Abstract
Background
The efficacy difference between the second‐ and third‐generation of anaplastic lymphoma kinase‐tyrosine kinase inhibitors (ALK‐TKIs) after crizotinib failure in advanced ALK‐positive non–small cell lung cancer (NSCLC) has not been clarified. This study evaluates the efficacy of different sequential patterns after crizotinib progression.
Methods
Data of patients who met the study criteria were retrospectively analyzed. The Kaplan–Meier method was used to draw survival curves, log‐rank method was used to compare the differences between groups, and Cox multivariate analysis was used to evaluate the significance of influencing factors.
Results
A total of 128 patients developed disease progression after crizotinib. The overall survival (OS) of 57 patients in the sequential second‐generation ALK‐TKIs group was significantly longer than that of 65 patients with other systemic treatment (58.5 months vs. 33.0 months, p < 0.001); The OS of the direct sequential lorlatinib group was significantly longer than the second‐generation ALK‐TKIs group (114.0 months vs. 58.5 months, p = 0.020). Similarly, of the 48 patients who developed disease progression after first‐ and second‐generation ALK‐TKIs treatment, 16 patients with sequential lorlatinib had significantly longer OS than the others (62.0 months vs. 43.0 months, p = 0.014). The progression‐free survival (PFS) of second‐line and third‐ or later‐line lorlatinib were statistically different (20.0 months vs. 5.5 months, p = 0.011).
Conclusions
The application of next‐generation ALK‐TKIs after crizotinib progression significantly prolonged survival, whereas direct sequencing lorlatinib seemed advantageous. Similarly, lorlatinib also prolonged survival in patients with first‐ and second‐generation ALK‐TKIs failure.
Keywords: anaplastic lymphoma kinase, crizotinib, non–small cell lung cancer, progression, sequential
In this retrospective analysis of 128 patients with crizotinib failure, we found that the application of next‐generation ALK‐TKIs after crizotinib progression significantly prolonged survival, whereas direct sequencing lorlatinib seemed advantageous. In addition, lorlatinib also prolonged survival in patients with first‐ and second‐generation ALK‐TKIs failure.

INTRODUCTION
Lung cancer is associated with high morbidity and mortality, and non–small cell lung cancer (NSCLC) accounts for about 80%–90% of cases of lung cancer. Anaplastic lymphoma kinase (ALK) gene rearrangement was identified in NSCLC in 2007 1 ; the application of tyrosine kinase inhibitors (TKIs) targeting ALK fusion mutation developed rapidly and is proven to have good efficacy and safety in various clinical trials.
Although TKIs demonstrated good tumor response, drug resistance during treatment was a concomitant problem. Despite the good efficacy of crizotinib, disease progression is inevitable at 10.9–12.7 months. 2 , 3 , 4 , 5 , 6 The most common mechanism of drug resistance is acquired point mutation in the ALK gene. The second‐generation ALK‐TKIs, ceritinib, alectinib, and brigatinib, have gradually become the standard treatment after the development of resistance to crizotinib and are even administered as first‐line treatment. 7 , 8 , 9 The third‐generation inhibitor lorlatinib is also proven to overcome the resistance to other ALK‐TKIs. 10 As more next‐generation ALK‐TKIs are being developed and marketed, more options are available for ALK‐positive NSCLC patients in China. Therefore, it is essential to clarify the various drug resistance mechanisms and accordingly choose ALK‐TKIs for each case. 11 , 12
Further, effective sequential administration of ALK‐TKIs to maximize patient survival is a challenge for clinicians. Currently, there are few large clinical trials comparing the efficacy of different next‐generation ALK‐TKIs after crizotinib resistance. The survival outcomes of patients with advanced ALK‐positive NSCLC following administration of different sequential patterns need to be supported by more real‐world data. Therefore, we mainly evaluated the efficacy of sequential ALK inhibitors after crizotinib resistance in clinical practice and analyzed the influence of clinical characteristics and different sequential patterns on overall survival (OS).
