Abstract
Purpose
Epithelial ovarian carcinoma (EOC) is the most lethal of all human gynecologic malignancies. We previously reported that vaccination of female mice with the extracellular domain of anti-Müllerian hormone receptor II (AMHR2-ED) in complete Freund’s adjuvant (CFA) generates AMHR2-ED specific immunoglobulin G (IgG) that provides prevention and therapy against murine EOCs. Although CFA is the “gold standard” adjuvant in animal studies, it is not approved for human use because it often induces painful granulomas and abscesses. Thus, the objective of this study is to identify an alternative adjuvant to CFA for use in our ovarian cancer vaccine clinical trials.
Materials and Methods
Because it has been used successfully without serious adverse effects in numerous human clinical trials, we selected the IgG-inducing squalene-based adjuvant, AddaVax™, for evaluation of its ability to facilitate vaccine-induced prevention and treatment of EOC in mice. To this end, we immunized female C57BL/6 mice with recombinant mouse AMHR2-ED emulsified with either AddaVax or CFA as adjuvant and compared the results.
Results
We found that formulation of the AMHR2-ED vaccine with AddaVax adjuvant induced high serum titers of IgG and significant inhibition of EOC growth with significantly enhanced overall survival of mice using both prevention and therapeutic protocols. These results were compared favorably with results obtained using CFA as an adjuvant in the AMHR2-ED vaccine.
Conclusion
Our data indicate that the AMHR2-ED vaccine formulated with AddaVax may be used in human clinical trials and thereby serve as a novel and effective way to control human EOC.
Keywords: Ovarian Cancer, Vaccine adjuvants, Anti-Müllerian hormone receptor II, Tumor immunity
Introduction
Epithelial ovarian carcinoma (EOC) is the most lethal of all gynecologic malignancies with postmenopausal women accounting for more than 75% of all cases [1,2]. An array of non-specific symptoms and a lack of effective biomarkers for early detection often result in late diagnoses at advanced disease stages with high rates of disease recurrence and poor prognoses following current standard of care [1,2,3,4,5,6,7]. Thus, there remains an unmet need for more effective ways to control this disease. The induction of ovarian tumor immunity through vaccination is a promising approach and finds support from the increased overall survival observed in patients whose ovarian tumors are infiltrated by T cells [8].
We and others have shown that anti-Müllerian hormone receptor type II (AMHR2) is expressed in approximately 92% of primary EOCs, 78% of borderline malignancies, 77%–86% of non-EOC ovarian tumors, and 56% of malignant ascites from grades III-IV ovarian cancers [9,10,11,12]. AMHR2 is a serine/threonine kinase receptor homologous to type II receptors of the transforming growth factor-beta superfamily [13]. Anti-Müllerian hormone (AMH) is the cognate ligand of AMHR2, and binding of AMH to the extracellular domain of AMHR2 (AMHR2-ED) signals cell cycle arrest and programmed cell death resulting in regression of the Müllerian ducts during male fetal development, as well as regulation of oocyte development and control of ovarian reserve and fertility in adult females [14,15].
In adult women, the longest AMHR2 transcript codes for a 573 amino acid protein expressed exclusively in the ovary and consists of a 403 amino acid cytoplasmic domain (AMHR2-CD) that has kinase activity and a 26 amino acid hydrophobic transmembrane domain, both of which show extra-ovarian expression [16]. The long ovarian-specific transcript also codes for a 127 amino acid ligand-binding extracellular domain (AMHR2-ED) expressed exclusively in the human premenopausal ovary [16], and this expression drops to non-autoimmunogenic levels in postmenopausal ovaries [17]. These ‘retired’ features of AMHR2-ED expression and protein synthesis in normal tissues and its expression in the majority of EOCs have led us to propose that AMHR2-ED vaccination may provide safe and effective prevention of human EOCs [18]. Such prophylactic vaccination for control of EOC would be a relatively non-invasive way to control this disease particularly in high-risk women due to mutations in their BRCA1 and BRCA2 genes [19,20,21] and eventually in postmenopausal women who account for 75% of all cases [22].
To this end, we have previously shown that AMHR2-ED vaccination inhibits the growth of murine EOCs through the helper function of CD4+ T cells that facilitates B cell production of AMHR2-ED-specific immunoglobulin G (IgG) and activation of a Bax/caspase-3 dependent programmed cell death signaling cascade [17]. However, in our prior mouse studies, we used complete Freund’s adjuvant (CFA) in the formulation of our vaccine to generate a robust response to the AMHR2-ED immunogen. Although CFA has long been considered the “gold standard” to which all other adjuvants are compared and has been long considered to be most useful in establishing proof-of-principle in immunologic studies in animals, its use in human vaccination is precluded due to its induction of unresolved chronic granulomas often associated with painful abscess formation [23,24,25]. Thus, we recognized the need to identify an alternative adjuvant to CFA for use in our planned human ovarian cancer vaccine clinical trials.
