagnostic Laboratory, Rijswijk, Netherlands). If wild-type RV infection could not be excluded, infants were eliminated from the per-protocol set (PPS) immunogenicity analysis.
Safety and Reactogenicity Assessment
Solicited adverse events (AEs) were recorded by parents/LARs on diary cards distributed at each vaccination and returned at the next visit. Parents/LARs were queried about AEs at each visit and at the telephone contact at the end of the extended safety follow-up period (7–8 months post first vaccination).
Solicited AEs (fever [body temperature ≥38.0°C, recorded daily], irritability/fussiness, diarrhea [passage of 3 or more looser than normal stools within a day], vomiting [1 or more episodes of forceful emptying of partially digested stomach contents ≥1 hour after feeding within a day], loss of appetite, cough/runny nose) were recorded within 8 days following each vaccine dose.
Unsolicited AEs were recorded within 31 days following each vaccine dose.
Any GE episodes occurring within 8 days following each vaccine dose were recorded as a solicited AE (diarrhea). GE episodes were recorded as an unsolicited AE if 3 or more loose stools occurred at more than 8 days following each vaccine dose.
Serious AEs (SAEs) and AEs/SAEs leading to withdrawal from the study were recorded throughout the study up to 6 months after dose 2. SAEs related to study participation or concurrent GSK medication/vaccine were recorded from the time consent was obtained up to study end.
An IDMC comprising clinical experts and a biostatistician reviewed the unblinded safety data gathered during the study after the enrollment of 5%, 10%, and 50% of participants and during additional meetings. Enrollment was put on hold during IDMC review of data of 10% of infants and was to be resumed if no safety concerns were identified.
Statistical Analyses
Safety was evaluated in the exposed set (ES), which included all infants who received at least 1 dose of study vaccine. Immunogenicity was evaluated in the PPS, which included infants who received all doses according to protocol and had available immunogenicity data. A full list of inclusion criteria in the PPS is detailed in Supplementary Text 2. As the percentage of participants excluded from the PPS for analysis of immunogenicity was ≥5%, a second analysis based on the ES was performed to complement it.
The target enrollment was 1600 infants (400 in each group) to obtain at least 1280 evaluable infants (320 in each group) for the evaluation of the coprimary objectives, assuming that approximately 20% of the enrolled infants would not be evaluable.
To control the risk of concluding erroneously due to the multiple coprimary objectives, a hierarchical procedure was used with a 2.5% type I error. Each objective was considered met only if the associated criterion was met and the previous objective was met too. The statistical analyses were performed using SAS Drug Development.
RESULTS
Demographics
One thousand six hundred and twelve infants were enrolled. Among the 1600 infants who received at least 1 dose of study vaccine (ES), 1313 were included in the PPS for immunogenicity and 1545 infants completed the study (Figure 2).
The mean age of the infants at first dose was 8.4 (SD 1.5) weeks, 50.7% of them were girls, and the majority of the infants were white. Overall, demographic characteristics were comparable across groups (Table 1). A total of 51.9% and 46.1% of children received routine pediatric vaccines (including vaccines against diphtheria, tetanus, pertussis, hepatitis B, Haemophilus influenzae type b, Neisseria meningitidis, and Streptococcus pneumoniae) concomitantly with the first and second dose of RV vaccines, respectively.
Table 1.