METHODS
Patients
Patients with ALK‐positive locally advanced or metastatic NSCLC with disease progression undergoing crizotinib treatment at the Lung Oncology Department of the Fifth Medical Center of The People's Liberation Army (PLA) General Hospital from 2011 to 2019 were screened. Accepted test methods for molecular profiling were fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), real time‐polymerase chain reaction (RT‐PCR), or next generation sequencing (NGS). This study was approved by the Ethics Committee of the Fifth Medical Center of the General Hospital of Chinese People's Liberation Army and was conducted according to the principles of the Declaration of Helsinki. Because this retrospective study did not harm the rights and health of patients, and protected their privacy and personal information, the ethics committee waived the requirement for informed consent.
Efficacy evaluation
The efficacy evaluation of all enrolled patients was based on the response evaluation criteria in solid tumors (RECIST) version 1.1. The objective response rate (ORR) is the percentage of complete response (CR) and partial response (PR) in evaluable cases. The disease control rate (DCR) is the percentage of cases with response (CR + PR) and stable disease (SD) in evaluable cases. Progression‐free survival (PFS) is defined as the time from the start of treatment with an ALK inhibitor to the onset of disease progression or death from any cause. OS was defined as the time from the start of treatment with an ALK inhibitor to death from any cause. The last follow‐up was on October 31, 2021.
Statistical analysis
Descriptive statistics were used to summarize patients' demographic and baseline clinical characteristics. The Kaplan–Meier method was used to draw survival curves. The log‐rank method was used to conduct univariate analysis on the differences in OS between groups. Variables with p < 0.05 and clinical significance were included in Cox multivariate analysis. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 25.0 (IBM). All tests were double tailed when p < 0.05 was considered statistically significant.
RESULTS
Clinical characteristics
There were 171 patients with ALK‐positive advanced NSCLC admitted to our department from 2011 to 2019, among which nine lacked complete case information, 11 were lost to follow‐up, nine were not administered crizotinib, and 14 patients received crizotinib treatment without disease progression. Finally, 128 patients were included in the study (Figure 1). Among them, 12 (9.4%) were over 65 years of age, 67 (52.3%) were women, 33 (25.8%) had a smoking history, 50 (39.1%) had bone metastasis at baseline, 79 (61.7%) received at least one cycle of chemotherapy before crizotinib treatment, and 19 patients (14.8%) received three or more ALK‐TKI treatments (Table 1).
FIGURE 1.
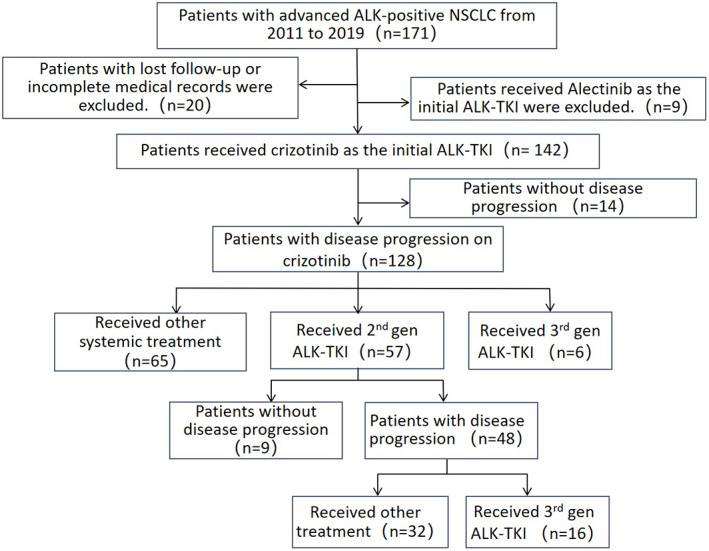
Patient flowchart.
TABLE 1.
