Abstract
In the agricultural domain, chemical pesticides are repetitively and harshly used to kill harmful pests, but they often pose a serious threat to microbial diversity, soil fertility and agricultural output. To deal with these problems, pesticide-tolerant plant growth promoting (PGP) rhizobacterial strains are often used to combat pesticidal toxicity. Here, Pseudomonas sp. PGR-11 (accession no. OM348534), recovered from a Vigna radiata (L.) rhizosphere, produced various growth regulating (GR) substances, including indole-3-acetic acid (IAA; 82.5 ± 9.2 μg mL−1), enzyme 1-aminocyclopropane 1-carboxylate (ACC) deaminase (μM α-ketobutyrate mg−1 protein h−1), siderophores and ammonia. Strain PGR-11 grew well when cultured in growth medium with added metalaxyl (MTXL; 1200 μg mL−1), carbendazim (CBZM; 800 μg mL−1) and tebuconazole (TBZL; 1600 μg mL−1). Pseudomonas sp. synthesized PGP substances even in the presence of increasing doses of pesticides. The phytotoxicity of the tested pesticides was assessed both in vitro and under pot-house conditions using a Vigna radiata (L.) crop. Increasing concentrations of chemical pesticides negatively impacted the growth, physiological and biochemical features. However, pesticide-tolerant Pseudomonas sp. relieved the toxicity and improved the biological attributes of the plant. Bio-inoculated plants showed significant enhancement in germination attributes, dry biomass, symbiotic features and yield features when compared to un-inoculated ones. Furthermore, with 100 μg metalaxyl kg−1 soil, strain PGR-11 increased the chl-a, chl-b, total chlorophyll, carotenoids, SPAD index, photosystem efficiency (Fv/Fm), PSII quantum yield (FPSII), photochemical quenching (qP) and non-photochemical quenching (NpQ) content by 12, 19, 16, 27, 34, 41, 26, 29 and 33%, respectively, over un-inoculated but pesticide-treated plants. Additionally, inoculation of Pseudomonas sp. with 100 μg tebuconazole kg−1 soil caused a significant (p ≤ 0.05) enhancement in transpiration rate (E), stomatal conductance (gs), photosynthetic rate (PN), vapor pressure deficit (kPa) and internal CO2 concentration (Ci) of 19, 26, 23, 28 and 34%, respectively. Conclusively, the power to tolerate abnormally high pesticide concentration, the capacity to produce/secrete PGP substances even in a pesticide-stressed medium and the potential for improving/increasing the growth and physiology of plants by pesticide detoxification makes Pseudomonas sp. PGR-11 a fascinating choice for augmenting the productivity of V. radiata (L.) even in pesticide-stressed soils. The current findings will be helpful for exploring pesticide-tolerant ACC-deaminase-positive microbial strains as gifted entities for the environmental bioremediation of pesticides.
In the agricultural domain, chemical pesticides are repetitively and harshly used to kill harmful pests, but they often pose a serious threat to microbial diversity, soil fertility and agricultural output.
1. Introduction
The population of the world is growing at an alarming rate, and by the end of 2050, it is predicted to be around nine to 10 billion people.1 With an increasing population, there will be a significant increase in food demand.2 Legumes are essential commercial crops that provide a variety of nutrients, including food, feed, protein, oil, and raw materials.3 Greengram (Vigna radiata L.) is grown in many parts of the world due to its high nutritional content, especially for persons who are malnourished.4 It forms a mutualistic association with Bradyrhizobium sp. in order to convert atmospheric N2 into organic nitrogen, hence contributing to nitrogen cycling in the food chain.5
Agricultural output has risen in the last four decades, owing to increased use of synthetic chemical fertilizers, pesticides including fungicides, and enhanced irrigation systems.6 To meet the increased need for food, chemical fertilizers and pesticides have surely polluted the environment and harmed soil creatures and insect pollinators.7 Despite this, over-utilization of chemical inputs has made crops more prone to disease and reduced soil fertility. In high-input agricultural practices, fungicides are often used to combat fungal diseases and hence to optimize crop production. On the other hand, fungicides have caused a loss of soil fertility and, as a result, a reduction in agricultural yield.8,9 Plant productivity can be influenced by fungicides that affect plant growth or rhizobium–legume interactions.10 Fungicides may also affect rhizobial activity or symbiotic partner interactions.11 The negative impacts of fungicide use are mostly attributable to a lack of awareness of a variety of elements, such as fungicide–inoculant compatibility, crop varieties, the environment, and their interactions. However, continued indiscriminate use of agrochemicals promotes their accumulation in the environment, which disrupts soil biological equilibrium and so creates pollution.12
To overcome this environmental concern, a vital approach has been introduced into agricultural practices that not only enhances agricultural productivity, but also aids in protecting environmental quality.13 So, there is need to find an alternative that could solve the toxicity problems of pesticides by restoring soil fertility. In this regard, plant growth promoting rhizobacteria (PGPR), a group of soil-dwelling bacteria capable of colonizing the root surface and augmenting the development of plants by secreting regulatory compounds in proximity to the rhizosphere, are used as bio-inoculants. They contribute to higher agricultural yields by stimulating plant growth in a variety of ways.14 Plant growth is influenced positively by PGPRs due to the generation of phytohormones, improved phosphorus availability, and the expansion of plant root systems to absorb more water and nutrients. A few soil bacteria demonstrate pesticide resistance after prolonged exposure, and they can be employed to efficiently remediate pesticide-polluted areas.15 In the presence of specific pesticides, microbes operate well by utilizing them as sources of energy and nutrition.16 These pesticides are degraded by efficient and tolerant PGPR and used as a carbon source.17 Their ability to break down pesticides is a significant phenomenon that allows harmful chemicals to be removed from the environment and pollution to be controlled.18 In modern agricultural practices, these pesticide-tolerant PGPR are often utilized as bio-inoculants in order to increase the agronomic yield in a stressful environment such as one under pesticide stress.10 As a result, those microorganisms are a key part of the recycling process and in maintaining soil fertility.19 The use of such microbes possessing multiple properties in pesticide-amended soils makes them one of the most suitable choices for the detoxification of fungicides.20 In this regard, various workers have applied pesticide-tolerant PGPR strains to legume crops grown in pesticide-polluted environments. For instance, a crop-based experiment conducted by Shahid et al.10 showed that bio-inoculation of fungicide-tolerant R. leguminosarum improved the dry biomass, chlorophyll formation, nutrients, symbiotic features and seed attributes of P. sativum even in a fungicide-stressed environment. Similarly, in a follow-up study, Sinorhizobium meliloti protected Medicago sativa (L.) plants from pesticidal nuisance21 and improved the overall performance of plants raised even in soil treated with different concentrations of pesticides.
Considering these points and unraveling the chemical-pesticide-induced phytotoxicity and potential role of pesticide-tolerant rhizobacterial strains, the present study was aimed at: (i) evaluating the phytotoxic impact of tested fungicides, namely carbendazim, metalaxyl and tebuconazole, on V. radiata (L.) seedlings sown in vitro (ii) isolating Pseudomonas sp. from Vigna radiata (L.), rhizosphere soil and assessing the fungicidal stress, (iii) assessing the plant growth promoting (PGP) activities of the PGR-11 strain under fungicidal stress, (iv) determining the inoculation impact of Pseudomonas sp. on the biological attributes of V. radiata (L.) raised in fungicide-stressed soils, (v) assessing the bio-inoculation effect of strain PGR-11 on the symbiosis and yield attributes and (vi) evaluating the effect of Pseudomonas sp. on chlorophyll fluorescence molecules and leaf-exchange parameters of fungicide-treated V. radiata (L.).
2. Material and methods
2.1. Bacterial isolation, morpho-biochemical characterization and screening for plant growth promoting (PGP) activities
To isolate plant growth promoting (PGP) strains, soil samples were collected from the rhizosphere of Vigna radiata (L.). Samples were then cleaned and serially diluted and then plated on King's B (KB) agar plates, incubated and observed for the growth of bacterial colonies. Isolates were purified by streaking three times on KB agar plates and maintained on the same medium for further use. Further, isolates were morphologically and biochemically characterized.22 Since the isolates were recovered from the rhizosphere, they were assessed for their plant growth promoting (PGP) traits. For the synthesis of phytohormones, indole-3-acetic acid (IAA) was determined using the method of Bric et al.23 For P-solubilization, Pikovskaya's (PVK) agar plates were spot inoculated in the middle with bacterial culture. The plates were next inoculated and incubated at 28 °C for 7 days, looking for the development of a distinct halo zone surrounding the bacterial colony. To investigate siderophore formation by bacterial isolates, 60.5 mg Chrome Azurol S was dissolved in 50 mL of water and added to 10 mL of iron(iii) solution (1 mM FeCl3·6H2O, 10 mM HCl). This solution was gently combined with 40 mL of water containing hexadecyl trimethyl ammonium (C19H42BrN). Bacterial strains were inoculated on the middle of blue agar plates and incubated for 48–72 hours at 28 °C. The development of siderophores was detected by the formation of a yellow to orange zone surrounding bacterial colonies following the method of Alexander and Zuberer.24 HCN and ammonia production activities were determined following the methods of Bakker and Shipper25 and Dye,26 respectively.
2.2. Fungicide tolerance and molecular identification of selected strain
The ability to tolerate fungicide of rhizobacterial isolates was determined using minimal salt agar (MSA) medium (g L−1: KH2PO4 1.0, K2HPO4 1.0, NH4NO3 1.0, MgSO4·7H2O 0.2, CaCl2·2H2O 0.02, FeSO4·7H2O 0.01, pH, 6.5). For this, fungicide, namely carbendazim (CBZM), metalaxyl (MTXL) and tebuconazole (TBZL), amended plates of MSA were prepared by supplementing them individually with 0, 50, 100, 200, 400, 800, 1200, 1600, 2000, 2400, 2800 and 3200 μg mL−1 of each test fungicide. The plates were spot inoculated with freshly grown 10 μL of (108 cells mL−1) of bacterial isolates and incubated at 28 ± 2 °C for a period of 72 h and the highest concentration of supporting isolates was defined as the maximum tolerance level (MTL).
2.3. Growth of strain PGR-11 under fungicide stress
For this, strain PGR-11 was grown in 10 mL of Luria-Bertani (LB) broth (g L−1: tryptone 10; yeast extract 5.0; NaCl 10 and pH 7.5) containing 100 μg mL−1 tryptophan (C11H12N2O2) and supplemented with 0, 50, 100, 200, 500 and 1000 μg mL−1 each of CBZM, MTXL and TBZL. Then LB broth was inoculated with 100 μL (108 cells mL−1) of PGR-11 strain and incubated at 28 ± 2 °C for 5 days with shaking at 125 rpm.
2.3.1. Investigation of the fungicide stress on the production of indole-3-acetic acid (IAA), siderophores, ammonia and cyanogenic compound (HCN)
Indole-3-acetic acid (IAA) was quantitatively determined by the modified method of Brick et al.23 From the growth of the strain cultured under fungicide stress, cultures removed at the exponential phase were centrifuged at 5433× g (10 000 rpm) for 15 min. An aliquot of 2 mL of supernatant was mixed with 100 μL of orthophosphoric acid (H3PO4) and 4.0 mL of Salkowsky reagent (2% 0.5 M FeCl3 in 35% perchloric acid, HClO4) and kept at room temperature (in darkness) for 1 h. At 530 nm, the absorbance of the pink color generated was measured using a UV-visible spectrophotometer. The concentration of IAA in the supernatant was calculated using a calibration curve of pure IAA as a reference. Furthermore, the siderophore-producing ability of pesticide-tolerant PGR-11 was checked by the ferric chloride (FeCl3) test method, as previously described by others.27 Further, the production of siderophores was quantitatively assessed by growing the bacterial cells in a liquid medium and the amount of produced phenolate siderophore i.e. salicylic acid (SA, C7H6O3) and 2,3-dihydroxy benzoic acid (DHBA, C7H6O4) was determined.24 For detection of ammonia, a freshly grown culture of PGR-11 strain was inoculated in 10 mL of peptone water supplemented separately with varying concentrations of fungicides and incubated at 28 ± 2 °C for 5 days. Following incubation, each tube received 1.0 mL of Nessler reagent [(potassium tetraiodomercurate(ii); K2)]. The transition from yellow to orange was noted and recorded.26 Bakker and Schipper's approach was used to assess hydrogen cyanide (HCN) production.25 Briefly, strain PGR-11 was streaked on glycine-amended King's B agar plates (g L−1: tryptic soy broth 30, glycine 4.4, agar 15) supplemented with variable doses of fungicides and incubated at 28 ± 2 °C for 4 days. On top of the plate, a Whatman filter paper no. 1 soaked in 2% sodium carbonate (Na2CO3) produced in 0.5% picric acid ((O2N)3C6H2OH) solution was placed, sealed with parafilm, and incubated for 4 days at 28 ± 2 °C. HCN production was detected by the color change from orange to red.
