Abstract
Studies of hemispheric specialization have traditionally cast the left hemisphere as specialized for language and the right hemisphere for spatial function. Much of the supporting evidence for this separation of function comes from studies of healthy adults and those who have sustained lesions to the right or left hemisphere. However, we know little about the developmental origins of lateralization. Recent evidence suggests that the young brain represents language bilaterally, with 4–6 year-olds activating the left-hemisphere regions known to support language in adults as well as homotopic regions in the right hemisphere. This bilateral pattern changes over development, converging on left-hemispheric activation in late childhood. In the present study, we ask whether this same developmental trajectory is observed in a spatial task that is strongly right-lateralized in adults—the line bisection (or “Landmark”) task. We examined fMRI activation among children ages 5–11 as they were asked to judge which end of a bisected vertical line was longer. We found that young children showed bilateral activation, with activation in the same areas of the right hemisphere as has been shown among adults, as well as in the left hemisphere homotopic regions. By age 10, activation was right-lateralized. This strongly resembles the developmental trajectory for language, moving from bilateral to lateralized activation. We discuss potential underlying mechanisms and suggest that understanding the development of lateralization for a range of cognitive functions can play a crucial role in understanding general principles of how and why the brain comes to lateralize certain functions.
Keywords: child development, visual-spatial functions, fMRI, line bisection task, landmark task, brain lateralization, parietal lobe
Introduction
Classically, it has been assumed that the two hemispheres of the brain are specialized for different functions: the left hemisphere for language and the right hemisphere for spatial functions (Broca, 1861; Wernicke, 1874; Bogen & Gazzaniga, 1965; Lenneberg, 1967; Sperry, 1969; Teuber, 1974; Hugdahl & Westerhausen, 2010). Abundant research has confirmed that language is, indeed, strongly left-lateralized among healthy adults. A range of research has also supported the idea that spatial functions are right-lateralized in adulthood. For example, adult patients with right-hemisphere lesions (most often in the parietal lobe) often show striking impairments in the visual-spatial domain, including hemispatial neglect (Bisiach & Luzzatti, 1978; Vallar & Perani, 1986) and difficulties in carrying out visual-spatial construction tasks (Hecaen et al., 1956). Additional evidence suggests that individuals who have had perinatal strokes to the right hemisphere show poorer outcomes in spatial functioning than those who have had left hemisphere strokes (Stiles et al., 2012). Furthermore, individuals with left hemisphere perinatal strokes may show deficits in spatial tasks when language is re-organized to the right hemisphere, possibly compromising right-localized spatial functions (Lidzba et al., 2006).
The idea that the brain’s hemispheres exhibit specialization of function is rooted in the general hypothesis that it is most efficient for each hemisphere to specialize in different kinds of processing (Levy, 1977; Vallortigara, 2006; Cai et al., 2013; Gerritts et al., 2020). In the domain of language, some have proposed that small biases in processing preferences may lead to larger system-wide preferences, with the right hemisphere biased towards processing characteristics of pitch (Zatorre et al., 1992; Zatorre & Belin, 2001) or more generally, aspects of the speech signal that span larger temporal windows (Poeppel, 2003). These initial biases could then lead to the division of labor between phonetics (typically left-lateralized) and prosody (typically right-lateralized; Zatorre & Gandour, 2007). In the domain of space, analogous proposals have been offered. For example, Ivry and Robertson (1998) proposed that the right hemisphere is biased to process low frequency visual information, leading to ‘global’ spatial processing preferences, while the left hemisphere is biased to process high frequency information, leading to ‘local’ processing preferences (see also Kosslyn et al., 1989).