To address this adjuvant issue, we selected the squalene-based adjuvant, AddaVax, for evaluation as a potential substitute for CFA because it was developed for inducing high titer IgG responses [26]. AddaVax is a sterile oil-in-water nano-emulsion of sorbitan trioleate in squalene oil with Tween 80 in sodium citrate buffer. It is based on the formulation of MF59, a squalene-based adjuvant that was licensed in Europe by Novartis International AG and first used in clinical trials 25 years ago to vaccinate over 20 million people against influenza [27,28]. AddaVax and MF59 are virtually identical but AddaVax has 5% squalene oil instead of the 4.3% found in MF59 [27]. Squalene-based emulsions consistently induce the activation of CD4+ T cells resulting in high antibody titers [26,29] and its immune inducing properties are due to a depot effect that enhances long-term persistence of antigen, recruitment of antigen-presenting cells at the site of injection, activation of CD4+ helper T cells, and subsequent production of IgG by B cells [27]. However, squalene oil is more readily metabolizable than paraffin oil used in the formulation of CFA resulting in a safety profile that precludes the formation of granulomas and has long been acceptable to the US Food and Drug Administration (FDA) for use in human clinical trials [29].
We found that a single AMHR2-ED vaccination using AddaVax as adjuvant compared favorably to the use of CFA as an adjuvant in inducing high titer IgG responses against AMHR2-ED and in mediating effective prevention and treatment of murine EOCs [17]. Our results indicate that AddaVax can substitute for CFA as adjuvant for determining efficacy of our AMHR2-ED ovarian cancer vaccine in human clinical trials.
Materials and Methods
Generation of recombinant mouse AMHR2-ED
The DNA coding sequence of the entire 125 amino acids of the mature native AMHR2-ED lacking the signal peptide was used to generate the expression construct as previously described [17] (National Center for Biotechnology Information reference sequence: NM_144547.2; Uniprot Q8K592). To optimize protein folding and enhance overall yield, substitutions for native codon sequences were made (Dapcel, Cleveland, OH, USA), and the optimized DNA was synthesized de novo to include an N-terminal methionine immediately followed by a FLAG tag, and a C-terminal 6xHis tag. The 6xHis-tagged AMHR2-ED was purified under denaturing conditions using nickel-nitrilotriacetic acid affinity chromatography (Qiagen, Valencia, CA, USA). Prior to use in vitro, the 6xHis-tagged AMHR2-ED was further purified by reverse-phase high performance liquid chromatography. Levels of endotoxin were determined to be <0.05 endotoxin units (<5 pg) per mg recombinant protein as previously described [17].
Mice and murine EOC cell lines
TgMISIIR-Tag (DR26) and TgMISIIR-Tag (low) transgenic mice were generously provided by Dr. Denise C. Connolly (Developmental Therapeutics Program, Fox Chase Cancer Center, Philadelphia, PA, USA). Female TgMISIIR-Tag (DR26) transgenic mice develop bilateral autochthonous EOCs due to expression of the large T antigen (Tag) of SV40 under control of the AMHR2 promoter [30]. TgMISIIR-Tag (low) transgenic female mice do not develop autochthonous EOCs due to errant transgene insertion, but these mice are immunologically tolerant to SV40-TAg and effectively serve as histocompatible recipients of transplantable mouse ovarian carcinoma (MOVCAR) cells derived from the ascites fluid of TgMISIIR-Tag (DR26) mice [31]. MOVCAR cells were cultured in DMEM (Media Preparation Core, Cleveland Clinic, Cleveland, OH, USA) supplemented with 5% fetal bovine serum (HyClone, Logan, UT, USA), 5% HEPES buffer (Sigma-Aldrich, St. Louis, MO, USA), 2 mM L-glutamine (Thermo Fisher Scientific, Waltham, MA, USA), and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). Colonies of transgenic mice were established and maintained by breeding male transgenic mice with wild-type syngeneic C57BL/6 females (Jackson Laboratory, Bar Harbor, ME, USA). The MOVCAR cells were used to develop EOCs by subcutaneous injection into histocompatible TgMISIIR-Tag (low) transgenic female mice. ID8 mouse ovarian surface epithelial cells were obtained commercially (Sigma-Aldrich) and cultured in DMEM (Media Preparation Core) containing 4% fetal bovine serum (HyClone), 2 mM L-glutamine (Thermo Fisher Scientific), 1% penicillin/streptomycin (Invitrogen), and insulin-transferrin-sodium selenite media supplement (Sigma-Aldrich). ID8 cells develop EOCs when injected subcutaneously into C57BL/6 female mice [32].
