Abstract
Lower respiratory infection was reported as the most common fatal infectious disease. Community-acquired pneumonia (CAP) and myocardial injury are associated; yet, true prevalence of myocardial injury is probably underestimated. We assessed the rate and severity of myocardial dysfunction in patients with CAP. Admitted patients diagnosed with CAP were prospectively recruited. All the patients had C-reactive protein (CRP), brain natriuretic peptide (BNP), and high-sensitivity cardiac troponin (hs-cTnl) tests added to their routine workup. 2D/3D Doppler echocardiography was done on a Siemens Acuson SC2000 machine ≤ 24 h of diagnosis. 3D datasets were blindly analyzed for 4-chamber volumes/strains using EchobuildR 3D-Volume Analysis prototype software, v3.0 2019, Siemens-Medical Solutions. Volume/strain parameters were correlated with admission clinical and laboratory findings. The cohort included 34 patients, median age 60 years (95% CI 55–72). The cohort included 18 (53%) patients had hypertension, 9 (25%) had diabetes mellitus, 7 (21%) were smokers, 7 (21%) had previous myocardial infarction, 4 (12%) had chronic renal failure, and 1 (3%) was on hemodialysis treatment. 2D/Doppler echocardiography findings showed normal ventricular size/function (LVEF 63 ± 9%), mild LV hypertrophy (104 ± 36 g/m2), and LA enlargement (41 ± 6 mm). 3D volumes/strains suggested bi-atrial and right ventricular dysfunction (global longitudinal strain RVGLS = − 8 ± 4%). Left ventricular strain was normal (LVGLS = − 18 ± 5%) and correlated with BNP (r = 0.40, p = 0.024). The patients with LVGLS > − 17% had higher admission blood pressure and lower SaO2 (144 ± 33 vs. 121 ± 20, systolic, mmHg, p = 0.02, and 89 ± 4 vs. 94 ± 4%, p = 0.006, respectively). hs-cTnl and CRP were not different. Using novel 3D volume/strain software in CAP patients, we demonstrated diffuse global myocardial dysfunction involving several chambers. The patients with worse LV GLS had lower SaO2 and higher blood pressure at presentation. LV GLS correlated with maximal BNP level and did not correlate with inflammation or myocardial damage markers.
Keywords: Community- acquired pneumonia, Myocardial injury, Echocardiography, Strain
Background
Lower respiratory infection was reported as the most common fatal infectious disease and the leading cause of death in lower income countries [1]. In spite of progress made in the laboratory evaluation, treatment and with supportive care, the mortality rate has remained similar for the last 70 years [2, 3]. Lately, myocardial involvement was acknowledged as playing a crucial role in the grim prognosis of these patients [4–6].
The pathogenesis of acute myocardial injury in patients hospitalized with community-acquired pneumonia (CAP) is variable and includes type-2 myocardial infarction with or without prior coronary artery disease (CAD) due to imbalance between demand and supply as well as non-CAD myocardial damage by toxins, direct myocardial infection, inflammatory mediators, and stress-induced cardiomyopathy. The risk of angina pectoris and myocardial infarction has been reported to be between 0.7 and 10.7%, and was the highest (50% of cases) in the first 24 h of admission for pneumonia, and up to 90% of cases are diagnosed within 7 days after admission [6–9]. The risk may also be related to the pathogenic etiologic agent of pneumonia that can be identified in 20–76% of cases.
The diagnosis of an acute coronary event is no different than in patients without pneumonia and includes elevated biomarkers of myocardial injury combined with clinical presentation, ECG findings, coronary angiogram, or echocardiography, including regional wall motion imaging. The association between CAP and myocardial injury is often assessed by myocardial biomarkers, ECG or cardiac imaging tests, which is clinically driven and is done only in specific patients. Accordingly, the true prevalence of myocardial damage is probably grossly underestimated. Most of the reports concentrate on the left ventricular myocardial function while the right ventricle, which is directly affected by the pulmonary pressure load and atria are hardly assessed.
Contemporary, highly sensitive, and specific serum biomarkers (i.e., hs-cTnl, natriuretic peptides), combined with myocardial mechanics assessed by strain imaging and 3D echocardiography, can identify early myocardial damage before functional deterioration becomes apparent [10].