Summary of Demographic Characteristics (Exposed Set)
| Group | PCV-Free Lot A HRV n = 400 | PCV-Free Lot B HRV n = 396 | PCV-Free Lot C HRV n = 402 | Pooled PCV -Free HRV n = 1198 | HRV n = 402 | Total n = 1600 |
|---|---|---|---|---|---|---|
| Age at first vaccine dose | ||||||
| Mean ± SD, wk | 8.5 ± 1.5 | 8.4 ± 1.5 | 8.4 ± 1.6 | 8.4 ± 1.5 | 8.5 ± 1.5 | 8.4 ± 1.5 |
| Median (min-max), wk | 9.0 (6–12) | 8.0 (6–12) | 9.0 (6–12) | 9.0 (6–12) | 8.5 (6–13) | 9.0 (6–13) |
| Age at second vaccine dose | ||||||
| Mean ± SD, wk | 15.1 ± 2.6 | 15.1 ± 2.6 | 15.0 ± 2.6 | 15.1 ± 2.6 | 15.0 ± 2.6 | 15.1 ± 2.6 |
| Median (min-max), wk | 15.0 (10–22) | 15.0 (10–22) | 15.0 (10–22) | 15.0 (10–22) | 15.0 (10–21) | 15.0 (10–22) |
| Sex, n (%) | ||||||
| Male | 192 (48.0) | 198 (50.0) | 197 (49.0) | 587 (49.0) | 199 (49.5) | 786 (49.1) |
| Geographic ancestry, n (%) | ||||||
| American Indian or Alaska Native | 6 (1.5) | 2 (0.5) | 3 (0.7) | 11 (0.9) | 5 (1.2) | 16 (1.0) |
| Asian | 95 (23.8) | 96 (24.2) | 98 (24.4) | 289 (24.1) | 95 (23.6) | 384 (24.0) |
| African or African American | 6 (1.5) | 9 (2.3) | 13 (3.2) | 28 (2.3) | 13 (3.2) | 41 (2.6) |
| Native Hawaiian or other Pacific Islander | 1 (0.3) | 0 (0) | 0 (0) | 1 (0.1) | 0 (0) | 1 (0.1) |
| Caucasian, Arabic, or North African | 263 (65.8) | 258 (65.2) | 262 (65.2) | 783 (65.4) | 262 (65.2) | 1045(65.3) |
| Other | 29 (7.3) | 31 (7.8) | 26 (6.5) | 86 (7.2) | 27 (6.7) | 113 (7.1) |
| Country, n (%) | ||||||
| Costa Rica | 23 (5.8) | 22 (5.6) | 22 (5.5) | 67 (5.6) | 23 (5.7) | 90 (5.6) |
| Finland | 29 (7.3) | 29 (7.3) | 29 (7.2) | 87 (7.3) | 30 (7.5) | 117 (7.3) |
| Germany | 18 (4.5) | 17 (4.3) | 18 (4.5) | 53 (4.4) | 18 (4.5) | 71 (4.4) |
| Japan | 40 (10.0) | 40 (10.1) | 40 (10.0) | 120 (10.0) | 40 (10.0) | 160 (10.0) |
| Republic of Korea | 15 (3.8) | 15 (3.8) | 15 (3.7) | 45 (3.8) | 15 (3.7) | 60 (3.8) |
| Spain | 125 (31.3) | 125 (31.6) | 126 (31.3) | 376 (31.4) | 125 (31.1) | 501 (31.3) |
| Taiwan | 37 (9.3) | 37 (9.3) | 38 (9.5) | 112 (9.3) | 38 (9.5) | 150 (9.4) |
| United States | 113 (28.3) | 111 (28.0) | 114 (28.4) | 338 (28.2) | 113 (28.1) | 451 (28.2) |
| Mean height ± SD at first vaccine dose, cm | 57.6 ± 2.6 | 57.6 ± 2.5 | 57.8 ± 2.6 | 57.7 ± 2.6 | 57.6 ± 2.7 | 57.6 ± 2.6 |
| Mean weight ± SD at first vaccine dose, kg | 5.2 ± 0.7 | 5.3 ± 0.7 | 5.3 ± 0.7 | 5.3 ± 0.7 | 5.2 ± 0.7 | 5.3 ± 0.7 |
| Mean BMI ± SD at first vaccine dose, kg/m2 | 15.8 ± 1.6 | 15.9 ± 1.6 | 15.8 ± 1.6 | 15.8 ± 1.6 | 15.8 ± 1.6 | 15.8 ± 1.6 |
Abbreviations: BMI, body mass index; HRV, human rotavirus vaccine licensed at the time of study conduct; min-max, minimum-maximum; n (%), number (percentage) of infants in a given category; PCV-free Lot A/B/C HRV, lots A, B, C of the porcine circovirus-free human rotavirus vaccine; Pooled PCV-free HRV, pooled PCV-free lot A/B/C HRV groups.
