Abstract
Two types of bacteriophage specific to Pseudomonas plecoglossicida, the causative agent of bacterial hemorrhagic ascites disease in cultured ayu fish (Plecoglossus altivelis), were isolated from diseased ayu and the rearing pond water. One type of phage, which formed small plaques, was tentatively classified as a member of the family Myoviridae, and the other type, which formed large plaques, was classified as a member of the family Podoviridae. All 27 strains of P. plecoglossicida examined, which were isolated from diseased ayu from geographically different areas in 1991 to 1999, exhibited quite similar sensitivities to either type of phage. One strain of P. plecoglossicida was highly virulent for ayu, and the 50% lethal dose (LD50) when intramuscular injection was used was 101.2 CFU fish−1; in contrast, phage-resistant variants of this organism were less virulent (LD50, >104 CFU fish−1). Oral administration of phage-impregnated feed to ayu resulted in protection against experimental infection with P. plecoglossicida. After oral administration of P. plecoglossicida cells of this bacterium were always detected in the kidneys of control fish that did not receive the phage treatment, while the cells quickly disappeared from the phage-treated fish. Bacterial growth in freshwater was lower in the presence of phage, and the number of phage PFU increased rapidly. These results suggest that it may be possible to use phage to control the disease caused by P. plecoglossicida.
Ayu, Plecoglossus altivelis, is the most popular freshwater fish for culture and sport fishing in Japan. For a long time vibriosis caused by Vibrio anguillarum was the most serious disease in cultured populations of ayu, but this disease was replaced by coldwater disease caused by Flavobacterium psychrophilum (4) in the late 1980s (5, 32). F. psychrophilum has been recognized as an important pathogen of salmonid fish worldwide for a long time (21). More recently, however, a new bacterial disease with a high level of mortality has prevailed among cultured ayu, and this disease causes serious damage to the ayu culture industry in Japan. The bacterium that causes this disease is similar to Pseudomonas putida, but some of its biochemical characteristics differ from characteristics of typical strains of P. putida (14, 31); a new species name, Pseudomonas plecoglossicida, has been proposed for this bacterium (15). The disease caused by P. plecoglossicida often occurs a short time after naturally or artificially produced fish are introduced into culture ponds, and it also occurs at any developmental stage during culture, particularly after chemotherapy for F. psychrophilum infection. At present, there are no licensed chemotheraputic compounds that are effective against the new disease and no procedures which can be used to control the disease other than reducing predisposing factors, such as overcrowding and overfeeding.
Theoretically, bacteriophages can be used to treat infectious disease, but little attention has been paid to phage therapy and prophylaxis after the early uncontrolled studies (3, 19). However, in the 1980s interesting studies on phage therapy were carried out by Smith and his colleagues, who used Escherichia coli infection models with mice and farm animals, and the results indicated that phage could be used for both treatment and prophylaxis (23–26). In addition, a series of successful phage therapies for human bacterial infections was described by Slopek et al. (22), although the clinical studies performed with humans did not provide scientifically important information. After this, many controlled experiments indicated that it may be possible to use phage therapy in animal models (2, 10, 27, 28). On the other hand, phages of some fish-pathogenic bacteria have been described (9, 18, 20, 30, 33), but there have been few attempts to use phage to control bacterial infections in fish. Our recent investigations of phage specific to Lactococcus garvieae (formerly Enterococcus seriolicida), a pathogen of yellowtail (Seriola quinqueradiata) and other marine fishes (7, 8), suggest that phage could be useful for controlling bacterial infections of fish (13, 16, 17).
In the present study, we isolated P. plecoglossicida-specific phages from diseased ayu and culture pond water, characterized these phages, and examined the effects of isolated phages on experimental infections with P. plecoglossicida. Below we discuss the potential for phage control of the disease caused by P. plecoglossicida.
MATERIALS AND METHODS
Bacteria and media.
Twenty-three bacterial strains which were isolated from diseased ayu obtained in seven prefectures in Japan from 1991 to 1999 were used in this study (Table 1). These strains were gram-negative, aerobic, motile or nonmotile rods and were identified as P. plecoglossicida based on other phenotypic characteristics of this species (15). The motility of the bacteria was examined directly (wet mount method) by using a microscope. Two motile strains (strains FPC941 [= ATCC 700384] and FPC951 [= ATCC 700383T]) and two nonmotile strains (strains PH-9501 and AK-9510) described previously (14, 15, 31) were used as reference strains. All 27 strains exhibited positive reactions in the slide agglutination test with an rabbit antiserum raised against P. plecoglossicida FPC951. Laboratory stock strains of other fish-pathogenic bacteria (Table 1) were used to determine the host specificity of phages that were isolated. Trypto-soy broth (TSB) (Nissui) and Trypto-soy agar (TSA) (Nissui) were used for bacterial culture and for a phage PFU assay.
TABLE 1.
