Abstract
The coronavirus disease 2019 (COVID-19) pandemic revolutionized the vaccine market and initiated the momentum for alternative routes of administration for vaccines. The intranasal route of immunization is one such possibility that appears to be the most promising since it has some significant advantages, particularly in the prevention of respiratory infection. To analyze and summarize the role of nasal vaccines over conventional vaccines during COVID-19 and the need for the nasal vaccine as a booster shot. In this narrative review, the required data was retrieved using keywords “COVID-19,” “Intranasal,” “Immunity,” “Nasal spray,” and “Mucosal” in databases including PubMed, Scopus, Embase, Science Direct, and Web of Sciences. The results of the study showed that the nasal vaccines were both effective and protective according to the current researches approaching during the COVID-19 period and the preclinical and clinical phase trials prove the intranasal vaccination elicits more robust and cross-protective immunity than conventional vaccines. In this narrative review article, mechanisms across the nasal mucosa will be briefly presented and the current status of nasal vaccines during the COVID-19 pandemic is summarized, and advantages over traditional vaccines are provided. Furthermore, after exploring the primary benefits and kinetics of nasal vaccine, the potential for consideration of nasal vaccine as a booster dose is also discussed.
Keywords: Nasal sprays, COVID-19, Immunity, Nasal cavity, Nasal vaccine
Introduction
Vaccines are biological agents that elicit an immune reaction to a particular antigen derived from a pathogen that causes an infectious disease [1]. They are considered to be one of the greatest public health achievements of the last century and work by triggering an innate immune response, which then triggers an antigen-specific adaptive immune response, similar to how normal infections do [2]. Vaccines come in a variety of forms, including active, inactivated, conjugate, component, and recombinant vaccines [3]. The nasal vaccine is one such type, where the administration has increasingly gained popularity as a viable alternative to injection [4]. Oral and intramuscular vaccinations have long been considered the best alternatives, but the nasal path has many benefits, including the convenience of delivery and the development of mucosal and systemic immunity [5]. and they are Needle-free vaccine delivery will help with the mass vaccines by making them easier and faster to administer, as well as improving protection and enforcement, lowering costs, and reducing the discomfort associated with vaccinations [6]. Nasal vaccine delivery has a number of advantages over most vaccine delivery methods. These benefits include the ease of administration without the use of needles, which decreases the risks of needle stick accidents and disposal [7]. The nasal route of vaccination can provide protection at a number of different mucosal areas making it more convenient that is both cost-effective and patient-friendly. Micro particulates, nano particulates, and liposomes make up the majority of nasal vaccine delivery systems [8]. After all, have benefits including ease of administration, fast onset of intervention, and the prevention of first-pass metabolism [9]. Intranasal vaccines are more effective than oral vaccines. Immunization via the nose is a popular choice. The advantages include being easily accessible, being highly vascularized, having a large number of microvilli, and so on [10]. The nose is a great way to administer vaccines because it allows for reduced doses and no susceptibility to high pH or intestinal issues [11]. The preparation of highly efficient coronavirus disease 2019 (COVID-19) vaccines in less than a year is a remarkable scientific achievement. However, some coronavirus variants have developed that might at least partially avoid the vaccine-induced immune response. These variations should serve as a wake-up call to avoid complacency—and to inspire us to investigate a new form of immunization that is administered by nasal spray. Intranasal immunizations may give an extra layer of protection and aid in the virus’s progress and intranasal vaccinations look to be a good prospect since they have the ability to prevent coronavirus infection while also requiring far less distribution and administration. It’s time to prioritize them and speed up their growth. This paper elucidates and explores the major physiological challenges and advantages over traditional vaccines that must be addressed for nasal vaccination to be successful, explain developments in the field of nasal administration of subunit vaccines, and explore new prospects for nasal vaccine improvement. Booster COVID-19 shots have become a debatable topic in recent times, with certain health institutions promoting their usage to extend vaccination-induced immunity. Novel nasal COVID-19 vaccines are being developed, and preliminary results in nonhuman primates have revealed that they are effective in preventing virus proliferation and shedding. The goal of this review is to provide an overview of intranasal vaccinations and their current status in comparison to conventional vaccines, as well as the possibility of using the intranasal vaccine as a booster dose.
This is a narrative review and does not include any human/animal subject; hence, no ethical approval is required.