2.3.2. ACC deaminase and P-solubilization
By employing the spot inoculation method with Dworkin and Foster (DF) salt minimum media, the bacterial enzyme ACC deaminase released by Pseudomonas sp. PGR-11 was quantified.28 The negative and positive controls were plates containing DF medium without ACC and plates containing (NH4)2SO4 (0.2% w/v). Every day, the bacterial growth on plates kept at 28 ± 2 °C for 72 hours was examined. Furthermore, the Honma and Shimomura29 and Penrose and Glick30 techniques were used to extract the amount of ACC deaminase from pesticide-treated bacterial cells. The amount of α-ketobutyrate (CH3CH2CCO2H) produced by ACC deaminase activity was quantified spectrophotometrically against an α-ketobutyrate reference curve. PSA was calculated using liquid PKV medium amended with 0–1000 g mL−1 concentrations of each fungicide. The chlorostannous reduced molybdophosphoric acid blue technique was used to determine the amount of solubilized P.31
2.4. Assessment of pesticide-induced morphological changes in Pseudomonas sp. strain PGR-11 using scanning electron microscopy (SEM)
Under SEM (JSM 6510 LV, JEOL, Japan), pesticide-induced cellular damage/distortion was detected/seen in bacterial strain PGR-11. To do so, bacterial strain PGR-11 was cultivated on nutrient broth (NB) medium with the addition of 1000 μg mL−1 each of carbendazim (CBZM), metalaxyl (MTXL) and tebuconazole (TBZL) for 24 hours at 28 ± 2 °C. A culture produced in pesticide-free media was used as a control (refer to ESI Section†).
2.5. Determination cellular permeability of Pseudomonas sp. strain PGR-11 under confocal laser scanning microscopy (CLSM)
Confocal laser scanning microscopy (CLSM) was utilized to examine the chemical pesticide-induced changes in the membrane integrity and mortality of Pseudomonas sp. PGR-11 as previously done by other workers32–34 (refer to ESI Section†).
2.6. Assessment of fungicide-induced toxicity to Vigna radiata (L.) seedlings in vitro
2.6.1. Seed germination, seedling vigor index (SVI) and plant length
V. radiata (L.) seeds were soaked in double-distilled water (DDW) to imbibe it for 24 h. Seeds were then surface sterilized with 3% sodium hypochlorite (NaOCl) solution followed by three successive washings with sterile water. Soft agar (0.7%) plates amended with different (0–1000 μg mL−1) concentrations of carbendazim (CBZM), metalaxyl (MTXL) and tebuconazole (TBZL) (Table S1†) were poured. Soft agar plates without any fungicide treatment served as controls to compare the impact of fungicides on seed germination. Seeds were placed on the soft agar plates and kept at room temperature for four to five days. The percent germination, vigor index (SVI), radicle, and plumule lengths of the plantlets were measured after 4 days of growth.
2.7. Assessment of inoculation impact of pesticide-tolerant Pseudomonas sp. PGR-11 on the performance of V. radiata (L.): pot-house studies
2.7.1. Seed inoculation, fungicide treatments and plant culture
V. radiata (var. K851) seeds were surface sterilized with 70% ethanol for 3 minutes, followed by 3% sodium hypochlorite (NaOCl) for 3 minutes, followed by six rinses with sterile water and drying. Greengram seeds were sterilized and coated with Pseudomonas sp. strain PGR-11 by dipping them in liquid culture medium for 2 hours and then sticking them with 10% gum acacia (as a glue) to achieve a density of 108 cells per seed. The seeds that had not been inoculated were used as a control and were just dipped in sterile water. Pot soils were treated individually with 0 (control), 50, 100, 250, 500 and 1000 μg fungicide kg−1 soils with CBZM, TBZL and MTXL. Soils were homogenized after each fungicide was added at the calculated concentration. A complete randomized block design was used with three pots for each treatment. The pots were irrigated on a regular basis with tap water and kept in an open-field environment.
2.7.2. Plant length, biomass and symbiotic features and assessment of photosynthetic pigments
Biological parameters, such as height of plant organs, dry matter accumulation in roots and shoots, and whole plant biomass, were measured at 50 and 80 DAS. The length (cm) of uprooted plants was measured using a measuring tape after they were split into roots and shoots. Plants were gently removed and rinsed repeatedly with tap water to determine root and shoot biomass (dry weight). The roots and shoots were dried for 30 minutes at 80 °C in a vented oven (Yorco, York Scientific Industries, Pvt. Ltd India) after drying on tissue paper. Nodules were carefully detached from each treatment, counted, and weighed for dry biomass (after drying in an oven for 24–48 h) and leghaemoglobin (LHb) content was determined.35
Photosynthetic pigments (such as chl a, chl b, total chlorophyll and carotenoid content determined following the universally adopted methods of Arnon36 and Kirk and Allen,37 respectively) accumulated in fungicide-exposed and PGPR-inoculated leaves of V. radiata were estimated (refer to ESI Method†).
2.7.3. Assessment of chlorophyll fluorescence and gas-exchange parameters in fungicide-treated and bio-inoculated V. radiata (L.)
By using a modulated fluorimeter (portable), the chlorophyll molecules/fluorescence accumulated in the upper branches of pesticide-treated and Pseudomonas sp. inoculated V. radiata (L.) was detected. At midday, the characteristics of dark- and light-adapted fluorescence were measured. A modulated pulse was used to determine the minimum fluorescence level in the dark-adapted condition (F0), which was insufficient to cause substantial physiological changes in the plant. The data was averaged over a 1.6 seconds period and retained. After administering a saturating actinic light pulse of 15 000 mol m−2 s−1 for 0.7 s, the maximum fluorescence in this state (Fm) was recorded. The highest average of two consecutive points was used to determine the value of Fm. The light-adapted characteristics of each plant were measured using the same leaf segment. After 30 minutes of acclimating plants to ambient light conditions, the steady-state fluorescence yield (Fs) was measured. After momentarily blocking photosystem II (PSII) photochemistry with a saturating actinic light pulse of 15 000 mol m−2 s−1 for 0.7 s, the maximal fluorescence yield (F′m) was obtained.38 The following equations were derived using fluorescence parameters determined in both light and dark-adapted regimes:
The maximum quantum efficiency of PSII photochemistry (Fv/Fm = (Fm − F0)/Fm) and quantum efficiency of PSII (ΦPSII = (F′m − Fs)/F′m) (refer to Table S2 in ESI†).
Furthermore, another important plant physiological trait (i.e. leaf-exchange parameters) was determined by taking the pesticide-treated and PGPR-inoculated primary branches of V. radiata from each treatment39,40 (refer to ESI Method†).
2.7.4. Seed features of pesticide-exposed and bio-inoculated V. radiata
At harvest, the yield attributes (number of pods, yield, number of seeds/plants, and seed yield and protein concentration) of fungicide-treated and Pseudomonas-primed plants were recorded.
2.8. Statistical analysis
The software SigmaPlot 12.0 and Minitab 17.0 were used to analyze the data statistically. For pot trials, three pots per individual test concentration were used in a complete randomized block design (CBRD). Crop-based tests were carried out for two years in a row to ensure that the data was consistent. Each year's data was gathered and examined. The results were compared to the control treatments after calculating the mean of the data in a single column. Figures and tables both show the mean standard deviation (SD) of at least three replicates (n = 3). Graphs and tables with different letters show a significant difference between treatments at a confidence level of p ≤ 0.05.
3. Results and discussion
3.1. Isolation, PGP features, pesticide tolerance and molecular identification
Based on biochemical and cultural testing, a total of fifteen (n = 15) Pseudomonas strains were identified from the rhizosphere soil of Vigna radiata (L.) roots in this investigation. The biochemical and microbiological characteristics of the recovered isolates differed significantly. Microscopic examination indicated that all of the isolates were Gram-negative short rods. Furthermore, the PGP activities of the bacterial strains were assessed. The majority of the isolates produced varied quantities of IAA when grown in broth (LB medium) containing different concentrations of tryptophan. In general, 400 μg mL−1 of tryptophan resulted in the maximum IAA synthesis. For example, among the isolates, PGR-11 was recorded as the maximum IAA-producing rhizobacterium (82.5 ± 9.2 μg mL−1 at 400 μg mL−1 tryptophan) (Table S3 in ESI†). Indole-3-acetic acid (IAA) synthesized by beneficial soil bacteria is one of the most prevalent and well-studied auxins, and most of the scientific literature uses the names auxin and IAA interchangeably.41 The division of cells, root elongation, differentiation, and extension are the main/primary functions of auxins (3-indole-acetic acid).42 However, plant susceptibility to IAA varies among crop plants and their species.
Likewise, recovered isolates exhibited the production of varied quantum yields of α-keto butyrate (ACC deaminase enzyme) when cultivated in Dwarkin and Foster (DF) salt medium amended with 3.0 mM of 1-aminocyclopropane 1-carboxylate (ACC) instead of (NH4)2SO4. Bacterial 1-aminocyclopropane-1-carboxylate deaminase (ACCD) performs a well-known function in the control of a plant hormone, ethylene, and as a result, plant growth and development are altered.43 Plant growth and development might be aided by PGPR inoculation combined with ACC deaminase activity in stressful situations.44 The ACCD activity of PGPR is hypothesized to reduce root ethylene synthesis by reducing the abundance of the ethylene precursor ACC, which can lessen the repressive impact of ethylene on root development.45 Here, the amount of ACC deaminase synthesized by bacterial isolates also varied. Additionally, in order to assess the P-solubilization activity, soil isolates were cultured in liquid Pikovskaya (tri-calcium phosphate containing) medium and strain PGR-11 was recorded as having the maximum amount (54.6 ± 7.8 μg mL−1) of TCP (tri-calcium phosphate) solubilizer among the isolates. Beneficial bacteria that hydrolyze insoluble inorganic phosphorus (iP) into soluble organic phosphorus (OP) which plants may take as a source of nutrition are known as phosphate-solubilizing bacteria (PSBs).46 Phosphate-solubilizing bacteria with the highest efficiency are found in the genera Pseudomonas and Bacillus.47 The capacity for phosphate solubilization by microbes is closely tied to the synthesis of siderophores, lytic enzymes, and phytohormones.48 The majority of the isolates recovered from the rhizosphere were positive for the production of ammonia and hydrogen cyanide (Table S3†).
Most microorganisms, including bacteria, algae, fungi, and plants, create poisonous cyanide in order to compete with their rivals for life.49 HCN, which is usually manufactured by Pseudomonas and Bacillus species, is also a secondary metabolite that functions as an efficient biocontrol agent for weeds.50 HCN is likely to block the electron transport chain (ETC) and the cell's energy source, resulting in cell death. It also appears that PGPR inhibits the correct functioning of enzymes and natural receptors through a reversible mechanism, and is known to block the action of cytochrome oxidase.51
Pesticide tolerance was tested by growing the isolates in different concentrations of fungicide (CBZM, TBZL and MTXL) treated minimal salt agar (MSA) medium. Among the isolates, Pseudomonas sp. PGR-11 was the only rhizobacterial isolate to survive up to 800, 1600 and 1200 μg mL−1 of each of CBZM, TBZL and MTXL, respectively (Table S4†). Pseudomonas sp. PGR-11 was specifically selected due to its highest ability to tolerate the tested fungicides and its maximum production of PGP substances (siderophores, IAA, EPS, HCN and ammonia). Pesticide tolerance, particularly fungicide tolerance, is regarded as a distinguishing feature among soil-dwelling microorganisms, and it is regulated by physiological and genetic variables. Therefore, microbes that are capable of developing resistance towards pesticides including fungicides are frequently degraded by them (soil microorganisms). Pesticide tolerance was discovered in almost all of the selected rhizobacteria. The ability of selected species to tolerate pesticides at various doses was most likely owing to the bacteria's ability to metabolize pesticides and use them as the sole carbon source for development in carbon-deficient minimum media. Because of the increase in metabolic potential, this may assist them to survive in pesticide-stressed conditions.52 Similar findings were reported by Castillo et al.53 in Azotobacter, in which the bacteria used the insecticide endosulfan as their sole source of C, S, and P for growth and other metabolic and physiological functions. Similar ability to tolerate a variety of pesticides has been documented in different soil isolates, viz., Pseudomonas, Azotobacter and phosphate-solubilizing bacteria.54 In profenofos-amended agricultural soils, Acinetobacter sp. and Comamonas sp. isolated from the rhizosphere soil solubilized an insoluble form of phosphorus and produced ACC deaminase, IAA, HCN, siderophores and NH3, thereby increasing the length of sprouts, shoots, roots, chlorophyll a and chlorophyll b in Vigna radiata (L.) plants by the efficient removal of pesticide.55
The selected PGR-11 strain was further subjected to different biochemical tests and showed varied responses toward them (Table S5†). The isolates were recognized as Pseudomonas sp. based on the information provided and a phylogenetic tree was constructed (Fig. 1). As a result, there may be minor discrepancies between the Pseudomonas sp. isolated in our investigation and those reported by other researchers. PGPR isolates have also been obtained and described using 16S rRNA partial gene sequence analysis and other cutting-edge molecular methods by a number of researchers.56–60
Fig. 1. Neighbor-joined phylogenetic tree of Pseudomonas sp. PGR-11 based on a 16S rRNA partial gene sequence of selected bacteria and closely related phylogenetic species derived using the NCBI BLAST search tool. Sequences were aligned using the Clustal W sequence alignment tool in MEGA 7.0 software.