We aimed to assess the rate and severity of myocardial dysfunction in patients admitted with CAP.
Methods
The study was designed as a prospective, single blind observational pilot study.
The patients were recruited consecutively, if they met the following inclusion criteria: ≥ 18 years of age, admitted to internal medicine ward/intensive care unit, and were diagnosed with CAP within 48 h as the cause of admission. Diagnosis of CAP required 3 of the following parameters: temperature > 38 °C or < 36 °C, coughing, purulent sputum, pleuritic chest pain, dyspnea, tachypnea > 16 breaths per minute, white cell count > 12,000/mm3 or < 4000/mm3 or > 10% polymorphonuclear band forms, and/or a new pulmonary infiltrate on the admission chest X-ray compatible with pneumonia. Exclusion criteria: immune suppression, pregnancy, nosocomial pneumonia (previous admissions within 28 days before current admission or pneumonia developing > 48 h during current admission), and/or aspiration pneumonia. The patients had to be able to sign an informed consent form to participate in this study. The study was approved by the institutional research ethics board of XXXXXE (0096–15-POR) XXXXX.
Laboratory evaluation included microbiologic testing — sputum and blood for cultures, serologic assessment for Q fever and Mycoplasma pneumonia, urine antigen for Legionella pneumophila, nasopharyngeal or sputum swab PCR for respiratory viruses and bacteria (RSV, Flu A, Flu B, H1N1, Streptococcus pneumonia, Haemophilus influenza). C-reactive protein was measured as an inflammatory marker, myocardial damage, and heart failure biomarkers (hs-cTnl, BNP) were examined within 24–48 h from CAP diagnosis.
The 2D-transthoracic echocardiography assessment was completed within 24–48 h of CAP diagnosis. All the studies were performed according to current echocardiography guidelines [11–13]. All the studies were performed on the Siemens Acuson SC2000 machine and stored for further evaluation on the Siemens SC2000 Workplace (Siemens Medical Solutions (SMS), Mountain View, CA, USA). Each study included 3D clips taken with 4Z1c transducer optimized for best temporal resolution.
3D strain analysis was done offline by a single viewer blinded to the clinical, laboratory, and conventional echocardiography study interpretation. All the 3D datasets were processed using a software package for automated volumetric strain analysis — EchobuildR 3D Volume Analysis prototype software, v3.0 2019, SMS [14–16]. The software generated 3D endocardial LV, RV, LA, and RA volumes (Fig. 1), global longitudinal strains (GLS), and LV and RV mid (papillary) level global circumferential strains (CGS).
Fig. 1.
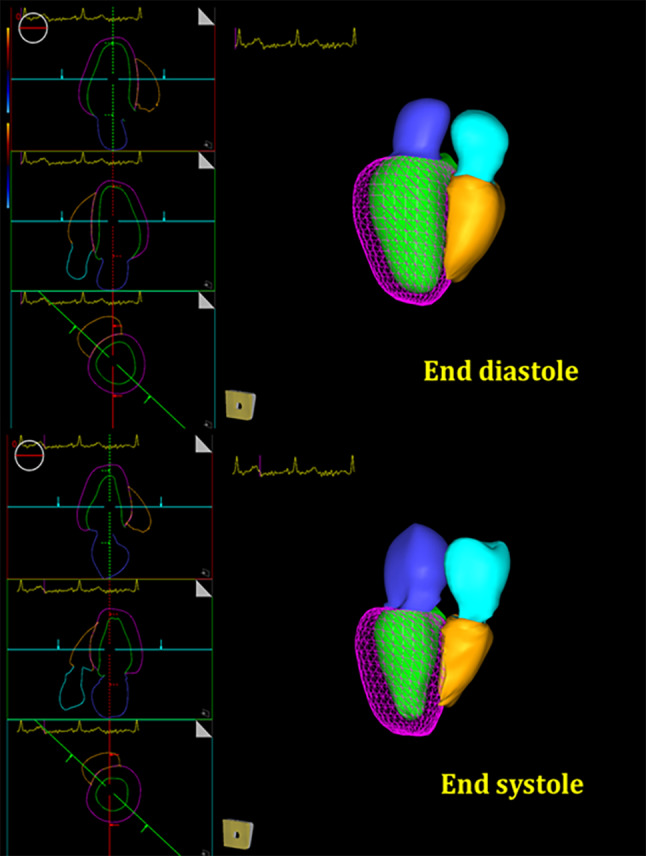
4 Chambers auto tracking with 3D Strain software. End-diastolic and end systolic representative images demonstrating endocardial contours of all chambers, and epicardial contour of the left ventricle. Tracking is done for all 3D clip frames, to generate volume and strain curves
Statistical analysis was done using MedCalc Statistical Software version 19.2 (MedCalc Software Ltd, Ostend, Belgium). Categorical variables were expressed as percentages and continuous variables as means ± standard deviations. The cohort was divided into two subgroups, according to the presence of reduced LV longitudinal shortening (GLS > − 17%). Anthropomorphic, clinical, laboratory, and echocardiographic results were compared using the Wilcoxon-Mann–Whitney non-parametric test, as normal distributions could not be assured. Chi-square analysis was used for categorical variables. Uni- and multivariable associates of reduced LV function (GLS < − 17%) were assessed by logistic regression analysis. Statistical significance was defined as a p value < 0.05.