P. plecoglossicida strains and other bacterial strains used in this study and their motility and sensitivity to two phage isolates
| Straina | Isolation
|
Motilityb | EOPc
|
||
|---|---|---|---|---|---|
| Location | Year | PPpW-3 | PPpW-4 | ||
| Pseudomonas plecoglossicida strains | |||||
| FPC941d | Shiga Prefecture | 1994 | + | 0.9 | 1.2 |
| AK-9510ef | Kyoto Prefecture | 1995 | − | 1.4 | 2.0 |
| PH-9501ef | Hiroshima Prefecture | 1995 | − | 1.2 | 1.0 |
| PY-9701 | Yamanashi Prefecture | 1997 | − | 1.1 | 1.4 |
| PM-9801 | Miyazaki Prefecture | 1998 | − | 2.0 | 2.3 |
| FPC951d | Tokushima Prefecture | 1994 | + | 1.0 | 1.8 |
| PT-97002f | Tokushima Prefecture | 1997 | + | 1.0 | 5.8 |
| PT-97034f | Tokushima Prefecture | 1997 | − | 1.0 | 3.5 |
| PT-98028f | Tokushima Prefecture | 1998 | − | 1.3 | 0.8 |
| PT-98058f | Tokushima Prefecture | 1998 | + | 1.0 | 2.4 |
| PTH-9801f | Tokushima Prefecture | 1998 | + | 1.2 | 1.0 |
| PTH-9802 | Tokushima Prefecture | 1998 | − | 1.0 | 1.0 |
| PTH-9805 | Tokushima Prefecture | 1998 | − | 4.7 | 6.7 |
| PTH-9811 | Tokushima Prefecture | 1998 | − | 3.6 | 1.2 |
| PTH-9817 | Tokushima Prefecture | 1998 | + | 4.2 | 3.3 |
| PTH-9824 | Tokushima Prefecture | 1998 | − | 2.9 | 5.8 |
| PTH-9901 | Tokushima Prefecture | 1999 | − | 1.3 | 0.8 |
| PTH-9902 | Tokushima Prefecture | 1999 | − | 1.3 | 1.1 |
| H3-16 | Wakayama Prefecture | 1991 | + | 1.5 | 4.8 |
| 92P3-0403 | Wakayama Prefecture | 1992 | + | 1.5 | 2.8 |
| 93P1-0225 | Wakayama Prefecture | 1993 | + | 2.0 | 2.3 |
| 94P1-0104 | Wakayama Prefecture | 1994 | + | 2.1 | 3.3 |
| 95P7-0502 | Wakayama Prefecture | 1995 | − | 1.8 | 3.3 |
| 96P25-0729 | Wakayama Prefecture | 1996 | − | 1.3 | 2.2 |
| 97P18-0617 | Wakayama Prefecture | 1997 | − | 1.4 | 4.1 |
| 98P3-0127 | Wakayama Prefecture | 1998 | − | 1.5 | 2.2 |
| 98P4-1015 | Wakayama Prefecture | 1998 | − | 1.7 | 3.3 |
| Aeromonas hydrophila ET-79059 | <10−9 | <10−9 | |||
| Aeromonas salmonicida ATCC 14174 | <10−9 | <10−9 | |||
| Edwardsiella tarda NUF251 | <10−9 | <10−9 | |||
| Pseudomonas putida ATCC 12633 | <10−9 | <10−9 | |||
| Pseudomonas fluorescens 03L | <10−9 | <10−9 | |||
| Pseudomonas anguilliseptica NCMB1949 | <10−9 | <10−9 | |||
| Vibrio anguillarum PT-24 | <10−9 | <10−9 | |||
| Vibrio ordalii PT-81024 | <10−9 | <10−9 | |||
ATCC, American Type Culture Collection; NCMB, National Collection of Marine Bacteria (United Kingdom).
Motility was determined by the standard wet mount method.
The number of PFU was determined after 1 day of incubation at 25°C, and the EOP was quantified by calculating the ratio of PFU obtained with each strain to those obtained with strain PTH-9802.
See reference 14.
This strain was used as an indicator strain for phage isolation.
Isolation of phage and PFU assay.
Phage were isolated from diseased ayu and culture pond water obtained at three fish farms in Tokushima Prefecture in September 1998 by using an enrichment method (29). Pooled kidney samples from six diseased fish were homogenized with sterile saline and centrifuged at 3,000 × g for 10 min, and 1 ml of the supernatant was inoculated into 100 ml of TSB which had been supplemented with a mixture of seven strains of P. plecoglossicida as indicator organisms (Table 1). For water samples, 100 ml of pond water was filtered through a 0.45-μm-pore-size membrane filter and mixed with 100 ml of double-strength TSB containing the indicator organisms. After 48 h of growth at 25°C with gentle agitation, the culture was centrifuged and filtered, and the supernatant was subjected to the following PFU assay performed by using a double-agar-layer method (18). The numbers of PFU was determined after 24 h of incubation at 25°C, and the efficiency of plating (EOP) was calculated. Strain PTH-9802 was used as an indicator strain for preparation of phage stock suspensions, which were stored at 5°C.
Electron microscopy of phage and bacteria.
A phage suspension (109 PFU ml−1) was centrifuged with a 20 and 60% discontinuous sucrose gradient at 100,000 × g for 90 min at 4°C. After dialysis against 50 mM Tris-HCl buffer (pH 7.2) containing 0.2 M NaCl overnight, phage samples were negatively stained with 4% uranyl acetate and examined with a Hitachi model H7500 electron microscope at 80 kV. For microscopy of bacterial flagella, four strains of P. plecoglossicida that had been grown on TSA overnight were negatively stained.
Analysis of phage nucleic acids.
Nucleic acids of phages were extracted with phenol saturated with TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA), and this was followed by extraction with a mixture of choloroform and isoamyl alcohol (24:1). The purified nucleic acids were tested for sensitivity to RNase and restriction enzyme EcoRI. The results were determined by 1% agarose gel electrophoresis.