Transplantable ovarian tumors and treatments
MOVCAR cells (4×106 cells) and ID8 cells (5×106 cells) were suspended in phosphate-buffered saline for subcutaneous inoculation in a total volume of 100 µL in the left dorsal flank of female TgMISIIR-Tag (low) and female C57BL/6 mice, respectively. For CFA-based formulation, mice were immunized with a single subcutaneous injection in the right abdominal flank with 200 µL of an emulsion containing 100 µg of recombinant mouse AMHR2-ED in 100 µL of sterile double-distilled deionized water (DDDH2O) and 100 µL of CFA (Difco, Detroit, MI, USA) containing 200 µg of Mycobacterium tuberculosis H37Ra. AddaVax emulsion was purchased from Invivogen (San Diego, CA, USA; catalog #vac-adx-10) and 100 µL was suspended with 100 µL of sterile DDDH2O containing 100 µg of recombinant mouse AMHR2-ED so that the final emulsion volume of 200 µL was injected into the abdominal flanks of female TgMISIIR-Tag (low) or female C57BL/6 mice. For prophylaxis, mice were vaccinated 15 days prior to tumor inoculation. For therapeutic intervention, mice were vaccinated with AMHR2-ED when tumors became palpable. All experiments started when mice were 6–7 weeks old. Tumor growth was assessed regularly using a Vernier caliper, and the endpoint for all experiments involving transplantable tumors was defined by a tumor measurement of 17 mm in any direction.
Immunohistochemistry
CD3+ T cells were immunostained in 5 µm sections of formalin-fixed paraffin-embedded ID8 tumor tissues using a 1:100 dilution of a primary CD3-specific rabbit monoclonal IgG antibody (Abcam, Cambridge, UK; catalog #ab16669) followed by a horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Abcam; catalog #ab214880). CD4+ T cells were immunostained in 5 µm sections of ovarian tumors by incubation with a 1:1,000 dilution of a primary CD4-specific rabbit monoclonal IgG antibody (Abcam; catalog #ab183685), followed by HRP-conjugated goat anti-rabbit IgG secondary antibody (Abcam; catalog #ab214880). Visualization was achieved using the DAB substrate kit (BD Phar-mingen, San Diego, CA, USA; catalog #550880), followed by hematoxylin counterstaining and mounting of sections in mounting medium (Richard-Allan Scientific, Kalamazoo, MI, USA; catalog #4112) for examination by light microscopy.
Real-time quantitative reverse transcription-PCR
Tissues were excised and stored frozen in RNAlater Stabilization Solution (Invitrogen; catalog #AM7021). RNA was extracted from each tissue by homogenization in TRIZOL reagent (Invitrogen: catalog #15596026). Quantitative reverse transcription-polymerase chain reaction (PCR) was performed using SYBR Green PCR mix (Applied Biosystems, Carlsbad, CA, USA; catalog #4309155) with gene-specific primer pairs (Invitrogen) including mouse AMHR2-CD primers: forward, CTGAGCCGCTGTTCCGATTTGA; reverse, ATGTTGGGGCGCTTCCTCTCCT; mouse AMHR2-ED primers: forward, GCGGGGAAGCACAAAGACACT; reverse, CCGGCCATGGGTAAGATTCC; mouse β-actin primers: forward, GGTCATCACTATTGGCAACG; reverse, ACGGATGTCAACGTCACACT. Relative gene expression was determined by normalization of each gene of interest to β-actin expression in each sample.
Enzyme-linked immunosorbent assays
Enzyme-linked immunosorbent assays were performed as previously described [33]. Briefly, serum samples were taken from vaccinated mice 8 weeks after vaccination, and after diluting each serum sample 1:10,000, were added into triplicate wells onto 96-well plates pre-coated with AMHR2-ED or with the control antigens ovalbumin (Sigma-Aldrich; catalog #A7641-250MG), and recombinant mouse β-casein, a protein generated in Escherichia coli in our laboratory in a manner similar to the generation of AMHR2-ED [33]. After completing the incubations at each step, absorbances at 405 nm were determined according to the manufacturer’s instructions. Isotype-specific serum antibody responses to AMHR2-ED at 1:10,000 dilutions were determined according to manufacturer’s instructions using the Mouse Typer Isotyping Panel (Bio-Rad, Hercules, CA, USA; catalog #1722051).
Biostatistical analysis
Differences between mRNA expression levels were compared using the Student t-test and two-way analysis of variance (ANOVA). Differences between tumor growth curves were compared using two-way ANOVA. Differences in mouse survival were determined by log-rank and chi-square using correlated samples. All experiments were repeated at least 3 times independently.
Ethics statement
All protocols for animal research met with the prior approval of the Institutional Animal Care and Use Committee of the Cleveland Clinic (IACUC) in compliance with the Public Health Service policy on humane care and use of laboratory animals.
The issued patents are as follows: Ovarian Cancer Vaccines; U.S. patent no., 11090284; issue date: August 17, 2021; application no., 15/757,151; publication no., 20190134173; and filing date: September 2, 2016.