Results
Patient Cohort
Our cohort included 34 patients admitted with CAP that met our inclusion and exclusion criteria. Median age was 60 years (95% CI 55–72), ranging from 19 to 95 years. In our cohort, 18 (53%) patients had hypertension, 9 (25%) had diabetes mellitus, 7 (21%) were smokers, 7 (21%) had previous myocardial infarction, 4 (12%) had chronic renal failure, and 1 (3%) was on hemodialysis treatment (Table 1).
Table 1.
Patients and admission characteristics
| All patients | n = 34 |
|---|---|
| Age (yr) | 61 ± 20 |
| Sex (M, %) | 19, 52 |
| Height (cm) | 167 ± 10 |
| Weight (kg) | 83 ± 21 |
| Diabetes mellitus (n, %) | 9 (25%) |
| Hyperlipidemia (n, %) | 16 (47%) |
| Hypertension (n, %) | 18 (53%) |
| Previous myocardial infarction (n, %) | 7 (21%) |
| Smoking (n, %) | 7 (21%) |
| Chronic renal failure | 4 (12%) |
| Hemodialysis (n, %) | 1 (3%) |
| Admission characteristics | |
| Sinus rhythm (n, %) | 32 (94%) |
| Heart rate (min−1) | 100 ± 19 |
| Systolic BP (mmHg) | 131 ± 28 |
| Diastolic BP (mmHg) | 76 ± 19 |
| Temperature (° Celsius) | 37.7 ± 0.9 |
| O2 saturation (%) | 92 ± 5 |
| Lactate (mg/dl) | 2 ± 0.8 |
| Arterial pH | 7.4 ± 0.1 |
| Arterial pCO2 | 47 ± 13 |
| Arterial bicarbonate (mmol/l) | 30 ± 7 |
| Hb (gr/dl) | 13 ± 2 |
| WBC (× 1000/ml) | 12.4 ± 5.1 |
| PLt (× 1000/ml) | 243 ± 91 |
| Creatinine (mg/dl) | 1.2 ± 1.3 |
| Blood urea nitrogen (mg/dl) | 19 ± 9 |
| Abnormal liver function tests (n, %) | 5 (15%) |
| INR | 1.7 ± 0.9 |
| PTT (s) | 31 ± 3 |
| BNP (pg/ml) | 279 ± 528 |
| CRP (mg/l) | 54 ± 41 |
| hs-cTnl (ng/l) (normal < 5 ng/l) | 36 ± 67 |
The patients had a mean heart rate of 100 ± 19 (beats/min), indicating mild tachycardia. The mean temperature was 37.7 ± 09 °C, with mean arterial O2 saturation was mildly reduced to 92 ± 5%, and mildly elevated mean pCO2 to 47 ± 13 mmHg. There was mild renal function impairment with mean creatinine of 1.2 ± 1.3 mg/dl, without metabolic signs of dehydration as defined by normal BUN/creatinine ratio. Blood count showed mild leukocytosis at 12.4 ± 5.1 (1000/ml), no thrombocytopenia at 243 ± 91 (1000/ml), and normal levels of hemoglobin 13 ± 2 g%. A minority of patients (15%) demonstrated hepatic dysfunction resulting in an abnormal liver function test and mildly elevated INR (mean 1.7 ± 0.9). The levels of CRP, a marker of inflammation, was 54 ± 41 mg/l, while hs-cTnl, a marker for myocardial damage was 36 ± 67 ng/l, both of which were elevated. Mean BNP was also elevated with a wide range of deviation (279 ± 528 pg/ml). An etiologic infectious agent was found in only 5 cases.