Preparation of phage-resistant variants of P. plecoglossicida.
A culture of P. plecoglossicida PTH-9802 was treated with an undiluted suspension (109 PFU ml−1) of phages PPpW-3 and PPpW-4. Colonies that appeared on the plate after 2 days of incubation at 25°C were purified on TSA, and selection for phage-resistant variants was repeated. Finally, cultures that produced no PFU with 109 PFU of one phage or both phages ml−1 were used as phage-resistant variants.
Pathogenicity test performed with P. plecoglossicida.
Phage-sensitive and phage-resistant cells of P. plecoglossicida PTH-9802 variants were used to study pathogenicity for ayu with an average weight of 10 g. Groups of 10 fish were injected intramuscularly with bacteria at doses of 100 to 104 CFU per fish and then kept in 40-liter plastic tanks with flowthrough water at 20 ± 1°C. Mortalities were recorded daily for 2 weeks, and the kidneys of dead fish were subjected to a bacterial isolation study to confirm that death was due to P. plecoglossicida infection; anti-FPC951 serum was used in this study.
Coculture of P. plecoglossicida and phage in water.
P. plecoglossicida PTH-9802 and phage PPpW-3 or PPpW-4 were inoculated into filter-sterilized (pore size, 0.45 μm) rearing pond water at doses of 102 CFU ml−1 and 103 PFU ml−1, respectively, and then the preparations were incubated with gentle agitation at 25°C. Preparations inoculated with either bacteria or phage were used as controls. The numbers of bacteria and phage in water were determined 1 to 72 h after inoculation.
Phage treatment of infected fish and kinetics of inoculated organisms.
In the first experiment, four groups of 20 ayu weighing 10 g (average weight) were fed commercial dry pellets that had been impregnated with a live culture of P. plecoglossicida PTH-9802 at a feeding rate of 1.5% based on body weight. After 15 min of feeding, two of the groups were immediately fed pellets that had been impregnated with a phage suspension containing PPpW-3 and PPpW-4. The concentrations of bacteria and phage were 107 CFU g−1 of pellets and 107 PFU g−1 of pellets, respectively. The other two groups received feed without phage and served as controls. In the second experiment, four groups of 50 or 40 ayu weighing 2.4 g (average weight) were similarly fed bacterium-impregnated pellets (107 CFU g−1), and then two of the groups were fed phage-impregnated pellets (107 PFU g−1) 1 or 24 h after they were fed the bacterium-impregnated pellets. The mortalities of the fish were recorded daily for 2 weeks, and the kidneys of dead fish or survivors at the end of the experiment (only in the first experiment) were subjected to a bacterial isolation study as described above. The water temperature of the fish tanks was 20 ± 1°C throughout the experiments.
Three groups of 30 ayu (average weight, 2.4 g) were used to study the kinetics of orally administered P. plecoglossicida and phage in fish. The fish were fed bacterium-impregnated pellets, phage-impregnated pellets, or bacterium-impregnated pellets followed by phage-impregnated pellets as described above. Groups of three fish were sacrificed in order to detect the inoculated phage and bacteria in their kidneys 3 to 168 h after inoculation (water temperature, 22 ± 1°C). The kidneys were homogenized with 9 volumes of saline, centrifuged at 7,000 × g for 3 min to enumerate phage particles, and then processed for CFU and PFU assays.
RESULTS
Isolation and characterization of phage.
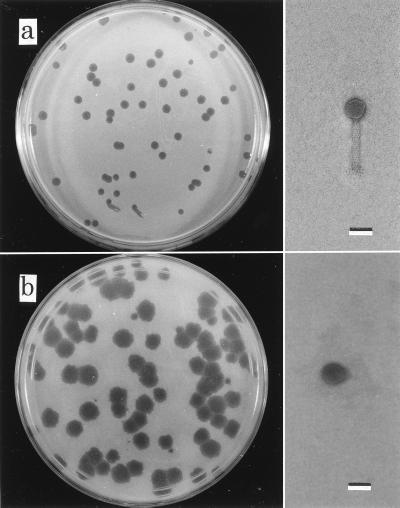
Eight phage isolates were obtained from nine samples (five pooled kidneys of diseased ayu and four pond water samples) by the enrichment method (Table 2). Only a kidney sample from farm C was negative for phage isolation. The phages were designated PPpA or PPpW. Two phage isolates (PPpW-1 and PPpW-3) from pond water formed small plaques (average diameter, 1.4 mm) after 24 h of incubation at 25°C with every type of indicator cells; these phage isolates had isometric heads that were 50 nm in diameter and long contractile tails (20 by 110 nm) with spikes (Fig. 1a). Another six phage isolates, represented by PPpW-4, formed large plaques that were 3.5 mm in diameter (average) after 24 h of incubation at 25°C; these isolates had isometric heads (diameter, 50 nm) and short tails (25 by 18 nm) (Fig. 1b). The nucleic acids of both types of phages were cleaved by EcoRI but not by RNase. The following two different restriction fragment length polymorphism profiles were produced by the phage isolates: type I (PPpW-1 and PPpW-3) and type II (the other six isolates).
TABLE 2.