3.2. PGP activities of Pseudomonas sp. PGR-11 under pesticide stress
3.2.1. IAA, siderophores and ACC deaminase under pesticide stress
Increasing pesticide concentration adversely affected the bacterial synthesis of IAA. In Luria-Bertani (LB) medium unsupplied with any pesticide stress i.e. the control, Pseudomonas sp. PGR-11 produced a maximum (82.5 ± 9.2 μg mL−1) amount of IAA. In contrast, as the concentration of each pesticide in the liquid broth was increased, the amount of released IAA dropped significantly. Of fungicides, the most severe effect on IAA synthesis was observed in the presence of CBZM. For example, 500 μg CBZM mL−1 decreased IAA production by 71%, compared to the control. At higher concentration (1000 μg mL−1), the decrease in the quantum yield of IAA was recorded as: CBZM (71%) > TBZL (59%) > MTXL (59%). Plant growth hormones are organic compounds that are not normally generated by plants, but are synthesized exogenously by natural and synthetic processes. Some of the plant hormones produced by PGPR operate as plant growth regulators both directly and indirectly.61 Auxin is a type of phytohormone that regulates plant growth and development by influencing a variety of cellular activities. Its biosynthesis can take place in both tryptophan-dependent and tryptophan-independent routes.62 Auxin is mostly produced in response to tryptophan. Auxins are involved in root and shoot growth orientation in response to light and gravity, vascular tissue differentiation, apical dominance, lateral and adventitious root initiation, stem and root cell division, and elongation. Auxins, especially indole-3-acetic acid (IAA), have also been implicated in host–parasite interactions.63 A high amount of IAA results in improved plant shoot development. A similar outcome was observed for many rhizobacteria.64–66 The higher degree of IAA synthesis in PGPR has been linked to tryptophan as a precursor. Other researchers have reported a comparable high degree of IAA generation.67 Similarly a pesticide-concentration-dependent decrease in IAA synthesis by a soil isolate Enterobacter cloacae has been reported.68
Production of siderophores (both qualitative and quantitative) by fungicide-tolerant Pseudomonas sp. was determined in liquid broth. The strain PGR-11 showed substantial siderophore-producing capacity, as seen by a color change from orange to reddish brown. The pure culture of the strain produced significantly less siderophore when tested pesticides were added to the medium. An increasing concentration of pesticide was inhibitory to siderophore production relative to an untreated control. The highest CBZM dose tested resulted in the greatest drop. As an example, production of SA and 2,3-DHBA greatly declined by 77 and 56%, respectively, at 1000 μg CBZM mL−1 over the untreated control (Table 2).
Plant growth promoting activities of Pseudomonas sp. strain PGR-11 under varying levels of fungicides stressa.
| Fungicides | Dose rate (μg mL−1) | IAA (μg mL−1) | Siderophore production | P-solubilization (μg mL−1) | ACC deaminase | Ammonia | HCN | ||
|---|---|---|---|---|---|---|---|---|---|
| FeCl3 test | Quantitative estimation | ||||||||
| SA (μg mL−1) | 2,3-DHBA (μg mL−1) | ||||||||
| Control | 0 | 82.5 (a) ± 9.2 | ++ | 37.5 (a) ± 3.7 | 46.2 (a) ± 7.2 | 54.6 (a) ± 7.8 | ++ | +++ | + |
| Carbendazim | 25 | 73.2 (b) ± 6.3 | + | 31.4 (b) ± 4.2 | 39.0 (b) ± 6.2 | 52.2 (a) ± 6.6 | + | ++ | + |
| 50 | 63.7 (c) ± 6.0 | + | 25.2 (c) ± 3.2 | 35.1 (c) ± 4.1 | 47.3 (b) ± 4.3 | + | ++ | + | |
| 100 | 51.9 (d) ± 5.2 | + | 21.1 (d) ± 1.8 | 31.5 (d) ± 3.2 | 41.2 (c) ± 3.7 | + | + | + | |
| 200 | 35.6 (f) ± 3.2 | + | 15.3 (e) ± 2.4 | 25.6 (e) ± 2.0 | 36.5 (d)± 4.4 | + | + | + | |
| 500 | 23.6 (g) ± 2.7 | — | 8.7 (h) ± 0.8 | 20.3 (f) ± 1.7 | 31.4 (f) ± 3.2 | + | + | + | |
| Metalaxyl | 25 | 76.5 (b) ±5.8 | + | 33.8 (b) ± 3.4 | 42.7 (a) ± 6.3 | 54.0 (a) ± 6.6 | + | + | — |
| 50 | 68.8 (c) ± 8.2 | + | 26.7 (c) ± 5.1 | 38.4 (b) ± 5.5 | 48.4 (b) ± 5.3 | + | + | + | |
| 100 | 62.7(c) ± 4.7 | + | 20.1 (d) ± 2.4 | 33.6 (c) ± 4.2 | 44.0 (b) ± 2.7 | + | + | + | |
| 200 | 55.6 (d) ± 5.1 | + | 15.3 (f) ± 1.6 | 28.1 (e) ± 2.7 | 34.9 (e) ± 4.6 | + | + | + | |
| 500 | 42.2 (e) ± 3.8 | — | 11.2 (g) ± 2.0 | 23.4 (e) ± 1.4 | 25.4 (g) ± 3.0 | + | + | + | |
| Tebuconazole | 25 | 79.2 (a) ± 6.4 | + | 34.0 (b) ± 2.8 | 44.0 (a) ± 5.3 | 54.0 (a) ± 4.6 | + | + | — |
| 50 | 71.7 (b) ± 7.3 | + | 32.0 (b) ± 3.2 | 39.9 (b) ± 4.5 | 48.3 (b) ± 5.3 | + | + | + | |
| 100 | 56.9 (d) ± 5.4 | + | 27.0 (c) ± 4.0 | 36.6 (c) ± 3.2 | 45.2 (c) ± 6.7 | + | + | + | |
| 200 | 42.6 (e) ± 5.2 | + | 23.3 (d) ± 1.5 | 29.6 (d) ± 3.7 | 39.2 (d) ± 3.4 | + | + | + | |
| 500 | 33.6 (f) ± 4.2 | — | 18.3 (e) ± 1.7 | 24.5(e) ± 2.4 | 35.6 (d) ± 2.2 | + | — | — | |
Each value is a mean of three replicates. Mean values (±) are significant at p ≤ 0.05. Means followed by the same letters are not significantly different from each other according to the DMRT test.
Under iron (Fe) deficiency, one of the biocontrol methods used by PGPR groups is the generation of siderophores. As a result, harmful organisms would be slowed by the iron deficiency in the environment.69 Under pesticide stress, from the production of a considerable amount of siderophores by Pseudomonas sp. in our research, it was concluded that this strain could be utilized to decrease plant-pathogen-mediated illnesses by giving bio-control agents a competitive advantage in the limited supply of key trace minerals seen in natural settings. Similar production and stress-induced reduction in siderophores synthesized by Enterobacter cloacae have recently been reported.70
A number of soil isolates synthesize ACC deaminase, which boosts plant development indirectly by reducing the extraordinarily high quantities of ethylene produced by plants in stressful environments. Here, Pseudomonas sp. exhibited a good response to the ACC deaminase activity when cultivated on DF medium supplemented with/without varying amounts of tested pesticides. In comparison to those treated with varied rates of pesticide, strain PGR-11 produced the most ACC deaminase in DF medium when no pesticide was present.
In addition, as the pesticide concentration was increased, ACC deaminase activity fell progressively. Carbendazim showed the most damaging effect on bacterial ACC deaminase production of all the HMs tested. When compared to an untreated control, 1000 μg CBZM mL−1 inhibited ACC deaminase activity by 72% in the PGR-11 strain. Tested pesticides were ranked in toxicity order as: CBZM > TBZL > MTXL. Similarly, various pesticides inhibited the ACC deaminase activity of the agriculturally beneficial soil bacterium Azotobacter vinelandii in a similar investigation.71
3.2.2. Investigation of the ability of PGR-11 strains in P-solubilization, HCN and ammonia production
The second most important nutrient for plants, phosphorus, has the potential to reduce the need for costly P fertilizers. Plants do not take up soil phosphorus in the form of insoluble phosphates, despite the fact that bio-inoculants have shown P-solubilization activity. Agricultural researchers are interested in bacteria's ability to solubilize the mineral phosphate because it improves the availability of phosphorus and iron for plant growth. Plant-growing rhizobacteria solubilize precipitated phosphate, making it more accessible to plants. Phosphate is released from spare soluble inorganic and organic phosphate complexes in soil by free-living phosphate-solubilizing bacteria, which then make phosphate available to plants.72 The P-solubilizing efficiency (PSA) of Pseudomonas PGR-11 decreased as the dose rates of all the tested fungicides increased. The maximum decrease in P-solubilization was recorded at 1000 μg mL−1 doses each of CBZM, TBZL and MTXL which diminished the solubilized-P by 42, 53 and 35%, respectively, over the control (Table 2).
At greater concentrations of all pesticides tested, bacterial strains did not produce cyanogenic compound (HCN) or ammonia. One of the plant-growth-boosting and disease-regulating qualities was HCN, a volatile metabolite produced by soil bacterial biocontrol agents (BCAs) against soil-borne diseases.73 Rhizobacteria, which create hydrogen cyanide, are used to manage infections biologically. Nitrogen is converted to ammonia by diazotrophic bacteria so that plants can absorb it.
3.3. Morphological features of Pseudomonas sp. under chemical pesticide stress
The use of electron microscopy to study the morphology of bacterial cells and changes in their cellular structure as a result of exposure to various pollutants, such as pesticides, can provide a new dimension to the research. SEM was used to assess the fatal impact of test chemical pesticides on the surface morphology of the PGR-11 strain. When bacterial cells were cultivated without pesticides, SEM micrographs revealed a distinct and unbroken bacterial cell surface (data not provided). The pesticide-untreated bacterial cells were smooth, rod-shaped, and had a healthy/unaffected surface morphology. Pesticide-treated bacterial cells, on the other hand, revealed disordered, ruptured, and shattered cells. For example, SEM pictures of TBZL-treated bacterial cells revealed a disordered rod-shaped bacterial cell. Carbendazim (CBZM), out of all the tested chemical pesticides, had the most damaging effect on the morphology of Pseudomonas sp. PGR-11. The pesticidal nuisance was therefore validated by SEM pictures in this investigation, which clearly indicated the growth inhibitory impact of test pesticides. Like our observation, Khan et al.74 recently demonstrated a comparable lethality of fungicides that caused considerable cellular damage to Pseudomonas sp. under SEM.