In particular, there is one experimental paradigm that provides an abundance of evidence supporting the right hemisphere’s specialization for spatial ability: This is the classic task known as the line bisection task and its perceptual version, the “Landmark Task” (Fink et al., 2000). Traditionally used for assessing hemispatial neglect in clinical settings (e.g., Karnath, 1988; Jansen et al., 2005; Vallar & Perani, 1986), this task has also been widely used to study lateralization of visual-spatial function in healthy adults. In the classic line bisection task, participants are asked to draw a hashmark at the perceived center of a horizontal line. In the corresponding Landmark Task, participants are asked to judge whether a short vertical line correctly bisects a horizontal line or whether it deviates from the midpoint. People with disrupted right parietal function (either due to lesion or transcranial magnetic stimulation) deviate to the right side of the line, presumably because they neglect the contralesional left side of space, representing that side as smaller than it really is (Karnath, 1988; Fierro et al., 2000; Schenkenberg et al., 1980). In healthy adults, neuroimaging studies using versions of the Landmark and line bisection tasks have revealed strong activation in right parietal cortex (Foxe et al., 2003; Waberski et al., 2008; Fink et al., 2000; Çiçek et al., 2009; Cavézian et al., 2012). Indeed, the task has high reliability, showing reproducible right hemispheric dominance in 93% of adult participants (Schuster et al., 2017). The strong tendency for this task to show right lateralization may be the reason why researchers have used it as a proxy of sorts for visual-spatial functions as a whole, particularly in studies that examine whether lateralization of language and space are organized across the hemispheres in complementary fashion (Flöel et al., 2005; Jansen et al., 2005; Badzakova-Trajkov et al., 2010; Rosch et al., 2012; Cai et al., 2013; Badzakova-Trajkov et al., 2016).
Interestingly, lateralization of activation to the right hemisphere that is associated with the line bisection task is not limited to horizontal presentation of the line. Fink et al. (2001) directly compared fMRI activation for line bisection performed on horizontal vs. vertical lines within the same healthy adult participants. They observed right-lateralized activation in posterior parietal cortex regardless of stimulus orientation, concluding that “orientation did not differentially affect the neural mechanisms underlying the visuospatial judgment per se” (p. S65-S66). Furthermore, no significant interactions between task (bisection judgment vs. control) and stimulus orientation (horizontal vs. vertical) were found. In a recent adaptation of the task that uses a vertical line with a horizontal bisector, strong right-lateralized parietal activation was also found in healthy adults at both the group and individual level (Seydell-Greenwald et al., 2019).
An important starting point for the present study is the recognition that, although there is robust evidence that the line bisection task is strongly right-lateralized in healthy adults, this may not hold true for all spatial functions. Indeed, lateralization patterns vary significantly across different spatial tasks, even among healthy adults. Perhaps surprisingly, research over the past decade has shown that some basic spatial-cognitive functions appear to be bilateral in healthy adults. For example, one classic spatial function—mental rotation—activates critical regions of both hemispheres to a similar degree (Carpenter et al., 1999; Cohen et al., 1996; Desrocher et al., 1995; Jordan et al., 2002; Kosslyn et al., 1998; Peronnet & Farah, 1989; Richter et al., 1997; Tagaris et al., 1996). Two recent meta-analyses of studies using mental rotation tasks support this (Zacks, 2008; Tomasino & Gremese, 2016). Similar findings have been shown for other widely-used spatial tasks. For example, visual-spatial construction, as tested by a puzzle task inspired by the well-known WASI-II Block Design sub-test (Wechsler, 2011), shows consistent activation of bilateral inferior and superior parietal regions in healthy adults (Seydell-Greenwald et al., 2017) and children (Ferrara et al., 2020; see Ebner et al., 2011, for similar results).
In contrast to the rich history and growing number of neuroimaging studies on hemispheric lateralization in adults, we know remarkably little about the developmental origins of hemispheric specialization. Importantly, research on the development of language is revealing a complex process in which adult lateralization of function is only the endpoint. By four months of age, infants show some degree of left lateralization of function for speech perception (Dehaene-Lambertz et al., 2002; Petitto et al., 2011; Perani et al., 2011). But there is also evidence that lateralization for language changes considerably over childhood. In healthy children, the degree of left lateralization for sentence and word processing increases significantly between the ages of 4 and 13; younger children show much more bilateral activation for sentences, with increasing left lateralization over age; the adult pattern of strong left lateralization is not reached until adolescence (Berl et al., 2014; Olulade et al., 2020). These findings may help to explain the unusually robust preservation of language in individuals who have sustained perinatal strokes: if both the left and right hemispheres are initially capable of representing language, then damage to the left hemisphere should leave the healthy right hemisphere to support language (Olulade et al. 2020; Newport et al, 2017). This point was originally made by Lenneberg (1967), who emphasized the high degree of plasticity in the young brain. Subsequent studies have documented the resiliency of language after perinatal stroke (Stiles et al., 2012; Newport et al., 2017; in preparation). In the spatial domain we know less about the typical developmental course of lateralization, but the findings for language raise the possibility that a similar pattern might be found, with early bilaterality for some spatial functions followed by increased lateralization towards one hemisphere (presumably the right). The present study examines this possibility, focusing on a task that is well-known for evoking right-lateralized brain activation in adults: the line bisection judgment or “Landmark Task” (Fink et al., 2000).