Results
Domains of mouse AMHR2 and their gene expression in mouse EOCs
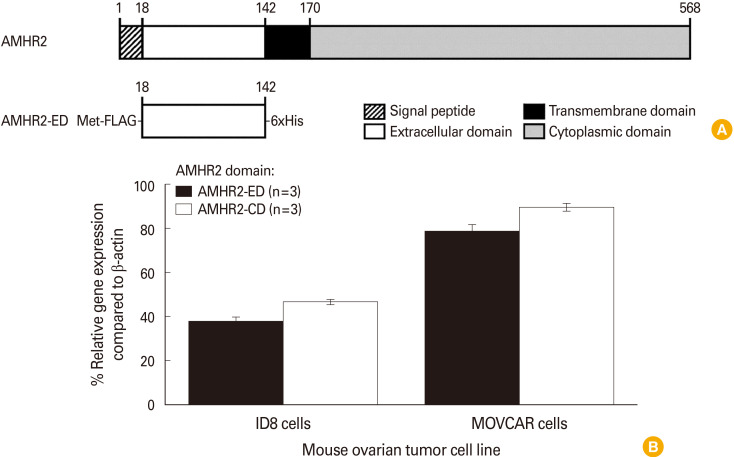
The schematic of various domains of mouse AMHR2, including the signal peptide, the extracellular domain (AMHR2-ED) for binding to the cognate ligand AMH, the transmembrane domain, and the cytoplasmic domain (AMHR2-CD) for mediating the kinase activity is represented (Fig. 1A). The recombinant AMHR2-ED with a FLAG tag at the N-terminus and a 6xHis tag at the C-terminus was generated as previously described [17] and used as our primary immunogen (Fig. 1A, lower panel). We determined the relative gene expression of both domains of AMHR2 (AMHR2-ED and AMHR2-CD) in ID8 and MOVCAR murine EOC cells. We found that although MOVCAR and ID8 cells express high levels of both AMHR2 domains, the expression levels of each domain were consistently about twice as high in MOVCAR cells compared to ID8 cells (Fig. 1B).
Fig. 1. Domains of mouse anti-Müllerian hormone receptor II (AMHR2) and their gene expression in mouse epithelial ovarian carcinomas (EOCs). (A) Depiction of the different domains of the full-length sequence of the mouse AMHR2 protein indicating the signal peptide, the extracellular domain (AMHR2-ED), the transmembrane domain, and the cytoplasmic domain (AMHR2-CD) with amino acid numbers (upper panel), and the recombinant AMHR2-ED used as immunogen in the current study (lower panel) with a FLAG tag at the N-terminus and a 6xHis tag at the C-terminus. (B) Quantitative reverse transcription-polymerase chain reaction analysis of the ID8 and mouse ovarian carcinoma (MOVCAR) EOC cell lines indicating that both cell lines express AMHR2-ED and AMHR2-CD domains, but the expression of each AMHR2 domain in MOVCAR cells was consistently about twice that of the expression levels measured in ID8 cells. Error bars indicate ±standard deviation.
Formulation of the AMHR2-ED vaccine with AddaVax induces high IgG responses
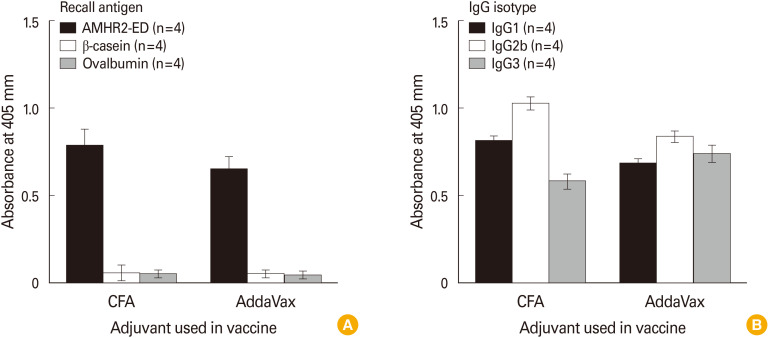
To determine if using AddaVax as adjuvant would induce a robust IgG response to AMHR2-ED, we immunized 6–7-week-old C57BL/6 female mice with 100 µg of recombinant mouse AMHR2-ED using either CFA or AddaVax as adjuvants. Eight weeks after immunization the serum IgG response at 1:10,000 dilution was assessed in response to AMHR2-ED and in response to the irrelevant antigens, ovalbumin and recombinant mouse β-casein. We found that the absorbance obtained using AddaVax as adjuvant was similar to the absorbance obtained using CFA as adjuvant (Fig. 2A). In addition, we found that the IgG isotype profile generated using AddaVax as adjuvant was also similar to that obtained using CFA as adjuvant (Fig. 2B).
Fig. 2. Formulation of the anti-Müllerian hormone receptor II (AMHR2)-extracellular domain (ED) vaccine with AddaVax induces high immunoglobulin G (IgG) responses. Enzyme-linked immunosorbent assay analyses show that the use of AddaVax as adjuvant when immunizing C57BL/6 female mice resulted in (A) an antigen-specific IgG response against the AMHR2-ED immunogen, and (B) an antigen-specific IgG isotype response similar to that obtained using complete Freund’s adjuvant (CFA) as adjuvant. Sera were analyzed 8 weeks after immunization using a 1:10,000 dilution. Error bars indicate ±standard deviation.
