Abstract
Background
A 25-mg dapivirine vaginal ring has been demonstrated to reduce risk of human immunodeficiency virus (HIV) acquisition in nonpregnant adult women. In this secondary analysis of studies conducted in US adolescent, lactating, and postmenopausal females, vaginal microbiota was assessed prior to and after ring use, and between dapivirine and placebo ring users.
Methods
Vaginal fluid swabs were collected before and after product use for the evaluation of microbiota using Nugent criteria, quantitative culture, and quantitative polymerase chain reaction.
Results
Vaginal ring use did not impact bacterial vaginosis prevalence among the 3 populations and was associated with minimal shifts in microbiota. Adolescents in both arms demonstrated an increased prevalence of Lactobacillus crispatus and a decrease in quantity of Megasphaera lornae. Postmenopausal active and placebo ring users demonstrated an increased prevalence of lactobacilli and non-albicans yeast, while dapivirine ring users demonstrated an increased prevalence of Candida albicans and increased quantity of group B Streptococcus and non-albicans yeasts. Prevotella species were increased in lactating women, whereas Prevotella timonensis increased in prevalence and concentration among adolescent and postmenopausal females and Prevotella bivia increased in prevalence among adolescent dapivirine ring users.
Conclusions
Dapivirine vaginal ring use was associated with minimal changes in the vaginal microbiota that are likely not clinically significant.
Keywords: dapivirine, vaginal ring, preexposure prophylaxis, microbiota
The impact of the dapivirine vaginal ring, a product for prevention of HIV in women, on the vaginal microbiota of adolescent, lactating, and postmenopausal women was evaluated. There was a minimal impact of ring use on the vaginal microbiota.
Globally, women are disproportionately burdened by the human immunodeficiency virus (HIV) epidemic, as they constitute more than half of all individuals living with HIV [1–3]. Socioeconomic structure, gender inequality, and constraints on reproductive rights restrict women from negotiating safe sexual practices. Developing safe and efficacious products that can be used discreetly by women are paramount for preventing the transmission and acquisition of HIV [4, 5]. Vaginal drug delivery systems are being developed as an alternative route for the administration of preexposure prophylaxis (PrEP) [6–8]. Vaginal rings containing the antiretroviral compound dapivirine (DPV) are currently being evaluated in African adolescents (ClinicalTrials.gov NCT03593655), pregnant African women (NCT03965923), and breastfeeding women (NCT04140266). Two completed phase 3 studies evaluating its efficacy in nonpregnant, reproductive-aged women suggests that the DPV vaginal ring is an acceptable and well-tolerated option for the prevention of HIV infection [9, 10]. The DPV ring received a positive scientific opinion from the European Medicines Agency and has been recommended for use by the World Health Organization.
The vaginal microbiome is a complex environment and its contribution to genital tract health and disease is well documented [11–18]. Studies assessing the impact of DPV drug release on vaginal microbiome stability are sparse [19–22]. Given that bacterial vaginosis (BV) is associated with an increased risk of acquiring HIV, drugs that are delivered locally ideally should not impact or alter the innate protection provided by a Lactobacillus-dominated vaginal microbiome. While Baeten et al noted that the protection afforded by the DPV vaginal ring did not differ by BV status [23], the impact among women in a hypoestrogenic state, including lactating and postmenopausal women where lactobacilli may be naturally depleted, is unknown. Understanding the impact of topical antiretroviral compounds on the vaginal microbiome in all women across the lifespan is an important measure of the safety profile and should be considered when developing and testing microbicide products [24].
The objective of this study was to perform an exploratory analysis comparing the prevalence and concentration of specific microbiota before and after DPV ring use in 3 trials conducted in adolescent, lactating, and postmenopausal women.
METHODS
We performed a longitudinal analysis on 208 women enrolled across 3 clinical trials conducted within the United States (US) to assess the impact of DPV ring (25 mg) or placebo ring use on specific vaginal microbiota compared to baseline. MTN-023/IPM 030 (NCT02028338) was a multisite, phase 2a, randomized, double-blind, placebo-controlled trial evaluating the safety and pharmacokinetics of DPV rings in sexually active cisgender women aged 15–17 years. DPV or placebo rings were inserted monthly for 6 months. Ninety-six participants were enrolled across 6 sites [25]. MTN-024/IPM 031 (NCT02010593) was also a phase 2a, multisite, randomized, double-blind, placebo-controlled trial evaluating the safety and pharmacokinetics of the DPV ring in postmenopausal women aged 45–65 years. DPV or placebo rings were inserted monthly for 3 months. Ninety-six participants were enrolled across 3 US sites [26]. MTN-029/IPM 039 (NCT02808949) was a 2-site, phase 1, open-label trial evaluating the pharmacokinetic profile of the DPV ring over 14 consecutive days of use in 16 healthy adult cisgender women who were at least 6 weeks postpartum and lactating (retaining the ability to express breast milk) but who were willing to abstain from breastfeeding their infant during study duration [27]. Premenopausal cisgender women were required to use a consistent and effective method of contraception prior to and throughout the study. All participants with urogenital complaints were excluded from enrollment. Protocols for each study were approved by local institutional review boards, and appropriate written consent and assent from adolescents were obtained prior to participation in study activities.
Vaginal fluid was collected using polyester-tipped Dacron swabs (Fisher Scientific, Pittsburgh, Pennsylvania) for the separate evaluation of microbiota using Nugent criteria, quantitative vaginal cultures, and quantitative polymerase chain reaction (qPCR) prior to ring insertion and at 4 and 12 weeks during ring use for each participant enrolled in both the MTN-023/IPM 031 and MTN-024/IPM 031 studies. Additional swabs were collected for each participant enrolled in MTN-023/IPM 031 at 24 weeks of ring use. Participants enrolled in MTN-029/IPM 039 had vaginal fluid swabs collected prior to ring insertion, on days 1, 7, and 14 during ring use, and 48 hours following ring removal.
Vaginal swabs collected for Nugent evaluation were immediately rolled onto glass slides, which were air-dried and shipped to a central laboratory at Magee-Womens Research Institute (Pittsburgh, Pennsylvania) for processing and testing. A Nugent score of 0–3 was considered optimal (Lactobacillus-dominant), 4–6 was considered intermediate (presence of lactobacilli and other bacterial morphotypes), and 7–10 was indicative of BV (predominance of other bacterial morphotypes and absence of lactobacilli) [28]. Vaginal swabs collected for quantitative cultures were placed in a commercially prepared transport collection system and shipped on ice packs to the laboratory. Quantitative cultures were performed as previously described [29]. All distinct colonies present in culture at their highest quantity were isolated, subcultured for purity, and identified using characteristic colony, Gram stain morphology, and appropriate biochemical testing. Yeast, Enterococcus faecalis, Escherichia coli, group B Streptococcus (GBS), and Staphylococcus aureus were selected for identification because of their associations with yeast vulvovaginitis, urinary tract infections, and/or toxic shock syndrome. Bacterial concentrations derived by culture were reported as log10 colony-forming units/mL.