Baseline characteristics
| Characteristics | Overall patients (n = 128) |
|---|---|
| Age (y) | |
| Median (range) | 51(23–77) |
| <65 | 116 (90.6) |
| ≥65 | 12 (9.4) |
| Sex, n (%) | |
| Male | 61 (47.7) |
| Female | 67 (52.3) |
| Smoking history, n (%) | |
| Yes | 33 (25.8) |
| No | 95 (74.2) |
| ECOGPS, n (%) | |
| 0 | 4 (3.2) |
| 1 | 122 (95.2) |
| 2 | 2 (1.6) |
| Pathological type, n (%) | |
| Adenocarcinoma | 120 (93.7) |
| Non‐adenocarcinoma | 8 (6.3) |
| Stage at diagnosis, n (%) | |
| III B | 14 (10.9) |
| IV | 114 (89.1) |
| Site of distant metastases, n (%) | |
| Bone | 50 (39.1) |
| Brain | 46 (35.9) |
| Liver | 23 (18.0) |
| Adrenal gland | 9 (7.0) |
| Number of distant metastases, n (%) | |
| <4 | 108 (84.4) |
| ≥4 | 20 (15.6) |
| Testing method | |
| FISH | 62 (48.5) |
| IHC | 22 (17.2) |
| RT‐PCR | 16 (12.5) |
| NGS | 3 (2.3) |
| Unknown | 25 (19.5) |
| ALK‐TKI as first‐line therapy, n (%) | |
| Yes | 49 (38.3) |
| No | 79 (61.7) |
| Number of ALK‐TKI, n (%) | |
| 1 | 65 (50.8) |
| 2 | 44 (34.4) |
| ≥3 | 19 (14.8) |
Abbreviations: y, years; ECOG‐PS, Eastern Cooperative Oncology Group‐performance status; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; RT‐PCR, real time‐polymerase chain reaction; NGS, next generation sequencing; ALK‐ TKI, anaplastic lymphoma kinase‐tyrosine kinase inhibitor.
Clinical efficacy of enrolled patients
After 128 patients were assessed according to RECIST 1.1 evaluation criteria, the ORR of crizotinib was 68.0%, and DCR was 93.0%. By the last follow‐up, disease progression occurred in enrolled patients, and the median PFS (mPFS) of crizotinib was 9.0 months (95% confidence interval [CI], 7.7–10.3 months) (Figure 2(a)).
FIGURE 2.
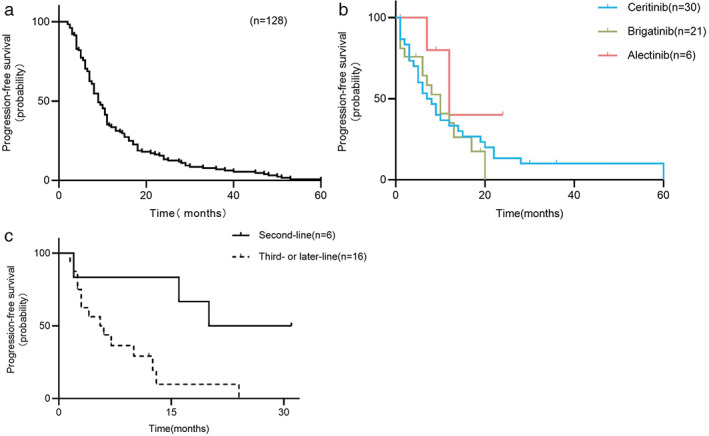
Kaplan–Meier curves for each ALK inhibitor (PFS). (a) The median PFS of crizotinib was 9.0 months (95% CI, 7.7–10.3 months). (b) The median PFS of ceritinib was 7.0 months (95% CI, 3.4–10.6 months); the median PFS of brigatinib was 10.0 months (95% CI, 6.1–13.9 months); the median PFS of alectinib was 12.0 months (95% CI, 4.7–19.3 months). (c) The median PFS of lorlatinib was 12.5 months (second‐line: 20.0 months vs. third‐ or later‐line: 5.5 months, p = 0.011). Abbreviations: ALK, anaplastic lymphoma kinase; PFS, progression‐free survival; CI, confidence interval; p‐values <0.05 were statistically significant
As per the last follow‐up date, 95 patients (74.2%) died. Overall, the median OS (mOS) was 43.0 months (95% CI, 36.9–49.1 months) (Figure 3(a)). Univariate analysis showed that patients age <65 years, without bone metastasis at baseline, and undergoing next‐generation ALK inhibitors had longer OS than other patients. Cox multivariate analysis showed that no smoking habit (hazard ratio [HR], 0.494; 95% CI, 0.258–0.946; p = 0.034), no bone metastasis (HR, 0.502; 95% CI, 0.295–0.854; p = 0.011), administration of second‐generation ALK inhibitors (HR, 0.584; 95% CI, 0.369–0.922; p = 0.021) and third‐generation ALK inhibitors (HR, 0.250; 95% CI, 0.115–0.547; p = 0.001) were related to longer OS (Table 2).
FIGURE 3.