AMHR2-ED vaccination using AddaVax as adjuvant is highly effective in preventing the growth of mouse EOCs
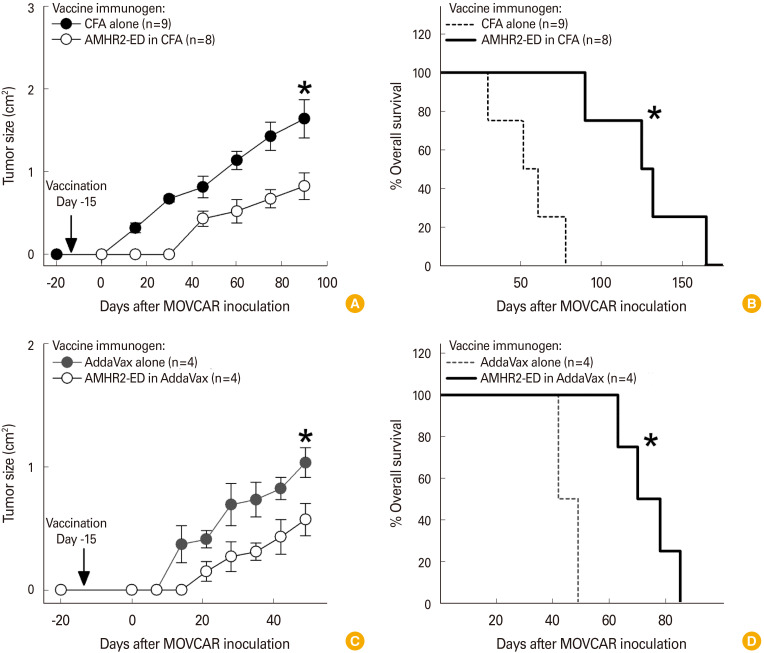
We next examined whether AMHR2-ED vaccination using AddaVax as adjuvant would compare favorably with the inhibition of murine EOC tumor growth induced when AMHR2-ED vaccination incorporated CFA as an adjuvant. We found that prophylactic vaccination against AMHR2-ED in CFA 15 days prior to inoculation of MOVCAR cells into TgMISIIR-Tag (low) female mice significantly inhibited the growth of MOVCAR-derived tumors (p<0.001) (Fig. 3A) and significantly enhanced overall survival (p<0.0001) (Fig. 3B) when compared to vaccination with CFA alone. Similarly, prophylactic vaccination against AMHR2-ED formulated with AddaVax adjuvant 15 days prior to inoculation of MOVCAR cells into TgMISIIR-Tag (low) female mice significantly inhibited the growth of the MOVCAR-derived tumors (p<0.001) (Fig. 3C) and significantly enhanced overall survival (p<0.002) (Fig. 3D) when compared to vaccination with AddaVax adjuvant alone.
Fig. 3. Anti-Müllerian hormone receptor II (AMHR2)-extracellular domain (ED) vaccination using AddaVax as adjuvant is highly effective in preventing the growth of mouse epithelial ovarian carcinomas (EOCs). Prophylactic AMHR2-ED vaccination of mouse ovarian carcinoma (MOVCAR) inoculated TgMISIIR-Tag (low) female mice using complete Freund’s adjuvant (CFA) as adjuvant resulted in (A) significant inhibition of MOVCAR tumor growth and (B) significant enhancement of overall survival. Likewise, prophylactic AMHR2-ED vaccination of MOVCAR inoculated TgMISIIR-Tag (low) female mice using AddaVax as adjuvant resulted in (C) significant inhibition of MOVCAR tumor growth and (D) significant enhancement of overall survival. Error bars indicate ±standard deviation and asterisks indicate significance.
AMHR2-ED vaccination formulated with AddaVax is highly effective in treating mouse EOCs
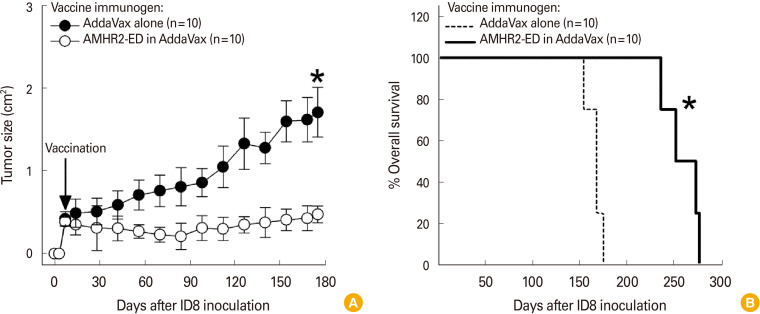
The effectiveness of AddaVax may be most clearly evident in female C57BL/6 mice that were vaccinated with either AddaVax alone or with AMHR2-ED using AddaVax as adjuvant after inoculated ID8 EOC cells became palpable tumors. Such therapeutic vaccination significantly inhibited the growth of the ID8 tumor (p<0.0001) (Fig. 4A) resulting in a significantly enhanced overall survival (p<0.001) (Fig. 4B).