Vaginal swabs collected for qPCR were immediately placed in a cryotube containing 400 µL of phosphate-buffered saline and frozen at –70°C and shipped to the testing laboratory on dry ice. Quantitative PCR was performed as previously described [30]. In brief, bacterial DNA was extracted using the QIAamp DNA mini kit (Qiagen, Valencia, California) with additional modifications to maximize bacterial yield. Previously developed primer sets targeting species-specific 16S ribosomal RNA genes were utilized for qPCR assays and included Lactobacillus crispatus, Lactobacillus iners, Lactobacillus jensenii, Lactobacillus gasseri, Limosilactobacillus vaginalis (previously classified as Lactobacillus vaginalis) [31], Gardnerella vaginalis, Atopobium vaginae, and Megasphaera lornae. Three additional primer sets for the detection of Prevotella bivia, Prevotella amnii, and Prevotella timonensis were referenced from published sequences [32, 33] and adapted and validated for our qPCR platform as previously described [30]. Lactobacillus crispatus, L. jensenii, and L. gasseri were chosen because of their association with low-diversity community state types, whereas L. iners was selected because of its high prevalence in women and because it represents the dominant microorganism in one vaginal community state [34, 35]. Little is known about the prevalence of Limosilactobacillus vaginalis colonization in adolescent, postmenopausal, and lactating women. The remaining targets were chosen because of their association with BV. Additionally, P. bivia and P. timonensis were chosen because of their association with increased genital tract inflammation [36, 37]. Furthermore, P. bivia was associated with an increased risk of HIV acquisition and poor outcomes observed in previous studies evaluating topical PrEP [36, 38, 39]. Standard curves ranging from 102 to 109 gene copies for absolute quantification were constructed from linearized plasmids. Vaginal swab samples, standards, and all controls were run in triplicate and detected and reported using a SYBR green platform. Bacterial concentrations were reported as log10 gene copies per swab.
Statistical Methods
One-way analysis of variance and Fisher exact tests were used to assess differences in demographic characteristics between the 3 cohorts. Prevalence and concentration of vaginal microbiota at enrollment were compared between the placebo and DPV arms and between the 3 study cohorts using Fisher exact test and the Mann–Whitney U test, respectively. Mixed-effects linear regression models were used to evaluate mean change in Nugent score and concentration of vaginal microbiota prior to and during placebo and DPV ring use. Modified Poisson regression models were used to compare the marginal prevalence of microbiota prior to and during placebo and DPV ring use. All swabs were analyzed for all participants. The median number of swabs collected per participant was 4, 3, and 3 for the MTN-023/IPM 031, MTN-024/IPM 031, MTN-029/IPM 031 studies, respectively. Change in prevalence was expressed as relative risk (with 95% confidence interval [CI] with values <1.0 [decrease] and values >1.0 [increase]). Change in concentration was expressed as the mean value (with 95% CI). The qPCR concentration was reported as zero if detection was outside the range of standard curves.
RESULTS
The mean age of participants in MTN-023 (adolescents), MTN-024 (postmenopausal), and MTN-029 (lactating) was 16.2, 56.8, and 29.9 years, respectively (P < .001). Mean body mass index (BMI) also differed with respect to participants. Adolescents (BMI, 25.0 kg/m2) and lactating women (BMI, 26.8 kg/m2) had significantly lower BMIs than postmenopausal women (BMI, 29.9 kg/m2) (P < .001). Adolescent females were more likely to self-identify as black (47.3%) or Hispanic (21.5%) compared with postmenopausal and lactating women who identified predominately as white (62.8% and 62.5%, respectively).
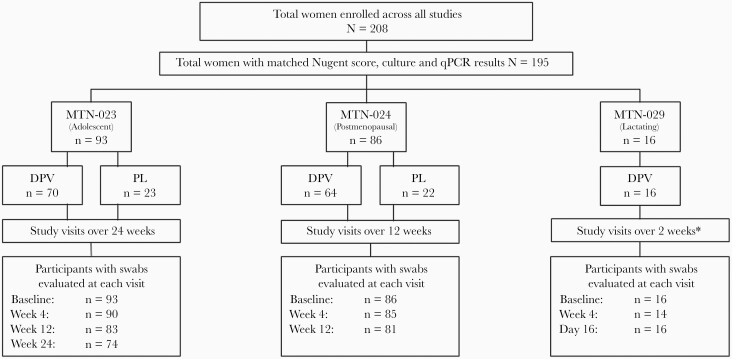
Figure 1 depicts the proportion of women stratified by ring group and number of swabs evaluated for Nugent score, culture, and qPCR at each visit as not all women initially enrolled into the study completed all visits. At baseline, women in each cohort differed with respect to Nugent score and vaginal microbiota (Table 1). Adolescent females (54.8%) were more likely to have a Lactobacillus-dominant Nugent score compared to postmenopausal (26.7%) and lactating women (37.5%). Postmenopausal women (51.2%) were more likely to have an intermediate Nugent score when compared to adolescent females (16.1%) or lactating women (25.0%), and BV was most prevalent in lactating women (37.5%) compared to adolescent females (29.0%) and postmenopausal women (22.1%). Compared with postmenopausal and lactating women, adolescent females were more likely to have L. iners (P < .001), L. jensenii (P = .025), Limosilactobacillus vaginalis (P = .018), M. lornae (P = .039), and Candida albicans (P < .001) at baseline.
Figure 1.
Total swabs evaluated for Nugent score, quantitative culture, and quantitative polymerase chain reaction, stratified by study and visit. ∗Includes visit at day 16 (2 days post–ring removal). Abbreviations: DPV, dapivirine vaginal ring; MTN-023, adolescent females; MTN-024, postmenopausal women; MTN-029, postpartum, lactating women; PL, placebo ring; qPCR, quantitative polymerase chain reaction.
Table 1.