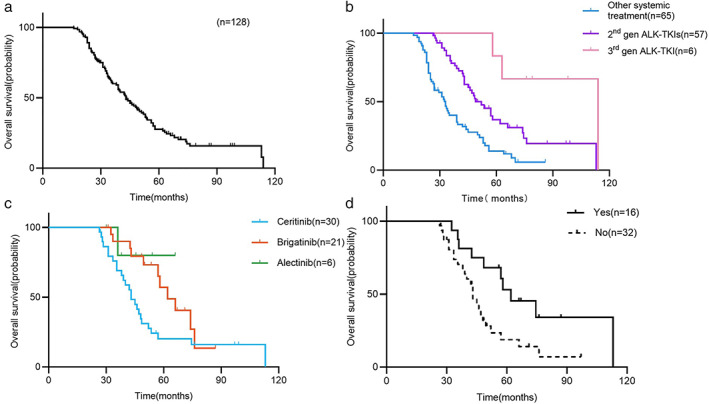
Kaplan–Meier curves for all patients and different sequential patterns after crizotinib progression (OS). (a) The median OS of 128 patients: 43.0 months (95% CI, 36.9–49.1 months). (b) Received other systemic treatment: 33.0 months (95% CI, 28.9–37.2 months); received the 2nd gen ALK‐TKI: 58.5 months (95% CI, 45.6–71.4 months); received the 3rd gen ALK‐TKI: 114.0 months (NR). (c) The median OS of ceritinib was 43.0 months (95% CI, 37.7–48.3 months); the median OS of brigatinib was 62.0 months (95% CI, 49.1–74.9 months); the median OS of alectinib was not reached. (d) The median OS with or without lorlatinib after first‐ and second‐generation drug resistance: 62.0 months versus 43.0 months, p = 0.014. Abbreviations: OS, overall survival; CI, confidence interval; 2nd gen, second generation; 3rd gen, third generation; ALK‐TKI, anaplastic lymphoma kinase‐tyrosine kinase inhibitor; NR, not reached; p‐values <0.05 were statistically significant
TABLE 2.
Cox multivariate analysis of overall survival of all patients (n = 128)
| Variables | Log‐rank test | Multivariate analysis | ||
|---|---|---|---|---|
| HR | 95% CI | p‐Value | ||
| Sex (male vs. female) | 0.850 | |||
| Age (≥65 y vs. <65 y) | 0.037 | |||
| Smoking history (yes vs. no) | 0.160 | 2.025 | 1.057–3.881 | 0.034 |
| Adenocarcinoma (yes vs. no) | 0.449 | |||
| Stage at diagnosis (IV vs. III) | 0.169 | |||
| Number of distant metastases (≥4 vs. <4) | 0.818 | |||
| Bone metastasis (yes vs. no) | 0.019 | 1.994 | 1.171–3.394 | 0.011 |
| Brain metastasis (yes vs. no) | 0.214 | |||
| Liver metastasis (yes vs. no) | 0.910 | |||
| Adrenal gland metastasis (yes vs. no) | 0.093 | |||
| ALK‐TKI as first‐line therapy (yes vs. no) | 0.090 | |||
| Received second‐generation ALK‐TKI (yes vs. no) | 0.003 | 0.691 | 0.369–0.992 | 0.021 |
| Received third‐generation ALK‐TKI (yes vs. no) | <0.001 | 0.250 | 0.115–0.547 | 0.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval; p‐values <0.05 were statistically significant; ALK‐ TKI, anaplastic lymphoma kinase‐tyrosine kinase inhibitor.
Efficacy of different sequential patterns in crizotinib‐resistant patients
Overall survival of different treatment patterns
In this study, 128 patients developed disease progression after crizotinib treatment, the mOS of 65 patients undergoing other systemic therapy was 33.0 months (95% CI, 28.9–37.2 months), and that of 57 patients administered sequential second‐generation ALK‐TKIs was 58.5 months (95% CI, 45.6–71.4 months). Further, mOS was 114.0 months (not reached [NR]) in six patients directly undergoing sequential third‐generation ALK‐TKI lorlatinib (Figure 3(b)).
Pairwise comparison between groups showed that the OS of sequential second‐generation ALK‐TKIs group was significantly longer than that of other systemic treatment group (p < 0.001); the OS of the directly sequential lorlatinib group was significantly longer than that of the sequential second‐generation ALK‐TKIs group (p = 0.020).