All the patients with previous MI or known CAD had documented normal LV systolic function assessed by conventional 2D echocardiography prior to pneumonia as part of their standard care.
Conventional 2D Doppler reports (Table 2) demonstrated normal size and function of the left and right ventricles, mild concentric hypertrophy (LV mass index > 100 g/m2), mildly enlarged LA, normal RA, and normal right ventricular and right atrial systolic pressures.
Table 2.
Conventional 2D-Doppler and 3D strain echocardiography
| 2D-Doppler | (n = 34) |
|---|---|
| LV end diastolic diameter (mm) | 51 ± 5 |
| LV ejection fraction (%) | 63 ± 9 |
| LV mass index (g/m2) | 104 ± 36 |
| Regional wall abnormalities (n, %) | 4 (11%) |
| RV function, abnormal (n, %) | 1 (3%) |
| RV size, abnormal (n, %) | 0 (0%) |
| LA systolic diameter | 41 ± 6 |
| Mitral valve regurgitation ≥ 3 | 1 (3%) |
| Aortic valve regurgitation ≥ 3 | 0 (0%) |
| Tricuspid valve regurgitation ≥ 3 | 1 (3%) |
| RV systolic pressure (mmHg) | 32 ± 13 |
| Right atrial pressure (mmHg) | 8 ± 6 |
| Mitral E velocity (cm/min) | 80 ± 23 |
| Mitral E to A ratio | 1.2 ± 0.5 |
| Mitral E to E′ ratio | 9 ± 4 |
| 3D-strain analysis | |
| Left ventricular | |
| End diastolic volume (ml) | 97 ± 33 |
| Ejection fraction (%) | 49 ± 8 |
| Global longitudinal strain (%) | − 18 ± 5 |
| Global circumferential strain (%) | − 23 ± 7 |
| Right ventricular | |
| End diastolic volume (ml) | 81 ± 23 |
| Ejection fraction (%) | 31 ± 13 |
| Global longitudinal strain (%) | − 8 ± 4 |
| Global circumferential strain (%) | − 9 ± 5 |
| TAPSE | 10 ± 5 |
| Left atrial | |
| End systolic volume (ml) | 52 ± 32 |
| Ejection fraction (%) | 24 ± 15 |
| Global longitudinal strain | 22 ± 13 |
| Right atrial | |
| End systolic volume (ml) | 36 ± 20 |
| Ejection fraction (%) | 18 ± 12 |
| Global longitudinal strain | 26 ± 16 |
| Heart rate during study (min−1) | 85 ± 15 |
3D volumes and strains are summarized in Table 2. While all the parameters demonstrated large variabilities, the mean LV volume and strain were found to be in the normal range, and LA, RV, and RA strains and volumes all demonstrated reduced function. The LA reservoir strain was nearly half normal as was the LA ejection fraction (reservoir function). The RA strain demonstrated similar results. Maximal volumes of all chambers were normal (end diastolic for ventricles, end systolic for atria) probably implying that functional impairment was not due to chronic pathology. LV GLS was the only strain parameter moderately correlating with maximal BNP level (r = 0.40, 95% CI 0.06–0.66, p = 0.024). Clinical, admission laboratory tests, and other volume/strain variables were not associated with elevated BNP levels. Neither LVGLS nor all other variables were associated with CRP or hs-cTnl serum levels.