Comparison of Nugent Score and Vaginal Microbiota Across All Populations of Women at Baseline
| Characteristic | Adolescent Females (n = 93) |
Postmenopausal Women (n = 86) |
Lactating Women (n = 16) |
P Value |
|---|---|---|---|---|
| Nugent score | <.001 | |||
| Normal | 51 (54.8) | 23 (26.7) | 6 (37.5) | |
| Intermediate | 15 (16.1) | 44 (51.2) | 4 (25.0) | |
| Bacterial vaginosis | 27 (29.0) | 19 (22.1) | 6 (37.5) | |
| Organism prevalence | ||||
| Lactobacillus crispatus | 33 (35.5) | 22 (25.6) | 2 (12.5) | .12 |
| Lactobacillus iners | 74 (79.6) | 37 (43.0) | 7 (43.8) | <.001 |
| Lactobacillus jensenii | 37 (39.8) | 19 (22.1) | 3 (18.8) | .025 |
| Lactobacillus gasseri | 31 (33.3) | 21 (24.4) | 5 (31.3) | .435 |
| Limosilactobacillus vaginalis | 32 (34.4) | 15 (17.7) | 2 (12.5) | .018 |
| Gardnerella vaginalis | 68 (73.1) | 49 (57.0) | 11 (68.8) | .072 |
| Atopobium vaginae | 44 (47.3) | 27 (31.4) | 6 (37.5) | .089 |
| Megasphaera lornae | 23 (24.7) | 10 (11.6) | 1 (6.3) | .039 |
| Prevotella bivia | 36 (38.7) | 21 (24.4) | 3 (18.8) | .077 |
| Prevotella amnii | 17 (18.3) | 6 (7.0) | 1 (6.3) | .056 |
| Prevotella timonensis | 56 (60.2) | 38 (44.2) | 6 (37.5) | .051 |
| Enterococcus faecalis | 25 (26.9) | 18 (20.9) | 2 (12.5) | .434 |
| Group B Streptococcus | 17 (18.3) | 7 (8.1) | 1 (6.3) | .106 |
| Staphylococcus aureus | 7 (7.5) | 1 (1.2) | 0 | .097 |
| Escherichia coli | 19 (20.4) | 19 (22.1) | 1 (6.3) | .357 |
| Candida albicans | 23 (24.7) | 2 (2.3) | 2 (12.5) | <.001 |
| Non-albicans yeast | 0 | 3 (3.5) | 0 | .207 |
Data are presented as No. (%) unless otherwise indicated. Bold values indicate significant differences between populations at baseline.
At baseline, there were no statistically significant differences observed in Nugent scores and vaginal microbiota when comparing adolescent females in the DPV or placebo ring groups (data not shown). As shown in Table 2, few changes were observed in Nugent scores and prevalence or concentration of vaginal microbiota between adolescent females following 6 months of DPV or placebo ring use. Although there was no increase in the prevalence of BV throughout ring use in either group, adolescent females using placebo rings had a marginal decrease in Nugent score while using the ring (P = .03). Adolescent females in both ring groups had an increase in prevalence of L. crispatus (DPV, P = .03; placebo, P = .03) and a decrease in the concentration of M. lornae (DPV, P = .002; placebo, P = .01), whereas adolescent females in the placebo ring group had a decreased prevalence of M. lornae (P = .03). Prevotella timonensis and P. bivia were the only bacteria that differed between study arms. Prevotella timonensis increased in prevalence and concentration among adolescents using the DPV ring from baseline through study duration (P = .01 and P = .02, respectively), and when compared to placebo ring users (P = .02) in whom there were slight decreases in P. timonensis during ring use (P > .23). The differences in P. bivia prevalence and concentration between the DPV and placebo ring users (P < .05) were due to the slight increase in P. bivia among DPV ring users (P > .24) and the slight decrease in placebo ring users (P > .23).
Table 2.
Change in Vaginal Microbiota Prevalence and Concentration Following Ring Use in Adolescent Females a
| Organism | Dapivirine Vaginal Ring-004 (n = 70) |
P Value DPV vs Baseline |
Placebo Ring (n = 23) |
P Value PL vs Baseline |
P Value PL vs DPV |
|---|---|---|---|---|---|
| BV prevalenceb | 0.94 (.66–1.24) | .73 | 0.63 (.36–1.11) | .11 | .21 |
| Nugent scorec | –0.12 (–.77 to .53) | .72 | –0.79 (–1.49 to –.09) | .03 | .12 |
| Lactobacillus crispatus | |||||
| Prevalenceb | 1.27 (1.02–1.58) | .03 | 1.37 (1.04–1.81) | .03 | .63 |
| Quantityc | 0.43 (–.05 to .91) | .08 | 0.69 (–.06 to 1.44) | .07 | .56 |
| Lactobacillus iners | |||||
| Prevalenceb | 1.04 (0.96–1.12) | .37 | 1.04 (.94–1.14) | .44 | .96 |
| Quantityc | –0.11 (–.56 to .34) | .63 | –0.12 (–.67 to .43) | .67 | .97 |
| Lactobacillus jensenii | |||||
| Prevalenceb | 0.98 (.83–1.15) | .82 | 0.89 (.62–1.26) | .50 | .60 |
| Quantityc | ––0.07 (–.47 to .33) | .72 | –0.15 (–.84 to .54) | .68 | .85 |
| Lactobacillus gasseri | |||||
| Prevalenceb | 1.22 (1.00–1.50) | .051 | 1.27 (1.00–1.63) | .055 | .78 |
| Quantityc | 0.36 (–.02 to .73) | .06 | 0.46 (–.03 to .96) | .07 | .72 |
| Limosilactobacillus vaginalis | |||||
| Prevalenceb | 1.11 (.88–1.40) | .36 | 1.35 (.97–1.88) | .07 | .27 |
| Quantityc | 0.25 (–.24 to .74) | .32 | 0.59 (–.26 to 1.44) | .17 | .46 |
| Gardnerella vaginalis | |||||