3.4. Cellular viability under pesticide stress
Similarly, confocal laser scanning microscopy (CLSM) is a useful method for determining the hazardous effects of chemical contaminants, such as pesticides, on bacterial cells. The pesticide-treated bacterial cells were stained with propidium iodide (PI) to see if there was any loss of membrane integrity. Under CLSM, any reduction in membrane permeability that occurred as a result of pesticide exposure was noticed. Pesticide-free cells were alive and had a black background, but pesticide-treated cells became red/orange, representing the chemicals' harm to the cells. The CBZM-treated cells, for example, showed red/orange rod-shaped cells due to PI uptake, indicating a change in cell viability (displaying the highly toxic impact on cellular viability). Bacterial cell membranes allow bacteria to communicate with their surroundings. The movement of molecules across cell membranes is controlled by their selective permeability. When pesticides and other stressor chemicals interact with bacterial cells, their toxicity increases, causing membrane damage and a microbial oxidative state. Due to its exclusion by the cell membrane of living cells, PI dye (a DNA intercalating dye) is most generally utilized as a cell death marker; hence, fluorescence imparted by the dye is usually connected to cells whose membrane integrity has been destroyed. As a result, when chemical substances (e.g., chemical pesticides) interact with bacterial cells, the current work provides a distinct toxicity profile. Under CLS microscopy, a comparable rise in the number of PI-stained dead cells of soil-beneficial microbes, namely Sinorhizobium saheli,75Enterobacter cloacae,68Bradyrhizobium japonicum76 and Azotobacter vinelandii71 was seen with a gradual increase in pesticidal concentration.
3.5. Crop-based studies
3.5.1. Phytotoxicity assessment of pesticides on V. radiata (L.) seedlings in vitro
Every plant's life revolves around the process of germination. Seedling germination and the vigor index are the most important and vital properties of seeds. As a result, seed germination and the seedling vigor index are the most significant characteristics of seeds to be used in agriculture. Under controlled circumstances, seeds that germinate quickly and aggressively are more likely to generate vigorous seedlings in the field. The germination efficiency of V. radiata (L.) seeds sown in vitro treated with various pesticide concentrations varies dramatically. Seeds put in controlled (untreated) conditions germinated to their full potential. However, pesticidal doses significantly decreased the efficiency of seeds to be germinated. Higher concentrations (500 μg mL−1) had a maximum negative effect on germination attributes. Among the fungicides tested, carbendazim (CBZM) had a highly detrimental effect and a higher CBZM concentration completely reduced the germination rate (i.e. no germination was recorded) (Table 1). Under fungicide-free conditions (control), %germination and the seedling vigor index (SVI) were found to be 100% and 1875 ± 21.3, respectively. Conversely, the selected doses of fungicides inhibited such parameters. For example, at 1000 μg mL−1 of each of CBZM, MTXL and TBZL, %germination was significantly (p < 0.05) decreased by 100, 60 and 50%, respectively, over the control (Table 1). In this context, it has been discovered that a disrupted germination metabolism is linked to delayed germination following pesticide treatment. This could be attributed to the uptake of fungicide toxicity in test plants, which may have damaged numerous physiological activities such as photosynthesis, eventually leading to plant mortality. Similarly, the seedling germination rate of Lycopersicon esculentum was negatively affected by exposure to pesticides.77 Different classes of pesticide reduced the efficacy of cowpea seed germination.78 In both in vitro and in vivo circumstances, raising the concentration of different pesticide groups had a negative impact on the germination efficiency of P. sativum.79
Effect of increasing concentrations of tested pesticides on seedling germination efficiency, vigor index, radicle and plumule length of V. radiata seedlings germinated in vitroa.
| Treatment/pesticides | Concentration (μg mL−1) | Number of seeds plated | Germination efficiency (%) | Radicle length (cm) | Plumule length (cm) | Whole plant length (cm) | Seedling vigor index (SVI) |
|---|---|---|---|---|---|---|---|
| Control | 0 | 10 | 100 | 11.6 ± 2.4 (a) | 10.0 ± 2.0 (a) | 21.6 ± 4.4 (a) | 1875 ± 21.3 (a) |
| Carbendazim | 25 | 10 | 85 | 8.2 ± 1.4 (d) | 7.2 ± 0.8 (d) | 15.4 ± 2.2 (c) | 1504 ± 17.7 (c) |
| 50 | 10 | 70 | 6.4 ± 2.0 (e) | 6.1 ± 0.7 (e) | 12.5 ± 2.7 (d) | 1413 ± 12.0 (d) | |
| 100 | 10 | 54 | 4.5 ± 0.9 (f) | 4.2 ± 0.4 (g) | 8.7 ± 1.3 (f) | 1116 ± 29.5 (e) | |
| 200 | 10 | 30 | 2.3 ± 0.3 (g) | 2.0 ± 0.1 (h) | 4.3 ± 0.4 (h) | 778 ± 13.9 (g) | |
| 500 | 10 | 0 | 00 ± 00 (h) | 00 ± 00 (i) | 00 ± 00 (i) | 00 ± 00 (h) | |
| Metalaxyl | 25 | 10 | 90 | 10.0 ± 1.6 (b) | 8.9 ± 1.1 (b) | 18.8 ± 2.7 (b) | 1684 ± 20.6 (c) |
| 50 | 10 | 80 | 9.5 ± 2.4 (c) | 8.0 ± 0.7 (c) | 17.5 ± 3.1 (b) | 1563 ± 18.4 (c) | |
| 100 | 10 | 70 | 8.3 ± 0.9 (d) | 6.3 ± 0.8 (e) | 14.9 ± 1.7 (d) | 1421 ± 19.5 (d) | |
| 200 | 10 | 55 | 6.0 ± 0.7 (e) | 5.0 ± 0.6 (f) | 11.0 ± 1.3 (e) | 1271 ± 13.8 (e) | |
| 500 | 10 | 40 | 4.4 ± 0.4 (f) | 3.6 ± 0.5 (g) | 8.0 ± 0.9 (f) | 956 ± 14.0 (f) | |
| Tebuconazole | 25 | 10 | 95 | 9.0 ± 1.8 (c) | 9.5 ± 1.3 (b) | 18.5 ± 3.1 (b) | 1714 ± 25.0 (b) |
| 50 | 10 | 85 | 7.5 ± 1.4 (d) | 8.5 ± 0.8 (c) | 16.0 ± 2.2 (c) | 1613 ± 23.0 (c) | |
| 100 | 10 | 75 | 6.4 ± 0.5 (e) | 6.9 ± 1.1 (d) | 12.4 ± 1.6 (d) | 1467 ± 17.7 (c) | |
| 200 | 10 | 65 | 4.0 ± 0.5 (f) | 5.8 ± 0.8 (f) | 9.8 ± 1.3 (f) | 1231 ± 18.2 (e) | |
| 500 | 10 | 50 | 2.5 ± 0.3 (g) | 3.9 ± 0.4 (g) | 6.4 ± 0.7 (g) | 1046 ± 17.0 (f) | |
| LSD (p ≤ 0.05) F value | — | — | 27.4 | 4.07 | 2.69 | 2.87 | 19.43 |
| — | — | 9.72 | 6.78 | 22.3 | 21.8 | 18.5 |
Each value is a mean of independent replicated experiments done in triplicate (mean ± SD). Mean values (±) are significant at p ≤ 0.05. Means denoted by different letters are significantly different from each other according to Duncan's multiple range (DMRT) test.
3.6. Inoculation impact of Pseudomonas sp. strain PGR-11 on pesticide-treated V. radiata (L.)
3.6.1. Germination efficiency
The germination efficiency of V. radiata raised in soil treated with varying concentrations of fungicides was variable. The germination was recorded at 8 DAS for seeds in clay pots. Little seedlings appeared from the dirt two weeks after seeding, with a distinct leaf. V. radiata plants that were grown in soil amended with TBZL and MTXL grew, but plants that were sown in the soil treated with CBZM were dead/dried, since leaf senescence took place and therefore the whole plants died, but plants that were sown in soil treated with the other two test pesticides, survived but were adversely affected. This could be related to the accumulation of fungicide toxicity in test plants, which then disrupted several physiological activities, including photosynthesis by the plants, leading eventually to the death of the plants. According to recent research, certain pesticides disrupt plant molecular connections, preventing the critical process of biological N2-fixation. Many biochemical reactions, including organic matter mineralization, nitrification, denitrification, ammonification, and redox reactions, may be influenced by chemical pesticides.80 Fungicides, for instance, block electron transport from compound Q to plastoquinone in photosystem II, inhibiting photosynthesis.81 and hence prevent the reduction of NADP+ required for CO2 fixation. Furthermore, photo-bleaching may be the cause of pigment deficiency in greengram plant leaves. In contrast, following seed coating and soil application, the pesticide-detoxifying PGPR strain Pseudomonas sp. improved the germination rate. Likewise, pesticide-resistant PGPR strains like S. rhizophila, B. cereus, B. licheniformis and Microbacterium hydrocarbonoxydans boosted germination attributes and crop development even in the presence of varying concentrations of chlorpyrifos and monocrotophos.82 This increased germination efficiency could have been changed, implying that seedling vigor has improved. Seedlings with higher vigor are better able to withstand pathogen infections and survive in tough environments. Because seed ageing is one of the key factors impacting seed quality, seed treatments that promote germination and vigor could be beneficial for carryover seeds that have been stored for a long period.
3.6.2. Biological attributes (length and biomass) of pesticide-treated V. radiata (L.) were improved by Pseudomonas sp.
In this study, tested pesticides and Pseudomonas sp. variably affect the biological attributes of V. radiata when raised in pot soil in the presence of both. Generally, the increasing concentrations (50–1000 μg fungicide kg−1 soil) of both tested fungicides decreased the biometric parameters of V. radiata. For example soil with 1000 μg MTXL kg−1 displayed the most toxic effect and decreased the root and shoot length by 71% (decreasing from 15.6 cm to 4.5 cm) and 61% (decreasing from 31.7 cm to 12.2 cm), respectively, at 50 DA (Table 3). Likewise, a maximum reduction of 58 and 66% in root and shoot dry biomass, respectively, was recorded in soil with 1000 μg MTXL kg−1 with respect to the untreated control (Table 3). But, a considerable increase/improvement in all these parameters was observed when pesticide-tolerant Pseudomonas sp. was inoculated into V. radiata plants. For example, in soil with 50 μg TBZL kg−1, PGR-11 greatly increased the root and shoot lengths, and root and shoot dry biomass by 20, 12, 25 and 30%, respectively, over un-inoculated plants and those treated with an identical dose of tebuconazole (Table 3). Even in the face of pesticide stress, increased production of IAA and other PGP active compounds was likely owing to Pseudomonas sp., which help plants by encouraging symbiosis and root morphogenesis in pesticide-stressed conditions. Furthermore, after inoculation with Pseudomonas sp. PGR-11, longer plant roots were recorded compared to un-inoculated treatments, which may benefit the absorption of substantially more water from the deep soil under adverse pesticide conditions. We strongly believe that the release of growth-regulating bioactive molecules by strain PGR-11 improved the overall performance of V. radiata. As an example, plant hormones (e.g., indole-3-acetic acid) are often involved in the expansion and proliferation of roots, whereas stress-relieving substances (ACC deaminase enzyme), siderophores and ammonia increase the performance of the overall crop.
Bio-inoculation effect of Pseudomonas sp. PGR-11 strain on biological attributes of Vigna radiata (L.) raised in tebuconazole and metalaxyl stressed soila.