Differences in efficacy among second‐generation ALK‐TKIs
Fifty‐seven patients were sequentially treated with second‐generation ALK‐TKIs after crizotinib resistance. Among them, 30 ceritinib patients had the mPFS of 7.0 months (95% CI, 3.4–10.6 months) and mOS of 43.0 months (95% CI, 37.7–48.3 months). The mPFS and mOS of 21 patients with brigatinib were 10.0 months (95% CI, 6.1–13.9 months) and 62.0 months (95% CI, 49.1–74.9 months), respectively. The mPFS of six alectinib patients was 12.0 months (95% CI, 4.7–19.3 months) and mOS was not reached (only one patient died) (Figures 2(b) and 3(c)). Pairwise comparison between groups showed that OS of the sequential brigatinib group was significantly longer than that of the ceritinib group (p = 0.034), and there was no statistically significant difference among the other groups.
Differences in the efficacy of lorlatinib at different lines
Forty‐eight patients developed disease progression after first‐ and second‐generation ALK‐TKIs therapy, 16 of whom were sequentially treated with lorlatinib. Subgroup analysis showed statistically significant differences in OS with or without lorlatinib after first‐ and second‐generation drug resistance (62.0 months vs. 43.0 months, p = 0.014) (Figure 3(d)). Of the enrolled patients, 22 were administered lorlatinib. In addition to the 16 above‐mentioned patients, six patients were directly sequential with lorlatinib after crizotinib progression. Subgroup analysis showed that different lines of lorlatinib treatment had a significant effect on the PFS (second‐line: 20.0 months vs. third‐line or later: 5.5 months, p = 0.011) (Figure 2(c)). The specific treatment and PFS of patients who received lorlatinib are shown in Figure 4.
FIGURE 4.
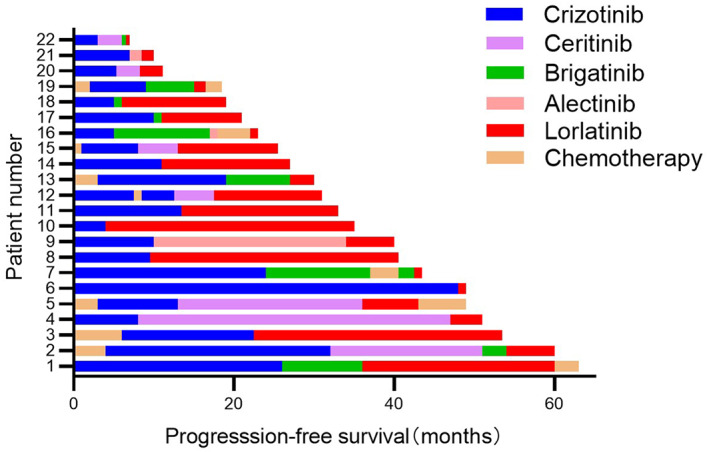
The specific treatment and progression‐free survival for patients who received lorlatinib.
DISCUSSION
To investigate the difference in survival of patients with advanced ALK‐positive NSCLC after crizotinib progression under different treatment patterns, we retrospectively analyzed the clinical data of 128 patients who received crizotinib as initial ALK‐TKI and demonstrated the clinical outcomes of different sequential patterns following crizotinib resistance.