| Prevalenceb | 1.02 (.91–1.13) | .78 | 1.02 (.83–1.26) | .86 | .97 |
| Quantityc | –0.30 (–.82 to .23) | .27 | –0.29 (–1.13 to .55) | .50 | .99 |
| Atopobium vaginae | |||||
| Prevalenceb | 0.84 (.67–1.06) | .14 | 0.85 (.58–1.23) | .39 | .98 |
| Quantityc | –0.58 (–1.19 to .03) | .06 | –0.69 (–1.62 to .24) | .15 | .84 |
| Megasphaera lornae | |||||
| Prevalenceb | 0.74 (.55–1.00) | .053 | 0.52 (.29–.93) | .03 | .28 |
| Quantityc | –0.53 (–.88 to –.19) | .002 | –0.70 (–1.25 to –.14) | .01 | .55 |
| Prevotella bivia | |||||
| Prevalenceb | 1.16 (.90–1.51) | .25 | 0.82 (.56–1.19) | .29 | .050 |
| Quantityc | 0.23 (–.15 to .60) | .24 | –0.27 (–.70 to .16) | .23 | .02 |
| Prevotella amnii | |||||
| Prevalenceb | 0.88 (.58–1.33) | .53 | 0.95 (.51–1.76) | .87 | .83 |
| Quantityc | –0.33 (–.83 to .17) | .20 | –0.17 (–.96 to .61) | .67 | .70 |
| Prevotella timonensis | |||||
| Prevalenceb | 1.20 (1.04–1.38) | .01 | 0.87 (.66–1.15) | .32 | .02 |
| Quantityc | 0.52 (.07–.97) | .02 | –0.38 (–1.13 to .36) | .32 | .02 |
| Enterococcus | |||||
| Prevalenceb | 0.99 (.73–1.34) | .96 | 0.90 (.54–1.50) | .69 | .70 |
| Quantityc | –0.10 (–.52 to .32) | .63 | –0.28 (–.87 to .31) | .35 | .53 |
| Group B Streptococcus | |||||
| Prevalenceb | 1.16 (.74–1.84) | .52 | 0.72 (.30–1.72) | .46 | .26 |
| Quantityc | 0.12 (–.33 to .57) | .60 | –0.44 (–1.12 to .24) | .20 | .10 |
| Staphylococcus aureus | |||||
| Prevalenceb | 0.85 (.39–1.88) | .69 | 0.61 (.16–2.24) | .45 | .64 |
| Quantityc | –0.01 (–.27 to .26) | .96 | –0.04 (–.41 to .32) | .82 | .86 |
| Escherichia coli | |||||
| Prevalenceb | 1.15 (.70–1.88) | .58 | 0.97 (.53–1.79) | .93 | .62 |
| Quantityc | –0.29 (–.84 to .26) | .30 | –0.43 (–1.00 to .14) | .14 | .61 |
| Candida albicans | |||||
| Prevalenceb | 1.05 (.80–1.39) | .71 | 1.12 (.72–1.76) | .62 | .80 |
| Quantityc | 0.18 (–.12 to .48) | .24 | 0.20 (–.36 to .75) | .48 | .96 |
| Other yeasts | |||||
| Prevalenceb | d | d | |||
| Quantityc | 0.17 (0–.38) | .051 | 0.21 (–.02– to .43) | .07 | .79 |
Sample sizes are 70 and 23 for DPV and placebo rings for qPCR samples, respectively. Bold values indicate statistical significance in either prevalence and/or quantity from baseline through ring use.
Abbreviations: BV, bacterial vaginosis; DPV, dapivirine vaginal ring-004; PL, placebo ring.
Microbiota prevalence and concentration evaluated at baseline and at 4, 12, and 24 weeks following ring use.
Change in prevalence expressed as relative risk (95% confidence interval [CI]); values <1.0 reflect a decrease, whereas values >1.0 reflect an increase; P value from modified Poisson regression model.
Change in Nugent score and log10 quantity expressed as mean (95% CI); values <0 reflect a decrease, whereas values >0 reflect an increase; P value from mixed-effects linear regression model.
Unable to provide estimates as model did not achieve convergence; 3 participants in each group were colonized by other yeasts at one follow-up visit and one participant in the DPV group was colonized by other yeasts at 2 follow-up visits.
At baseline, postmenopausal women randomized to the placebo group had a higher prevalence of P. amnii (P = .04), whereas women randomized to DPV ring group had a higher concentration of P. amnii (P = .02). No other differences were observed in Nugent score or organism prevalence and/or concentration among postmenopausal women at baseline (data not shown). As shown in Table 3, no changes were observed in prevalence of BV or Nugent scores among postmenopausal women in either arm over 3 months of use. Minimal changes in vaginal microbiota were observed among women in their respective ring group during the study. Postmenopausal women using placebo rings had an increased prevalence of L. iners (P = .02) and non-albicans yeast (P = .03). DPV ring users had an increase in prevalence of L. gasseri (P = .02), increased concentration of GBS (P = .009), and an increase in both prevalence and concentration for C. albicans (P = .02 for both, respectively) and non-albicans yeasts (P = .03 and P = .02, respectively). Over the study duration, the prevalence of yeast was lower than reproductive-aged women. Escherichia coli concentration differed between study arms (P = .047) since placebo ring users experienced a nonsignificant decrease and DPV ring users experienced a nonsignificant increase. Similar to adolescent females, detection of P. timonensis differed significantly over study duration and between study arms with an increased prevalence (P = .003) and concentration (P = .001) among DPV users only.
Table 3.