Vaccines against Viral Infections
Every year, influenza disease affects the world’s population up to 10%, or up to 500 million individuals [12]. Vaccination is one of the viable options to restrict any outbreaks, present seasonal influenza vaccines, and are only effective against closely related circulating strains [13]. There are currently offered trivalent vaccines that contain two A viruses and one B virus. Recently, quadrivalent vaccines have been developed [14]. The live attenuated influenza vaccine (LAIV) has been used as an alternative to standard inactivated influenza vaccines since 2003 in the United States and Europe in 2012 [15].
Trivalent and Quadrivalent Vaccines
The trivalent inactivated vaccine (TIV) is the most often used kind of influenza vaccine and the typical vaccine comprises three currently circulating seasonal influenza virus strains: two strains of influenza A viruses (H3N2 and H1N1) and one type of influenza B virus. TIVs confer immunity by producing antibodies that target the hemagglutinin (HA) protective epitopes. Some formulations may also stimulate the formation of NA-specific antibodies, which do not protect against infection but may change the sickness that occurs. TIV is administered as a single dose to children aged 9 years old and contains 15 g of HA per strain (total HA concentration of 45 g). Younger children (between the ages of 6 and 8 years) require two doses delivered 4 weeks apart [16].
The same strains as the TIV are included in the quadrivalent influenza vaccine (QIV), as well as an extra B strain from another B lineage [17]. Between 2007 and 2017, there was a mismatch between the circulating B lineage and the one in the vaccine in four out of ten seasons in Spain, despite the fact that the World Health Organization (WHO) currently advocates TIV [18]. The WHO recommends the influenza virus strains that should be included in the influenza vaccine for the following epidemic season twice a year. QIV, which comprises two influenza A subtypes (H1N1 and H3N2) and two influenza B lineages (B/Victoria and B/Yamagata), has been used since 2013–2014 season in addition to the TIV. For the first time in the 2018–2019 season, the WHO classified QIV as a first-line recommendation. Furthermore, the European Centre for Disease Prevention and Control has advised QIV for influenza elimination since 2017. The importance of QIV has also been acknowledged by other European nations. Between 2007 and 2017, the WHO still advised TIV in Spain [19].
The US Food and Drug Administration (FDA) initially approved one new B antigen in 2012, and it has just recently hit the market (registered in 2014 in Israel and marketed in 2015) [20]. Although the trivalent seasonal influenza vaccination contains one strain of influenza B, it is not always feasible to forecast which influenza B lineage would prevail during the next influenza season [21]. When compared to trivalent immunization, quadrivalent vaccinations have been proven to lower morbidity, mortality, and the use of healthcare services. The comparative cost-effectiveness of the extended vaccination against the trivalent vaccine, on the other hand, is strongly reliant on the influenza B population burden. Both a LAIV and an inactivated flu vaccine are obtainable for the quadrivalent and trivalent vaccines [22]. In addition to H1N1 and H3N2, the decreased efficacy of a mismatched vaccination may be prevented by adding four virus strains in the vaccine one from each B lineage. In the first decade of the 21 century, the use of quadrivalent influenza vaccination was proposed [23]. The LAIV is given intranasal, whereas the Inactivated influenza vaccine (IIV) is given intramuscularly. The attenuated virus replicates in the nasopharyngeal mucosal tissues, simulating physiological infection. When compared to intramuscular IIV, LAIV nasal delivery results in greater secretory immunoglobulin A (IgA) titers. While both vaccine delivery strategies rely on humoral and cell-mediated immunity, mimicking natural infection may have immunological benefits [24].