Fig. 4. Anti-Müllerian hormone receptor II (AMHR2)-extracellular domain (ED) vaccination formulated with AddaVax is highly effective in treating mouse epithelial ovarian carcinomas (EOCs). Therapeutic AMHR2-ED vaccination of ID8 inoculated C57BL/6 female mice using AddaVax as adjuvant resulted in (A) significant inhibition of ID8 tumor growth and (B) significant enhancement of overall survival. Error bars indicate ±standard deviation and asterisks indicate significance.
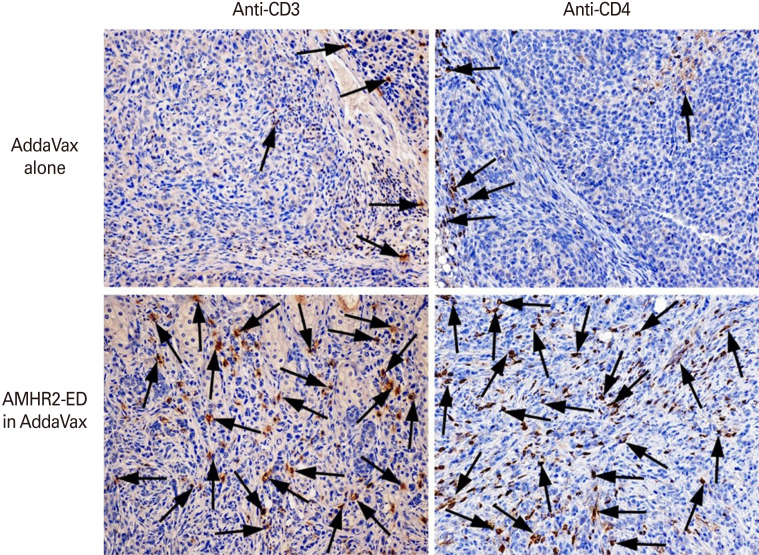
AMHR2-ED vaccination using AddaVax as adjuvant results in infiltration of CD4+ T cells in the murine EOC tumor beds
We next examined the tumor beds of female C57BL/6 mice 175 days after inoculation with ID8 tumor cells and 168 days after treatment of the palpable tumor with the AMHR2-ED vaccine in AddaVax. We observed substantial infiltration of CD3+ (Fig. 5, left lower panel) and CD4+ (Fig. 5, right lower panel) T cells in the tumor beds from mice vaccinated with AMHR2-ED in AddaVax compared to tumor beds from mice vaccinated with AddaVax alone (Fig. 5, corresponding left and right upper panel). These results were similar to our observations from prior studies when CFA was used as adjuvant [17].
Fig. 5. Anti-Müllerian hormone receptor II (AMHR2)-extracellular domain (ED) vaccination using AddaVax as adjuvant results in infiltration of CD4+ T cells in the murine epithelial ovarian carcinoma (EOC) tumor beds. AMHR2-ED vaccination using AddaVax as adjuvant resulted in substantial infiltration of CD3+ (arrows, bottom row, left), and CD4+ (arrows, bottom row, right) T cells compared to corresponding EOC tissues from mice vaccinated with AddaVax alone (arrows, upper row). Tissues are representative of several examined from several different mice (tissue magnification, ×20).
Discussion
The current study shows that formulation of our AMHR2-ED vaccine with AddaVax as adjuvant induces a robust IgG response and inhibits the growth of mouse EOCs in a manner similar to using CFA as adjuvant. Thus, our data support the substitution of AddaVax for CFA as an adjuvant in our planned clinical trials aimed at the prevention of EOC in women at high genetic risk for developing EOC.
Studies have shown that 39%–44% of women with a harmful BRCA1 variant and 11%–17% of women with a harmful BRCA2 variant develop ovarian cancer by 70–80 years of age [19,20,21]. The availability of such high-risk populations of women with a high incidence of EOC and with the greatest need for an EOC preventive vaccine makes it feasible to perform such prevention trials that are very expensive to complete often because of the large numbers of test subjects needed for enrollment and always due to the long-term follow-up needed for establishing safety.
However, there are reasons other than financial feasibility why we will be able to perform EOC prevention trials on women with high genetic risk for this disease. First, prior studies have shown that in normal mouse and human tissues, AMHR2-ED is expressed exclusively in the premenopausal ovary [16,17,18]. Such confined expression precludes the development of systemic autoimmunity following vaccination with AMHR2-ED, and the only oophoritis we observed was in young fertile mice. However, this mild ovarian inflammation was transient and benign because it was observed at 4 months but not at 8 months after vaccination and had absolutely no effect on ovarian function as measured by normal fertility through four complete mating cycles [17]. Thus, AMHR2-ED vaccination proved to be quite safe even in young mice that show ovarian expression of AMHR2-ED.