Change in Vaginal Microbiota Prevalence and Concentration Following Ring Use in Postmenopausal Women a
| Organism | Dapivirine Vaginal Ring-004 (n = 64) |
P Value DPV vs Baseline |
Placebo Ring (n = 22) |
P Value PL vs Baseline |
P Value PL vs DPV |
|---|---|---|---|---|---|
| BV prevalenceb | 1.11 (.73–1.67) | .64 | 1.01 (.68–1.50) | .95 | .74 |
| Nugent scorec | –0.23 (–.80 to .34) | .42 | –0.48 (–1.18 to .23) | .18 | .58 |
| Lactobacillus crispatus | |||||
| Prevalenceb | 1.19 (.94–1.49) | .15 | 1.02 (.70–1.49) | .92 | .49 |
| Quantityc | 0.15 (–.19 to .48) | .38 | 0.02 (–.37 to .40) | .93 | .61 |
| Lactobacillus iners | |||||
| Prevalenceb | 1.10 (.96–1.26) | .18 | 1.22 (1.03–1.45) | .02 | .29 |
| Quantityc | 0.10 (–.21 to .42) | .51 | 0.39 (–.16 to .93) | .16 | .36 |
| Lactobacillus jensenii | |||||
| Prevalenceb | 1.19 (.96–1.47) | .12 | 0.80 (.48–1.32) | .38 | .15 |
| Quantityc | 0.20 (–.14 to .55) | .25 | –0.02 (–.42 to .38) | .92 | .42 |
| Lactobacillus gasseri | |||||
| Prevalenceb | 1.39 (1.06–1.82) | .02 | 1.11 (.92–1.35) | .28 | .16 |
| Quantityc | 0.34 (–.04 to .73) | .08 | 0.18 (–.11 to .48) | .23 | .51 |
| Limosilactobacillus vaginalis | |||||
| Prevalenceb | 1.03 (.72–1.48) | .87 | 1.40 (.91–2.15) | .12 | .25 |
| Quantityc | 0.02 (–.32 to .37) | .89 | 0.36 (–.18 to .90) | .19 | .30 |
| Gardnerella vaginalis | |||||
| Prevalenceb | 1.11 (.92–1.34) | .27 | 1.01 (.78–1.30) | .96 | .50 |
| Quantityc | 0.13 (–.36 to .63) | .59 | –0.35 (–1.15 to .45) | .39 | .28 |
| Atopobium vaginae | |||||
| Prevalenceb | 1.02 (.82–1.27) | .88 | 1.03 (.60–1.78) | .92 | .97 |
| Quantityc | –0.01 (–.39 to .37) | .95 | –0.08 (–1.08 to .91) | .87 | .90 |
| Megasphaera lornae | |||||
| Prevalenceb | 1.20 (.90–1.60) | .21 | 0.83 (.56–1.22) | .34 | .13 |
| Quantityc | 0.12 (–.07 to .30) | .22 | –0.16 (–.39 to .08) | .19 | .06 |
| Prevotella bivia | |||||
| Prevalenceb | 0.99 (.70–1.40) | .96 | 0.43 (.14–1.38) | .16 | .16 |
| Quantityc | 0.02 (–.38 to .43) | .90 | –0.55 (–1.23 to .14) | .12 | .11 |
| Prevotella amnii | |||||
| Prevalenceb | 1.15 (.64–2.07) | .64 | 0.72 (.17–2.99) | .65 | .55 |
| Quantityc | 0.06 (–.22 to .35) | .67 | –0.26 (–.73 to .21) | .29 | .18 |
| Prevotella timonensis | |||||
| Prevalenceb | 1.42 (1.13–1.79) | .003 | 0.91 (.61–1.35) | .64 | .03 |
| Quantityc | 0.94 (.38–1.50) | .001 | –0.39 (–1.04 to .27) | .25 | .001 |
| Enterococcus | |||||
| Prevalenceb | 1.01 (.68–1.50) | .95 | 1.19 (.76–1.87) | .45 | .55 |
| Quantityc | 0 (–.38 to .37) | .99 | 0.01 (–.40 to .42) | .96 | .96 |
| Group B Streptococcus | |||||
| Prevalenceb | 1.72 (.94–3.18) | .08 | 1.05 (.53–2.10) | .88 | .24 |
| Quantityc | 0.47 (.12–.82) | .009 | 0.14 (–.21 to .50) | .44 | .22 |
| Staphylococcus aureus | |||||
| Prevalenceb | 2.09 (.22–20.20) | .52 | 4.05 (.37–44.33) | .25 | .46 |
| Quantityc | 0.06 (–.09 to .20) | .44 | 0.20 (–.16 to .55) | .28 | .45 |
| Escherichia coli | |||||
| Prevalenceb | 1.23 (.82–1.83) | .31 | 0.81 (.44–1.50) | .51 | .26 |
| Quantityc | 0.34 (–.16 to .83) | .18 | –0.28 (–.72 to .15) | .21 | .05 |
| Candida albicans | |||||
| Prevalenceb | 4.41 (1.26–15.44) | .02 | 1.34 (.22–8.01) | .75 | .14 |
| Quantityc | 0.33 (.06–.60) | .02 | 0.09 (–.22 to .41) | .56 | .26 |
| Other yeasts | |||||
| Prevalenceb | 2.06 (1.09–6.22) | .03 | 3.88 (1.14–13.22) | .03 | .43 |
| Quantityc | 0.39 (.07–.70) | .02 | 0.59 (–.04 to 1.21) | .07 | .59 |
Sample sizes are 64 and 22 for DPV and placebo rings for qPCR samples, respectively. Bold values indicate statistical significance in either prevalence and/or quantity from baseline through ring use.
Abbreviations: BV, bacterial vaginosis; DPV, dapivirine vaginal ring-004; PL, placebo ring.
Microbiota prevalence and concentration evaluated at baseline and at 4 and 12 weeks following ring use.
Change in prevalence expressed as relative risk (95% confidence interval [CI]); values <1.0 reflect a decrease, whereas values >1.0 reflect an increase; P value from modified Poisson regression model.
Change in Nugent score and log10 quantity expressed as mean (95% CI); values <0 reflect a decrease, whereas values >0 reflect an increase; P value from mixed-effects linear regression model.
For lactating women, changes in prevalence and concentration of vaginal microbiota and Nugent score were evaluated from baseline through 2 days post–ring removal after 2 weeks of DPV ring use (Table 4). The concentration of P. bivia increased marginally over DPV ring use (P = .005); however, no other microbiota had statistically significant shifts in prevalence or concentration over the study duration. Nugent scores remained stable and no increases in BV prevalence were observed.
Table 4.