Nasal Vaccine
Injected vaccines activate the systemic immune response, however may not have a mucosal immune defense. Vaccines for the mucosa, however, elicit not only strong local immune defense, but also systemic reaction close to that of injection [4]. Nasal administration is available in a range of sizes and formulations, including granular and sprays delivery. Particles bigger than 5 mm in diameter settle in the nasal cavity mostly when air is driven through the anterior surfaces of the nasal turbinates, and to a lesser proportion when the air flow shifts to the posterior nasal cavity [25]. The mucous membrane of the nasal cavity is the initial line of defense against inhaled antigens, and the presence of nasal-associated lymphoid tissue (NALT) around the root of the nasal canal is critical for mucosa surface protection. Furthermore, the nasal membrane is porous, and there are blood vessels and lymphatic vessels to which the antigen may have immediate entry if it is delivered efficiently through the epithelial tissue [26]. And providing effective and long-lasting defenses from pathogen invasion. Most commercial vaccinations, on the other hand, are delivered systemically, ensuing in just a humoral immune response and no protection against pathogens in the mucosa. As a result, mucosal vaccination is extremely beneficial for infectious diseases that are inhaled, swallowed, or sexually transmitted, such as measles, coronaviruses, and human immunodeficiency virus (HIV) [27]. Nasal medication administration is also noninvasive, which adds to its simplicity and safety. Furthermore, as compared to oral, sublingual, and transdermal drug delivery, nasal medication delivery resulted in a rapid onset of effect [28]. To be successful after intranasal delivery, a vaccine’s formulation must maintain antigen safety, provide the antigen enough time to connect with the lymphatic system, and activate both innate and cellular processes with or without the use of the healthy chemicals by attacking particular parts of the body, effective adjuvants immune cells that provide long-term protection from pathogens [11]. Nasal vaccination has a number of intriguing benefits. The nasal cavity includes a high number of dendritic cells, which can trigger potent systemic and local immune responses to infections that enter the body through the respiratory tract [29]. Any medications can be absorbed in a very fast rate after being applied nasally. It is often associated with high bioavailability (depending on the physicochemical properties of the drug [30]. For example, if a medicine is required to have a quick start of effect if gastro stasis occurs (e.g., migraine), or if a medication is poorly absorbed throughout the gastrointestinal tract or is extensively destroyed by natural pH conditions or enzymes inside the lumen of the intestine, and/or by first-pass liver metabolism, then oral administration is not practicable [31].
Kinetics of Intranasal Aerosols
Medication distribution in a controlled dose, decreased droplet or particle size, excellent penetration within the nasal cavity with limited inadvertent delivery through the lungs, maintenance of dose-to-dose sterility, better patient compliance; reduced mucosal irritability, and greater substance design flexibility are the ascendant properties of intra-nasal aerosols [32]. Lipophilic drugs are generally well absorbed through the nose, with pharmacokinetic profiles that are often identical to those obtained following an intravenous injection and bio availabilities approaching 100%. The Tmax for both intravenous and nasal delivery of these fentanyls has been demonstrated to be relatively quick (7 minutes or less) and the bioavailability for nasal administration was approximately 80% [33]. Nasal products can be single-dose or bi-dose, although they frequently include numerous doses, which necessitates storage. In general, formulations designed for local or systemic action must have no irritating or toxic effects on mucosa and cilia, be isotonic, and contain excipients to modify pH, viscosity, solubility, and stability. Apart from dosage content uniformity testing for systemic preparations and the fairly vague condition that the particle size should allow predominant nasal deposition, the present standard does not offer specific characterization procedures for such nasal formulations [34]. To begin with, the adjuvant must not just enhance any immune response, but it must also be linked to improved therapeutic effect. Second, in the development of new vaccine adjuvants, safety has been a key issue, with the goal of providing sufficient progressive efficacy while avoiding or eliminating reactogenicity or toxicity [35]. Despite the many benefits of the nasal path, drug absorption through the nasal mucosa can be inhibited by limitations such as high molecular weight [36]. The spray frequently drips out of or is wiped from the nose as a result of this anterior deposition. Patients frequently sniff to avoid drip-out. However, this permits the medication to be absorbed via the nasal floor and into the throat and gastrointestinal tract. Before entering the gastrointestinal tract, the drug travels past taste receptors at the base of the tongue. It causes an unpleasant/bitter taste sensation and inhibits delivery to the desired nasal areas [37]. Furthermore, nasal vaccines increase the synthesis of secretory IgA, a key component of the mucosal defense system that acts as a local barrier against infections that enter the body via other mucosal membranes. Indeed, mucosal vaccination is founded on the concept of a unified mucosal immune system, which claims for developing mucosal immunity on one mucus layer might lead to the development of effector cells on other mucosal surfaces [38].
Advantages of Nasal Vaccines over Traditional Vaccines
Cross-reactive antibodies have also been observed as a result of intranasal vaccination, which might indicate cross-protection. Cross-protective immunizations can elicit cross-reactive antibodies that detect several antigens, which can enhance vaccine effectiveness by lowering the number of doses needed. Given the high cost of various antigen manufacturing methods, this offers significant cost savings over alternative options [7]. The intranasal vaccination is non-invasive, as it does not require injection. Needles and syringes are not required for nasal protection [39]. Patient compliance is improved. Antigenic dosage is little [40]. It is suitable and safe for youngsters, the elderly, HIV-positive patients, and patients with many comorbidities, and it removes the danger of needle-related infections and discomfort [41]. Oral, intranasal, pulmonary, rectal, and vaginal immunization are all options for mucosal vaccination. The nasal route is the simplest and is appropriate for vaccination delivery [40].
