Abstract
A 38-year-old female patient, with healthy history, was vaccinated with ChAdOx1 nCoV-19 (Astra Zeneca Cambridge, UK). Five days after the second injection, the patient presented headache, vertigo, then fatigue, nervousness, palpitations, shortness of breath, small amplitude tremors, and sweating episodes. Laboratory investigation revealed a suppressed thyroid-stimulating hormone (TSH), with elevated free thyroxine. However, the TSH receptor antibody and anti-thyroid peroxidase antibody were normal and thyroid-stimulating immunoglobulin negative. The patient was maintained on Metoprolol, and no specific treatment was added. After 3 months of following, the patient now feels comfortable. Our literature review found that 21 cases of subacute thyroiditis (SAT) following coronavirus disease 2019 (COVID-19) vaccines were reported. Most patients were young women who presented neck pain and systemic symptoms, with or without fever. These symptoms can appear as early (3 to 5 days), or later (1 month) after vaccination, regardless of vaccine type and mechanism of action. Laboratory tests showed decreased levels of TSH and elevated thyroid hormone. The mechanism of this event remains unknown. Further study is recommended to investigate the possible predisposing factors to developing SAT after receiving the COVID-19 vaccine.
Keywords: Thyroiditis, SARS-CoV-2, COVID-19, Vaccine, AstraZeneca, Case report
Introduction
Although 2 years have passed, the coronavirus disease 2019 (COVID-19) epidemic is still developing very complicatedly. Variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, particular variants of concerns, continuously create new waves of infection in many countries around the world (https://covariants.org/). As of November 3, 2021, approximately 250 million persons were infected, and over 5 million died (https://www.worldometers.info/coronavirus/). Since the introduction of vaccines, the mortality rate due to COVID-19 tends to decrease (https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov). At the time of writing, 7.1 billion doses have been administered globally, so 49.6% of the world population has received at least one dose of a COVID-19 vaccine (https://ourworldindata.org/), and 28.05 million are now administered each day.
However, the vaccine against COVID-19 has caused some undesirable effects that have been reported with all vaccines produced by different technologies such as mRNA, adenovirus vectored, or inactivated vaccine (https://www.who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines). The main and most common side effects disappear without sequelae including fever, pain at the injection site, headache, and tiredness [1]. Important but very rare side effects including allergic reactions, blood clotting, or cardiac complications have been reported [1]. Recently, case reports on thyroiditis following COVID-19 vaccination were published [2,3]. Among them, subacute thyroiditis (SAT) is known to be an inflammatory disorder of the thyroid gland, and it is often self-limiting. Nowadays, at least 10 cases of SAT after receiving the vaccine for COVID-19 have been reported in the world.
Here, we report one case of SAT a few days after receiving the second dose of the ChAdOx1 nCoV-19 (Astra Zeneca, Cambridge, UK) vaccine, and summarize the relevant data reported in the literature.
Case Report
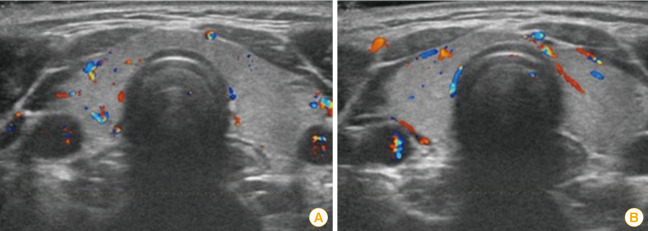
A 38-year-old female patient, with healthy history, was vaccinated with the Astra Zeneca vaccine against COVID-19. A few days after the first dose, the patient felt a slight pain in the injection area, she had no other symptoms. One month later, the patient presented fatigue, palpitations, shortness of breath, small amplitude tremors, and sweating episodes. Her symptoms decreased slightly with the treatment by Metoprolol 25 mg×1 tablet/day. Twelve weeks after the first dose of vaccination, the patient received the second while maintaining Metoprolol. Ten hours after the injection, the patient presented headache, vertigo, then much fatigue and nervousness. Five days later, these symptoms did not improve; hence, she consulted the doctor. Her nasopharyngeal swab test for SARS-CoV-2 was negative. The electrocardiogram results showed a sinus rhythm of 75 cycles/min, with atrial extrasystoles (under treatment with Metoprolol). Thyroid ultrasound results showed that the thyroid gland is not enlarged, and the parenchyma is slightly hyperechoic (Fig. 1).