Second, in a recent prevention study involving women with mutations in their BRCA1 or BRCA2 genes, two prevention options conforming to international guidelines [34,35,36] were offered to each test subject including: (1) risk-reducing salpingo-oophorectomy for women 35 years or older who have completed childbearing or (2) intense surveillance involving transvaginal pelvic ultrasound with serum testing for carcinoma antigen 125 (CA-125; also known as mucin 16 or MUC16) every 6 months starting at age 30 years of age. The vast majority (42 of 53 [79.2%]) of these high-risk women elected intense surveillance over salpingo-oophorectomy [37]. Since most women are averse to surgical prevention strategies and the many adverse events associated with surgical prevention including, but not limited to, instant menopause and the lasting effects of inadequate hormone replacement therapy, we expect that many high-risk women for EOC may be even more interested in intense surveillance rather than the much more invasive salpingo-oophorectomy risk-reducing option if AMHR2-ED vaccination accompanies the surveillance option.
Third, the use of AddaVax as adjuvant in our AMHR2-ED vaccine facilitates obtaining permission from the FDA to perform such EOC prevention clinical trials because the virtually identical MF59 squalene-based adjuvant has been found to have an acceptable safety profile and has been approved for use by the FDA in numerous clinical trials including but certainly not limited to a phase II trial for prevention of cytomegalovirus [38], a phase III trial for prevention of influenza virus [39], and a phase II/III trial for prevention of HIV-1 virus [40]. Thus, we believe that our study forms the basis for successfully obtaining permission from the FDA to use our AMHR2-ED vaccine investigational new drug in human clinical trials for providing better control of EOC.
Footnotes
The vaccine technology discussed in this manuscript has been licensed to Anixa Biosciences Inc. (San Jose, CA). SM, JMJ, and VKT are the inventors on issued and pending patents related to the vaccine technology discussed in this manuscript and may earn royalties for such if the vaccine becomes commercially successful. In addition, SM, JMJ, and VKT have received equity in Anixa Biosciences Inc. in the form of stock options. The manuscript was prepared without any input or coercion whatsoever from the licensee. VS and ND have no conflicts of interest and no financial interest to be gained from the publication of this manuscript.
This study was funded in part from a Cleveland Clinic Velosano Award (SM), a Cleveland Clinic Center of Excellence Award (VKT), a Sponsored Research Grant from Anixa Biosciences Inc. (VKT), and from Philanthropic Funding (VKT).
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2018 [Internet] Bethesda (MD): National Cancer Institute; 2021. [cited 2022 Feb 20]. Available from: https://seer.cancer.gov/csr/1975_2018/ [Google Scholar]
- 3.Pignata S, Pisano C, Di Napoli M, Cecere SC, Tambaro R, Attademo L. Treatment of recurrent epithelial ovarian cancer. Cancer. 2019;125 Suppl 24:4609–4615. doi: 10.1002/cncr.32500. [DOI] [PubMed] [Google Scholar]
- 4.Pfisterer J, Ledermann JA. Management of platinum-sensitive recurrent ovarian cancer. Semin Oncol. 2006;33(2 Suppl 6):S12–S16. doi: 10.1053/j.seminoncol.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Fung-Kee-Fung M, Oliver T, Elit L, Oza A, Hirte HW, Bryson P. Optimal chemotherapy treatment for women with recurrent ovarian cancer. Curr Oncol. 2007;14:195–208. doi: 10.3747/co.2007.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montemorano L, Lightfoot MD, Bixel K. Role of olaparib as maintenance treatment for ovarian cancer: the evidence to date. Onco Targets Ther. 2019;12:11497–11506. doi: 10.2147/OTT.S195552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubalanza K, Konecny GE. Mechanisms of PARP inhibitor resistance in ovarian cancer. Curr Opin Obstet Gynecol. 2020;32:36–41. doi: 10.1097/GCO.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 9.Masiakos PT, MacLaughlin DT, Maheswaran S, et al. Human ovarian cancer, cell lines, and primary ascites cells express the human Mullerian inhibiting substance (MIS) type II receptor, bind, and are responsive to MIS. Clin Cancer Res. 1999;5:3488–3499. [PubMed] [Google Scholar]
- 10.Bakkum-Gamez JN, Aletti G, Lewis KA, et al. Mullerian inhibiting substance type II receptor (MISIIR): a novel, tissue-specific target expressed by gynecologic cancers. Gynecol Oncol. 2008;108:141–148. doi: 10.1016/j.ygyno.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Song JY, Chen KY, Kim SY, et al. The expression of Müllerian inhibiting substance/anti-Mullerian hormone type II receptor protein and mRNA in benign, borderline and malignant ovarian neoplasia. Int J Oncol. 2009;34:1583–1591. doi: 10.3892/ijo_00000288. [DOI] [PubMed] [Google Scholar]