Crizotinib was the only available ALK inhibitor before the second‐generation ALK‐TKIs was approved in China in December 2018; therefore, all patients in this study received crizotinib as the initial ALK‐TKI. Crizotinib was proven effective in the PROFILE series, and the mOS of 43.0 months in this study. Similarly, a previous study was reported the mOS of 48 months in the 121 patients treated with ALK‐TKIs. 13 , 14 Correlation analysis of clinical characteristics and efficacy of patients enrolled in the study showed that smoking history and bone metastasis at baseline had poor OS. The presence of brain and liver metastases at baseline and the number of metastases had no significant impact on OS, which was considered due partly to better control of multiple sites of metastases by the next‐generation of ALK inhibitors, and partly because of the small number of cases or local therapies. Ceritinib has a good effect on the liver, bone, and brain metastases in patients with resistance to crizotinib. 15 , 16 , 17 Alectinib, brigatinib, and lorlatinib showed good intracranial response rates in the population treated with crizotinib. 10 , 18 , 19 , 20
After crizotinib resistance, survival was significantly better in 63 patients who were sequentially treated with other ALK‐TKIs than in 65 patients who were treated with other systemic therapies. Of these, the survival following treatment with second‐line directly sequential lorlatinib was significantly longer than with second‐generation ALK inhibitors. Previous real‐world analysis showed that the 5‐year OS rate of second‐line application of lorlatinib could reach 81.8%. 21 Although there were only six patients administered lorlatinib after crizotinib in this study, the long‐term survival advantage of this prioritized lorlatinib pattern requires further investigation. Therefore, some randomized double‐blind clinical trials should be conducted analyzing ALK‐TKI administration after crizotinib resistance. Additionally, 16 of 48 patients with first‐and second‐generation ALK inhibitor progression had significantly longer survival with sequential lorlatinib. Some prospective studies showed good clinical efficacy of lorlatinib in second‐generation ALK‐TKIs resistant patients. 22 , 23 , 24 , 25 Previous real‐world studies in Japan (WJOG9516L) and France (IFCT‐1302 CLINALK) demonstrated the importance of sequential therapy. 26 , 27 However, the third‐generation ALK‐TKI still cannot overcome partial resistance mechanisms of second‐generation drugs. Therefore, more accurate genetic sequencing after ALK‐TKI resistance and development of new drugs against more resistance mechanisms are essential.
Until the next‐generation ALK‐TKI was recommended as a first‐line priority, sequential second‐generation ALK inhibitors following crizotinib failure was the standard mode of treatment. However, the efficacy of the sequential second‐generation ALK inhibitors varied between them. Randomized double‐blind trials comparing the efficacy of second‐generation ALK‐TKIs in crizotinib‐resistant patients with advanced ALK‐positive NSCLC are still lacking. Intergroup comparisons in this study showed that OS of the 21 sequential brigatinib patients was significantly longer than that of the 30 sequential ceritinib patients (intergroup comparisons with the alectinib group were not performed because only one patient died). However, a deeper look at the treatment after progression in the two groups showed that nine patients (42.9%) in the brigatinib group were subsequently treated with lorlatinib, compared with only five patients (16.7%) in the ceritinib group. Confounding factors for survival intervention cannot be completely excluded in retrospective analysis. Therefore, whether there is a difference in the survival following crizotinib resistance sequentially with different second‐generation ALK‐TKIs needs to be further verified by high‐standard clinical trials.
In recent years, the second‐generation ALK‐TKI has become the first‐line drug of choice for advanced ALK‐positive NSCLC. However, whether the PFS advantage of the next‐generation drugs can be translated into OS advantage remains to be verified. OS data from the recently published J‐Alex study in Japan showed no significant difference in 5‐year OS rates between patients receiving first‐line treatment with alectinib and crizotinib (60.85% vs. 64.11%). 28 Unlike the ALEX study, 29 the J‐Alex study allowed patients in the crizotinib group to switch to alectinib group before disease progression (such patients accounted for 78.8%), which was one of the reasons for the similar 5‐year OS rates of the two drugs. Further, the first‐line treatment with lorlatinib in the CROWN study yielded unexpected results. 30
Our study has several limitations. This was a single‐center, retrospective study, with limited number of patients in each group and inevitable population bias. Additionally, the follow‐up time was not sufficiently long, and the follow‐up treatment and OS data of the enrolled patients need further improvement. However, dynamic circulating tumor DNA (ctDNA) detection of 35 patients receiving ALK‐TKIs of various generations is being conducted in our follow‐up study, which may provide further important information. The purpose of the follow‐up study is to further explore the correlation between genomic characteristics and the efficacy of ALK‐TKI before and after treatment and to clarify the mechanism of resistance to ALK‐TKIs among Chinese patients with advanced ALK‐positive NSCLC. Thereafter, a preliminary profile of genomic cloning evolution will be drawn.
In conclusion, through preliminary analysis of real‐world data, we increased our understanding of the clinical efficacy and factors influencing OS following administration of various generations of ALK inhibitors. The application of next‐generation ALK‐TKI after crizotinib failure significantly prolonged survival and direct sequencing lorlatinib seemed advantageous. Similarly, lorlatinib also prolonged survival in patients with first‐ and second‐generation ALK‐TKIs failure. The results of dynamic ctDNA molecular variation characteristics are expected to help develop precise treatments for advanced NSCLC.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
We thank the patients and their families for participating in the survey. This work was supported by the Chinese National Instrumentation Program (2011YQ170067) and Beijing Municipal Science and Technology Commission, PR China (Z181100001718074). We would like to thank Editage (https://www.editage.com) for their writing support.