P. plecoglossicida-specific phages isolated from ayu and rearing pond water
| Fish farm | Sample from which phage was isolated | Type of plaque | Designation of phage | Tail | RFLP typea |
|---|---|---|---|---|---|
| A | Diseased ayu, kidney | Large | PPpA-1 | Short | II |
| B | Diseased ayu, kidney | Large | PPpA-2 | Short | II |
| B | Diseased ayu, kidney | Large | PPpA-3 | Short | II |
| C | Diseased ayu, kidney | Large | PPpA-4 | Short | II |
| B | Pond water | Small | PPpW-1 | Long | I |
| C | Pond water | Large | PPpW-2 | Short | II |
| C | Pond water | Small | PPpW-3 | Long | I |
| A | Pond water | Large | PPpW-4 | Short | II |
RFLP, restriction fragment length polymorphism. The types were based on EcoRI digestion data.
FIG. 1.
Phage plaques with P. plecoglossicida PTH-9802 and electron micrographs of the phages. (a) PPpW-3. (b) PPpW-4. Bars = 50 nm.
The EOPs of phages PPpW-3 and PPpW-4 with P. plecoglossicida PTH-9802 at different incubation temperatures are shown in Table 3. Bacteria that were not infected with phage grew well at 10 to 30°C but not at 35°C. During a 3-day incubation period, lytic activity was observed at a temperature of 30°C or less for PPpW-3 and at a temperature of 25°C or less for PPpW-4, without any significant change in the EOP.
TABLE 3.
Effect of incubation temperature on the lytic activities of phage isolates PPpW-3 and PPpW-4
| Incubation temp (°C) | EOPa
|
|
|---|---|---|
| PPpW-3 | PPpW-4 | |
| 10 | 0.9 | 0.9 |
| 15 | 1.1 | 1.3 |
| 20 | 1.0 | 1.4 |
| 25 | 1.0 | 1.0 |
| 30 | 0.9 | <10−9 |
| 35 | NGb | NG |
P. plecoglossicida PTH-9802 infected with phage was incubated at each temperature for 3 days, and the EOP was quantified by calculating the ratio of the PFU obtained at each temperature to those obtained at 25°C.
NG, no growth of bacteria.
All 27 strains of P. plecoglossicida examined were sensitive to both types of phage (PPpW-3 and PPpW-4), and the EOPs at 25°C ranged from 0.8 to 6.7; however, the other eight strains of fish-pathogenic bacteria tested were not susceptible to the phages (Table 1). Similar results were obtained with the other six phage isolates.
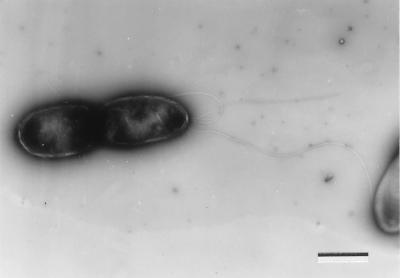
When the motility of P. plecoglossicida strains was examined by the wet mount method, 10 of the 27 strains examined were motile (Table 1). As determined by electron microscopy, lophotrichous flagella were observed in motile strain FPC941 (Fig. 2) but not in three nonmotile strains (PTH-9802, AK-9510, and PH-9501).
FIG. 2.
Flagella of P. plecoglossicida FPC941. Bar = 1 μm.
Phage-resistant bacterial variants and pathogenicity in fish.
Variants of PTH-9802 that emerged after exposure to PPpW-3 were also resistant to PPpW-4, while variants that emerged after exposure to PPpW-4 were sensitive to PPpW-3. Four bacterial variants, designated R1, R2, and R3 (resistant to both phage PPpW-3 and phage PPpW-4) and R4 (resistant to only phage PPpW-4), were used in the pathogenicity test. All of the phage-resistant variants reacted positively in slide agglutination tests with anti-FPC951 serum. The pathogenicities of these four variants and their parent, strain PTH-9802, for ayu are shown in Table 4. A phage-sensitive parent PTH-9802 was highly virulent for ayu, and its 50% lethal dose (LD50) by intramuscular injection was 101.2 CFU fish−1. Fish died 5 to 10 days after injection and produced bloody ascites, and the bacteria inoculated were isolated in pure culture from kidneys of dead fish. By contrast, variants that were resistant to PPpW-3 and/or PPpW-4 were not pathogenic for fish even at a dose of 104 CFU fish−1.
TABLE 4.
Pathogenicities of phage-sensitive parent cells and phage-resistant variants of P. plecoglossicida PTH-9802 for ayua
| Strain injected | Dose (CFU/fish) | % Mortality (no. dead/no. tested) |
|---|---|---|
| Parent PTH-9802 | 3.9 × 103 | 100 (10/10) |
| 102 | 100 (10/10) | |
| 101 | 80 (8/10) | |
| 100 | 0 (0/9) | |
| Variant R1 | 2.4 × 104 | 0 (0/10) |
| 103 | 0 (0/10) | |
| 102 | 0 (0/10) | |
| Variant R2 | 4.0 × 104 | 0 (0/10) |
| 103 | 0 (0/10) | |
| 102 | 0 (0/10) | |
| Variant R3 | 1.6 × 104 | 0 (0/10) |
| 103 | 0 (0/10) | |
| 102 | 0 (0/10) | |
| Variant R4 | 1.5 × 104 | 0 (0/10) |
| 103 | 0 (0/10) | |
| 102 | 0 (0/10) |
Fish were injected intramuscularly with bacteria and were cultivated at 20°C and observed for 2 weeks. Variants R1, R2, and R3 were resistant to both phage PPpW-3 and phage PPpW-4; variant R4 was resistant only to phage PPpW-4.