Patient Subgroups According to LV Longitudinal Strain
As the LV strain was the only parameter that was not uniformly subnormal, we divided our cohort to patients with normal LV longitudinal strain (GLS ≤ − 17%, where < means larger negative value, better shortening) and abnormal (GLS > − 17%) 10,17. The patients with LV longitudinal dysfunction had similar clinical characteristics (Table 3), significantly higher systolic and diastolic blood pressures, and lower arterial O2 saturation on admission (Fig. 2). There were no significant differences in other laboratory test results. BNP and troponin levels were elevated in the LV systolic dysfunction group, a difference that did not reach statistical significance. Conventional 2D echocardiography, as shown in Table 2, demonstrated normal mean LVEF (63 ± 9%), and only one patient had RV dysfunction. However, when evaluated with 3D volume and strain analysis, LVEF and RVEF were both significantly abnormal (49 ± 8% and 31 ± 13, respectively). It is important to emphasize that echocardiographic 3D volume assessment is considered more accurate compared to the 2D echocardiography and correlates better with MRI and CT assessments [17].
Table 3.
Patient and admission characteristics grouped by LV GLS
| Patient | LV GLS ≤ − 17 (good) n = 21 | LV GLS > − 17 (bad) n = 13 | p-Value |
|---|---|---|---|
| Age (yr) | 63 ± 23 | 62 ± 18 | 0.9 |
| Sex (M, %) | 10, 62 | 8, 48 | 0.43 |
| Height (cm) | 168 ± 12 | 166 ± 10 | 0.65 |
| Weight (kg) | 78 ± 16 | 82 ± 17 | 0.53 |
| Diabetes mellitus (n, %) | 4 (19%) | 5 (38%) | 0.22 |
| Hyperlipidemia (n, %) | 9 (43%) | 7 (54%) | 0.54 |
| Hypertension (n, %) | 10 (48%) | 8 (62%) | 0.44 |
| Previous MI (n, %) | 6 (29%) | 1 (8%) | 0.15 |
| Smoking (n, %) | 3 (14%) | 4 (30%) | 0.26 |
| Chronic renal failure (n, %) | 2 (10%) | 2 (15%) | 0.61 |
| Hemodialysis (n, %) | 0 (0%) | 1 (8%) | 0.20 |
| Admission characteristics | |||
| Sinus rhythm (n, %) | 19 (90) | 12 (92) | 0.86 |
| Heart rate (min−1) | 98 ± 21 | 105 ± 17 | 0.37 |
| Systolic BP (mmHg) | 121 ± 20 | 144 ± 33 | 0.02 |
| Diastolic BP (mmHg) | 70 ± 16 | 84 ± 20 | 0.025 |
| Temperature (° Celsius) | 37.7 ± 1.0 | 37.7 ± 0.9 | 0.96 |
| O2 saturation (%) | 94 ± 4 | 89 ± 4 | 0.006 |
| Lactate (mg/dl) | 1.8 ± 0.8 | 2.2 ± 1.0 | 0.313 |
| Arterial pH | 7.39 ± 0.08 | 7.40 ± 0.08 | 0.65 |
| Arterial pCO2 | 50 ± 16 | 44 ± 7 | 0.27 |
| Arterial bicarbonate (mmol/l) | 30 ± 5 | 30 ± 9 | 0.96 |
| Hb (gr/dl) | 13 ± 2 | 13 ± 2 | 0.46 |
| WBC (× 1000/ml) | 13 ± 6 | 12 ± 4 | 0.61 |
| PLt (× 1000/ml) | 252 ± 111 | 245 ± 50 | 0.60 |
| Creatinine (mg/dl) | 1.0 ± 0.4 | 1.5 ± 2.0 | 0.24 |
| Blood urea nitrogen (mg/dl) | 17 ± 7 | 21 ± 11 | 0.21 |
| Abnormal liver function tests (n, %) | 2 (10%) | 3 (23%) | 0.45 |
| INR | 2.0 ± 1.2 | 1.3 ± 0.3 | 0.07 |
| PTT (s) | 35 ± 10 | 27 ± 5 | 0.18 |
| Maximal BNP (mg/l) | 199 ± 166 | 423 ± 852 | 0.26 |
| CRP (pg/ml) | 58 ± 41 | 42 ± 39 | 0.28 |
| Maximal hs-cTnl (ng/l) | 22 ± 38 | 58 ± 93 | 0.145 |
Fig. 2.