Change in Vaginal Microbiota Prevalence and Concentration Following Ring Use in Lactating Women a
| Organism | Dapivirine Vaginal Ring-004 (n = 16) |
P Value DPV vs Baseline |
|---|---|---|
| BV prevalenceb | 0.55 (.22–1.41) | .22 |
| Nugent scorec | 0.03 (–1.07 to 1.13) | .95 |
| Lactobacillus crispatus | ||
| Prevalenceb | d | |
| Quantityc | 0 (0–.01) | .31 |
| Lactobacillus iners | ||
| Prevalenceb | 1.07 (.92–1.24) | .36 |
| Quantityc | 0.08 (–.20 to .35) | .59 |
| Lactobacillus jensenii | ||
| Prevalenceb | 1.52 (.79–2.91) | .21 |
| Quantityc | 0.64 (–.43 to 1.70) | .24 |
| Lactobacillus gasseri | ||
| Prevalenceb | 0.80 (.50–1.28) | .35 |
| Quantityc | –0.18 (–.62 to .26) | .43 |
| Limosilactobacillus vaginalis | ||
| Prevalenceb | 1.01 (.23–4.40) | .98 |
| Quantityc | 0.18 (–.80 to 1.16) | .72 |
| Gardnerella vaginalis | ||
| Prevalenceb | d | |
| Quantityc | 0.11 (–.39 to .62) | .66 |
| Atopobium vaginae | ||
| Prevalenceb | 1.02 (.52–2.00) | .94 |
| Quantityc | –0.10 (–1.55 to 1.35) | .89 |
| Megasphaera lornae | ||
| Prevalenceb | d | |
| Quantityc | 0.01 (–.01 to –.04) | .32 |
| Prevotella bivia | ||
| Prevalenceb | 2.27 (.98–5.27) | .06 |
| Quantityc | 0.85 (.26–1.44) | .005 |
| Prevotella amnii | ||
| Prevalenceb | d | |
| Quantityc | 0 (0–.01) | .32 |
| Prevotella timonensis | ||
| Prevalenceb | 1.26 (.60–2.66) | .54 |
| Quantityc | 0.63 (–.66 to 1.92) | .34 |
| Enterococcus | ||
| Prevalenceb | 1.82 (.47–7.06) | .39 |
| Quantityc | 0.35 (–.32 to 1.02) | .31 |
| Group B Streptococcus | ||
| Prevalenceb | 1.52 (.57–4.03) | .40 |
| Quantityc | 0.26 (–.10 to .62) | .16 |
| Staphylococcus aureus | ||
| Prevalenceb | e | |
| Quantityc | e | |
| Escherichia coli | ||
| Prevalenceb | 0.52 (.03–9.24) | .66 |
| Quantityc | –0.03 (–.36 to .30) | .87 |
| Candida albicans | ||
| Prevalenceb | 1.79 (.54–5.92) | .34 |
| Quantityc | 0.55 (–.29 to 1.38) | .20 |
| Other yeasts | ||
| Prevalenceb | e | |
| Quantityc | e | |
Abbreviations: BV, bacterial vaginosis, DPV, dapivirine vaginal ring-004. Bold values indicate statistical significance in either prevalence and/or quantity from baseline through ring use.
Microbiota prevalence and concentration evaluated at baseline and 14 and 16 days of use.
Change in prevalence expressed as relative risk (95% confidence interval [CI]); values <1.0 reflect a decrease, whereas values >1.0 reflect an increase; P value from modified Poisson regression model.
Change in Nugent score and log10 quantity expressed as mean (95% CI); values <0 reflect a decrease, whereas values >0 reflect an increase; P value from mixed-effects linear regression model.
Unable to provide estimates as model did not achieve convergence; 2 participants remained colonized by L. crispatus at each follow-up visit; 11 participants remained colonized by G. vaginalis at each follow-up visit; 1 participant remained colonized by M. lornae and 1 by P. amnii.
S. aureus and other yeasts were not detected at any visit in this cohort.
DISCUSSION
Adolescent, postmenopausal, and lactating females using the DPV ring for 6 months, 3 months, and 2 weeks, respectively, experienced minimal perturbations in their vaginal microbiota. BV incidence did not increase following ring use for any of the 3 cohorts. Although postmenopausal women randomized to the DPV ring arm experienced increases in the concentrations of GBS and prevalence and concentration of all yeasts, levels of incident vaginitis remained low in this cohort with only 1.4% of women in the DPV ring group and 2.8% of women in the placebo ring groups having vulvovaginitis over the 3-month product use period [26]. The incidence of Candida is generally low in postmenopausal women (~6%) [40]. Given the lower incidence of vaginitis observed in this study compared to the general population, it seems unlikely that significantly increased incidences of vulvovaginitis from baseline would have been observed in this cohort with >3 months of ring use.
In adolescent females and postmenopausal women, there was an increased prevalence and concentration of P. timonensis among DPV ring users only. Adolescent females and lactating women using DPV rings also experienced minimal increases in P. bivia prevalence and concentration, respectively, following use. However, no increase in BV prevalence was observed in either cohort. Previous phase 1 studies assessing changes in genital tract microbiota with DPV ring use over time have also reported increases in anaerobic gram-negative rods without changes in Nugent scores [20, 41]. The clinical significance of these findings is still unknown. In their study assessing the impact of the vaginal microbiome on PrEP, Cheu et al poignantly concluded that diagnosis of BV through species delineation may be a better predictor than Nugent score for shifts in vaginal microbiota and may better explain variable host response to topical PrEP [42].
Prevotella bivia and P. timonensis were found to be associated with increased genital tract inflammation, a known factor for increased risk of HIV acquisition [36–39]. Lipopolysaccharide is posited to be how gram-negative rods may modulate inflammation in the genital tract [12]. Increases in the prevalence and concentration of P. timonensis could modify susceptibility to infection similarly observed with P. bivia and warrants further study. Recently, Cheu et al revealed that organisms in cervicovaginal lavages collected from women with BV can metabolize dapivirine and alter HIV infection in cells in vitro [42]. To our knowledge, the effects of dapivirine metabolism on bacterial growth have not yet been studied in vitro and it is unclear if dapivirine or other antiretroviral drugs increase prevalence or concentration of BV-associated organisms.
Adolescents in both arms experienced an increased prevalence of L. crispatus. Farr Zuend et al [19], in an independent parallel study on a subset of samples from the adolescent cohort, used proteomics to identify shifts in the microbiota, and also found a statistically significant increase in L. crispatus following ring use. It is possible that the enhanced access to care and testing and treatment of sexually transmitted infections during the study could have accounted for the shift toward an L. crispatus–dominant microbiome during ring use since this was not observed among postmenopausal or lactating women. While postmenopausal women randomized to the placebo arm had an increased prevalence of L. iners and those randomized to the DPV arm had an increased prevalence of L. gasseri over 3 months of use, the relevance, if any, of this finding is unknown since these species are not considered to be as beneficial as L. crispatus [43, 44]. The prevalence and concentration of Limosilactobacillus vaginalis, previously known as Lactobacillus vaginalis and recently emended, was evaluated among 685 healthy, asymptomatic, nonpregnant, sexually active women aged 18–45 years across 5 US studies. It was detected in approximately half of women (49.5%) with Lactobacillus-dominant microbiota (Nugent score 0–3) but in lower concentration (interquartile range, 0–5.4) [31, 45]. In the present study this microorganism was detected in 34% of US adolescents, 18% of lactating women, and 13% of postmenopausal women at baseline, but there were no changes in the prevalence or concentration of this organism during ring use. The role of this lactic acid bacteria in the health of the vaginal microbiome remains to be determined.