Interaction of P. plecoglossicida and phage in water.
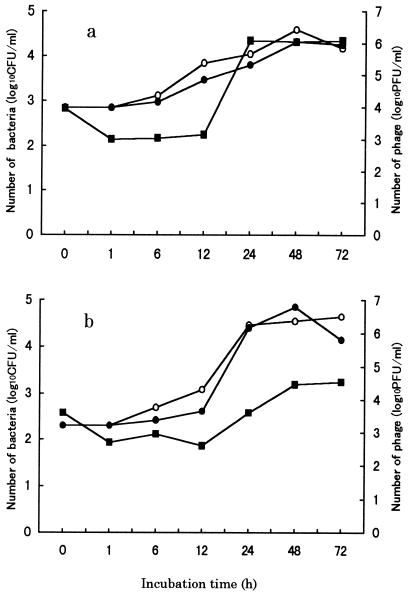
Bacteria inoculated into water without phage began to grow at 6 h postinoculation (p.i.) and reached a concentration of 104 CFU ml−1 at 48 h p.i. (Fig. 3). In the presence of phage, bacterial growth decreased until 24 or 48 h p.i., while the number of inoculated phage temporarily decreased at 12 h p.i. and then rapidly increased to 106 PFU ml−1 (PPpW-3) or 104 PFU ml−1 (PPpW-4) at 24 or 48 h p.i. There were no changes in the number (PFU) of phage in the absence of P. plecoglossicida.
FIG. 3.
Interaction of P. plecoglossicida and phage particles in water. Phage and P. plecoglossicida PTH-9802 were coinoculated into rearing pond water and incubated at 25°C. (a) PPpW-3. (b) PPpW-4. Symbols: ○, bacteria only; ●, bacteria coinoculated with phage; ■, phage coinoculated with bacteria.
Protective effect of phage administration.
The protective effects resulting from oral administration of phage against experimental P. plecoglossicida infection are shown in Table 5. In the first trial, fish were orally challenged with live P. plecoglossicida cells and received phage-impregnated or phage-free feed. Fish in the control groups that received feed without phage began to die 7 days after the bacterial challenge, and the cumulative mortality in 2 weeks was 65.0% (average for the two groups); in contrast, fish that received phage-impregnated feed died later, and the average cumulative mortality was 22.5%. The mortality of phage-treated fish was significantly lower than the mortality of control fish that were not treated with phage (significance level, α = 0.05 as determined by a chi-square test). Inoculated P. plecoglossicida strains were isolated from all of the kidneys of dead fish (n = 35) irrespective of the phage treatment and from 3 of 14 surviving fish in control groups but not from any of the fish that received phage and survived (n = 31). In addition, bacteria that were isolated from fish that had received phage and died were still susceptible to both phages. Similar protective effects of phage treatment, including significantly (α = 0.05) lower and delayed mortality, were observed with fish that received phage 1 or 24 h after bacterial challenge in the second trial, in which smaller fish were used (Table 5).
TABLE 5.
Phage treatment of ayu infected with P. plecoglossicidaa
| Expt | Avg fish wt (g) | Phage treatment | No. of dead fish/no. tested | % Mortality | Mean time to death (days) | Reisolation of P. plecoglossicida from survivors (no. positive/ no. examined) |
|---|---|---|---|---|---|---|
| 1 | 10 | Yes (0 h) | 5/20 | 25 | 11.4 | 0/15 |
| Yes (0 h) | 4/20 | 20 | 11.5 | 0/16 | ||
| No | 14/20 | 70 | 9.8 | 1/6 | ||
| No | 12/20 | 60 | 10.0 | 2/8 | ||
| 2 | 2.4 | Yes (1 h) | 0/50 | 0 | NDb | |
| No | 39/50 | 78 | 11.3 | ND | ||
| Yes (24 h) | 5/40 | 13 | 8.8 | ND | ||
| No | 32/40 | 80 | 5.7 | ND |
Fish were challenged by oral administration of bacterium (PTH-9802)-impregnated pellets and immediately (0 h) or 1 or 24 h later received phage (mixture of PPpW-3 and PPpW-4)-impregnated pellets. Fish were cultivated at 20°C and observed for 2 weeks, and the kidneys of survivors were examined for the presence of P. plecoglossicida.
ND, not done.
Kinetics of P. plecoglossicida and phage inoculated into fish.
P. plecoglossicida cells in fish that received bacterium-impregnated feed first appeared at a concentration of 103.5 CFU g−1 in the kidney of one fish 3 h after feeding and were detected at levels of 103.9 to 106.3 CFU g−1 in all of the kidneys examined up to 168 h later (Table 6). In fish that received phage-impregnated feed, the inoculated phages were detected at concentrations of 103.1 to 105.7 PFU g−1 in kidneys after 3 and 12 h but disappeared after 24 h. On the other hand, when fish received phage-impregnated feed after a bacterial challenge, P. plecoglossicida cells were not detected in kidneys of fish 12 h after challenge or later, and phages were detected in kidneys of some fish 3 to 24 h after challenge.
TABLE 6.