The nasal epithelium’s many microvilli provide a much greater absorption surface. Through mucosal antibody production. Intranasal immunization may give protection against disease in other mucosal areas as well as cross-protection against different strains [41]. Intranasal vaccinations may be especially beneficial for certain groups—youngsters, patients who are old, HIV-positive patients, and patients with several comorbidities, and it is critical to have someone who can administer them in a cost-effective manner. It makes a difference whether the injections must be administered by a physician or if nurses or pharmacists are capable of doing so. Self-administration of intranasal vaccinations is a viable approach [5].
Immunological Features of Mucosal Vaccines
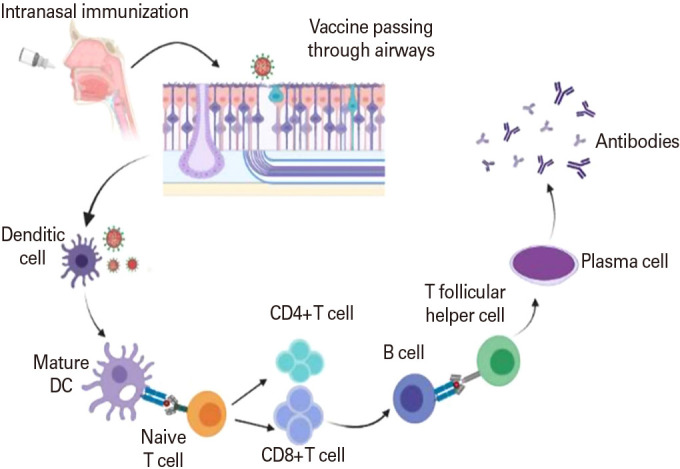
Most vaccinations have traditionally been administered subcutaneously to generate systemic protection against infection; however, this method of administration is ineffective for causing antigen-specific mucosal immune responses [42]. The mucosal immune system is the most effective region for vaccination and acts as the first layer of defense against infection. Nasal and oral vaccines are the most effective treatments for mucosal infections (Fig. 1) [8]. Dendritic cells, T cells, and B cells make up NALT, which is enclosed by an epithelium cell layer that contains M cells, which are unique cells [43]. M cells in NALT serve as antigen absorption sites for mucosal immune induction (Fig. 1) [44]. Particle antigens are preferentially undertaken by M cells in NALT, despite the fact that tiny soluble antigens can pass through the nasal mucosa. M cells deliver antigen to dendritic cells, macrophages, and B cells, where it is processed and presented [45]. CD4+ T helper cells become activated and communicate with B cells, causing IgA into (IgA+). IgA+ B cells migrate to effector locations like the nasal passage, where they develop into IgA-producing plasma cells and form IgA dimers. When dimeric IgA interacts with the polymeric Ig receptor, which transports IgA to effector locations, S-IgA is produced (Fig. 1) [46]. Intranasal vaccination formulations that work retain the antigen stable and guarantee that it stays in the nasopharyngeal region prolonged period to interact with the lymphatic system with or without adjuvants, boosting the immune system to give long-term immunity [11].
Fig. 1. Mechanism of intranasal vaccine.
Nasal Vaccine as Boosting Dose for COVID-19
Boosting the dose or third dose for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has proven to be effective in restricting severe clinical outcomes of COVID-19 [47,48]. The key importance of considering the boosting dose is to restrict the resurgence of coronavirus influenced by Omicron variant B1.1.529, and Delta variant B1.617.2 among others. Boosting may be acceptable for a certain group of the population whose initial immunization, was through single or multiple dose vaccines which may not provide enough protection for individuals with low-efficacy potential or immune compromised populations. Additionally, numerous mutations and the host compatibility to the virus-induced immune reactions may vary, and the immune response of existing vaccine candidates may not be enough to tackle the restriction of newer mutations. It’s still unclear if an additional dose of the same vaccine or an alternative vaccine that complements the first immune reaction is more beneficial to the high-risk populations.
The efficiency of COVID vaccines seems to increase with the number of doses to induce sufficient immunity against the virus. A single dosage of neither the Oxford-AstraZeneca (AZD1222; (AstraZeneca, Cambridge, UK) or Pfizer-BioNTech vaccine (Pfizer, New York, NY, USA) is only about 30% protective towards delta variant of SARS-CoV-2, according to scientists; however, dual dose vaccination showed the efficiency of 88 % for Pfizer and 67% for AstraZeneca [49]. A multicenter randomized phase II trial of 2,878 participants has shown promising results in using a booster dose for COVID-19 using Intramuscular Vaccine [50]. The clear implications of protocols regarding the boosting dose must only be implicated once there is adequate information about the requirement of boosting dose and the targeted population, to avoid unwanted significant boosting reactions.