This study has several strengths and important limitations. The study of adolescents included females 15–17 years of age with >6 months of use and thus provides robust data on stability of microbiota during ring use in younger adolescents. This study is also strengthened by the inclusion of both lactating women and adolescents, who are routinely overlooked when new HIV prevention products are being evaluated. A strength of the present study was that it included cultivation-based methods for traditional pathogens, which are generally present at low quantities and as minority members of the vaginal microbiome and not detected using 16S sequencing methods. A limitation of this study is the use of targeted qPCR for detection of selected species associated with vaginal dysbiosis and health rather than sequencing of the vaginal microbiome to identify community states. However, the combination of evaluation of vaginal fluid using Nugent criteria, which is well correlated with community states in women of reproductive age, and the qPCR targets should minimize the risk of missing major community shifts. A second limitation of the present study is that these cohorts of women were followed for variable lengths of time. Lactating women were followed for only 2 weeks, which is a limitation related to this cohort’s participation in a short-term study primarily focusing on measurement of drug transfer into breast milk. The present study was dependent on samples collected in the primary trials; however, it was reassuring that minimal changes were observed in the vaginal microbiota across all 3 groups of women. This was an exploratory analysis and not powered to make adjustments for multiple comparisons. We acknowledge the possibility of false-positive findings; thus, significant findings should be interpreted with caution and used to inform larger future studies.
Vaginal microbiota and BV prevalence differed between these cohorts at baseline. Except for P. timonensis and P. bivia, any minimal changes in microbiota observed following ring use were also not consistent between cohorts, highlighting the importance of studying how topical PrEP impacts the vaginal milieu across the lifespan. Most studies completed to date have focused on adult, reproductive-age cisgender women and these findings are not generalizable to adolescent, pregnant, postpartum, and postmenopausal genital tract physiology. Moreover, data establishing the safety in transgender women are lacking. Life phases are characterized by fluctuations in estrogen. Decreased estrogen can result in modulation of the genital tract microbiome and immune function and contribute to increased susceptibility to HIV [46–50]. This exploratory analysis assessing vaginal microbiota before and after ring use demonstrated that use of the rings did not cause concerning increases in pathogens associated with urinary tract infections, toxic shock, or vaginitis. The rings had favorable safety profiles and excellent tolerability in adolescents, lactating, and postmenopausal women [25–27], and the present study extends those studies to provide reassuring data on the impact of these rings on the vaginal microbiome.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. The MTN is funded by the National Institute of Allergy and Infectious Diseases (grant numbers UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the NIH. The vaginal rings used in this study were developed and supplied by the International Partnership for Microbicides.
Potential conflicts of interest. B. A. C. has received research grants from Medicines360 and Sebela, which are managed by Magee-Womens Research Institute. K. S. is an employee of Merck & Co, Rahway, New Jersey. S. L. H. has received honoraria from Merck and Gilead, and her institution receives grant funding from Merck. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: HIV Research for Prevention Meeting, Madrid, Spain, 23–25 October 2018. Poster P17.02.
Contributor Information
Michele N Austin, Magee-Womens Research Institute, Pittsburgh, Pennsylvania, USA.
Leslie A Meyn, Magee-Womens Research Institute, Pittsburgh, Pennsylvania, USA; University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Hilary A Avolia, Magee-Womens Research Institute, Pittsburgh, Pennsylvania, USA.
Melinda A Petrina, Magee-Womens Research Institute, Pittsburgh, Pennsylvania, USA.
Lisa A Cosentino, Magee-Womens Research Institute, Pittsburgh, Pennsylvania, USA.
Calins Alphonse, Fred Hutchinson Cancer Research Center, Seattle, Washington, USA.
Beatrice A Chen, Magee-Womens Research Institute, Pittsburgh, Pennsylvania, USA; University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Katherine Bunge, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Lisa Noguchi, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Richard Beigi, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Kathleen Squires, Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
Sharon L Hillier, Magee-Womens Research Institute, Pittsburgh, Pennsylvania, USA; University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
REFERENCES
- 1. Girum T, Wasie A, Lentiro K, et al. Gender disparity in epidemiological trend of HIV/AIDS infection and treatment in Ethiopia. Arch Public Health 2018; 76:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Foundation for AIDS Research. Statistics: women and HIV/AIDS. https://www.amfar.org/About-HIV-and-AIDS/Facts-and-Stats/Statistics--Women-and-HIV-AIDS/. Accessed 18 April 2020.
- 3. Meyer JP, Womack JA, Gibson B.. Beyond the pap smear: gender-responsive HIV care for women. Yale J Biol Med 2016; 89:193–203. [PMC free article] [PubMed] [Google Scholar]
- 4. Chen NE, Meyer JP, Springer SA.. Advances in the prevention of heterosexual transmission of HIV/AIDS among women in the United States. Infect Dis Rep 2011; 3:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heumann CL. Biomedical approaches to HIV prevention in women. Curr Infect Dis Rep 2018; 20:11. [DOI] [PubMed] [Google Scholar]
- 6. Thurman AR, Clark MR, Hurlburt JA, Doncel GF.. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. Int J Womens Health 2013; 5:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marrazzo JM. Biomedical prevention of HIV in women: challenges and approaches, with particular reference to the vaginal microbiome. Trans Am Clin Climatol Assoc 2018; 129:63–73. [PMC free article] [PubMed] [Google Scholar]
- 8. Baeten JM, Hendrix CW, Hillier SL.. Topical microbicides in HIV prevention: state of the promise. Annu Rev Med 2020; 71:361–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention. N Engl J Med 2016; 375:2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nel A, van Niekerk N, Kapiga S, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 2016; 375:2133–43. [DOI] [PubMed] [Google Scholar]
- 11. Bayigga L, Kateete DP, Anderson DJ, Sekikubo M, Nakanjako D.. Diversity of vaginal microbiota in sub-Saharan Africa and its effects on HIV transmission and prevention. Am J Obstet Gynecol 2019; 220:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015; 42:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eastment MC, McClelland RS.. Vaginal microbiota and susceptibility to HIV. AIDS 2018; 32:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdool Karim SS, Passmore JS, Baxter C.. The microbiome and HIV prevention strategies in women. Curr Opin HIV AIDS 2018; 13:81–7. [DOI] [PubMed] [Google Scholar]