Kinetics of P. plecoglossicida and phage administered to ayua
| Time after inoculation (h) | Fish no. |
P. plecoglossicida count (log10 CFU g−1) in fish fed:
|
Phage count (log10 PFU g−1) in fish fed:
|
||
|---|---|---|---|---|---|
| Bacteria alone | Bacteria and phage | Phage alone | Bacteria and phage | ||
| 0 | 1 | <2 | <2 | <2 | <2 |
| 2 | <2 | <2 | <2 | <2 | |
| 3 | <2 | <2 | <2 | <2 | |
| 3 | 1 | <2 | <2 | 5.0 | <2 |
| 2 | 3.5 | <2 | 4.9 | 3.1 | |
| 3 | <2 | 4.8 | 5.7 | 4.8 | |
| 12 | 1 | 5.3 | <2 | 3.8 | 3.1 |
| 2 | 4.8 | <2 | 3.1 | 3.9 | |
| 3 | 4.0 | <2 | <2 | 3.9 | |
| 24 | 1 | 4.0 | <2 | <2 | <2 |
| 2 | 4.9 | <2 | <2 | 3.5 | |
| 3 | 6.3 | <2 | <2 | <2 | |
| 48 | 1 | 4.9 | <2 | <2 | <2 |
| 2 | 4.2 | <2 | <2 | <2 | |
| 3 | 4.7 | <2 | <2 | <2 | |
| 72 | 1 | 4.3 | <2 | <2 | <2 |
| 2 | 4.1 | <2 | <2 | <2 | |
| 3 | 4.2 | <2 | <2 | <2 | |
| 120 | 1 | 4.3 | —b | — | — |
| 2 | 4.1 | — | — | — | |
| 3 | 4.2 | — | — | — | |
| 168 | 1 | 4.2 | — | — | — |
| 2 | 4.2 | — | — | — | |
| 3 | 3.9 | — | — | — | |
Three groups of fish (average weight, 2.4 g) were fed bacterium (PTH-9802)-impregnated pellets, pellets impregnated with a mixture of phages PPpW-3 and PPpW-4, or phage-impregnated pellets after bacterial challenge (water temperature, 22°C).
—, not done.
DISCUSSION
Because of the lack of effective chemotherapy, a way to control P. plecoglossicida infections in cultured ayu is urgently needed. Some antimicrobial agents, such as florfenicol and sulfisozole, are used to treat coldwater disease; after such treatment, particularly when it is coupled with overfeeding, P. plecoglossicida infection abruptly emerges and results in high mortality levels. This is a typical example of microorganism substitution in fish disease. Thus, it has been hypothesized that P. plecoglossicida is an opportunistic pathogen, but our infection experiment in which intramuscular injection was used revealed that this bacterium is highly virulent for ayu, with an LD50 of 101.2 CFU fish−1. This virulence is equivalent to the reported virulence of V. anguillarum (6), which was the most devastating pathogen of cultured ayu in the 1980s. In contrast to V. anguillarum, which is not able to grow in medium that does not contain sodium chloride or to survive in freshwater (1, 11), P. plecoglossicida survived and proliferated well in freshwater in which ayu was reared (Fig. 3). This indicates that this bacterium can be present in all ayu culture environments and that there can be rapid horizontal transmission of the disease, although the precise mechanisms of infection remain unknown.
Previously, we described isolation of L. garvieae-specific phage belonging to the family Siphoviridae from fish culture environments (seawater and sediment) by the enrichment method (16). This method was also very useful for isolating phage specific to P. plecoglossicida in the present study, and two types of phages were isolated from diseased ayu and the rearing pond water. Based on morphological and genotypic characteristics, one type of phage, which has a long contractile tail and forms small plaques, can be classified as a member of the family Myoviridae, and the other type, which has a very short tail and forms large plaques, can be classified as a member of the family Podoviridae (12).
P. plecoglossicida strains are homogeneous with respect to biochemical characteristics (14, 15), and our study demonstrated that all of the bacterial isolates obtained from geographically and chronologically different sources which we examined were members of a single serotype and a single phage type. This makes it possible to identify the bacterium by phage analysis. The fact that P. plecoglossicida infection has been restricted to cultured ayu so far may have led to the low level of diversity of the organism, although some marine fish species, such as Japanese flounder (Paralichthys olivaceus) and red seabream (Pagrus major), were susceptible to the bacterium in experimental infection studies (14). However, in two previous papers (14, 31) the authors described contrasting results for in vitro motility of the bacterium and the presence of bloody ascites in affected fish; both of these characteristics were positive in one study (31), and both were negative in the other study (14). According to our observations during disease outbreaks in Tokushima Prefecture in 1998 and 1999, the presence of bloody ascites was a characteristic clinical sign in diseased fish. Of the 27 isolates of P. plecoglossicida examined in the present study, 10, including FPC941 and FPC951 (31), were motile and 17, including AK-9510 and PH-9501 (14), were not motile. The relationship between the motility of the bacterium and different clinical conditions (bloody ascites) in affected fish remains unclear.