Immunogenicity
All primary objectives were met.
Lot-to-lot consistency for PCV-free HRV was demonstrated, as the 95% CIs for the between-group ratio of anti-RV IgA antibody GMCs among groups receiving 1 of the 3 lots, 1–2 months after the second vaccine dose were within the .5–2 interval (Table 2 and Supplementary Table 1).
Table 2.
Summary of Results for the Coprimary Objectives, Evaluated at 1–2 Months After the Second Vaccine Dose (Per-Protocol Set)
| Group | n | GMC/SC Rate | Assessed Outcome | Value (95% CI) |
|---|---|---|---|---|
| Lot-to-lot consistency between PCV-free HRV lots | ||||
| PCV-free Lot A HRV | 332 | 156.1 U/mL | Lot A/Lot B GMC ratio | 1.07 (.79–1.44) |
| PCV-free Lot B HRV | 326 | 145.9 U/mL | Lot A/Lot C GMC ratio | 0.88 (.65–1.19) |
| PCV-free Lot C HRV | 326 | 177.1 U/mL | Lot B/Lot C GMC ratio | 0.82 (.61–1.11) |
| Noninferiority of PCV-free HRV compared to HRV in terms of SC rates | ||||
| Pooled PCV-free HRV | 984 | 79.27% | Difference in SC rate (pooled PCV-free HRV–HRV) | −2.49 (−7.15 to 2.63) |
| HRV | 329 | 81.76% | ||
| Noninferiority of PCV-free HRV compared to HRV in terms of GMCs | ||||
| Pooled PCV-free HRV | 984 | 159.5 U/mL | GMC ratio (pooled PCV-free HRV/HRV) | 1.04 (.82–1.33) |
| HRV | 329 | 152.8 U/mL |
The adjusted GMC ratio was computed with an ANOVA model including the vaccine group (lot A, lot B, and lot C) and the country as fixed effects.
The seroconversion rate was defined as the percentage of infants who were initially seronegative (ie, with anti-RV IgA antibody concentration <20 U/mL prior the first dose) and developed anti-RV IgA antibody concentration ≥20 U/mL after the second dose.
Bolded values indicate that the lot-to-to consistency or noninferiority criteria were met.
Abbreviations: CI, confidence interval; GMC, geometric mean concentration estimated from the ANOVA model; HRV, human rotavirus vaccine licensed at the time of study conduct; n, number of infants with available results; PCV-free Lot A HRV, PCV-free lot B HRV, PCV-free Lot C HRV, lots A, B, and C of the porcine circovirus-free HRV; Pooled PCV-free, porcine circovirus-free HRV liquid formulation of pooled lot A/B/C groups; SC, seroconversion.
The PCV-free HRV was shown to be noninferior to HRV in terms of seroconversion rates and GMCs, 1–2 months after the second vaccine dose. The lower limit of the 95% CI for the difference in seroconversion rates between the pooled PCV-free HRV group and the HRV group was greater than the predefined criterion of −10% (Table 2). The lower limit of the 95% CI for the ratio of anti-RV IgA antibody GMCs (pooled PCV-free HRV/HRV) was greater than the predefined criterion of .67 (Table 2).
Seroconversion rates and anti-RV IgA GMCs are shown in Supplementary Table 1 for the 3 different lots of PCV-free HRV, for the pooled PCV-free HRV group, and the HRV group. Results of the analyses performed on the ES were in agreement with results of the analyses performed on the PPS (data not shown).
Of the 123 stool samples analyzed, potential wild-type RV infection was identified in 2 infants in the pooled PCV-free HRV group. The identified RV types were G2P[4] (in addition to G1 vaccine type) and G1 vaccine type/nontypeable P, respectively. As a consequence, the 2 infants were excluded from the PPS for immunogenicity.