Discussion
The nasal route is highly advantageous for simple immunization, and most COVID-19 vaccine developers are testing the nasal-based vaccination platforms as a booster shot that majority of current efforts may lead the way for next-generation vaccines in the near future. Intranasal vaccinations are extremely useful since the nasal mucosal area is frequently the first area of infection. According to preclinical and clinical studies, intranasal vaccination induces the production of neutralizing antibodies as well as mucosa IgA and T cell responses, which protect against serious complications of respiratory disease in respiratory tracts. Intranasal vaccine development, on the other hand, has its own set of obstacles and potential.
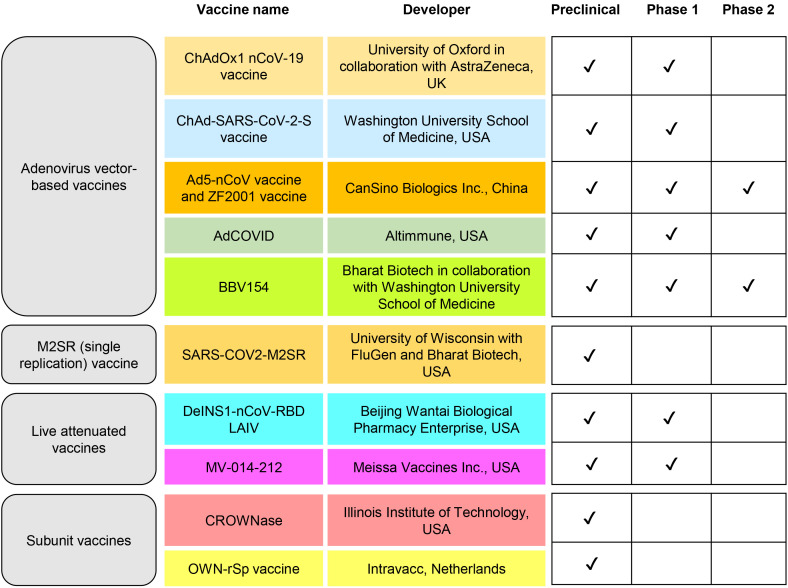
Many clinical and preclinical studies successfully explored the safety and efficacy parameters of the nasal vaccine for COVID-19 (Table 1, Fig. 2), where the use of the nasal vaccine as a booster dose needs to be investigated further. In a year when a drifting influenza-A virus predominated, Nichol et al. [51] illustrated that intranasal vaccinations are safe and effective in healthy, working persons. The participants were randomly randomized to receive either an intranasally administered LAIV vaccine (n=3,041) or a placebo (n=1,520) in the fall of 1997. Following the discovery that LAIV vaccination recipients had less febrile illnesses than placebo receivers during epidemic periods (13.2% for vaccine versus 14.6% for placebo; p=0.19), vaccine candidates have reported some side effects that are not statistically significant. The match between the type A (H3N2) vaccine strain and the circulating virus strain (A/Sydney/05/97[H3N2]) was poor during the 1997–1998 season, showing that LAIV conferred significant cross-protection against variant influenza A virus strains [51].
Table 1. Current status of worldwide intranasal vaccine candidates.