As pointed out previously (3, 23), it is thought that the reasons why phages are of little value in controlling bacterial infections in humans and animals include the apparent low activity of phages in vivo, the very narrow host specificities of phages, and the rapid emergence of phage-resistant bacterial variants during treatment. These hypotheses concerning phage therapy were reinforced after successful chemotherapy with antibiotics began in the 1940s. However, these characteristics may not be disadvantages for phage treatment in some cases (3), including phage treatment of P. plecoglossicida infection of ayu. In this study, oral administration of phage with feed clearly protected fish against experimental infection, indicating that the level of phage activity in vivo was high (Table 5). The kinetics of inoculated bacteria and phage resulted in in vivo survival of phage and in vivo killing of bacterial cells by phage (Table 6). Interestingly, phage that were administered orally appeared in the kidneys of fish without host bacterial cells as a transport vehicle, although the time that the phage remained in the organ was relatively short. Easy movement of phage from the alimentary tract to the blood circulation system was also observed during human phage therapy (22). These results indicate that orally administered phage can be expected to kill bacterial cells in internal organs, as well as bacterial cells in the intestine, which means that phage therapy can be effective at the systemic infection stage. This conclusion is supported by the finding that phage treatment was effective even 24 h after bacterial challenge, when the infection was systemic (Table 6). If a similar effect occurs in naturally infected fish, bacterial cells that are disrupted by phage infection may serve as a good unattenuated bacterin; that is, there could be two effects of phage therapy, treatment and vaccination. In addition, the incubation temperature required for lytic activity of the phage ranged from 10 to 25 or 30°C, which covers the entire range of rearing water temperatures during ayu culture.
All phage isolates obtained in this study were P. plecoglossicida specific but not strain specific, suggesting that a single phage strain or a few phage strains could provide effective phage therapy. Further studies should be performed to select the most effective phage strain or effective combination of phage strains for therapeutic applications. The fact that infection was established by oral route also suggests that the intestine is an important portal of entry for the pathogen, and the narrow host range of phage should be an advantage in phage treatment because the phage do not harm the normal intestinal microflora.
Phage-resistant variants of P. plecoglossicida, which were induced in vitro, lacked virulence for ayu, and no phage-resistant variants were obtained from fish that died after phage treatment. In successful phage control of E. coli infections in mice and calves, the phage-resistant organisms that emerged during phage treatment were the less virulent K− type organisms (23, 24). Considering the general importance of the bacterial cell surface as a virulence factor, a surface component associated with bacterial virulence also seems to be the receptor for phage attachment, and consequently phage-resistant variants of a virulent organism would not be pathogenic. As stated previously (2), adaptation of a pathogen and a phage in which the bacterial surface virulence determinant is the attachment site for the phage may be essential for successful phage therapy or control. If this is true, P. plecoglossicida must have such a cell surface component that is a virulence factor; this hypothesis is supported by the fact that no toxins lethal to ayu were detected in P. plecoglossicida cultures (data not shown). From a different point of view, phage should be useful for obtaining mutants that have different surface characteristics as virulence factors for given pathogenic bacteria.
In conclusion, our successful phage treatment of experimentally induced P. plecoglossicida infection suggests that phage could be used to control this disease. Moreover, the finding that phage can inhibit bacterial growth in water (Fig. 3) suggests that the phage could be used prophylactically to prevent horizontal transmission of the pathogen. Experiments to determine the effectiveness of phage against natural infections should be performed in order to develop a phage control treatment for the disease.
ACKNOWLEDGMENTS
We thank the staffs of the Yamanashi, Wakayama, Kyoto, Shiga, Hiroshima, and Miyazaki Prefectural Fisheries Experiment Stations and H. Wakabayashi of The University of Tokyo for providing P. plecoglossicida strains and a rabbit antiserum used in this study.
This study was supported in part by grants from the Ministry of Education and Tokushima Prefecture, Japan.
REFERENCES
- 1.Austin B, Austin D A. Bacterial fish pathogens. Disease in farmed and wild fish. 2nd ed. London, United Kingdom: Ellis Horwood Ltd.; 1993. Vibrionaceae representatives; pp. 265–307. [Google Scholar]
- 2.Barrow P, Lovell M, Berchieri A., Jr Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin Diagn Lab Immunol. 1998;5:294–298. doi: 10.1128/cdli.5.3.294-298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrow P A, Soothill J S. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol. 1997;5:268–271. doi: 10.1016/S0966-842X(97)01054-8. [DOI] [PubMed] [Google Scholar]