Fig. 1. Ultrasound image of the thyroid gland at the time of examination (A) and after treatment (B).
Laboratory investigation revealed a suppressed thyroid-stimulating hormone (TSH), with elevated free thyroxine (FT4). However, TSH receptor antibody and anti-thyroid peroxidase antibody were normal and thyroid-stimulating immunoglobulin negative (Table 1).
Table 1. Laboratory results.
| Markers | Initial | Follow-up | Reference rang |
|---|---|---|---|
| FT4: free thyroxine (pmol/L) | 24.14 | 15.23 | 9.01–19.05 |
| TSH: thyroid-stimulating hormone (µU/mL) | 0.018 | 2.318 | 0.350–4.940 |
| TRAb: TSH receptor antibody (U/L) | <0.8 | <0.9 | 0–1.75 |
| Anti-TPO antibody: anti-thyroid peroxidase antibody (U/mL) | 1.35 | 1.46 | 0–5.61 |
| TSI: thyroid-stimulating immunoglobulin | Negative | Negative | - |
The patient was maintained on Metoprolol, and no specific treatment was added. After 3 months of following, the patient now feels comfortable. All clinical symptoms disappeared. Thyroid hormone tests came back normal (Table 1).
The patient provided written informed consent for publication of the research details.
Discussion
SAT is a self-limiting inflammatory thyroid disease. It is characterized by neck pain accompanied by general symptoms such as fever, malaise, asthenia, high levels of inflammatory biomarkers, and thyrotoxicosis [4]. The incidence of this thyroid disorder is relatively low and likely to be underestimated [5]. SAT is associated with various etiologies, nonetheless, viral infection is the most frequent. Several respiratory viruses, including rhinovirus and adenovirus, are thought to be associated with SAT [5]. SARS-CoV-2 infection can also cause thyroiditis [6]. After the first report in early March 2020 [7], several cases of SAT associated with SARS-CoV-2 infection were described [8].
Rare cases of subacute thyroiditis have also been described following vaccination. In 2016, Cases of SAT occurring after receiving influenza vaccination were published [9,10,11,12]. Furthermore, since the introduction of COVID-19 vaccines, SAT following COVID-19 vaccination has been occasionally reported [2,3,13] (Supplement 1).
Most patients of SAT following COVID-19 vaccination were young women who presented neck pain and systemic symptoms, with or without fever. These symptoms can appear as early (3 to 5 days), or later (1 month) after vaccination, regardless of vaccine type and mechanism of action [2,3]. Laboratory tests showed decreased levels of TSH and elevated free triiodothyronine and FT4. Moreover, markers reflecting inflammation (C-reactive protein, white blood cells, or erythrocyte sedimentation rate) also increased [2,3,13]. Most patients were resolved with symptomatic treatment or with a non-steroidal anti-inflammatory drug (NSAID). A small number of patients must be treated with corticosteroids. More rarely, severe hypothyroidism appeared after 6 weeks of treatment with NSAID and propranolol, requiring levothyroxine treatment [14].
The mechanism of SAT following the SARS-CoV-2 vaccination remains unknown. Probable mechanisms include the activation of autoimmune cascades, B-lymphocytes polyclonal activation, and molecular mimicry. The mechanism of angiotensin-converting enzyme 2 (ACE2) receptor stimulation is also plausible considering the fact that the ChAdOx1 nCoV-19 (Astra Zeneca) is a vector-like spike protein of SARS-CoV-2 virus. It binds to ACE2, and the thyroid is known to have one of the highest expressions of ACE2 in follicular cells [15]. In addition, Chatzi et al. [3] described a report of SAT after receiving the COVID-19 vaccine among two sisters, which suggests the potential role of genetic predisposition and needs to be investigated in further studies.