| Products name | Developer | Delivery platforms | Mechanism against COVID-19 | Current status | Country of origin |
|---|---|---|---|---|---|
| NONS | SaNOtize | NORS targets upper airways | S protein adhesion as well as viral RNA production are reduced. | Phase 3 completed | Canada |
| Beyond Air | Lung Fit | S protein adhesion as well as viral RNA production are reduced. | FDA approval pending | USA | |
| Vero Biotech LLC | GeNOsyl Chronic DS | S protein adhesion as well as viral RNA production are reduced. | FDA approval for emergency | Georgia | |
| Inhalation nebulizer suspension powder | APEPTICO | synthesized peptide solnatide | Repairs the pulmonary alveolar endothelial–epithelial barrier | Ongoing phase 3 | Australia |
| Micronized cellulose powder | Nasus Pharma | Taffix spray | Blocks viruses with nasal mucosa | Approved | Israel |
| polysaccharides-containing nasal spray | University of Brimingham | Mucoadhesive nasal spray | Prevents spread of virus and shield the lining of respiratory system | Approved | UK |
| Inhaled mRNA-based antibody | Neurimmune and Ethris | SNIM RNA technology | Neutralizing SARS-CoV-2 antibodies | Phase 2 | Germany |
| Peptide KL4 surfactant (Sinapultide) | Windtree Therapeutics Inc. | AEROSURF delivery technology | Lowers the occurrence of nasal continuous positive airway pressure | Phase 2 | USA |
| Alvesco HFA inhaler | Covis Pharma | Ciclesonide | Inhibits the viral responses\replication | Phase 3 | Luxemburg |
| Nasal spray | Marinomed Biotech | Carragelose spray | Encases viral particles | Phase 2 | Australia |
| Peptide Nebulization | NeuroRX Inc. | Aviptadil synthetic vasoactive polypeptide | SARS-CoV-2 replication is inhibited & cytokine production is prevented | FDA emergency use IND authorization | USA |
| Leukine sargramostim nebuliser | Partner Therapeutics | iLeukPulm | For acute hypoxemia in COVID-19 | Phase 2 | USA |
| Neuroactive spray | VistaGen Therapeutics Inc. | PH94B | Reduces anxiety by activating synaptic pathways | Phase 3 | USA |
| Anti-IL-6 receptor nebulized monoclonal antibody | Tiziana Life sciences, UK & STC Biologics, USA, and Sciarra Laboratories, USA | TZLS-501 | Depletes circulating levels of interleukin 6 in blood | Phase 1 | USA and UK |
| Nebulized Aspartyl- alanyl diketopiperazine | Ampio Pharmaceuticals | Ampion | Interrupts inflammatory response & respiratory disease associated with COVID-19 | Phase 2 | USA |
| Nebulised Interferon beta | Synairgen | SNF001 | Up regulates pulmonary antiviral defenses | Phase 2 | UK |
| Nasal spray | Biohaven Pharmaceuticals, Inc. | Zavegepant (BHV-3500) | Calcitonin gene-related peptide receptor antagonist | Phase 2/3 | USA |
COVID-19, coronavirus disease 2019; FDA, Food and Drug Administration; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IND, Investigational New Drug.
Fig. 2. Update of nasal vaccine candidates for coronavirus disease 2019.
Booster COVID-19 injections have been the focus of attention in recent months, with certain countries pressing for their usage to extend COVID-19 vaccine immunity and safety. Despite several findings strongly supporting the appearance of a probable third wave and waning vaccine immunity, novel booster vaccines, and their efficiency, despite India’s recent achievement of a significant milestone of billion immunizations. Bharat Biotech has mentioned the possibility of giving a third dosage of Covaxin as a booster shot, which will be very different from the existing vaccination.
Conclusion
Vaccine administration via the nasal route appears to be a viable option given the advantages of nasal vaccine over traditional vaccines, which improves patient compliance and reduces the requirement for specialized healthcare staff to give the vaccine. However, the growing number of clinical studies shows that there is a clear demand for nasal vaccinations that are simple to administer and provide better benefits than the additional mucosal lines in terms of formulation cost and the requirement for qualified staff to deliver them. Intranasal vaccination has the capability to produce a durable and cross-protective immune reaction at the respiratory pathogen’s point of entry. As a result, it is an effective technique for preventing infections caused by the influenza A virus which is very variable. Intranasal given vaccinations that are mucosally active have the potential to confer immunization against a wide range of infectious illnesses. When it comes to the availability of these booster doses in India, the next few months will be crucial, since these are the months when seroprevalence, or immunity to COVID-19, may begin to diminish. People are beginning to recover as the second wave peaks.
Footnotes
No potential conflict of interest relevant to this article was reported.
Authors would like to express their gratitude to the Department of Pharmaceuticals, Ministry of Chemicals & Fertilizers, Government of India.