Developmental evidence for patterns of lateralization associated with spatial tasks is quite limited, and the findings are mixed, with some studies finding evidence of right-lateralized function, and others finding bilateral activation. Lidzba and colleagues (Lidzba et al., 2013) used a complex visual search task that involved side-by-side comparison of two Rey-Osterrieth Complex Figures (Rey, 1941). Participants were required to indicate whether one detail was missing from one of the figures. Interestingly, individuals who were high-performers on the behavioral task in the scanner (regardless of age) had stronger activation in right superior parietal cortex, suggesting a more mature visual search network that is right-lateralized. Everts et al. (2009) used the same task and also evaluated performance on the Rey-Osterrieth Complex Figure outside of the scanner (participants were required to draw reproductions of the figure as accurately as they could). Performance on this task outside of the scanner was found to correlate with the degree of right-lateralized activation during the in-scanner visual search task. Using the same task with 8–20-year-olds, Everts et al. (2009) found right lateralization of parietal and frontal activation that increased with age. These studies suggest that the right hemisphere plays a privileged role in some aspects of spatial processing, which may become further strengthened as children develop. Conversely, the opposite developmental pattern is found in some studies that used different spatial tasks. Kucian et al. (2007) found right-lateralized activation in children during a mental rotation task, and bilateral activation in adults. Nagel and colleagues measured brain activation in 10–16 year-olds during a spatial working memory task and found that older participants showed greater bilateral activation in posterior parietal cortex than younger participants (Nagel et al., 2013). Still other studies have found that children show consistent bilateral processing of spatial information. For example, Ebner et al. (2011) asked participants to determine whether an abstract, colored shape could fit with another “like a piece in a puzzle.” Activation in right and left superior parietal areas were reported in participants between 7–17 years of age (no age effects were evaluated). Lastly, Ferrara et al. (2020) tested children between the ages of 5 and 11 and found consistent and robust bilateral parietal activation associated with a visual-spatial construction task.
In sum, the little we know about the developmental course of lateralization for spatial functions suggests that the pattern is mixed, with some tasks consistently activating bilateral regions of the parietal lobe from early childhood and into adolescence and adulthood, and others appearing to show patterns of right lateralization that are linked with maturation and performance. These mixed results are likely in part due to the different computational requirements of the different tasks. This highlights how little is known about the origins and developmental trajectories for neural lateralization of spatial tasks, which may vary depending on the computational requirements of the functions involved.
Here we aim to discover whether certain spatial functions may show a developmental lateralization pattern that is similar to what has been found for language (i.e., initially bilateral and becoming increasingly lateralized to the hemisphere that is dominant for the task in adulthood). This calls for a task that is definitively right-lateralized among healthy adults. Fortunately, a number of neuroimaging studies provide a strong basis for the conclusion that the line bisection task is consistently right-lateralized in adults. This suggests that it is a good candidate for testing whether the developmental trajectory for lateralization of spatial function can, in principle, parallel the trajectory found in language.
Our understanding of the neural underpinnings of the line bisection and/or Landmark Task in early development is, at present, quite limited. The classic line bisection task has been used to study visual-spatial attention, spatial acuity, and neglect in both typically and atypically developing children (e.g., Ferro et al., 1984; Johnston & Shapiro, 1986; Bradshaw et al., 1987, 1988; Paquier et al., 1999; Dellatolas et al., 1996; Chokron & De Agostini, 1995; Hausmann et al., 2003; Sheppard et al., 1999, 2002; Failla et al., 2003; Patro et al., 2018; Saj et al., 2020). Although these behavioral studies alone cannot address the issue of lateralization or its developmental profile, they can provide a picture of developmental change in accuracy. In perhaps the most comprehensive investigation of behavioral performance, van Vugt et al. (2000) tested 650 typically developing children aged 7–12 years on both horizontal and vertical versions of the task. Participants were instructed to mark the midpoint of the line with their preferred hand using a pen. Performance systematically improved with age, with older children more accurately marking the midpoint of the line than younger children. Recently, Hoyos et al. (2020) used a perceptual version of the horizontal line bisection task to understand the development of frontal-parietal attentional networks. In this behavioral task, they found that participants marked the bisection point in an overall leftward direction. This leftward bias was found among all ages (6–14 years), largest in the youngest children (grades 1–3) and decreasing among older children (grades 6–8), who showed the same very small bias as adults (i.e., close to zero).