- 12.Mazumder S, Swank V, Komar AA, Johnson JM, Tuohy VK. Immunotherapy of ovarian cancer with a monoclonal antibody specific for the extracellular domain of anti-Mullerian hormone receptor II. Oncotarget. 2020;11:1894–1910. doi: 10.18632/oncotarget.27585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristensen SG, Andersen K, Clement CA, Franks S, Hardy K, Andersen CY. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol Hum Reprod. 2014;20:293–308. doi: 10.1093/molehr/gat089. [DOI] [PubMed] [Google Scholar]
- 14.Josso N, di Clemente N, Gouedard L. Anti-Mullerian hormone and its receptors. Mol Cell Endocrinol. 2001;179:25–32. doi: 10.1016/s0303-7207(01)00467-1. [DOI] [PubMed] [Google Scholar]
- 15.Gruijters MJ, Visser JA, Durlinger AL, Themmen AP. Anti-Mullerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003;211:85–90. doi: 10.1016/j.mce.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 17.Mazumder S, Johnson JM, Swank V, et al. Primary immunoprevention of epithelial ovarian carcinoma by vaccination against the extracellular domain of anti-Mullerian hormone receptor II. Cancer Prev Res (Phila) 2017;10:612–624. doi: 10.1158/1940-6207.CAPR-17-0154. [DOI] [PubMed] [Google Scholar]
- 18.Tuohy VK, Johnson JM, Mazumder S. Primary immunoprevention of adult onset cancers by vaccinating against retired tissue-specific self-proteins. Semin Immunol. 2020;47:101392. doi: 10.1016/j.smim.2020.101392. [DOI] [PubMed] [Google Scholar]
- 19.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 22.Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145:124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 23.Chapel HM, August PJ. Report of nine cases of accidental injury to man with Freund’s complete adjuvant. Clin Exp Immunol. 1976;24:538–541. [PMC free article] [PubMed] [Google Scholar]
- 24.Stills HF., Jr Adjuvants and antibody production: dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J. 2005;46:280–293. doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 25.Apostolico Jde S, Lunardelli VA, Coirada FC, Boscardin SB, Rosa DS. Adjuvants: classification, modus operandi, and licensing. J Immunol Res. 2016;2016:1459394. doi: 10.1155/2016/1459394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59: Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 27.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Kommareddy S, Singh M, O’Hagan DT. In: Immunopotentiators in modern vaccines. 2nd ed. Schijns VE, O’Hagan DT, editors. Amsterdam: Academic Press; 2017. MF59: A safe and potent adjuvant for human use; pp. 154–196. [Google Scholar]
- 29.Calabro S, Tritto E, Pezzotti A, et al. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine. 2013;31:3363–3369. doi: 10.1016/j.vaccine.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Connolly DC, Bao R, Nikitin AY, et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 31.Quinn BA, Xiao F, Bickel L, et al. Development of a syngeneic mouse model of epithelial ovarian cancer. J Ovarian Res. 2010;3:24. doi: 10.1186/1757-2215-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roby KF, Taylor CC, Sweetwood JP, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 33.Sakalar C, Mazumder S, Johnson JM, et al. Regulation of murine ovarian epithelial carcinoma by vaccination against the cytoplasmic domain of anti-Mullerian hormone receptor II. J Immunol Res. 2015;2015:630287. doi: 10.1155/2015/630287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strasser-Weippl K, Goss PE. Suitable trial designs and cohorts for preventive breast cancer agents. Nat Rev Clin Oncol. 2013;10:677–687. doi: 10.1038/nrclinonc.2013.174. [DOI] [PubMed] [Google Scholar]
- 35.Temkin SM, Bergstrom J, Samimi G, Minasian L. Ovarian cancer prevention in high-risk women. Clin Obstet Gynecol. 2017;60:738–757. doi: 10.1097/GRF.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1.2020 [Internet] Plymouth Meeting (PA): National Comprehensive Cancer Network; 2020. [cited 2022 Feb 20]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf . [Google Scholar]
- 37.Manoukian S, Alfieri S, Bianchi E, Peissel B, Azzollini J, Borreani C. Risk-reducing surgery in BRCA1/BRCA2 mutation carriers: are there factors associated with the choice? Psychooncology. 2019;28:1871–1878. doi: 10.1002/pon.5166. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein DI, Munoz FM, Callahan ST, et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: a randomized clinical trial. Vaccine. 2016;34:313–319. doi: 10.1016/j.vaccine.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Essink B, Fierro C, Rosen J, et al. Immunogenicity and safety of MF59-adjuvanted quadrivalent influenza vaccine versus standard and alternate B strain MF59-adjuvanted trivalent influenza vaccines in older adults. Vaccine. 2020;38:242–250. doi: 10.1016/j.vaccine.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Gray GE, Bekker LG, Laher F, et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N Engl J Med. 2021;384:1089–1100. doi: 10.1056/NEJMoa2031499. [DOI] [PMC free article] [PubMed] [Google Scholar]