References
- 1.Czochor J, Turchick A. Introduction: vaccines. Yale J Biol Med. 2014;87:401–402. [PMC free article] [PubMed] [Google Scholar]
- 2.Vetter V, Denizer G, Friedland LR, Krishnan J, Shapiro M. Understanding modern-day vaccines: what you need to know. Ann Med. 2018;50:110–120. doi: 10.1080/07853890.2017.1407035. [DOI] [PubMed] [Google Scholar]
- 3.Lahariya C. Vaccine epidemiology: a review. J Family Med Prim Care. 2016;5:7–15. doi: 10.4103/2249-4863.184616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djupesland PG. Nasal delivery of vaccines: EPC (2003 Jan 29) Munich: European Patent Convention; 2003. [Google Scholar]
- 5.Birkhoff M, Leitz M, Marx D. Advantages of intranasal vaccination and considerations on device selection. Indian J Pharm Sci. 2009;71:729–731. [Google Scholar]
- 6.Giudice EL, Campbell JD. Needle-free vaccine delivery. Adv Drug Deliv Rev. 2006;58:68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf H, Kett V. Current prospects and future challenges for nasal vaccine delivery. Hum Vaccin Immunother. 2017;13:34–45. doi: 10.1080/21645515.2016.1239668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramvikas M, Arumugam M, Chakrabarti SR, Jaganathan KS. In: Micro and nanotechnology in vaccine development. Skwarczynski M, Toth I, editors. Oxford: William Andrew Publishing; 2017. Nasal vaccine delivery; pp. 279–301. [Google Scholar]
- 9.Grassin-Delyle S, Buenestado A, Naline E, et al. Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacol Ther. 2012;134:366–379. doi: 10.1016/j.pharmthera.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Illum L, Davis SS. Nasal vaccination: a non-invasive vaccine delivery method that holds great promise for the future. Adv Drug Deliv Rev. 2001;51:1–3. [PubMed] [Google Scholar]
- 11.Jabbal-Gill I. Nasal vaccine innovation. J Drug Target. 2010;18:771–786. doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- 12.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–1779. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 13.Chen JR, Liu YM, Tseng YC, Ma C. Better influenza vaccines: an industry perspective. J Biomed Sci. 2020;27:33. doi: 10.1186/s12929-020-0626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasparini R, Amicizia D, Lai PL, Panatto D. Influenza vaccination: from epidemiological aspects and advances in research to dissent and vaccination policies. J Prev Med Hyg. 2016;57:E1–E4. [PMC free article] [PubMed] [Google Scholar]
- 15.Mohn KG, Smith I, Sjursen H, Cox RJ. Immune responses after live attenuated influenza vaccination. Hum Vaccin Immunother. 2018;14:571–578. doi: 10.1080/21645515.2017.1377376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013;26:476–492. doi: 10.1128/CMR.00097-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crepey P, Redondo E, Diez-Domingo J, et al. From trivalent to quadrivalent influenza vaccines: Public health and economic burden for different immunization strategies in Spain. PLoS One. 2020;15:e0233526. doi: 10.1371/journal.pone.0233526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shasha D, Valinsky L, Hershkowitz Sikron F, et al. Quadrivalent versus trivalent influenza vaccine: clinical outcomes in two influenza seasons, historical cohort study. Clin Microbiol Infect. 2020;26:101–106. doi: 10.1016/j.cmi.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz de Lejarazu R, Diez Domingo J, Gil de Miguel A, et al. Description of influenza B in seasonal epidemics in Spain. Rev Esp Quimioter. 2018;31:511–519. [PMC free article] [PubMed] [Google Scholar]
- 20.Tanner AR, Dorey RB, Brendish NJ, Clark TW. Influenza vaccination: protecting the most vulnerable. Eur Respir Rev. 2021;30:200258. doi: 10.1183/16000617.0258-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudenko L, Kiseleva I, Krutikova E, et al. Rationale for vaccination with trivalent or quadrivalent live attenuated influenza vaccines: protective vaccine efficacy in the ferret model. PLoS One. 2018;13:e0208028. doi: 10.1371/journal.pone.0208028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoft DF, Lottenbach KR, Blazevic A, et al. Comparisons of the humoral and cellular immune responses induced by live attenuated influenza vaccine and inactivated influenza vaccine in adults. Clin Vaccine Immunol. 2017;24:e00414–e00416. doi: 10.1128/CVI.00414-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Swart RL, de Vries RD, Rennick LJ, et al. Needle-free delivery of measles virus vaccine to the lower respiratory tract of non-human primates elicits optimal immunity and protection. NPJ Vaccines. 2017;2:22. doi: 10.1038/s41541-017-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Low N, Kraemer S, Schneider M, Restrepo AM. Immunogenicity and safety of aerosolized measles vaccine: systematic review and meta-analysis. Vaccine. 2008;26:383–398. doi: 10.1016/j.vaccine.