- 4.Bernardet J-F, Segers P, Vancanneyt M, Berthe F, Kersters K, Vandamme P. Cutting a gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978) Int J Syst Bacteriol. 1996;46:128–148. [Google Scholar]
- 5.Iida Y, Mizokami A. Outbreaks of coldwater disease in wild ayu and pale chub. Fish Pathol. 1996;31:157–164. [Google Scholar]
- 6.Jo Y. Studies on Vibrio anguillarum infection in cultured ayu and its immunological prophylaxis. Shikoku Acta Med. 1981;37:82–110. [Google Scholar]
- 7.Kitao T. Streptococcal infections. In: Inglis V, Roberts R J, Bromage N R, editors. Bacterial diseases of fish. London, United Kingdom: Blackwell Scientific Publications Ltd.; 1993. pp. 196–210. [Google Scholar]
- 8.Kusuda R, Kawai K, Salati F, Banner C R, Fryer J L. Enterococcus seriolicida sp. nov., a fish pathogen. Int J Syst Bacteriol. 1991;41:406–409. doi: 10.1099/00207713-41-3-406. [DOI] [PubMed] [Google Scholar]
- 9.Merino S, Camprubi S, Tomas J M. Isolation and characterization of bacteriophage PM2 from Aeromonas hydrophila. FEMS Microbiol Lett. 1990;68:239–244. doi: 10.1016/s0378-1097(05)80047-3. [DOI] [PubMed] [Google Scholar]
- 10.Merril C R, Biswas B, Carlton R, Jensen N C, Creed G J, Zullo S, Adhya S. Long-circulating bacteriophage as antibacterial agents. Proc Natl Acad Sci USA. 1996;93:3188–3192. doi: 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muroga K, Iida M, Matsumoto H, Nakai T. Detection of Vibrio anguillarum from waters. Bull Jpn Soc Sci Fish. 1986;52:641–647. [Google Scholar]
- 12.Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy—6th report of ICTV. New York, N.Y: Springer-Verlag; 1995. Tailed phages; pp. 49–63. [Google Scholar]
- 13.Nakai T, Sugimoto R, Park K-H, Matsuoka S, Mori K, Nishioka T, Maruyama K. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis Aquat Org. 1999;37:33–41. doi: 10.3354/dao037033. [DOI] [PubMed] [Google Scholar]
- 14.Nakatsugawa T, Iida Y. Pseudomonas sp. isolated from diseased ayu, Plecoglossus altivelis. Fish Pathol. 1996;31:221–227. [Google Scholar]
- 15.Nishimori E, Kita-Tsukamoto K, Wakabayashi H. Pseudomonas plecoglossicida sp. nov., the causative agent of bacterial hemorrhagic ascites of ayu, Plecoglossus altivelis. Int J Syst Evol Microbiol. 2000;50:83–89. doi: 10.1099/00207713-50-1-83. [DOI] [PubMed] [Google Scholar]
- 16.Park K-H, Kato H, Nakai T, Muroga K. Phage typing of Lactococcus garvieae (formerly Enterococcus seriolicida), a pathogen of cultured yellowtail. Fish Sci. 1998;64:62–64. [Google Scholar]
- 17.Park K-H, Matsuoka S, Nakai T, Muroga K. A virulent bacteriophage of Lactococcus garvieae (formerly Enterococcus seriolicida) isolated from yellowtail, Seriola quinqueradiata. Dis Aquat Org. 1997;29:145–149. [Google Scholar]
- 18.Paterson W D, Douglas R J, Grinyer I, McDermott L A. Isolation and preliminary characterization of some Aeromonas salmonicida bacteriophages. J Fish Res Can. 1969;26:629–632. [Google Scholar]
- 19.Radetsky P. The good virus. Discover. 1996;17:50–58. [Google Scholar]
- 20.Rodgers C J, Pringle J H, McCarthy D H, Austin B. Quantitative and qualitative studies of Aeromonas salmonicida bacteriophage. J Gen Microbiol. 1981;125:335–345. [Google Scholar]
- 21.Shotts E B, Jr, Starliper C E. Flavobacterial diseases: columnaris disease, cold-water disease and bacterial gill disease. In: Woo P T K, Bruno D W, editors. Fish diseases and disorders, vol. 3. Viral, bacterial and fungal infections. I Publishing, New York: CAB; 1999. pp. 559–576. , N.Y. [Google Scholar]
- 22.Slopek S, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981–1986. Arch Immunol Ther Exp. 1987;35:569–583. [PubMed] [Google Scholar]
- 23.Smith H W, Huggins M B. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol. 1982;128:307–318. doi: 10.1099/00221287-128-2-307. [DOI] [PubMed] [Google Scholar]
- 24.Smith H W, Huggins M B. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J Gen Microbiol. 1983;129:2659–2675. doi: 10.1099/00221287-129-8-2659. [DOI] [PubMed] [Google Scholar]
- 25.Smith H W, Huggins M B, Shaw K M. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J Gen Microbiol. 1987;133:1111–1126. doi: 10.1099/00221287-133-5-1111. [DOI] [PubMed] [Google Scholar]
- 26.Smith H W, Huggins M B, Shaw K M. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J Gen Microbiol. 1987;133:1127–1135. doi: 10.1099/00221287-133-5-1127. [DOI] [PubMed] [Google Scholar]
- 27.Soothill J S. Treatment of experimental infections of mice with bacteriophages. J Med Microbiol. 1992;37:258–261. doi: 10.1099/00222615-37-4-258. [DOI] [PubMed] [Google Scholar]
- 28.Soothill J S. Bacteriophage prevents destruction of skin grafts by Pseudomonas aeruginosa. Burns. 1994;20:209–211. doi: 10.1016/0305-4179(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 29.Spencer R. Indigenous marine bacteriophages. J Bacteriol. 1960;79:614. doi: 10.1128/jb.79.4.614-614.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson R M W, Airdrie D W. Isolation of Yersinia ruckeri bacteriophages. Appl Environ Microbiol. 1984;47:1201–1205. doi: 10.1128/aem.47.6.1201-1205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakabayashi H, Sawada K, Ninomiya K, Nishimori E. Bacterial hemorrhagic ascites of ayu caused by Pseudomonas sp. Fish Pathol. 1996;31:239–240. [Google Scholar]
- 32.Wakabayashi H, Toyama T, Iida T. A study on serotyping of Cytophaga psychrophila isolated from fishes in Japan. Fish Pathol. 1994;29:101–104. [Google Scholar]
- 33.Wu J-L, Lin H-M, Jan L, Hsu Y-L, Chang L-H. Biological control of fish bacterial pathogen, Aeromonas hydrophila, by bacteriophage AH1. Fish Pathol. 1981;15:271–276. [Google Scholar]