| Treatment | Concentration | Plant length (cm) | Fresh weight (g) | Dry biomass (g) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Root | Shoot | Total | Root | Shoot | Total | Root | Shoot | ||
| Control (UC) | — | 15.6 (b) | 31.7 (b) | 47.3 (b) | 4.6 (b) | 12.6 (c) | 17.2 (b) | 1.0 (c) | 2.7 (b) |
| Control (IC) | Pseudomonas sp. PGR-11 | 19.4 (a) | 37.2 (a) | 56.6 (a) | 6.9 (a) | 17.9 (a) | 24.8 (a) | 1.76 (a) | 3.8 (a) |
| Tebuconazole | 50 μg TBZL kg−1 | 14.2 (c) | 29.3 (b) | 43.5 (c) | 3.3 (c) | 11.7 (c) | 15.0 (c) | 0.92 (c) | 2.4 (b) |
| 100 μg TBZL kg−1 | 13.0 (d) | 25.1 (d) | 38.1 (d) | 3.0 (c) | 10.2 (d) | 13.2 (d) | 0.81 (d) | 2.0 (c) | |
| 200 μg TBZL kg−1 | 11.2 (e) | 21.4 (e) | 32.6 (e) | 2.4 (d) | 8.5 (e) | 10.9 (e) | 0.65 (d) | 1.5 (d) | |
| 500 μg TBZL kg−1 | 9.0 (f) | 17.6 (f) | 26.6 (f) | 2.0 (d) | 8.0 (f) | 10.0 (f) | 0.47 (f) | 1.2 (e) | |
| 1000 μg TBZL kg−1 | 5.2 (j) | 12.0 (h) | 17.2 (h) | 1.4 (g) | 6.2 (h) | 7.6 (g) | 0.32 (g) | 0.89 (f) | |
| 50 μg TBZL kg−1 + PGR-11 | 17.4 (b) | 32.4 (b) | 49.8 (b) | 4.5 (b) | 14.6 (b) | 19.1 (b) | 1.21 (b) | 3.4 (a) | |
| 100 μg TBZL kg−1 + PGR-11 | 15.2 (c) | 28.5 (c) | 43.7 (c) | 4.0 (b) | 13.8 (b) | 17.8 (b) | 0.97 (c) | 2.9 (b) | |
| 200 μg TBZL kg−1 + PGR-11 | 14.0 (c) | 24.0 (d) | 38.0 (d) | 2.9 (c) | 10.5 (d) | 13.4 (d) | 0.75 (d) | 2.1 (c) | |
| 500 μg TBZL kg−1 + PGR-11 | 11.0 (d) | 20.3 (e) | 31.3 (e) | 2.6 (d) | 9.5 (e) | 12.1 (e) | 0.67 (e) | 1.8 (d) | |
| 1000 μg TBZL kg−1 + PGR-11 | 7.1 (i) | 14.0 (g) | 21.1(g) | 1.8 (f) | 7.7 (g) | 9.5 (f) | 0.42 (f) | 0.97 (e) | |
| Metalaxyl | 50 μg MTXL kg−1 | 12.6 (d) | 23.5 (d) | 36.1 (d) | 4.2 (b) | 10.6 (d) | 14.8 (c) | 0.94 (c) | 2.0 (c) |
| 100 μg MTXL kg−1 | 10.4 (e) | 21.2 (e) | 31.6 (e) | 3.7 (c) | 9.6 (e) | 13.3 (d) | 0.83 (d) | 1.7 (d) | |
| 200 μg MTXL kg−1 | 8.5 (f) | 17.8 (f) | 26.3 (f) | 2.9 (c) | 8.0 (f) | 10.9 (e) | 0.81 (d) | 1.4 (d) | |
| 500 μg MTXL kg−1 | 7.2 (f) | 14.8 (g) | 22.0 (g) | 2.5 (d) | 7.0 (g) | 9.5 (f) | 0.70 (d) | 1.0 (e) | |
| 1000 μg MTXL kg−1 | 3.1 (k) | 10.0 (i) | 13.1 (i) | 1.7 (f) | 5.4 (i) | 7.1 (g) | 0.42 (f) | 0.7 (f) | |
| 50 μg MTXL kg−1 + PGR-11 | 15.3 (c) | 27.7 (c) | 43.0 (c) | 4.9 (b) | 14.0 (b) | 18.9 (b) | 1.43 (b) | 2.9 (b) | |
| 100 μg MTXL kg−1 + PGR-11 | 12.8 (d) | 25.6 (d) | 38.4 (d) | 4.3 (b) | 11.7 (c) | 16.0 (c) | 0.90 (c) | 2.4 (c) | |
| 200 μg MTXL kg−1 + PGR-11 | 10.2 (e) | 20.0 (e) | 30.2 (e) | 3.4 (c) | 9.8 (e) | 13.2 (d) | 0.83 (d) | 1.99 (c) | |
| 500 μg MTXL kg−1 + PGR-11 | 9.1 (e) | 17.9 (f) | 27.0 (f) | 3.0 (c) | 8.3 (f) | 11.3 (e) | 0.77 (d) | 1.4 (d) | |
| 100 μg MTXL kg−1 + PGR-11 | 4.5 (j) | 12.2 (h) | 16.7 (h) | 2.2 (d) | 6.6 (h) | 8.8 (g) | 0.52 (f) | 0.9 (e) | |
Each value is a mean of independent replicated experiments done in triplicate (mean ± SD). Mean values (±) are significant at p ≤ 0.05. Means denoted by different letters are significantly different from each other according to Duncan's multiple range (DMRT) test.
Pesticide-tolerant microbes used in a soil system with chemical pesticides have been shown to increase root development and root architecture in plants. It has also been suggested that bacteria-induced changes in root architecture could lead to an increase in total root surface area, resulting in better water and nutrient absorption, which would be beneficial to overall plant development.83 Similarly, different pesticide-tolerant PGPR strains belonging to numerous genera have been documented to augment the growth and dry biomass of edible/food crops, such as cereals, when grown in pesticide-treated soils.16,20 In pot-house studies with radish seedlings, Khan et al.74 employed fungicide-tolerant PGPR strains (Azotobacter sp. and Pseudomonas sp.). Fungicide-treated but PGPR-inoculated plants grew 2–3-fold taller than stressed but un-inoculated control plants in that study. They found that PGPR treatment enhanced root length and biomass, resulting in improved water absorption and the ability of treated plants to withstand pesticides.
3.6.3. Bio-inoculation impact of Pseudomonas sp. on V. radiata (L.) symbiosis
The number (NN), biomass (NDB) and leghaemoglobin (LHb) content of V. radiata were considerably decreased with increasing rates of both pesticides. As an example, NN, NDB and LHb content greatly decreased by 84, 63 and 68%, respectively, when plants were detached from pot-soils treated with 1000 μg MTXL kg−1. (Fig. S2† panels A–C). The harmful action of pesticides on photosynthates may have reduced the availability of photosynthates to the roots, resulting in a loss in functional symbiosis. Conversely, inoculation of pesticide-tolerant Pseudomonas sp. significantly (p ≤ 0.05) increased V. radiata's symbiotic characteristics. For example, PGR-11 strain maximally and significantly (p ≤ 0.05) enhanced NN, NDB and LHb by 13, 22 and 17%, respectively, in the presence of soils with 50 μg TBZL kg−1 at 50 DAS (Fig. S2† panels A–C). The improvement in V. radiata symbiosis following soil inoculation by pesticide-tolerant PGPR is a strong indicator of microbial colonization and presence in fungicide-contaminated soil. Further, the findings imply that increased nodulation following inoculation by pesticide-tolerant, ACC-deaminase-positive, indole-3-acetic-acid-producing, and other PGP-regulating Pseudomonas sp. PGR-11 strain reversed the symbiotic effectiveness (number, biomass, and LHb content of nodules) of V. radiata which, however, showed a considerable decrease in the absence of bio-inoculants. It is also likely that successful bacterium deployment in the rhizosphere of V. radiata improved plant symbiotic characteristics via local and systemic hormone transmission. Fungicide-tolerant PGPR strains; Bradyrhizobium japonicum76 and Bradyrhizobium sp.84 isolated/obtained from mung-bean root nodules are reported to increase the nodulation and the symbiotic characteristics of V. radiata (L.) plants cultivated under pesticide-stressed conditions. Furthermore, inoculation of greengram plants grown in pesticide-treated soils with the IAA-producing and P-solubilizing non-symbiotic PGPR strains Azotobacter sp. and Bacillus sp. increased plant development, corroborating our findings.34 They stated that PGPR-inoculated greengram had higher seed germination efficiency, dry biomass, LHb content, chlorophyll pigment and seed output as well as restored nodulation, which had been suppressed due to fungicidal stress. Similarly, bio-inoculated legumes grown on fungicide-stressed soils have shown improvements in a variety of developmental characteristics.85
3.6.4. Physiological state of V. radiata in the presence of Pseudomonas sp. and pesticides
3.6.4.1. Chlorophyll content
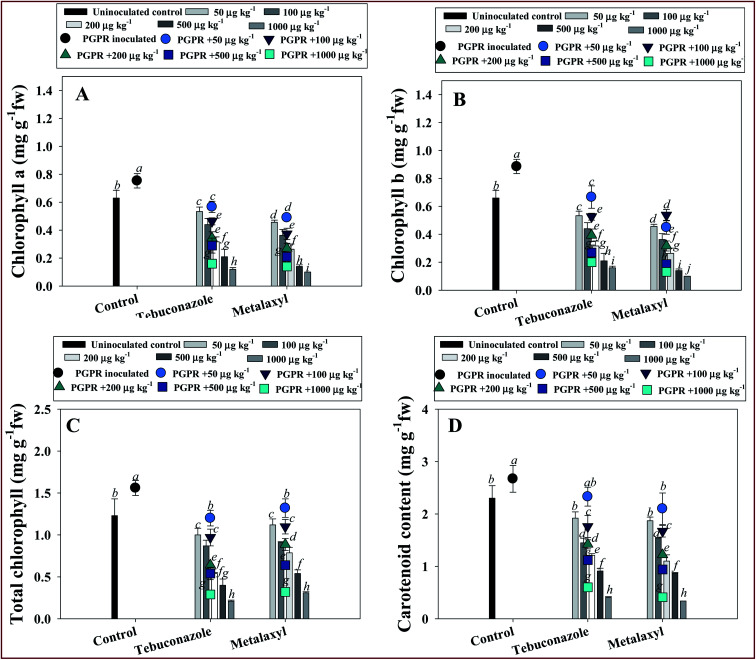
The process of chlorophyll formation (photosynthesis) is a vital biochemical practice in higher plants that has an undeviating effect on plant biomass production; it is often susceptible to abiotic stresses. Therefore, the effect of increasing rates of TBZL and MTXL on photosynthetic pigments accumulated in fresh leaves of V. radiata was calculated. The chl a, chl b and total chlorophyll content consistently declined with increasing rates of fungicides either in the presence or absence of inoculants. For example, MTXL at 1000 μg kg−1 soil decreased the chl a, chl b, total chlorophyll and carotenoid content by 84, 83, 74 and 8%, respectively, relative to the control (Fig. 1 panels A–D). However, the application of tolerant Pseudomonas sp. to pesticide-treated V. radiata detoxified the toxic action of the chemicals and considerably increased the photosynthetic pigments. For instance, when applied to soil treated with 50 μg TBZL kg−1, strain PGR-11 increased the chl-a (Fig. 2 panel A), chl-b (Fig. 2 panel B), total chlorophyll (Fig. 2 panel C), and carotenoid contents (Fig. 2 panel D) by 12, 19, 16 and 20%, respectively, with respect to un-inoculated and treated plants. The increase in these parameters suggests that in the presence of pesticide stress, the PGPR strain directly regulates the physiological activities of V. radiata by producing various plant growth promoting substances, such as indole-3-acetic acid (IAA) and other PGP molecules, and by increasing the availability of nutritional content to the plants. The improvement in chlorophyll content by pesticide-tolerant Pseudomonas sp. applied as a bio-inoculant in this study might possibly be linked to chloroplast development. Similarly, increased photosynthetic pigment in chickpea plants has been observed after inoculating the fungicide-tolerant Mesorhizobium ciceri into chickpea plants treated with increasing kitazin concentrations.86 Furthermore, inoculation of Rhizobium leguminosarum increased tolerance to fungicide and considerably enhanced the concentrations of chlorophyll pigments in Pisum sativum (pea) plants cultured in pot soils with added fungicides.10
Fig. 2. Impacts of pesticide-tolerant Pseudomonas sp. on V. radiata (L.) grown in pot soils supplemented with increasing concentrations (50–1000 μg kg−1) of tebuconazole and metalaxyl. Effect on (A) chl a, (B) chl b, (C) total chlorophyll and (D) carotenoid content. Bar and scatter diagrams represent the mean values of three replicates (n = 3) of three plants/pot. Mean values followed by different letters are significantly different (p ≤ 0.05) as determined by the DMRT test. Vertical and scattered bars represent means ± SDs (n = 3).