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Vila A, Sanchez A, Janes K, et al. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm. 2004;57:123–131. doi: 10.1016/j.ejpb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005;57:1640–1665. doi: 10.1016/j.addr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Liu H, Zhang X, Qian F. Intranasal and oral vaccination with protein-based antigens: advantages, challenges and formulation strategies. Protein Cell. 2015;6:480–503. doi: 10.1007/s13238-015-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed S, Sileno AP, deMeireles JC, et al. Effects of pH and dose on nasal absorption of scopolamine hydrobromide in human subjects. Pharm Res. 2000;17:974–977. doi: 10.1023/a:1007551927177. [DOI] [PubMed] [Google Scholar]
- 29.Davis SS. Nasal vaccines. Adv Drug Deliv Rev. 2001;51:21–42. doi: 10.1016/s0169-409x(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 30.Slutter B, Hagenaars N, Jiskoot W. Rational design of nasal vaccines. J Drug Target. 2008;16:1–17. doi: 10.1080/10611860701637966. [DOI] [PubMed] [Google Scholar]
- 31.Davis SS, Illum L. Absorption enhancers for nasal drug delivery. Clin Pharmacokinet. 2003;42:1107–1128. doi: 10.2165/00003088-200342130-00003. [DOI] [PubMed] [Google Scholar]
- 32.Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmacol. 2001;53:3–21. doi: 10.1211/0022357011775145. [DOI] [PubMed] [Google Scholar]
- 33.Illum L. Nasal drug delivery: possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 34.Scherließ R. Nasal formulations for drug administration and characterization of nasal preparations in drug delivery. Ther Deliv. 2020;11:183–191. doi: 10.4155/tde-2019-0086. [DOI] [PubMed] [Google Scholar]
- 35.Smith A, Perelman M, Hinchcliffe M. Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum Vaccin Immunother. 2014;10:797–807. doi: 10.4161/hv.27449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozsoy Y, Gungor S, Cevher E. Nasal delivery of high molecular weight drugs. Molecules. 2009;14:3754–3779. doi: 10.3390/molecules14093754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djupesland PG, Skretting A. Nasal deposition and clearance in man: comparison of a bidirectional powder device and a traditional liquid spray pump. J Aerosol Med Pulm Drug Deliv. 2012;25:280–289. doi: 10.1089/jamp.2011.0924. [DOI] [PubMed] [Google Scholar]
- 38.Koping-Hoggard M, Sanchez A, Alonso MJ. Nanoparticles as carriers for nasal vaccine delivery. Expert Rev Vaccines. 2005;4:185–196. doi: 10.1586/14760584.4.2.185. [DOI] [PubMed] [Google Scholar]
- 39.Guo C. Oxford-AstraZeneca COVID-19 vaccine (AZD1222), an approved, non-replicating chimpanzee adenovirus-vectored vaccine for the COVID-19 pandemic. J Appl Med Sci. 2021;10:1–12. [Google Scholar]
- 40.Zaman M, Chandrudu S, Toth I. Strategies for intranasal delivery of vaccines. Drug Deliv Transl Res. 2013;3:100–109. doi: 10.1007/s13346-012-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah SS, Patel CM, Patel DH, Vadgama PH, Patel M, Trivedi R. A Review on modern use of intranasal vaccination in the treatment of SARS-COV-2. J Drug Deliv Ther. 2021;11(4-S):263–270. [Google Scholar]
- 42.Azegami T, Yuki Y, Kiyono H. Challenges in mucosal vaccines for the control of infectious diseases. Int Immunol. 2014;26:517–528. doi: 10.1093/intimm/dxu063. [DOI] [PubMed] [Google Scholar]
- 43.Wu HY, Nguyen HH, Russell MW. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 44.Fujimura Y. Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch. 2000;436:560–566. doi: 10.1007/s004289900177. [DOI] [PubMed] [Google Scholar]
- 45.Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011;4:598–602. doi: 10.1038/mi.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogasawara N, Kojima T, Go M, et al. Epithelial barrier and antigen uptake in lymphoepithelium of human adenoids. Acta Otolaryngol. 2011;131:116–123. doi: 10.3109/00016489.2010.520022. [DOI] [PubMed] [Google Scholar]
- 47.Hung IFN, Poland GA. Single-dose Oxford-AstraZeneca COVID-19 vaccine followed by a 12-week booster. Lancet. 2021;397:854–855. doi: 10.1016/S0140-6736(21)00528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bar-On YM, Goldberg Y, Mandel M, et al. Protection across age groups of BNT162b2 vaccine booster against COVID-19. medRxiv [Preprint] 2021 Jan 01; doi: 10.1101/2021.10.07.21264626. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahase E. COVID-19: two vaccine doses are crucial for protection against delta, study finds. BMJ. 2021;374:n2029. doi: 10.1136/bmj.n2029. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Shaw RH, Stuart AS, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nichol KL, Mendelman PM, Mallon KP, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999;282:137–144. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]