Admission O2 saturation and left ventricular global longitudinal strain (LVGLS). Cut-off value for normal GLS was selected as − 17%. Patients with left ventricular dysfunction (LVGLS > − 17% demonstrated lower admission arterial O2 saturation
3D volume/strain analysis revealed that the patients with reduced LV longitudinal function had a statistically significant lower 3D analyzed–LV ejection fraction (in the mildly to moderately reduced range), a worse longitudinal strain (not surprising, as it was the categorizing variable) and a worse LV circumferential strain. The reduced longitudinal strain was not compensated by increased circumferential strain as may be seen in other pathologies. All the other parameters generated by the automatic 4-chamber–3D strain analysis were similar among patient groups (Table 4).
Table 4.
Strain analysis grouped by LV GLS
| LV GLS ≤ − 17 (good) n = 21 | LV GLS > − 17 (bad) n = 13 | p-Value | |
|---|---|---|---|
| Left ventricular | |||
| End diastolic volume (ml) | 96 ± 24 | 94 ± 46 | 0.84 |
| 3D ejection fraction (%) | 54 ± 7 | 42 ± 9 | < 0.001 |
| Global longitudinal strain (%) | − 21 ± 3 | − 14 ± 4 | < 0.001 |
| Global circumferential strain (%) | − 26 ± 6 | − 19 ± 7 | 0.006 |
| Right ventricular | |||
| End diastolic volume (ml) | 81 ± 18 | 77 ± 31 | 0.65 |
| Ejection fraction (%) | 33 ± 13 | 30 ± 12 | 0.53 |
| Global longitudinal strain (%) | − 8 ± 4 | − 8 ± 3 | 0.83 |
| Global circumferential strain (%) | − 9 ± 5 | − 10 ± 7 | 0.77 |
| TAPSE | 10 ± 6 | 10 ± 4 | 0.75 |
| Left atrial | |||
| End systolic volume (ml) | 50 ± 31 | 50 ± 31 | 1.0 |
| Ejection fraction (%) | 24 ± 14 | 24 ± 15 | 0.85 |
| Global longitudinal strain | 23 ± 14 | 19 ± 8 | 0.40 |
| Right atrial | |||
| End systolic volume (ml) | 37 ± 19 | 32 ± 17 | 0.52 |
| Ejection fraction (%) | 15 ± 13 | 22 ± 9 | 0.15 |
| Global longitudinal strain | 25 ± 16 | 26 ± 14 | 0.90 |
| Heart rate during study (min−1) | 83 ± 13 | 86 ± 17 | 0.52 |
Discussion
We have demonstrated that the patients with CAP, severe enough to be admitted, exhibited 4-chamber dysfunction that was detected with 3D strain echocardiography and not detected with conventional 2D echocardiography. The 3D volume/strain echocardiography provides a more accurate assessment of volume and function compared to the 2D conventional echocardiography [17]. Furthermore, since changes in the strain occur before the changes in volume and function, 3D volume/strain echocardiography allows for early detection of myocardial dysfunction and for prompt intervention to restore cardiac function. The implication of early detection of myocardial dysfunction is that it promotes early intervention, which may improve clinical outcomes in patients with community-acquired pneumonia. Possible interventions may include enhanced hemodynamic monitoring. This intervention may include transferring patient to the cardiac ICU or pharmaceutical intervention to restore cardiac function, including beta-blockers, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers/angiotensin and aldosterone receptor antagonists.
In addition, left ventricular dysfunction, detected with 3D volume and strain analysis was found to be associated with lower admission O2 saturation, elevated BNP levels, and higher blood pressure in CAP patients. The 3D volume/strain echocardiograph can be used for risk stratification and may trigger early medical intervention. The possibility of using 3D volume/strain echocardiography for early intervention and risk stratification in patients with CAP on clinical outcomes should be examined in larger longitudinal studies. This intervention may include transferring patients to the cardiac ICU or pharmaceutical intervention to restore cardiac function.
The atria and RV exhibited uniform dysfunction in all patients while LV function was reduced in a third of the patients. Decreased LV longitudinal strain correlated with elevated BNP levels, lower admission arterial O2 saturation, and higher blood pressure. Circumferential strain was similarly reduced in both ventricles.