3.6.4.2. Chlorophyll fluorescence parameters in the presence of PGPR strain and pesticide
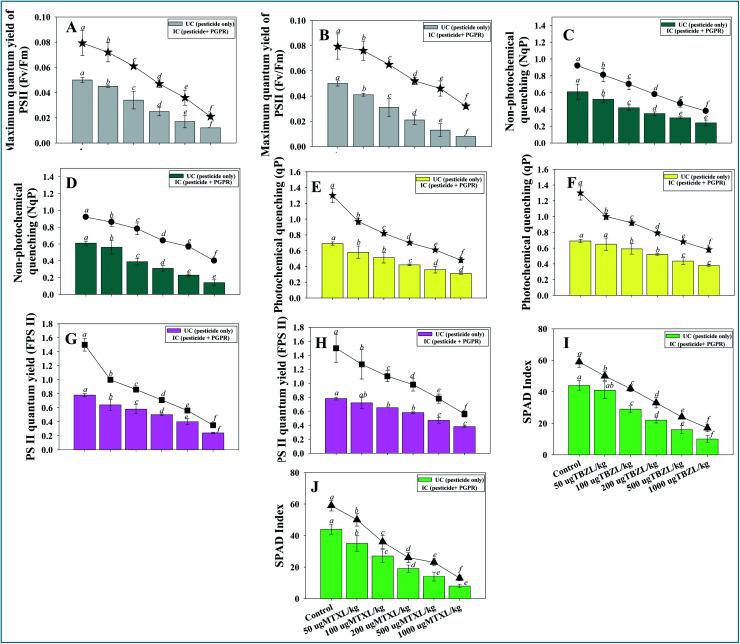
The chlorophyll fluorescence (CF) process has been proven to be a responsive method for identifying and evaluating different photosynthetic mechanisms. The plant-stress response has been studied using CF analysis, which can detect minor variations in photosynthetic activity. Alteration in chlorophyll fluorescence has frequently been recommended as a susceptible plant stress biomarker, with the assumption that any plant stress severe enough to impair plant development will also influence chlorophyll synthesis. The efficiency of electron transport (ET) via the distinct phases of the photosystem-II (PSII) and photosystem-I electron transport chains can be studied by a variety of methods in chlorophyll. As a result, if a stressor interferes with ET, changes in one or more fluorescence characteristics can be recognized instantly. Furthermore, indirect effects on photosynthesis are predicted to be identified in changing fluorescence characteristics, interfering with the downstream usage of the reductants created by the photosystems. This is because a quick reduction in reductant sink capacity is likely to induce an accumulation of electrons and reductants in the chloroplasts, resulting in a rise in harmful reactive oxygen species. Considering these, the effect of tested pesticides was evaluated to assess the effect of increasing concentration of both MTXL and TBZL on chlorophyll molecules of V. radiata. The increasing pesticide doses significantly (p ≤ 0.05) reduced the fluorescence parameters of V. radiata. For instance, for soil with 1000 μg MTXL kg−1, the SPAD index, photosystem efficiency (Fv/Fm), PSII quantum yield (FPSII), photochemical quenching (qP), and non-photochemical quenching (NpQ) were maximal and significantly reduced by 71, 76, 69, 55 and 60%, respectively, over the untreated control (Fig. 3 panels A–J). Quite the opposite, inoculation of tolerant Pseudomonas sp. into pesticide-treated V. radiata modulated the photosynthetic apparatus (Fv/Fm, Fv/F0, qP, and NPQ). The application of strain PGR-11 compensated for the negative and toxic effects of pesticides. Here, a maximum increase in leaf chlorophyll fluorescence parameters was recorded when pesticide-detoxifying Pseudomonas sp. was applied to plants in the presence of soil with 50 μg pesticide kg−1. These findings suggest that the pesticide-tolerant PGPR strain can improve the leaf fluorescence molecules and have a protective effect on the photosynthetic machinery of V. radiata cultivated in soils contaminated with pesticides. Increased chlorophyll fluorescence could be ascribed to the generation of growth-regulating molecules, increased photosynthesis, better root development, improved water and nutrient translocation and uptake, and efficient utilization. Similarly, the stress-tolerant PGPR strains increased the chlorophyll fluorescence in different plant species, including Cicer aritienum87 and Populus deltoides88 by increasing their tolerance to environmental stresses.
Fig. 3. Bio-inoculation effect of pesticide-tolerant Pseudomonas sp. on measurement of physiological status (chlorophyll fluorescence); maximum quantum yield of PSII (A and B), non-photochemical quenching (C and D), photochemical quenching (E and F), PSII quantum yield (G and H) and SPAD index (I and J) of V. radiata (L.) leaf exposed to 50, 100, 200, 500 and 1000 μg kg−1 soil with tebuconazole and metalaxyl. Here, bar and line diagrams represent the mean values of three replicates (n = 3) of three plants/pot. Mean values followed by different letters are significantly different (p ≤ 0.05) as determined by DMRT test. Vertical and scattered bars represent means ± SDs (n = 3).
3.6.5. Gas-exchange attributes of V. radiata in the presence of pesticides and Pseudomonas sp.
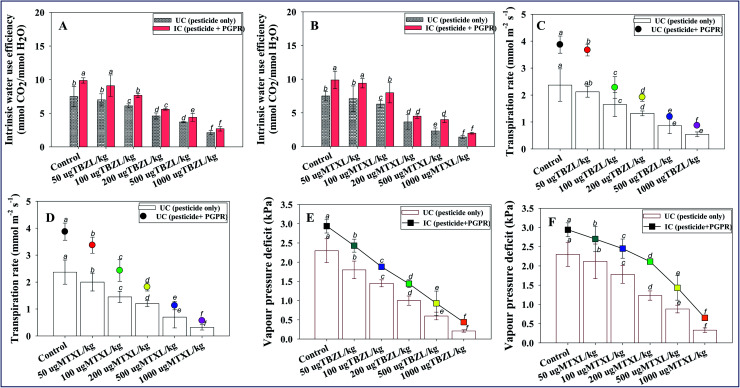
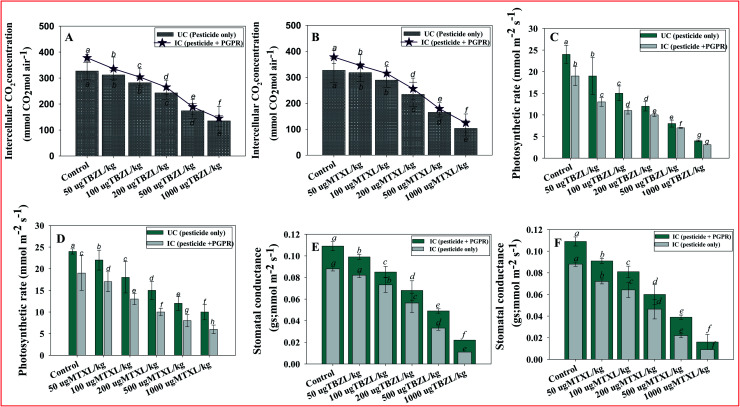
Physiological parameters (such as stomatal conductance, intercellular CO2 concentration, rate of transpiration and photosynthesis, intrinsic water use efficiency and vapor pressure deficit) can be used to investigate how drought stress affects a range of plants. This is due to a quick reduction in reductant sink capacity being likely to induce an accumulation of electrons and reductants in the chloroplasts, resulting in a rise in harmful reactive oxygen species (ROS). Because of the close relationship between photosystem electron transport efficiency (ETE) and carbon assimilation, the operational efficiency of PSII and gas exchange, evaluated as the quantum yield of CO2 assimilation, is usually linear. Here, all these above-mentioned physiological parameters of pesticide-treated and Pseudomonas sp. inoculated plants were assessed. Like other biometric plant parameters, the stomatal conductance (gs) of V. radiata was greatly (89% reduction) affected in soil with 1000 μg MTXL kg−1 in un-inoculated plants which, however, maximally increased by 20% when Pseudomonas sp. was inoculated into treated V. radiata treated with 50 μg TBZL kg−1 soil with respect to un-inoculated but treated plants (Fig. 4 panels E and F). Similarly, the rate of transpiration (E), vapor pressure deficit (VpDL), intrinsic water use efficiency (iWUE), and photosynthetic rate (PN) were significantly (p ≤ 0.05) increased by 43, 26, 23 and 31%, respectively, when Pseudomonas sp. was inoculated into V. radiata plants cultivated with 50 μg MTXL kg−1 soil (Fig. 5 panels A–E). An opposite pattern was recorded for intercellular CO2 concentration (Ci), where Ci was consistently greater in un-inoculated plants, indicating lower C-fixing rates. Inoculation with pesticide-resistant strains reversed the condition, drastically lowering Ci levels. It is suggested that the pesticide-tolerant PGPR strain improves the physiological state of the plant by detoxifying/remediating the toxic action of chemical pesticides. This improvement may possibly be due to the pesticide-degrading/detoxifying ability of the PGP bio-molecule synthesizing and effective rhizosphere colonizing behavior. Furthermore, these improvements in physiological features could lead to alterations in CO2 assimilation for photosynthesis. The results obtained in this study show that the pesticide-tolerant strain PGR-11 enhances stomatal conductance, which helps to mitigate/alleviate the negative impacts of the tested fungicides. In response to pesticide pressure, Pseudomonas sp. PGR-11 inoculated plants showed lower potentials and improved gas-exchange characteristics, permitting the bacterized and fungicide-supplemented V. radiata to continue high organ hydration and turgor levels, that keep up the overall physiological activities of the cells, particularly those related to the photosynthetic apparatus. Pesticides lowered all physiological indicators in non-infected plants, but they did not affect the physiological status of inoculated plants. Furthermore, better root development, and an increased chlorophyll pigment strong source–sink relationship are possible reasons for the improved stomatal conductance, transpiration rate and other leaf-exchange attributes. The considerable increase in the physiological state of the plant could be attributed to the ability of PGPR to produce plant growth hormones, improved water and nutrient translocation, and efficient water and nutrient uptake and use. Likewise, the PGPR strain Serratia liquefaciens improved stress tolerance in Zea mays (L.) by increasing the leaf-exchange parameters of the plants.89
Fig. 4. Bio-inoculation effect of pesticide-tolerant Pseudomonas sp. PGR-11 on leaf-exchange parameters; intrinsic water use efficiency (A and B), transpiration rate (C and D) and vapour pressure deficit (E and F) measured in leaves of V. radiata (L.) exposed to 50, 100, 200, 500 and 1000 μg kg−1 soil with tebuconazole and metalaxyl. Here, bar and line diagrams represent the mean values of three replicates (n = 3) of three plants/pot. Mean values followed by different letters are significantly different (p ≤ 0.05) as determined by the DMRT test. Vertical and scattered bars represent means ± SDs (n = 3).
Fig. 5. Bio-inoculation effect of pesticide-tolerant Pseudomonas sp. PGR-11 on leaf-exchange parameters; intercellular CO2 concentrations (A and B), photosynthetic rate and (C and D) stomatal conductance (E and F) measured in leaves of V. radiata (L.) exposed to 50, 100, 200, 500 and 1000 μg kg−1 soil with tebuconazole and metalaxyl. Here, bar and line diagrams represent the mean values of three replicates (n = 3) of three plants/pot. Mean values followed by different letters are significantly different (p ≤ 0.05) as determined by the DMRT test. Vertical and scattered bars represent means ± SDs (n = 3).
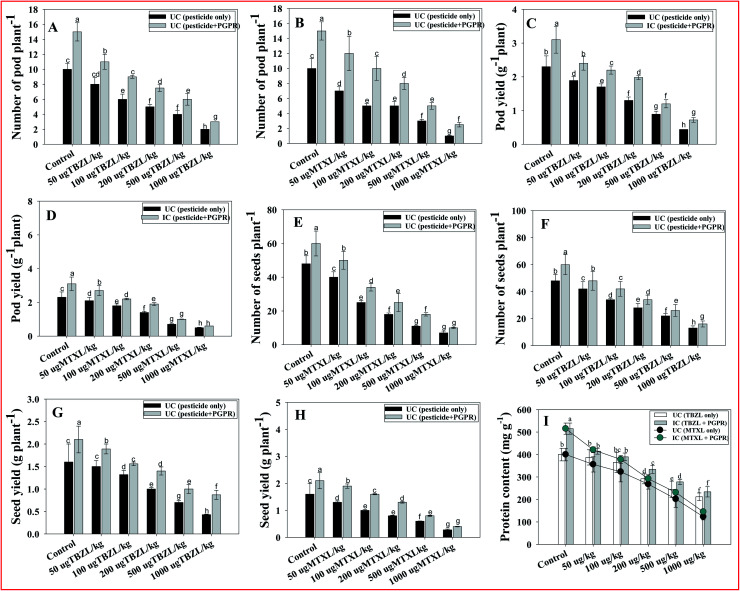
3.7. Yield attributes were improved by Pseudomonas sp. PGR-11 inoculation
The yield attributes of V. radiata exhibited a substantial decline with increasing concentrations of both test pesticides. A greater decrease in yield attributes was recorded at higher concentrations. For example, with 1000 μg MTXL kg−1 soil, the number of pods, yield of pods, number and yield of seeds, and protein content of V. radiata were maximally decreased by 90, 78, 85, 82 and 69%, respectively, compared to un-inoculated but MTXL-treated plants (Fig. 6 panels A–I). However, following soil inoculation with Pseudomonas sp. PGR-11 into plants, all these parameters were significantly increased (Fig. 6 panels A–I). Here, Pseudomonas sp. showed a significant synergistic effect (bio-inoculum × pesticides) on the yield attributes of V. radiata. It is possible that synthesis of plant hormones (such as auxin) by a tolerant bacterial strain positively influenced directly/indirectly cell division, floral initiation and plant development, resulting in a higher number of seeds and higher seed yield. Plant adaptation is associated with increased solute concentrations (such as soluble proteins and carbohydrates), which change the osmotic potential of the cells, resulting in an increase in water absorption under adverse conditions, allowing plants to withstand abiotic stimuli such as pesticides. Similar to this study, when plant bioactive molecule (ACC, IAA and siderophores) secreting Burkholderia cepacia was utilized in field trials, pesticide stress showed no effect on the biological attributes and seed yield of the chickpea plants.90
Fig. 6. Bio-inoculation effect of pesticide-tolerant Pseudomonas sp. PGR-11 on yield attributes: number of pod/plants (A and B), pod yield (C and D), number of seeds/plants (E and F), seed yield (G and H) and protein content (I) in V. radiata (L.) plants exposed to 50, 100, 200, 500 and 1000 μg kg−1 soil with tebuconazole and metalaxyl. Here, bar and line diagrams represent the mean values of three replicates (n = 3) of three plants/pot. Mean values followed by different letters are significantly different (p ≤ 0.05) as determined by the DMRT test. Vertical and scattered bars represent means ± SDs (n = 3).