Ma X, Yang S, Zhang K, Xu J, Lv P, Gao H, et al. Efficacy of different sequential patterns after crizotinib progression in advanced anaplastic lymphoma kinase‐positive non–small cell lung cancer. Thorac Cancer. 2022;13(12):1788–1794. 10.1111/1759-7714.14455
Funding informationThis work was supported by the Chinese National Instrumentation Program (2011YQ170067) and Beijing Municipal Science and Technology Commission, PR China (Z181100001718074).
Xiya Ma and Shaoxing Yang contributed equally to this work as first co‐authors.
REFERENCES
- 1. Shaw AT, Yeap BY, Mino‐Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol. 2009;27:4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crino L, Kim D, Riely G, et al. Initial phase II results with crizotinib in advanced ALK‐positive non‐small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29:7514. [Google Scholar]
- 3. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371:2167–77. [DOI] [PubMed] [Google Scholar]
- 4. Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al. Results of PROFILE 1029, a phase III comparison of first‐line Crizotinib versus chemotherapy in east Asian patients with ALK‐positive advanced non‐small cell lung cancer. J Thorac Oncol. 2018;13:1539–48. [DOI] [PubMed] [Google Scholar]
- 5. Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK‐rearranged non small‐cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Selvaggi G, Wakelee HA, Mok T, Wu YL, Reck M, Chiappori A, et al. ID: 1882 phase III randomized study of ensartinib vs crizotinib in anaplastic lymphoma kinase (ALK) positive NSCLC patients: eXalt3. J Thor Oncol. 2020;15:e41–2. [Google Scholar]
- 7. Gainor JF, Tan DS, De Pas T, et al. Progression‐free and overall survival in ALK‐positive NSCLC patients treated with sequential crizotinib and ceritinib. Clin Cancer Res. 2015;21:2745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib‐refractory ALK‐rearranged non‐small‐cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661–8. [DOI] [PubMed] [Google Scholar]
- 9. Gettinger SN, Bazhenova LA, Langer CJ, Salgia R, Gold KA, Rosell R, et al. Activity and safety of brigatinib in ALK‐rearranged non‐small‐cell lung cancer and other malignancies: a single‐arm, open‐label, phase 1/2 trial. Lancet Oncol. 2016;17:1683–96. [DOI] [PubMed] [Google Scholar]
- 10. Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in patients with ALK‐positive non‐small‐cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–67. [DOI] [PubMed] [Google Scholar]
- 11. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular mechanisms of resistance to first‐ and second‐generation ALK inhibitors in ALK‐rearranged lung cancer. Cancer Discov. 2016;6:1118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Addeo A, Tabbo F, Robinson T, Buffoni L, Novello S. Precision medicine in ALK rearranged NSCLC: a rapidly evolving scenario. Crit Rev Oncol Hematol. 2018;122:150–6. [DOI] [PubMed] [Google Scholar]
- 13. Britschgi C, Addeo A, Rechsteiner M, Delaloye R, Früh M, Metro G, et al. Real‐world treatment patterns and survival outcome in advanced anaplastic lymphoma kinase (ALK) rearranged non‐small‐cell lung cancer patients. Front Oncol. 2020;10:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu C, Yu H, Long Q, Chen H, Li Y, Zhao W, et al. Real‐world experience of crizotinib in 104 patients with ALK rearrangement non‐small‐cell lung cancer in a single Chinese cancer center. Front Oncol. 2019;9:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bendaly E, Dalal AA, Culver K, Galebach P, Bocharova I, Foster R, et al. Treatment patterns and early outcomes of ALK‐positive non‐small cell lung cancer patients receiving Ceritinib: a chart review study. Adv Ther. 2017;34:1145–56. [DOI] [PubMed] [Google Scholar]
- 16. Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐1): updated results from the multicentre, open‐label, phase 1 trial. Lancet Oncol. 2016;17:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crino L, Ahn MJ, De Marinis F, et al. Multicenter phase II study of whole‐body and intracranial activity with Ceritinib in patients with ALK‐rearranged non‐small‐cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND‐2. J Clin Oncol. 2016;34:2866–73. [DOI] [PubMed] [Google Scholar]