Safety and Reactogenicity
Solicited AEs
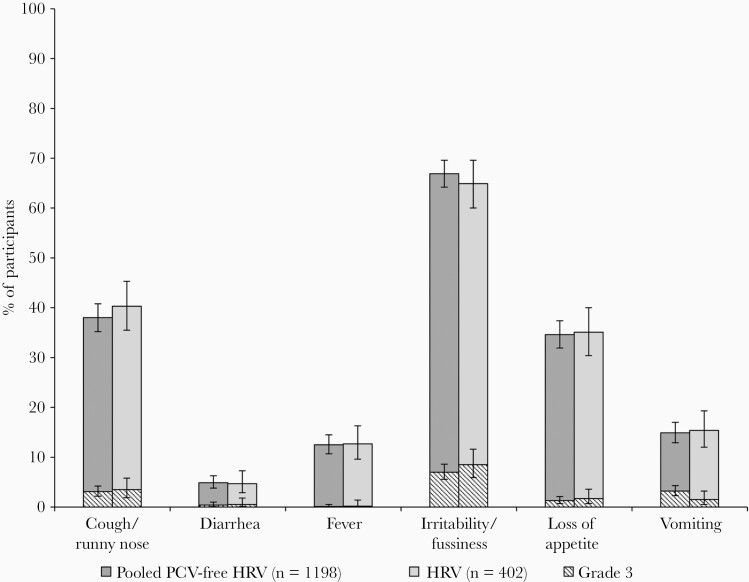
The occurrence of solicited AEs was similar in the pooled PCV-free HRV group and the HRV group (Figure 3 and Supplementary Table 2). Irritability/fussiness was the most commonly reported solicited AE (66.9% in the pooled PCV-free HRV group and 64.9% in the HRV group). Cough/runny nose was the most frequently reported AE leading to medically attended visits and was reported by similar percentages of infants in the pooled PCV-free HRV group and the HRV group.
Figure 3.
Solicited general adverse events after HRV vaccination, overall per infant (exposed set). Grade 3 was defined as preventing normal daily activity (irritability/fussiness and cough/runny nose), did not eat at all (loss of appetite), ≥6 looser than normal stools/day (diarrhea), ≥3 episodes of vomiting/day (vomiting), and body temperature >39.5°C (fever). Abbreviations: HRV, human rotavirus vaccine licensed at the time of study conduct; Pooled PCV-free HRV, pooled groups receiving the porcine circovirus-free human rotavirus vaccine.
Unsolicited AEs
The most commonly reported unsolicited AEs following each vaccine dose were upper respiratory tract infection, reported in 8.0% of infants in the pooled PCV-free HRV group and 7.5% of infants in the HRV group, and nasopharyngitis reported in 7.1% of infants in the pooled PCV-free HRV group and 7.2% of infants in the HRV group (Supplementary Table 3). Grade 3 unsolicited AEs were reported in 3.3% of infants in the pooled PCV-free HRV group and 2.0% of infants in the HRV group.
Unsolicited AEs considered causally related to vaccination were reported in 6.7% of infants (in the pooled PCV-free HRV group and the HRV group). The most frequently reported were diarrhea (2.8% of infants in pooled PCV-free HRV group and 3.0% of infants in HRV group) and flatulence (0.5% of infants in pooled PCV-free HRV group and 1.0% of infants in HRV group). Unsolicited AEs requiring medical attention were reported in 35.0% of infants in the pooled PCV-free HRV group and 33.1% of infants in the HRV group.
Throughout the study, SAEs were reported in 5.0% of infants in the pooled PCV-free HRV group and 4.5% of infants in the HRV group. None of the SAEs were considered by the investigator causally related to vaccination. For 1 infant in PCV-free lot C HRV group, intussusception was reported 132 days after receiving the second vaccine dose. The event was resolved after 3 days and was not considered as causally related to vaccination by the investigator. No AEs or SAEs leading to withdrawal and no deaths were recorded in this study.
DISCUSSION
In 2010, the discovery of PCV material in HRV and HBRV generated a series of tests by both independent health authorities and pharmaceutical companies. Extensive investigations have been conducted to identify the source, amount, and nature of the contamination as well as the potential clinical implications in vaccinated children. The most likely source of PCV-1 contamination of HRV appeared to be contaminated porcine-derived trypsin used to manufacture the Vero cell banks, the cell substrate used to produce HRV [13, 14]. PCV-1 is known to asymptomatically infect pigs and humans are frequently exposed to PCV-1 through dietary meat products. Existing evidence supports the view that PCV-1 does not cause infection in humans. The presence of adventitious agents in biological products is not unusual when animal or plant-derived materials are used during manufacturing process [19]. Its presence is typically detected when new technologies become available [20, 22].