Actually, there are no reports of total cases of SAT following COVID-19 vaccination in a defined population; therefore, estimating the incidence of this side effect is difficult. However, based on the data available to date, we can estimate that SAT after COVID-19 vaccination is rare. In the context of vaccine safety monitoring, clinicians should be aware that SAT might be a probably adverse effect of COVID-19 vaccines, especially among patients presenting with neck pain following a SARS-CoV-2 vaccination. Further study is recommended to investigate the possible predisposing factors to developing SAT after receiving the COVID-19 vaccine.
Footnotes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Supplementary materials are available at Clinical and Experimental Vaccine Research website (http://www.ecevr.org).
Previously reported cases of subacute thyroiditis after the coronavirus disease 2019 vaccination
References
- 1.National Health Service. Coronavirus (COVID-19) vaccines side effects and safety [Internet] London: National Health Service; c2022. [cited 2022 Jan 18]. Available from: https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/safety-and-side-effects/ [Google Scholar]
- 2.Siolos A, Gartzonika K, Tigas S. Thyroiditis following vaccination against COVID-19: report of two cases and review of the literature. Metabol Open. 2021;12:100136. doi: 10.1016/j.metop.2021.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatzi S, Karampela A, Spiliopoulou C, Boutzios G. Subacute thyroiditis after SARS-CoV-2 vaccination: a report of two sisters and summary of the literature. Hormones (Athens) 2022;21:177–179. doi: 10.1007/s42000-021-00332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishihara E, Ohye H, Amino N, et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med. 2008;47:725–729. doi: 10.2169/internalmedicine.47.0740. [DOI] [PubMed] [Google Scholar]
- 5.Domin R, Szczepanek-Parulska E, Dadej D, Ruchala M. Subacute thyroiditis: literature overview and COVID-19. J Med Sci. 2020;89:e472 [Google Scholar]
- 6.Khatri A, Charlap E, Kim A. Subacute thyroiditis from COVID-19 infection: a case report and review of literature. Eur Thyroid J. 2021;9:324–328. doi: 10.1159/000511872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brancatella A, Ricci D, Viola N, Sgro D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. 2020;105:dgaa276. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brancatella A, Viola N, Rutigliano G, Sgro D, Santini F, Latrofa F. Subacute thyroiditis during the SARS-CoV-2 pandemic. J Endocr Soc. 2021;5:bvab130. doi: 10.1210/jendso/bvab130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altay FA, Guz G, Altay M. Subacute thyroiditis following seasonal influenza vaccination. Hum Vaccin Immunother. 2016;12:1033–1034. doi: 10.1080/21645515.2015.1117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girgis CM, Russo RR, Benson K. Subacute thyroiditis following the H1N1 vaccine. J Endocrinol Invest. 2010;33:506. doi: 10.1007/BF03346633. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao JY, Hsin SC, Hsieh MC, Hsia PJ, Shin SJ. Subacute thyroiditis following influenza vaccine (Vaxigrip) in a young female. Kaohsiung J Med Sci. 2006;22:297–300. doi: 10.1016/s1607-551x(09)70315-8. [DOI] [PubMed] [Google Scholar]
- 12.Passah A, Arora S, Damle NA, Reddy KS, Khandelwal D, Aggarwal S. Occurrence of subacute thyroiditis following influenza vaccination. Indian J Endocrinol Metab. 2018;22:713–714. doi: 10.4103/ijem.IJEM_237_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bornemann C, Woyk K, Bouter C. Case report: two cases of subacute thyroiditis following SARS-CoV-2 vaccination. Front Med (Lausanne) 2021;8:737142. doi: 10.3389/fmed.2021.737142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oyibo SO. Subacute thyroiditis after receiving the adenovirus-vectored vaccine for coronavirus disease (COVID-19) Cureus. 2021;13:e16045. doi: 10.7759/cureus.16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das L, Bhadada SK, Sood A. Post-COVID-vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J Endocrinol Invest. 2022;45:465–467. doi: 10.1007/s40618-021-01681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Previously reported cases of subacute thyroiditis after the coronavirus disease 2019 vaccination