Diagnosis of Chamber Dysfunction
The most accurate and reproducible method of chamber volume assessment by echocardiography as endorsed by guidelines is the 3D approach [11–16, 18], as it encompasses all endocardial borders, analyzes all the acquired frames of the acquired clip, and does not incorporate geometric chamber models in the calculations. This is even more pronounced in RV and RA that are not optimally imaged in 2D echocardiography [19]. Mild and unexpected global dysfunction is even more difficult to identify especially when it is longitudinal, and there is a misleading “normal” or even “hyper-normal” EF on MMode that represents preserved or compensatory circumferential shortening. Strain imaging is becoming the method of choice to assess subtle changes in ventricular function especially for pathologies that may affect global function such as immune-mediated myocarditis and chemotherapy-induced toxic cardiomyopathy [20, 21]. We have demonstrated that patients with CAP, severe enough to be admitted exhibited 4-chamber dysfunction detected only by 3D strain echocardiography, that was clinically associated with lower admission O2, higher blood pressure, and higher BNP. Larger studies are required to validate our findings that abnormal LVGLS was associated with reduced strain and lower arterial O2 saturation and higher BNP serum levels. Future study should determine the clinical role of 3D volume/strain echocardiography as a tool in risk stratification and management of CAP.
It is important to note that since RV dysfunction was not clinically suspected by 2D conventional echocardiography, CT angiography was not performed in these patients. RV dysfunction was only identified after performing 3D volume and strain analysis.
Potential Mechanisms of Chamber Dysfunction
The main mechanisms suggested mediating cardiac dysfunction in infectious diseases include: direct myocardial damage by the etiologic pathogen per se, myocardial inflammation, and cardio-depressant effects of cytokines, and stress-induced cardiomyopathy, which are similar to Tako-Tsubo syndrome [22]. Of note, some of these mechanisms have also been implicated in the recent SARS CoV-2 pandemic [23–25] affecting the severity of presentation and mortality in COVID-19. Our findings of elevated serum CRP and troponin accompanied with higher blood pressures upon admission may support hypoxemia, inflammation and a hyper adrenergic status and stress-induced cardiomyopathy as potential mechanisms [26]. Unfortunately, due to a very low yield of pathogen isolation, we were not able to relate the findings to any of the infectious agents. Yet, this suggests that myocardial dysfunction may be a universal finding in severe viral CAP.
Limitations
This is a proof-of-concept study with a limited number of patients. Larger longitudinal studies with adequate power are needed to detect differences in clinical outcomes between patients who were assessed by 3D echocardiography versus those who were assessed by 2D echocardiography. Although this study included patients with CAP severe enough to be hospitalized, it did not include patients with respiratory failure and mechanical ventilation that may have introduced a selection bias against severe myocardial dysfunction. The 3D volume/strain software was previously validated, but it has yet to be released commercially. More experience is still needed to improve its user interface and to use it to its full extent.
Future studies should compare the clinical outcomes of patients with reduced strain and those with preserved strain in community-acquired pneumonia patients. In addition, future studies should investigate whether early medical interventions in patients with reduced strain aimed at restoring myocardial function improve outcomes.
Conclusion
Using a novel 3D volume/strain software, we were able to demonstrate that patients hospitalized for CAP had significant myocardial dysfunction that was not observed using conventional echocardiography. Since the 3D volume/strain echocardiography provides accurate assessment of volume and function and allows for early detection of myocardial dysfunction, it has the potential to improve clinical outcomes by risk stratification and early intervention in community-acquired pneumonia patients. Future larger studies are required to evaluate 3D volume/strain echocardiography as a tool in the management of CAP.
Author Contribution
MK performed the research and contributed to the manuscript writing. GEG contributed to the manuscript writing and performed the final revision. ON performed the research and contributed to the manuscript writing. SS performed the research. AP designed the study and performed the research. YH recruited the study participants and contributed to the drafting of the manuscript. WK recruited the study participants and contributed to the drafting of the manuscript. YH recruited the study participants and performed the research. LGR contributed to the manuscript writing. HH performed the data analysis. OA designed the study and contributed to the manuscript writing. SC designed the study, performed the data analysis, statistics, and contributed to the manuscript writing. All the authors have read and approved the final manuscript.
Availability of Data and Material
Data are available upon request from the instigators.
Code Availability
Not applicable.
Declarations
Ethics Approval
Approval was obtained from our institutional ethic board. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. All the patients signed an informed consent form before enrolment in this study.