In the context of PCV-1 contamination of HRV, retrospective testing identified PCV-1 in vaccine lots since its initial stages of development and in pivotal prelicensure clinical trials, which means that the acceptable safety profile of HRV reflects exposure to PCV-1 [15, 23]. Moreover, analysis of 40 archived stool samples from HRV-vaccinated children detected PCV-1 in 4 samples, only at earliest postvaccination timepoints, suggesting an absence of viral replication of PCV-1 in the gastrointestinal tract [13]. Similarly, postvaccination serologic response to PCV-1 in a large number of archived serum samples from HRV-vaccinated children did not reveal a statistical difference compared to the placebo arm (HRV group, 1% [90% CI, .3–2.6]; placebo group, 0.3% [90% CI, .0–1.6]) [15]. After thorough assessment and evaluation by health authorities, it has been concluded that there is no evidence that the presence of a small amount of PCV-1 in HRV poses a safety risk to vaccinated children [16, 18, 19] and PCV-1 presence in HRV has been recognized as a manufacturing quality issue [15]. However, GSK committed to regulatory authorities worldwide to develop a PCV-free HRV. New starting materials (including cell banks and seeds) have been introduced for the preparation of the PCV-free HRV, which follows the same process as that used for HRV [13].
Of note, in this study 2 different formulations of HRV (PCV-free HRV liquid versus HRV lyophilized) were used. A comparable immunological and safety profile between liquid and lyophilized formulations of the HRV has been demonstrated in previous clinical studies [24, 25]. The production of liquid HRV follows general recommendations from several health organizations encouraging development of liquid formulations due to their easier handling for administration, lower logistical costs, and higher manufacturing capacity [9, 25]. In view of this switching strategy in long-term vaccine supply, the PCV-free vaccine assessed in the present study is a liquid formulation.
The present study demonstrated that the PCV-free HRV can be consistently produced, which is a regulatory requirement for vaccine production. It was also shown to be immunologically noninferior to the vaccine licensed at the time of study conduct. The seroconversion rate following vaccination with the PCV-free HRV was 79.3%, which is in line with results from previous HRV studies reporting seroconversion rates ranging from 61.4% to 93.9% after 2-dose HRV [26]. Although no recognized immunological correlate of protection has been established to date, postvaccination anti-RV IgA seropositivity (antibody concentration ≥20 U/mL) is considered as a useful correlate of efficacy in clinical trials of HRV [26–30]. In the present study, the seroconversion rate was approximately 80% with anti-RV IgA GMC almost 8 times higher than the 20 U/mL threshold in both study groups after the second vaccine dose. Because infection with RV could influence immunogenicity results after RV vaccination, stool samples of GE episodes occurring up to 1–2 months post dose 2 were tested to identify wild-type RV and positive samples were excluded from the analyses. Based on the immunogenicity results in this trial, the same level of protection against RV GE disease is expected with the PCV-free HRV compared to HRV. HRV was shown to be efficacious against severe RV GE-associated hospitalizations in several large clinical trials conducted worldwide, with a vaccine efficacy ranging from 58.7% to 96.1% [6, 7, 31–35].
No marked imbalance in safety data was reported for the infants receiving PCV-free HRV and those receiving HRV. The incidence of infants reporting AEs and SAEs were similar between PCV-free HRV and HRV groups. Although the present study was not powered to draw confirmative conclusions on safety end points, the results are in agreement with the acceptable safety profile of HRV as reported in the integrated safety analyses including over 100 000 infants worldwide and showing that HRV was well tolerated [36]. The safety of the PCV-free HRV is being monitored in other clinical studies, which will contribute to a larger safety database.