In the present study, we used functional magnetic resonance imaging (fMRI) to examine the lateralization trajectory for a version of the line bisection/Landmark Task. We adapted an experimental paradigm from previous work that is suitable for use in the scanner and has shown robust right-lateralized parietal activation in healthy adults (Seydell-Greenwald et al., 2019). We asked whether this task also shows the same pattern in children between ages 5 and 11, or whether, in parallel with findings in the language domain, the youngest children show a predominantly bilateral pattern of activation that becomes right-lateralized in the oldest children. If the developmental profile for lateralization shows a pattern similar to that found for language, these findings would suggest a common mechanism of hemispheric specialization that holds for different domains of cognitive function.
Materials and Methods
Participants
The data presented here are from thirty-six typically developing children between the ages of 5 and 11 (5.10–10.98 years, Mean age = 8.17 years, SE = 0.30 years, 18 females). An additional 5 children participated in the study, but their data were excluded due to excessive motion in the scanner (greater than 3 mm in any direction). Participants were recruited via local parent groups and preschools and were compensated for their time. All participants had normal or corrected-to-normal vision with no history of neurological impairment or abnormality, and all were fluent English speakers. The study was approved by the university Institutional Review Board. Informed consent was provided by parents and written assent was provided by all children before the study procedures began.
Stimuli, Design, and Procedure
Participants performed a visual-spatial line bisection task while lying on their backs in the scanner. They were presented with a vertical line at the center of the screen that was divided into two segments and were asked to report which of the two segments was longer (Figure 1). The task is similar to that used by Hoyos et al. (2020), eliminating the traditional manual measure of line bisection and instead presenting participants with a line that is bisected slightly off-center and asking them to make judgements of relative length of the two line sections. We opted to use vertical lines instead of horizontal for several reasons. First, as reviewed above, it has been shown that this task elicits robust right-lateralized parietal activation, regardless of whether the stimulus lines are presented in vertical or horizontal orientations (Fink et al., 2001). Second, we aimed to build upon the findings of a previous study of adults (Seydell-Greenwald et al., 2019), which also used vertical lines. Third, as reviewed by Seydell-Greenwald et al. (2019), using a vertical line presented at screen center minimizes activations associated with shifts in spatial attention to left or right hemispace, which could influence lateralization of activation patterns (Heilman & van den Abell, 1980; Mesulam, 1981; Corbetta et al., 1993; Corbetta & Shulman, 2011). Thus, we are able to isolate right-lateralized activation associated with the spatial computations involved in comparison of relative length and proportion, without also drawing in activation that may be associated with left/right shifts in attention.
Figure 1.
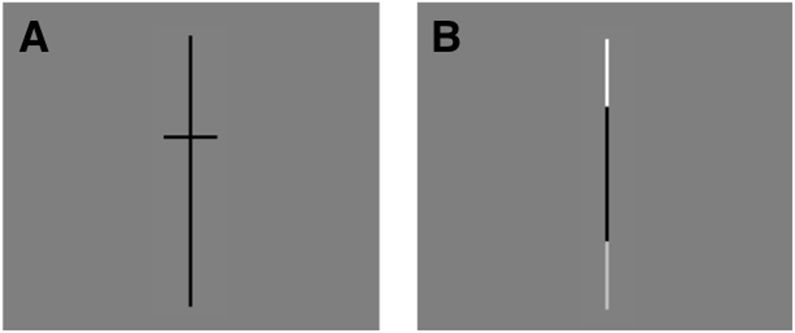
Experimental Design. A) Spatial condition: participants indicated whether the bottom or top part of the line was longer. B) Luminance condition: participants indicated whether the bottom or top part of the line was brighter.