- 18. Gadgeel SM, Shaw AT, Govindan R, Gandhi L, Socinski MA, Camidge DR, et al. Pooled analysis of CNS response to Alectinib in two studies of pretreated patients with ALK‐positive non‐small‐cell lung cancer. J Clin Oncol. 2016;34:4079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim DW, Tiseo M, Ahn MJ, Reckamp KL, Hansen KH, Kim SW, et al. Brigatinib in patients with crizotinib‐refractory anaplastic lymphoma kinase‐positive non‐small‐cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017;35:2490–8. [DOI] [PubMed] [Google Scholar]
- 20. Felip E, Shaw AT, Bearz A, Camidge DR, Solomon BJ, Bauman JR, et al. Intracranial and extracranial efficacy of lorlatinib in patients with ALK‐positive non‐small‐cell lung cancer previously treated with second‐generation ALK TKIs. Ann Oncol. 2021;32:620–30. [DOI] [PubMed] [Google Scholar]
- 21. Zhu VW, Lin YT, Kim DW, Loong HH, Nagasaka M, To H, et al. An international real‐world analysis of the efficacy and safety of lorlatinib through early or expanded access programs in patients with tyrosine kinase inhibitor‐refractory ALK‐positive or ROS1‐positive NSCLC. J Thorac Oncol. 2020;15:1484–96. [DOI] [PubMed] [Google Scholar]
- 22. Orlov SV, Iyevleva AG, Filippova EA, Lozhkina AM, Odintsova SV, Sokolova TN, et al. Efficacy of lorlatinib in lung carcinomas carrying distinct ALK translocation variants: the results of a single‐center study. Transl Oncol. 2021;14:101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hochmair MJ, Fabikan H, Illini O, Weinlinger C, Setinek U, Krenbek D, et al. Later‐line treatment with Lorlatinib in ALK‐ and ROS1‐rearrangement‐positive NSCLC: a retrospective, multicenter analysis. Pharmaceuticals (Basel). 2020;13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaw AT, Felip E, Bauer TM, Besse B, Navarro A, Postel‐Vinay S, et al. Lorlatinib in non‐small‐cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open‐label, single‐arm first‐in‐man phase 1 trial. Lancet Oncol. 2017;18:1590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee J, Sun JM, Lee SH, Ahn JS, Park K, Choi YL, et al. Efficacy and safety of Lorlatinib in Korean non‐small‐cell lung cancer patients with ALK or ROS1 rearrangement whose disease failed to respond to a previous tyrosine kinase inhibitor. Clin Lung Cancer. 2019;20:215–21. [DOI] [PubMed] [Google Scholar]
- 26. Ito K, Yamanaka T, Hayashi H, Hattori Y, Nishino K, Kobayashi H, et al. Sequential therapy of crizotinib followed by alectinib for non‐small cell lung cancer harbouring anaplastic lymphoma kinase rearrangement (WJOG9516L): a multicenter retrospective cohort study. Eur J Cancer. 2021;145:183–93. [DOI] [PubMed] [Google Scholar]
- 27. Duruisseaux M, Besse B, Cadranel J, Pérol M, Mennecier B, Bigay‐Game L, et al. Overall survival with crizotinib and next‐generation ALK inhibitors in ALK‐positive non‐small‐cell lung cancer (IFCT‐1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;8:21903–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshioka H, Hida T, Nokihara H, Morise M, Kim YH, Azuma K, et al. Final OS analysis from the phase III j‐alex study of alectinib (ALC) versus crizotinib (CRZ) in Japanese ALK‐inhibitor naive ALK‐positive non‐small cell lung cancer (ALK+ NSCLC). J Clin Oncol. 2021;39(15_suppl):9022. 10.1200/JCO.2021.39.15_suppl.9022 [DOI] [Google Scholar]
- 29. Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. Updated overall survival and final progression‐free survival data for patients with treatment‐naive advanced ALK‐positive non‐small‐cell lung cancer in the ALEX study. Ann Oncol. 2020;31:1056–64. [DOI] [PubMed] [Google Scholar]
- 30. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First‐line Lorlatinib or crizotinib in advanced ALK‐positive lung cancer. N Engl J Med. 2020;383:2018–29. [DOI] [PubMed] [Google Scholar]