Consent to Participate
Informed consent to participate in the study was obtained from each patient.
Consent for Publication
Informed consent to publish finding of the study while keeping confidentiality was obtained from each patient.
Conflict of Interest
Helene Houle is an employee of Siemens Medical Solutions. The other authors have no financial relationships or conflicts of interest regarding the content of this manuscript. No funding was provided for this study.
Additional Declarations
Not application.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Moayad Khatib, Gabby Elbaz-Greener, Offer Amir and Shemy Carasso equally contributed to this study and manuscript.
Contributor Information
Offer Amir, Email: oamir@hadassah.org.il.
Shemy Carasso, Email: scarasso@poria.health.gov.il.
References
- 1.WHO. Web site 2018:https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 18 April 2020.
- 2.Waterer GW, Kessler LA, Wunderink RG. Medium-term survival after hospitalization with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169(8):910–914. doi: 10.1164/rccm.200310-1448OC. [DOI] [PubMed] [Google Scholar]
- 3.Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162(9):1059–1064. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 4.Bordon J, Wiemken T, Peyrani P, et al. Decrease in long-term survival for hospitalized patients with community-acquired pneumonia. Chest. 2010;138(2):279–283. doi: 10.1378/chest.09-2702. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen EM, Kapoor WN, Chang CC, Fine MJ. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis. 2003;37(12):1617–1624. doi: 10.1086/379712. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone J, Eurich DT, Majumdar SR, Jin Y, Marrie TJ. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine (Baltimore). 2008;87(6):329–334. doi: 10.1097/MD.0b013e318190f444. [DOI] [PubMed] [Google Scholar]
- 7.Viasus D, Garcia-Vidal C, Manresa F, Dorca J, Gudiol F, Carratala J. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J Infect. 2013;66(1):27–33. doi: 10.1016/j.jinf.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125(6):773–781. doi: 10.1161/CIRCULATIONAHA.111.040766. [DOI] [PubMed] [Google Scholar]
- 9.Corrales-Medina VF, Taljaard M, Fine MJ, et al. Risk stratification for cardiac complications in patients hospitalized for community-acquired pneumonia. Mayo Clin Proc. 2014;89(1):60–68. doi: 10.1016/j.mayocp.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Carasso S, Biaggi P, Rakowski H, et al. Velocity vector imaging: standard tissue-tracking results acquired in normals-the VVI-STRAIN study. J Am Soc Echocardiogr. 2012;25(5):543–552. doi: 10.1016/j.echo.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28(1):1–39 e14. [DOI] [PubMed]
- 12.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 13.Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Georgescu B, Zheng Y, Wang Y, Meer P, Comaniciu D. Prediction based collaborative trackers (PCT): a robust and accurate approach toward 3D medical object tracking. IEEE Trans Med Imaging. 2011;30(11):1921–1932. doi: 10.1109/TMI.2011.2158440. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Thavendiranathan P, Chen Y, et al. Feasibility of automated three-dimensional rotational mechanics by real-time volume transthoracic echocardiography: preliminary accuracy and reproducibility data compared with cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2016;29(1):62–73. doi: 10.1016/j.echo.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Bouchez S, Heyde B, Barbosa D, et al. In-vivo validation of a new clinical tool to quantify three-dimensional myocardial strain using ultrasound. Int J Cardiovasc Imaging. 2016;32(12):1707–1714. doi: 10.1007/s10554-016-0962-5. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Lessick J, Abadi S, Agmon Y, et al. Multidetector computed tomography predictors of late ventricular remodeling and function after acute myocardial infarction. European journal of radiology. 2011. [DOI] [PubMed]
- 19.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 786–688. [DOI] [PubMed]
- 20.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J. 2017;38(35):2649–2662. doi: 10.1093/eurheartj/ehx321. [DOI] [PubMed] [Google Scholar]
- 22.Aliberti S, Ramirez JA. Cardiac diseases complicating community-acquired pneumonia. Curr Opin Infect Dis. 2014;27(3):295–301. doi: 10.1097/QCO.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 23.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed]
- 26.Richard C. Stress-related cardiomyopathies. Ann Intensive Care. 2011;1(1):39. doi: 10.1186/2110-5820-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the instigators.
Not applicable.