The present study included a Spatial condition and a Luminance condition (Figure 1). In the Spatial condition, as described above, a vertical line was presented with a small horizontal line segmenting it roughly in half. Participants were asked to decide which portion of the line was longer, either the top portion above the bisector or the bottom portion below the bisector. In the Luminance condition, which served as the control condition, the same vertical line was presented, but now the ends were different shades of grey. Participants were asked to decide if the top or the bottom part of the line was brighter. Both conditions thus required comparison of the ends of the vertical line, ensuring that any shifts of spatial attention would contribute to activation to a similar degree. The Luminance control condition was intentionally designed to be highly visually similar to the Spatial condition, but importantly it did not involve spatial judgements of relative length. Comparison of the Spatial and Luminance conditions should therefore reveal the critical cortical areas that are uniquely involved in the Spatial condition. Both the Spatial and Luminance conditions were calibrated to produce high accuracy. This was done to avoid the potential influence that task difficulty can have on lateralization of neural activation (Yeatman et al., 2010; Murphy & Garavan, 2004; Just et al., 1996). By choosing stimuli for which judgments would be relatively easy, we achieved high and similar levels of accuracy for the two conditions while keeping our participants actively engaged across a wide age range (see Ferrara et al., 2020 for a similar method).
Following consenting and MRI safety screening, participants were shown the task, called “The Line Game,” on a laptop outside of the scanner. They then completed a 20-minute training session to familiarize them with the scanning environment, first in a fabric play tunnel and then in a mock scanner.
In the scanner, visual stimuli were projected onto a screen and viewed by participants through a slanted mirror mounted on the head coil. Stimuli were vertical black lines presented on a gray background (RGB 127, 127, 127). The lines were 8 cm long and 1 mm thick on the computer screen, corresponding to a length of 6.4° visual angle and a thickness of 0.08° visual angle given the projection magnification and effective viewing distance. For the Spatial condition, each vertical line was bisected by a short horizontal line (length: 128°, thickness: 0.08°). The bisector was located 0.8° (12.5% of the line length) above or below the veridical line center. For the Luminance condition, there was no bisecting line, but the uppermost and lowermost tips (length: 1.6°) of each vertical line were lighter than the rest of the line. One of the tips was white (RGB 255, 255, 255) and the other was gray (RGB 190, 190, 190). To prevent participants from basing their judgments on the location of the line tips on the screen, the vertical position of the stimuli on the screen was varied randomly within a range of 2.4° around screen center. To prevent participants from comparing lines across trials, a longer, dashed line (length: 12.64°) was presented during the 200 ms inter-stimulus interval to visually mask the preceding stimulus and any potential afterimages. Mask and location randomization were applied to all conditions to keep visual stimulation as similar as possible across tasks.
Both the Spatial and Luminance conditions required participants to push one of two buttons. Button number 1 was held in the left hand and button number 2 was held in the right hand. Participants pushed button number 1 when they judged that the line was longer on the top (in the Spatial condition) and when they judged that the line was brighter on the top (in the Luminance condition). Participants pushed button number 2 when they judged that the line was longer or brighter on the bottom in the respective conditions.
Functional MRI Paradigm
Participants completed 4 three-minute runs of “The Line Game.” Each run contained two blocks of the Spatial condition and two blocks of the Luminance condition; order of presentation was counterbalanced across the 4 runs. Blocks were 24 seconds long and each block was followed by a 9-second fixation rest period. To remind participants of the different tasks for the two conditions, recorded auditory instructions were presented at the start of each block (9-seconds). At the start of Spatial blocks, participants heard a voice that asked, “Is the line longer on the top or the bottom?” At the start of the Luminance blocks, participants were asked “Is the line brighter on the top or the bottom?” These instructions were accompanied by an image of a cartoon giraffe or a polar bear with a thought bubble containing examples of the stimuli from the relevant condition (see Supporting Information Figure S1).
Two Cedrus fiber optic button boxes were used to record participant responses. All trials were self-paced; participants completed as many trials as they could within each block. This ensured that children were not rushed while at the same time preventing idle periods between trials. The next trial appeared immediately following a button push or if a response had not occurred within 3 seconds. Piloting indicated that this response time limit was generous even for the youngest participants.
Image Acquisition
A research-dedicated Siemens Trio Tim 3-Tesla magnetic resonance imaging scanner with a 12-channel birdcage head coil was used to acquire imaging data. Participants’ heads were stabilized with foam padding and they wore headphones mounted in Bilsom ear defenders. This protected them from the scanner noise and enabled them to hear the auditory recordings played during the instruction periods. Visual stimuli were presented using E-Prime 2.0 and were projected onto a screen at the back of the scanner via an Epson PowerLite 5000 projector which participants were able to view via a mirror mounted on the head coil.