CONCLUSIONS
The study demonstrated the lot-to-lot consistency of 3 production lots of PCV-free HRV and its noninferiority compared to HRV in terms of immunogenicity. In addition, the study showed that PCV-free HRV and HRV have a similar reactogenicity and safety profile.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all participants involved in the study, study nurses, coordinators, and study investigators; members of the Independent Data Monitoring Committee (Carlo Giaquinto, Shabir Madhi, Nigel Cunliffe, Catherine Legrand, and Dennis Conrad) for providing a valuable contribution in the review of the safety data; and Professor Timo Vesikari (FN) for his role as study coordinator and Elena García Sánchez (SP), Maria del Carmen Garcia (CR), and Silvia Guevara (CR) for their subinvestigator role. The authors also thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK (Maria Cornelia Maior and Silviu Bercea provided medical writing support and Emmanuelle Ghys coordinated manuscript development and editorial support).
Rota-081 group members . Javier Álvarez Aldeán (SP), Manuel Baca (SP), Mariano Miranda Valdivieso (SP), Agnes Schultz (US), Robyn Hartvickson (US), Randall Schumacher (US), Elizabeth Reyes (US), Nicola Klein (US), Peter Silas (US), Shelly Senders (US), Susan Moore (US), Kota Saito (JP), Akihito Sasaki (JP), Hiroshi Suzuki (JP), Hidetoshi Fujita (JP), Po-Yen Chen (TW), Cheng-Hsun Chiu (TW), Kao-Pin Hwang (TW), Ming-Ta Lee (TW), Aino Forsten (FN), Anitta Ahonen (FN), Ilkka Seppa (FN), Thomas Adelt (DE), Thorsten Krause (DE), Lothar Maurer (DE), Son Moon Shin (KR), Yong Sung Choi (KR), Ki-Hwan Kim (KR), and Hwang Min Kim (KR).
Trademark statement. Rotarix is a trademark owned by or licensed to the GSK group of companies. RotaTeq is a trademark of Merck.
Author contributions. All authors either participated in the design, implementation, or analysis, and interpretation of the study, as well as the development of this manuscript. All authors had full access to the data and granted their final approval of the paper before submission.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA. GlaxoSmithKline Biologicals SA was involved in all stages of the conduct and analysis of the studies, and covered the costs associated with the development and the publishing of the present article.
Potential conflicts of interest. D. B., C. D., L. M., and J. P. are employees of the GSK group of companies, and D. B. and C. D. hold shares in the GSK group of companies as part of the employee remuneration. A. U. was employed by the GSK group of companies during the conduct of this study and is a current employee of the Janssen Pharmaceutical companies of Johnson and Johnson. The institutions of M. H., M. L., and L.-M. H. received grants from the GSK group of companies for the conduct of this trial. J.-H. K. reports grants from GC Pharma, SK Bioscience, II-Yang Pharm, Boryung Pharma, and Sanofi-Pasteur outside the submitted work. P. B. received grants from various pharmaceutical companies to perform clinical trials and received nonbranded consulting fees from Sanofi-Pasteur, Pfizer, and Sequirus. M. L. reports grants from Merck, MedImmune, and Novartis outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Ignacio Salamanca de la Cueva, Instituto Hispalense de Pediatría, Sevilla, Spain.
Barbara Pahud, Children’s Mercy Hospitals, Kansas City, Missouri, USA.
Li-Min Huang, National Taiwan University Hospital, Taipei, Taiwan.
Michael Leonardi, Palmetto Pediatrics, North Charleston, South Carolina, USA.
José Garcia-Sicilia, Hospital Universitario Madrid Sanchinarro, Madrid, Spain.
Javier Céspedes, Clínica San Agustín, San José, Costa Rica.
Arturo Abdelnour, Instituto de Investigación en Ciencias Médicas, San José, Costa Rica.
Tsuyoshi Tamura, Hashimoto Clinic, Tokyo, Japan.
Haruo Kuroki, Sotobo Children’s Clinic, Chiba, Japan.
Nan-Chang Chiu, Mackay Memorial Hospital, Taipei, Taiwan.
Miia Virta, Tampere Vaccine Research Center, Tampere University, Tampere, Finland.
Satu Kokko, Tampere Vaccine Research Center, Tampere University, Tampere, Finland.
Michael Horn, Pediatric Office, Schoenau am Koenigssee, Germany.