4. Conclusion
Farmers' fields have previously been envenomed with a large number of pesticide residues as a result of the unrelenting usage of chemical pesticides. Most fungicides have a short-term effect and require multiple treatments, exposing the environment to many dosages of fungicides and raising the danger of residues accumulating in the environment. Biological control takes longer to activate in order to lessen disease, but the effect lasts longer. The majority of bio-control chemicals are non-toxic to naturally occurring species and hence are considered safe. As a result, finding effective native bio-inoculants that mitigate pesticide toxicity and promote plant development in pesticide-saturated soil is critical. Therefore, in the current work, pesticide-tolerant Pseudomonas sp. PGR-11 produced considerable amounts of plant growth regulators in both conventional and stressed media and promoted/increased the symbiosis and biological attributes of Vigna radiata (L.). Furthermore, the physiological state (chlorophyll fluorescence and leaf-exchange attributes) of V. radiata showed better performance under fungicide stress, after inoculating with strain PGR-11. Thus, these indigenous bio-inoculants have huge potential in the future in widening the spectrum of PGPR available for field use, with multifunctional PGPR activities and many benefits of remediation potential sustained in various pesticides, plant nutrition delivery, and growth promotion. As a result, in the future, these could be used as microbial bio-pesticides and bio-fertilizers for disease management, plant growth promotion, and soil bio-remediation for low-cost, environmentally friendly agriculture.
Conflicts of interest
None.
Supplementary Material
Acknowledgments
This work was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PUNRSP2022R317), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01570f
References
- Searchinger T., Waite R., Hanson C., Ranganathan J., Dumas P., Matthews E., and Klirs C. , Creating a sustainable food future: A menu of solutions to feed nearly10 billion people by 2050. Final report, 2019 [Google Scholar]
- Waraich E. A. Ahmad R. Ashraf M. Y. Ahmad S. M. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011;61(4):291–304. [Google Scholar]
- Voisin A. S. Guéguen J. Huyghe C. Jeuffroy M. H. Magrini M. B. Meynard J. M. Mougel C. Pellerin S. Pelzer E. Legumes for feed, food, biomaterials and bioenergy in Europe: a review. Agron. Sustainable Dev. 2014;34(2):361–380. [Google Scholar]
- Allahmoradi P. Ghobadi M. Taherabadi S. Taherabadi S. Physiological aspects of mungbean (Vigna radiata L.) in response to drought stress. Int. Conf. Food Eng. Biotech. 2011;9:272–275. [Google Scholar]
- Tang D. Dong Y. Ren H. Li L. He C. A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata) Chem. Cent. J. 2014;8(1):1–9. doi: 10.1186/1752-153X-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E. Gulledge J. Engelhaupt E. Burow M. E. McLachlan J. A. Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proc. Natl. Acad. Sci. 2007;104:10282–10287. doi: 10.1073/pnas.0611710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshman J. Blay-Palmer A. Landman K. Anthropocene crisis: climate change, pollinators, and food security. Environ. 2019;6(2):22. [Google Scholar]
- Shahid M., Khan M. S. and Zaidi A., A. Fungicide toxicity to legumes and its microbial remediation: A current perspective, Pesticides in Crop Production: Physiological and Biochemical Action, 2020, pp. 15–33 [Google Scholar]
- Sivasakthi S. Usharani G. Saranraj P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014;9(16):1265–1277. [Google Scholar]
- Shahid M. Khan M. S. Kumar M. Kitazin-pea interaction: understanding the fungicide induced nodule alteration, cytotoxicity, oxidative damage and toxicity alleviation by Rhizobium leguminosarum. RSC Adv. 2019a;9(30):16929–16947. doi: 10.1039/c9ra01253b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunohara Y. Shirai S. Wongkantrakorn N. Matsumoto H. Sensitivity and physiological responses of Eleusine indica and Digitaria adscendens to herbicide quinclorac and 2, 4-D. Environ. Exp. Bot. 2010;68(2):157–164. [Google Scholar]
- Chennappa G., Sreenivasa M. Y. and Nagaraja H., Azotobacter salinestris: a novel pesticide-degrading and prominent biocontrol PGPR bacteria, in Microorganisms for Green Revolution, Springer, Singapore, 2018, pp. 23–43 [Google Scholar]
- Saeidnejad A. H. Kafi M. Khazaei H. R. Pessarakli M. Effects of drought stress on quantitative and qualitative yield and antioxidative activity of Bunium persicum. Turk. J. Bot. 2013;37(5):930–939. [Google Scholar]
- Danish M. Shahid M. Zeyad M. T. Bukhari N. Al-Khattaf F. S. Ali S. Bacillus mojavensis, a Metal-Tolerant Plant Growth-Promoting Bacterium, Improves Growth, Photosynthetic Attributes, Gas Exchange Parameters, and Alkalo-Polyphenol Contents in Silver Nanoparticle (Ag-NP)-Treated Withania somnifera L. (Ashwagandha) ACS Omega. 2022;7:13878–13893. doi: 10.1021/acsomega.2c00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randika J. L. P. C. Bandara P. K. G. S. S. Soysa H. S. M. Ruwandeepika H. A. D. Gunatilake S. K. Bioremediation of pesticide-contaminated soil: a review on indispensable role of soil bacteria. J. Agric. Sci. - Sri Lanka. 2022;17:19–43. [Google Scholar]
- Nathiya S. Janani R. Kannan V. R. Potential of plant growth promoting Rhizobacteria to overcome the exposure of pesticide in Trigonella foenum-graecum (fenugreek leaves) Biocatal. Agric. Biotechnol. 2020;23:101493. [Google Scholar]
- Ahmad S. Chaudhary H. J. Damalas C. A. Microbial detoxification of dimethoate through mediated hydrolysis by Brucella sp. PS4: molecular profiling and plant growth-promoting traits. Environ. Sci. Pollut. Res. 2021;2021:1–12. doi: 10.1007/s11356-021-15806-1. [DOI] [PubMed] [Google Scholar]
- Saleh I. A. Zouari N. Al-Ghouti M. A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innovation. 2020:101026. [Google Scholar]
- Prasad S., Malav L. C., Choudhary J., Kannojiya S., Kundu M., Kumar S. and Yadav A. N., Soil microbiomes for healthy nutrient recycling, in Current Trends in Microbial Biotechnology for Sustainable Agriculture, Springer, Singapore, 2021, pp. 1–21 [Google Scholar]
- Mansotra P. Sharma P. Sirari A. Aggarwal N. Ecological performance of multifunctional pesticide tolerant strains of Mesorhizobium sp. in chickpea with recommended pendimethalin, ready-mix of pendimethalin and imazethpyr, carbendazim and chlorpyrifos application. Arch. Microbiol. 2022;204(1):1–25. doi: 10.1007/s00203-021-02628-5. [DOI] [PubMed] [Google Scholar]
- Aroua I. Abid G. Souissi F. Mannai K. Nebli H. Hattab S. Borgi Z. Jebara M. Identification of two pesticide-tolerant bacteria isolated from Medicago sativa nodule useful for organic soil phytostabilization. Int. Microbiol. 2019;22:111–120. doi: 10.1007/s10123-018-0033-y. [DOI] [PubMed] [Google Scholar]
- Holt J., Krieg N. R., Sneath P. H. A. and Staley J. T., Bergey's manual of determinative bacteriology, Williams and Wilkins. Baltimore, Maryland, USA, 9th edn, 1994 [Google Scholar]
- Bric J. M. Bostock R. M. Silverstone S. E. Rapid in situ assay for indole acetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991;57:535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. B. Zuberer D. A. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fertil. Soils. 1991;12:39–45. [Google Scholar]
- Bakker A. W. Schippers B. O. B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth-stimulation. Soil Biol. Biochem. 1987;19:451–457. [Google Scholar]
- Dye D. W. The inadequacy of the usual determinative tests for the identification of Xanthomonas spp. N. Z. J. Sci. 1962;5:393–416. [Google Scholar]
- Gull M. Hafeez F. Y. Characterization of siderophore producing bacterial strain Pseudomonas fluorescens Mst 8.2 as plant growth promoting and biocontrol agent in wheat. Afr. J. Microbiol. Res. 2012;6:6308–6318. [Google Scholar]
- Dworkin M. Foster J. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958;75:592–603. doi: 10.1128/jb.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M. Shimomura T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 1978;42(10):1825–1831. [Google Scholar]
- Penrose D. M. Glick B. R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003;118(1):10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem. J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M. Zaidi A. Khan M. S. Modulations in growth, structure, cell viability and antioxidant enzyme of a nodule bacterium Mesorhizobium ciceri induced by pesticides. Environ. Dev. Sustain. 2021a;23(3):4103–4119. [Google Scholar]
- Shahid M. Khan M. S. Cellular destruction, phytohormones and growth modulating enzymes production by Bacillus subtilis strain BC8 impacted by fungicides. Pestic. Biochem. Physiol. 2018a;149:8–19. doi: 10.1016/j.pestbp.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Shahid M. Ahmed B. Khan M. S. Evaluation of microbiological management strategy of herbicide toxicity to greengram plants. Biocatal. Agric. Biotechnol. 2018;14:96–108. [Google Scholar]
- Sadasivum S. and Manickam A., Biochemical methods, New Age International Publishers (P) Ltd, New Delhi, 1992 [Google Scholar]
- Arnon D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T. O. Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem. Biophys. Res. Commun. 1965;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- Chávez-Arias C. C. Gómez-Caro S. Restrepo-Díaz H. Physiological, biochemical and chlorophyll fluorescence parameters of Physalis peruviana L. seedlings exposed to different short-term water logging periods and Fusarium wilt infection. Agronomy. 2019;9(5):213. [Google Scholar]
- Wagner D. DeFoliart L. Doak P. Schneiderheinze J. Impact of epidermal leaf mining by the aspen leaf miner (Phyllocnistis populiella) on the growth, physiology, and leaf longevity of quaking aspen. Oecologia. 2008;157(2):259–267. doi: 10.1007/s00442-008-1067-1. [DOI] [PubMed] [Google Scholar]
- Ullah N. Ditta A. Imtiaz M. Li X. Jan A. U. Mehmood S. Rizwan M. S. Rizwan M. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agron. Crop Sci. 2021;207(5):783–802. [Google Scholar]
- Yue J. Hu X. Huang J. Origin of plant auxin biosynthesis. Trends Plant Sci. 2014;19(12):764–770. doi: 10.1016/j.tplants.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Perrot-Rechenmann C. Cellular responses to auxin: division versus expansion. Cold Spring Harbor Perspect. Biol. 2010;2(5):001446. doi: 10.1101/cshperspect.a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. Dodd I. C. Belimov A. A. Jiang F. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct. Plant Biol. 2016;43(2):161–172. doi: 10.1071/FP15200. [DOI] [PubMed] [Google Scholar]
- Saleem M. Arshad M. Hussain S. Bhatti A. S. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Ind. Microbiol. Biotechnol. 2007;34(10):635–648. doi: 10.1007/s10295-007-0240-6. [DOI] [PubMed] [Google Scholar]
- Chen L., Probing Roles of Ethylene in Leaf Gas Exchange, Growth and Development Using ACC-Deaminase Containing Rhizobacteria and 1-Methylcyclopropene (1-MCP), Lancaster University, (United Kingdom: ), 2012 [Google Scholar]
- Wan W. Qin Y. Wu H. Zuo W. He H. Tan J. Wang Y. He D. Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front. Microbiol. 2020;11:752. doi: 10.3389/fmicb.2020.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayu G. Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int. J. Agron. 2019:1–7. [Google Scholar]