A low-resolution anatomical image to aid volume placement for subsequent scans was first acquired during a 1-minute localizer scan. Four 3-minute functional runs were next acquired to measure blood-oxygen-level-dependent (BOLD) signal changes associated with the Spatial and Luminance conditions. Functional images were acquired with a gradient echo-planar T2* sequence (50 horizontal slices acquired in descending order, voxel size 3 × 3 × 2.8 mm3 with a distance factor of 7% between slices, TR = 3 s, TE = 30 ms, flip angle = 90 degrees, matrix 64 × 64, duration 3 minutes, 60 volume acquisitions). Lastly, a high-resolution structural scan was acquired, during which children watched a movie. Structural T1-weighted images were acquired using magnetization-prepared rapid-acquisition gradient echo (MPRAGE) (176 sagittal slices, voxel size 1 × 1 × 1 mm3, TR = 2530 ms, TE = 3.5 ms, inversion time (TI) = 1100 ms, flip angle = 7 degrees, matrix 256 × 256, duration 4 minutes).
Imaging analysis
Preprocessing
Brain Voyager QX software (Brain Innovation, Maastricht, the Netherlands) was used to conduct the imaging analyses. Anatomical data underwent inhomogeneity correction and transformation into Talaraich space using 9-parameter affine transformation. Manual definition of the landmarks was performed for the Talaraich transformation. Functional data were preprocessed, including removal of the first two volume acquisitions to allow for T1 saturation, slice scan time correction, linear trend removal, 3D motion correction using rigid-body transformation, co-registration to the anatomical scan using 9-parameter gradient-based alignment, and spatial smoothing with a 3 mm full-width at half-maximum (FWHM) Gaussian kernel.1 Functional data were transformed into Talaraich space using the same transformation applied to the anatomical data.
Statistical analysis
Voxel time courses from the four functional runs were combined and fitted with a general linear model (GLM) to investigate whole-brain activation. Rest periods served as the model’s baseline. The GLM contained two condition predictors: one for Spatial blocks and one for Luminance blocks. The time course for each predictor was determined by convolving the time course of the stimulation (a boxcar predictor that was “on” during the condition and “off” otherwise) with the hemodynamic response function (two gamma HRF, time to peak 5 s, time to undershoot peak 15 s). The model also contained a predictor for the instruction periods that occurred at the start of each block, z-transformed motion estimates, and a constant predictor for each functional run as nuisance regressors. Voxel time courses were normalized (percent signal change transformation) and corrected for serial autocorrelations (second-order model). For group-level analyses, beta maps for all participants were combined into a random effects (RFX) analysis. For single-subject analyses, data from a given participant’s four functional runs were combined in a fixed effects (FFX) analysis. All activation maps were thresholded using a single-voxel threshold of p < .001 in combination with a cluster-size threshold of k < 0.05 (as determined by Monte-Carlo simulation with 1000 iterations, using the Cluster-Level Statistical Threshold Estimator plugin for BrainVoyager QX).
Regions of interest
In addition to analyses of whole-brain activation, we also focused on activation within specific regions of interest (ROIs). We defined ROIs using two different methods: the first followed an anatomical definition approach and the second followed a functional definition approach. First, we created an anatomically-defined bilateral posterior parietal ROI that was made up of Brodmann Areas (BAs) 7, 40, and 39 (see Supporting Information, Figure S2). These BAs are commonly cited in the literature as important for visual-spatial processing. This anatomical ROI has been used in previous studies to analyze lateralization of visual-spatial function in healthy adults (Seydell-Greenwald et al., 2017; 2019) and healthy children (Ferrara et al., 2020). We also followed a second approach, using a functionally-defined ROI derived from independent adult data (these results are reported in the Supplemental Information).
Laterality Indices
We investigated lateralization of brain activation within the two ROIs described above to determine whether activation occurs more on the right than the left. We employed a measure commonly used in the neuroimaging literature on language, the lateralization index (LI), which quantifies activation in the left and right hemispheres and then computes the ratio of left vs. right (left activation – right activation)/(left activation + right activation). An LI of −1 indicates complete lateralization to the right hemisphere and an LI of 1 indicates complete lateralization to the left hemisphere.