Falko Panzer, Pediatric Office Dres. Panzer and Colleagues, Mannheim, Germany.
Jong-Hyun Kim, St Vincent’s Hospital, Catholic University of Korea, Suwon, Republic of Korea.
Jin Lee, Hanil General Hospital, Seoul, Republic of Korea.
Leentje Moerman, GlaxoSmithKline, Wavre, Belgium.
Christophe Debacq, GlaxoSmithKline, Wavre, Belgium.
Jose Parra, GlaxoSmithKline, Wavre, Belgium.
Ana Ugarte, Janssen Pharmaceutica companies of Johnson and Johnson, Beerse, Belgium.
Dan Bi, GlaxoSmithKline, Wavre, Belgium.
Rota-081 Study Group, Instituto Hispalense de Pediatría, Sevilla, Spain.
References
- 1. Khandoker N, Thongprachum A, Takanashi S, et al. Molecular epidemiology of rotavirus gastroenteritis in Japan during 2014–2015: Characterization of re-emerging G2P[4] after rotavirus vaccine introduction. J Med Virol 2018; 90:1040–6. [DOI] [PubMed] [Google Scholar]
- 2. Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 2018; 172:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Meeting of the Immunization Strategic Advisory Group of Experts, April 2009--conclusions and recommendations. Wkly Epidemiol Rec 2009; 84:220–36. [PubMed] [Google Scholar]
- 4. International Vaccine Access Center. VIEW-hub report: global vaccine introduction and implementation. a report on current global access to new childhood vaccines.https://view-hub.org/resourcesfile/VIEW-hubReports_Resources/2018_03/IVAC_VIEW-hub_Report%202018Mar.pdf. Accessed 27 March 2020.
- 5. Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu-Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics 2008; 122:e1062–6. [DOI] [PubMed] [Google Scholar]
- 6. Linhares AC, Velázquez FR, Pérez-Schael I, et al. ; Human Rotavirus Vaccine Study Group . Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181–9. [DOI] [PubMed] [Google Scholar]
- 7. Phua KB, Lim FS, Lau YL, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 2009; 27:5936–41. [DOI] [PubMed] [Google Scholar]
- 8. Steele AD, De Vos B, Tumbo J, et al. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine 2010; 28:6542–8. [DOI] [PubMed] [Google Scholar]
- 9. Anh DD, Carlos CC, Thiem DV, et al. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 (Rotarix™) oral suspension (liquid formulation) when co-administered with Expanded Program on Immunization (EPI) vaccines in Vietnam and the Philippines in 2006–2007. Vaccine 2011; 29:2029–36. [DOI] [PubMed] [Google Scholar]
- 10. Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J 2011; 30:e120–5. [DOI] [PubMed] [Google Scholar]
- 11. Standaert B, Alwan A, Strens D, Raes M, Postma MJ. Improvement in hospital quality of care (QoC) after the introduction of rotavirus vaccination: an evaluation study in Belgium. Hum Vaccin Immunother 2015; 11:2266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Standaert B, Strens D, Alwan A, Raes M. Medium- to long-term impact of rotavirus vaccination on hospital care in Belgium: a 7-year follow-up of the Rotavirus Belgium Impact Study (RotaBIS). Infect Dis Ther 2016; 5:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubin G, Toussaint JF, Cassart JP, et al. Investigation of a regulatory agency enquiry into potential porcine circovirus type 1 contamination of the human rotavirus vaccine, Rotarix: approach and outcome. Hum Vaccin Immunother 2013; 9:2398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petricciani J, Sheets R, Griffiths E, Knezevic I. Adventitious agents in viral vaccines: lessons learned from 4 case studies. Biologicals 2014; 42:223–36. [DOI] [PubMed] [Google Scholar]
- 15. Han HH, Karkada N, Jayadeva G, Dubin G. Serologic response to porcine circovirus type 1 (PCV1) in infants vaccinated with the human rotavirus vaccine, Rotarix™: a retrospective laboratory analysis. Hum Vaccin Immunother 2016; 13:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuehn BM. FDA: Benefits of rotavirus vaccination outweigh potential contamination risk. JAMA 2010; 304:30–1. [DOI] [PubMed] [Google Scholar]
- 17. Mijatovic-Rustempasic S, Immergluck LC, Parker TC, et al. Shedding of porcine circovirus type 1 DNA and rotavirus RNA by infants vaccinated with Rotarix®. Hum Vaccin Immunother 2017; 13:928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. European Medicines Agency. European Medicines Agency confirms positive benefit-risk balance of Rotarix: porcine circovirus type 1 in the oral vaccine poses no risk to public health.http://www.ema.europa.eu/en/documents/press-release/european-medicines-agency-confirms-positive-benefit-risk-balance-rotarix_en.pdf. Accessed 27 March 2020.