- Kenneth O. C. Nwadibe E. C. Kalu A. U. Unah U. V. Plant growth promoting rhizobacteria (PGPR): a novel agent for sustainable food production. Am. J. Agric. Biol. Sci. 2019;14:35–54. [Google Scholar]
- Gupta N. Balomajumder C. Agarwal V. K. Enzymatic mechanism and biochemistry for cyanide degradation: a review. J. Hazard. Mater. 2010;176(1–3):1–13. doi: 10.1016/j.jhazmat.2009.11.038. [DOI] [PubMed] [Google Scholar]
- Sivasakthi S. Usharani G. Saranraj P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014;9(16):1265–1277. [Google Scholar]
- Kundan R. Pant G. Jadon N. Agrawal P. K. Plant growth promoting rhizobacteria: mechanism and current prospective. J. fertil. pestic. 2015;6(2):9. [Google Scholar]
- Roy T. Bandopadhyay A. Sonawane P. J. Majumdar S. Mahapatra N. R. Alam S. Das N. Bio-effective disease control and plant growth promotion in lentil by two pesticide degrading strains of Bacillus sp. Biol. Control. 2018;127:55–63. [Google Scholar]
- Castillo J. M. Casas J. Romero E. Isolation of an endosulfan-degrading bacterium from a coffee farm soil: Persistence and inhibitory effect on its biological functions. Sci. Total Environ. 2011;412:20–27. doi: 10.1016/j.scitotenv.2011.09.062. [DOI] [PubMed] [Google Scholar]
- Shahid M. Khan M. S. Tolerance of pesticides and antibiotics among beneficial soil microbes recovered from contaminated rhizosphere of edible crops. Curr. Res. Microb. Sci. 2022;3:100091. doi: 10.1016/j.crmicr.2021.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. Sharma N. Maitra S. S. Lakkaboyana S. K. in vivo removal of profenofos in agricultural soil and plant growth promoting activity on Vigna radiata by efficient bacterial formulation. Int. J. Phytorem. 2020;22(6):585–593. doi: 10.1080/15226514.2019.1696743. [DOI] [PubMed] [Google Scholar]
- da Silva Rovida A. F. Costa G. Santos M. I. Silva C. R. Freitas P. N. N. P Oliveira E. Pileggi S. A. V. Olchanheski R. L. Pileggi M. Herbicides tolerance in a Pseudomonas strain is associated with metabolic plasticity of antioxidative enzymes regardless of selection. Front. Microbiol. 2021;12:1–15. doi: 10.3389/fmicb.2021.673211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambreen S. Yasmin A. Isolation, characterization and identification of organophosphate pesticide degrading bacterial isolates and optimization of their potential to degrade chlorpyrifos. Int. J. Agric. Biol. 2020;24:699–706. [Google Scholar]
- Mohanty S. S. Jena H. M. Degradation kinetics and mechanistic study on herbicide bioremediation using hyper butachlor-tolerant Pseudomonas putida G3. Process Saf. Environ. Prot. 2019;125:172–181. [Google Scholar]
- Sharma A. Kasture A. Pandit J. Shirkot P. Isolation, characterization and identification of chlorpyrifos tolerant Pseudomonas strain exhibiting extracellular OPH activity from apple orchard soils of Himachal Pradesh. Asian J. Microbiol., Biotechnol. Environ. Sci. 2017;19(4):158–167. [Google Scholar]
- Rajasankar R. Gayathry G. M. Sathiavelu A. Ramalingam C. aravanan V. S. Pesticide tolerant and phosphorus solubilizing Pseudomonas sp. strain SGRAJ09 isolated from pesticides treated Achillea clavennae rhizosphere soil. Ecotoxicol. 2013;22(4):707–717. doi: 10.1007/s10646-013-1062-0. [DOI] [PubMed] [Google Scholar]
- Khan N. Bano A. Ali S. Babar M. A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020;90:189–203. [Google Scholar]
- Sitbon F. Åstot C. Edlund A. Crozier A. Sandberg G. The relative importance of tryptophan-dependent and tryptophan-independent biosynthesis of indole-3-acetic acid in tobacco during vegetative growth. Planta. 2000;211(5):515–721. doi: 10.1007/s004250000338. [DOI] [PubMed] [Google Scholar]
- Löffler C. Czygan F. C. Proksch P. Role of indole-3-acetic acid in the interaction of the phanerogamic parasite Cuscuta and host plants. Plant Biol. 1991;1(06):613–617. [Google Scholar]
- Al-Shwaaiman H. A. Shahid M. Elgorban A. M. Siddique K. H. Syed A. Beijerinckia fluminensis BFC-33, a novel multi-stress-tolerant soil bacterium: Deciphering the stress amelioration, phytopathogenic inhibition and growth promotion in Triticum aestivum L. (wheat) Chemosphere. 2022:133843. doi: 10.1016/j.chemosphere.2022.133843. [DOI] [PubMed] [Google Scholar]
- Marathe R. Phatake Y. Shaikh A. Shinde B. Gajbhiye M. Effect of IAA produced by Pseudomonas aeruginosa 6a (bc4) on seed germination and plant growth of Glycin max. J. Exp. Biol. Agric. Sci. 2017;5:351–358. [Google Scholar]
- Naveed M. Qureshi M. A. Zahir Z. A. Hussain M. B. Sessitsch A. Mitter B. L-Tryptophan-dependent biosynthesis of indole-3-acetic acid (IAA) improves plant growth and colonization of maize by Burkholderia phytofirmans PsJN. Ann. Microbiol. 2015;65(3):1381–1389. [Google Scholar]
- Yousef N. M. Capability of plant growth-promoting rhizobacteria (PGPR) for producing indole acetic acid (IAA) under extreme conditions. Eur. J. Biol. Res. 2018;8(4):174–182. [Google Scholar]
- Shahid M. Manoharadas S. Altaf M. Alrefaei A. F. Organochlorine pesticides negatively influenced the cellular growth, morphostructure, cell viability, and biofilm-formation and phosphate-solubilization activities of Enterobacter cloacae strain EAM 35. ACS Omega. 2021b;6(8):5548–5559. doi: 10.1021/acsomega.0c05931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavino A. F. and Pedraza R. O., The role of siderophores in plant growth-promoting bacteria, in Bacteria in agrobiology: crop productivity, Springer, Berlin, Heidelberg, 2013, pp. 265–285 [Google Scholar]
- Syed A. Zeyad M. T. Shahid M. Elgorban A. M. Alkhulaifi M. M. Ansari I. A. Heavy metals induced modulations in growth, physiology, cellular viability, and biofilm formation of an identified bacterial isolate. ACS Omega. 2021;6(38):25076–25088. doi: 10.1021/acsomega.1c04396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M. Zaidi A. Ehtram A. Khan M. S. In vitro investigation to explore the toxicity of different groups of pesticides for an agronomically important rhizosphere isolate Azotobacter vinelandii. Pestic. Biochem. Physiol. 2019b;157:33–44. doi: 10.1016/j.pestbp.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Li Y. Liu X. Hao T. Chen S. Colonization and maize growth promotion induced by phosphate solubilizing bacterial isolates. Int. J. Mol. Sci. 2017;18(7):1253. doi: 10.3390/ijms18071253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakashvel M. Muthezhilan R. Srinivasan R. Hussain A. J. Gobalakrishnan S. Bhagat J. Kaarthikeyan C. Muthulakshmi R. Hydrogen cyanide mediated biocontrol potential of Pseudomonas sp. AMET1055 isolated from the rhizosphere of coastal sand dune vegetation. Adv. Biotechnol. 2010;9:39–42. [Google Scholar]
- Khan S. Shahid M. Khan M. S. Syed A. Bahkali A. H. Elgorban A. M. J. Pichtel. Fungicide-tolerant plant growth-promoting rhizobacteria mitigate physiological disruption of white radish caused by fungicides used in the field cultivation. Int. J. Environ. Res. Public Health. 2020;17(19):7251. doi: 10.3390/ijerph17197251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M. Manoharadas S. Chakdar H. Alrefaei A. F. Albeshr M. F. Almutairi M. H. Biological toxicity assessment of carbamate pesticides using bacterial and plant bioassays: An in vitro approach. Chemosphere. 2021c;278:130372. doi: 10.1016/j.chemosphere.2021.130372. [DOI] [PubMed] [Google Scholar]
- Shahid M. Khan M. S. Fungicide tolerant Bradyrhizobium japonicum mitigate toxicity and enhance greengram production under hexaconazole stress. J. Environ. Sci. 2019;78:92–108. doi: 10.1016/j.jes.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Shakir S. K. Kanwal M. Murad W. ur Rehman Z. ur Rehman S. Daud M. K. Azizullah A. Effect of some commonly used pesticides on seed germination, biomass production and photosynthetic pigments in tomato (Lycopersicon esculentum) Ecotoxicology. 2016;25(2):329–341. doi: 10.1007/s10646-015-1591-9. [DOI] [PubMed] [Google Scholar]
- Dhungana S. K. Kim I. D. Kwak H. S. Shin D. H. Unraveling the effect of structurally different classes of insecticide on germination and early plant growth of soybean [Glycine max (L.) Merr.] Pestic. Biochem. Physiol. 2016;130:39–43. doi: 10.1016/j.pestbp.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Shahid M. Ahmed B. Zaidi A. Khan M. S. Toxicity of fungicides to Pisum sativum: a study of oxidative damage, growth suppression, cellular death and morpho-anatomical changes. RSC Adv. 2018c;8(67):38483–38498. doi: 10.1039/c8ra03923b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parween T., Bhandari P., Jan S. and Raza S. K., Interaction Between Pesticide And Soil Microorganisms And Their Degradation: A Molecular Approach, in Plant, Soil and Microbes, Springer, Cham, 2016, pp. 23–43 [Google Scholar]
- Petit A. N. Fontaine F. Vatsa P. Clément C. Vaillant-Gaveau N. Fungicide Impacts On Photosynthesis In Crop Plants. Photosynth. Res. 2012;111:315–326. doi: 10.1007/s11120-012-9719-8. [DOI] [PubMed] [Google Scholar]
- Jaiswal D. K. Verma J. P. Krishna R. Gaurav A. K. Yadav J. Molecular characterization of monocrotophos and chlorpyrifos tolerant bacterial strain for enhancing seed germination of vegetable crops. Chemosphere. 2019;223:636–650. doi: 10.1016/j.chemosphere.2019.02.053. [DOI] [PubMed] [Google Scholar]
- Baldan E. Nigris S. Romualdi C. D’Alessandro S. Clocchiatti A. Zottini M. Stevanato P. Squartini A. Baldan B. Beneficial bacteria isolated from grapevine inner tissues shape Arabidopsis thaliana roots. PLoS One. 2015;10(10):e0140252. doi: 10.1371/journal.pone.0140252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M. Khan M. S. Assessment of glyphosate and quizalofop mediated toxicity to greengram [Vigna radiata (L.) Wilczek], stress abatement and growth promotion by herbicide tolerant Bradyrhizobium and Pseudomonas species. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(12):3001–3016. [Google Scholar]
- Sarkar A. Mukherjee P. K. Bhattacharya P. M. Bio-efficacy of pendimethalin and fluchloralin in mustard. Indian J. Weed Sci. 2005;37:275–276. [Google Scholar]
- Shahid M. Khan M. S. Syed A. Marraiki N. Elgorban A. M. Mesorhizobium ciceri as biological tool for improving physiological, biochemical and antioxidant state of Cicer aritienum (L.) under fungicide stress. Sci. Rep. 2021d;11(1):1–18. doi: 10.1038/s41598-021-89103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. Bano A. Babar M. A. Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS One. 2019;14(3):e0213040. doi: 10.1371/journal.pone.0213040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan K. Wu X. Ren J. Ye J. Photosynthetic characteristics of Populus deltoides seedlings inoculated ectomycorrhizal fungi and plant growth promoting rhizobacteria under salt stress. Acta Bot. Boreali-Occident. Sin. 2011;31:1216–1222. [Google Scholar]
- El-Esawi M. A. Alaraidh I. A. Alsahli A. A. Alzahrani S. M. Ali H. M. Alayafi A. A. Ahmad M. Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int. J. Mol. Sci. 2018;19:3310. doi: 10.3390/ijms19113310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M. Khan M. S. Glyphosate induced toxicity to chickpea plants and stress alleviation by herbicide tolerant phosphate solubilizing Burkholderia cepacia PSBB1 carrying multifarious plant growth promoting activities. 3 Biotech. 2018b;8(2):1–17. doi: 10.1007/s13205-018-1145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.