- 15. Sabo MC, Lehman DA, Wang B, et al. Associations between vaginal bacteria implicated in HIV acquisition risk and proinflammatory cytokines and chemokines. Sex Transm Infect 2019; 96:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McClelland RS, Lingappa JR, Srinivasan S, et al. Key vaginal bacteria associated with increased risk of HIV acquisition in African women: a nested case-control study. Lancet Infect Dis 2018; 18:554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gossman C, Anahtar MN, Handley SA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017; 46:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farcasanu M, Kwon DS.. The influence of cervicovaginal microbiota on mucosal immunity and prophylaxis in the battle against HIV. Curr HIV/AIDS Rep 2018; 15:30–8. [DOI] [PubMed] [Google Scholar]
- 19. Farr Zuend C, Noel-Romas L, Hoger S, et al. Influence of dapivirine vaginal ring use on cervicovaginal immunity and functional microbiome in adolescent girls. AIDS 2021; 35:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen BA, Panther L, Marzinke MA, et al. Phase 1 safety, pharmacokinetics and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr 2015; 70:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nel A, Haazen W, Nuttall JP, Romano JW, Rosenberg Z, van Niekerk N.. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in health women. AIDS 2014; 28:1479–87. [DOI] [PubMed] [Google Scholar]
- 22. Nel A, Haazen W, Nuttall JP, Romano JW, et al. Pharmacokinetics and safety assessment of anti-HIV dapivirine vaginal microbicide rings with multiple dosing. J AIDS Clin Res 2014; 5:355. [Google Scholar]
- 23. Baeten JM, Brown ER, Hillier SL; MTN-020/ASPIRE Study Team. . Dapivirine vaginal ring for HIV-1 prevention. N Engl J Med 2017; 376:994–5. [DOI] [PubMed] [Google Scholar]
- 24. Nicol MR, Corbino JA, Cottrell ML.. Pharmacology of antiretrovirals in the female genital tract for HIV prevention. J Clin Pharmacol 2018; 58:1381–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bunge KE, Levy L, Szydlo DW, et al. Brief report: phase IIa safety study of a vaginal ring containing dapivirine in adolescent young women. J Acquir Immune Defic Syndr 2020; 83:135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen BA, Zhang J, Gundacker HM, et al. Phase 2a safety, pharmacokinetics, and acceptability of dapivirine vaginal rings in US postmenopausal women. Clin Infect Dis 2019; 68:1144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noguchi LM, Hoesley C, Kelly C, et al. Pharmacokinetics of dapivirine transfer into blood plasma, breast milk and cervicovaginal fluid of lactating women using the dapivirine vaginal ring. Antimicrob Agents Chemother 2019; 63:e01930–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nugent RP, Krohn MA, Hillier SL.. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clarke JG, Peipert JF, Hillier SL, et al. Microflora changes with the use of a vaginal microbicide. Sex Trans Dis 2002; 29:288–93. [DOI] [PubMed] [Google Scholar]
- 30. Achilles SL, Austin MN, Meyn LA, Mhlanga F, Chirenje ZM, Hillier SL.. Impact of contraceptive initiation on vaginal microbiota. Am J Obstet Gynecol 2018; 218:e1–622.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 2020; 70:2782–858. [DOI] [PubMed] [Google Scholar]
- 32. Zozaya-Hinchcliffe M, Lillis R, Martin DH, Ferris MJ.. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J Clin Microbiol 2010; 48:1812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srinivasan S, Morgan MT, Fiedler TL, et al. Metabolic signatures of bacterial vaginosis. mBio 2015; 6:e00204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011; 108(Suppl 1):4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaneechoutte M. Lactobacillus iners, the usual suspect. Res Microbiol 2017;168:826–836. [DOI] [PubMed] [Google Scholar]
- 36. McKinnon LR, Liebenberg LJ, Yende-Zuma N, et al. Genital inflammation undermines the effectiveness of tenofovir gel in preventing HIV acquisition in women. Nat Med 2018; 24:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Teijlingen NH, Helgers LC, et al. Vaginal dysbiosis associated-bacteria Megasphaera elsdenii and Prevotella timonensis induce immune activation via dendritic cells. J Reprod Immunol 2020; 138:103085. [DOI] [PubMed] [Google Scholar]
- 38. Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017; 356:938–45. [DOI] [PubMed] [Google Scholar]
- 39. Vellozza J, Heffron R.. The vaginal microbiome and its potential to impact efficacy of HIV pre-exposure prophylaxis for women. Curr HIV/AIDS Rep 2017; 14:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoffman JN, You HM, Hedberg EC, Jordan JA, McClintock MK.. Prevalence of bacterial vaginosis and Candida among postmenopausal women in the United States. J Gerontol B Psychol Sci Soc Sci 2014: 69(Suppl 2): S205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang Y, Merkatz RB, Hillier SL, et al. Effects of a one-year reusable contraceptive vaginal ring on vaginal microflora and the risk of vaginal infection: an open-label prospective evaluation. PLoS One 2015; 10:e0134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheu RK, Gustin AT, Lee C, et al. Impact of vaginal microbiome communities on HIV antiretroviral-based pre-exposure prophylaxis (PrEP) drug metabolism. PLoS Pathog 2020; 16:e1009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amabebe E, Anumba DOC.. The vaginal microenvironment: the physiologic role of lactobacilli. Front Med (Lausanne) 2018; 5:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verhelst R, Verstraelen H, Claeys G, et al. Comparison between Gram stain and culture for the characterization of vaginal microflora: definition of a distinct grade that resembles grade I microflora and revised categorization of grade I microflora. BMC Microbiol 2005; 5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meyn LA, Beamer MA, Petrina MA, et al. When normal isn’t normal: heterogeneity in dominant Lactobacillus species among women having a Nugent score of 0–3. Am J Obstet Gynecol 2019; 221:683–84. [Google Scholar]
- 46. Thurman AR, Yousefieh N, Chandra N.. Comparison of mucosal markers of human immunodeficiency virus susceptibility in health premenopausal versus postmenopausal women. AIDS Res Hum Retroviruses 2017; 33:807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jais M, Younes N, Chapman S, Cu-Uvin S, Ghosh M.. Reduced levels of genital tract immune biomarkers in postmenopausal women: implications for HIV acquisition. Am J Obstet Gynecol 2016; 215:324.e1–10. [DOI] [PubMed] [Google Scholar]
- 48. Madan RP, Carpenter C, Fiedler T, et al. Altered biomarkers of mucosal immunity and reduced vaginal Lactobacillus concentrations in sexually female adolescents. PLoS One 2012; 7:e40415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson KA, Hughes J, Baeten JM, et al. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis 2018; 218:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Porter KA, Turpin J, Begg L, et al. Understanding the intersection of young age, mucosal injury and HIV susceptibility. AIDS Res Hum Retroviruses 2016; 32:1149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]