- 19. World Health Organization. Statement of the global advisory committee on vaccine safety on Rotarix.http://www.who.int/vaccine_safety/committee/topics/rotavirus/rotarix_statement_march_2010/en/. Accessed 27 March 2020.
- 20. European Medicines Agency. Summary of product characteristics. Rotarix. http://www.ema.europa.eu/en/documents/product-information/rotarix-epar-product-information_en.pdf. Accessed 27 March 2020.
- 21. Bernstein DI, Smith VE, Sherwood JR, et al. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89-12. Vaccine 1998; 16:381–7. [DOI] [PubMed] [Google Scholar]
- 22. Bernstein DI, Sack DA, Rothstein E, et al. Efficacy of live, attenuated, human rotavirus vaccine 89-12 in infants: a randomised placebo-controlled trial. Lancet 1999; 354:287–90. [DOI] [PubMed] [Google Scholar]
- 23. Aliabadi N, Tate JE, Parashar UD. Potential safety issues and other factors that may affect the introduction and uptake of rotavirus vaccines. Clin Microbiol Infect 2016; 22(suppl 5):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Velasquez AA, Dominguez E, Suryakiran PV, Delem A, Ortega-Barria E, Han HH. Immunogenicity of the oral live attenuated human rotavirus vaccine RIX4414 (Rotarix™) oral suspension (liquid formulation) co-administered with childhood vaccinations in Panama. Int J Infect Dis 2008; 12(suppl 1):e148. [Google Scholar]
- 25. Vesikari T, Karvonen A, Bouckenooghe A, Suryakiran PV, Smolenov I, Han HH. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 oral suspension (liquid formulation) in Finnish infants. Vaccine 2011; 29:2079–84. [DOI] [PubMed] [Google Scholar]
- 26. Cheuvart B, Neuzil KM, Steele AD, et al. Association of serum anti-rotavirus immunoglobulin A antibody seropositivity and protection against severe rotavirus gastroenteritis: analysis of clinical trials of human rotavirus vaccine. Hum Vaccin Immunother 2014; 10:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine 2006; 24:2718–31. [DOI] [PubMed] [Google Scholar]
- 28. Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis 2013; 208:284–94. [DOI] [PubMed] [Google Scholar]
- 29. Velázquez FR, Matson DO, Guerrero ML, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis 2000; 182:1602–9. [DOI] [PubMed] [Google Scholar]
- 30. Baker JM, Tate JE, Leon J, Haber MJ, Pitzer VE, Lopman BA. Post-vaccination serum anti-rotavirus immunoglobulin A as a correlate of protection against rotavirus gastroenteritis across settings [published online ahead of print 15 February 2020]. J Infect Dis doi: 10.1093/infdis/jiaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawamura N, Tokoeda Y, Oshima M, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011; 29:6335–41. [DOI] [PubMed] [Google Scholar]
- 32. Li RC, Huang T, Li Y, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother 2014; 10:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 34. Tregnaghi MW, Abate HJ, Valencia A, et al. ; Rota-024 Study Group . Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J 2011; 30:e103–8. [DOI] [PubMed] [Google Scholar]
- 35. Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757–63. [DOI] [PubMed] [Google Scholar]
- 36. Buyse H, Vinals C, Karkada N, Han HH. The human rotavirus vaccine Rotarix™ in infants: an integrated analysis of safety and reactogenicity. Hum Vaccin Immunother 2014; 10:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.