An in-house script (Matlab 2015b and BVQXtools v0.8d) was used for LI computation and mask generation. A bootstrapping approach was used to compute a weighted mean of LIs obtained at different activation thresholds (Wilke & Lidzba, 2007; Wilke & Schmithorst, 2006). Bootstrapping is a statistical technique whereby multiple resamples are taken from an original sample “in order to estimate a bootstrap distribution that allows approximating the ‘real’ distribution of the original sample” (p. 522, Wilke & Schmithorst, 2006; Davison & Hinkley, 1997; Hesterberg et al., 2005; Janssen & Pauls, 2003, Moore et al., 2002). Twenty-five different t-threshold values were applied to the activation maps, ranging in equal intervals from 0.1 to the maximum t-value in the participant’s map. To prevent distortion of the results by outliers, LI computation was aborted for thresholds at which there were fewer than 10 active voxels on either side. Otherwise, 10,000 LI estimates were computed from the sum of t-values from randomly-drawn active voxels on each side (0.25 of all active voxels were sampled per estimate). A trimmed mean (including only the central 50% of estimates) of the 10,000 estimates was used to provide a robust LI estimate that limits the influence of statistical outliers. Following LI computation at each threshold, each LI was multiplied by its t-threshold value, such that LIs for stricter thresholds received higher weights. This approach enables us to account for the “meaningfulness” of voxels obtained at different thresholds, because voxels that are significant at higher thresholds have a higher correlation with the task (Wilke & Schmithorst, 2006). Lastly, the sum of all weighted LIs was divided by the sum of all t-thresholds.
Results
Behavioral Performance
Overall, children showed highly accurate task performance in both conditions (Figure 2). The Spatial and Luminance conditions did not significantly differ in terms of accuracy (proportion of correct responses) (Spatial: M = .94, SE = .01; Luminance: M = .95, SE = .01, t(35) = −0.65, p = .52, 95% confidence interval, CI = [−.02, .01]) or response time (RT) (Spatial: M = 1082.47, SE = 45.70; Luminance: M = 1039.26, SE = 47.38, t(35) = −.03, p = .98, 95% CI = [−92.41, 89.92]). No gender differences for accuracy or RT were found (p’s > .41).
Figure 2.
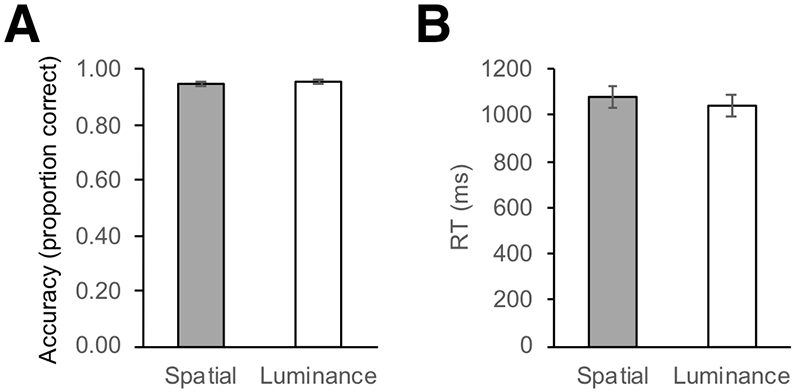
Behavioral performance on the “Line Game.” A) Average proportion of correct responses for the Spatial and Luminance conditions. B) Average reaction time (RT) for the Spatial and Luminance conditions. Error bars represent +/− standard error of the mean.
Pearson product-moment correlation coefficients (two-tailed) were computed to explore relationships among age, accuracy, and RT. Accuracy in each condition was not significantly correlated with age (Spatial: r = .19, p = .26; Luminance: r = .26, p = .17). The same was found for RT in each condition (Spatial: r = −.06, p = .72; Luminance: r = −.26, p = .23). Within each condition, RT and accuracy were not significantly correlated, indicating that participants who were quicker to respond were not necessarily more accurate (Spatial: r = −.06, p = .74; Luminance: r = .06, p = .71). Overall, analyses of behavioral performance demonstrate that the task was appropriately designed and could be completed by children across the age range.
Imaging
We first carried out whole-brain group analyses to determine the areas of significant activation for the Spatial condition compared to the Luminance condition. We then investigated developmental changes in lateralization patterns of activation.
Areas of activation: Whole brain and ROI analyses
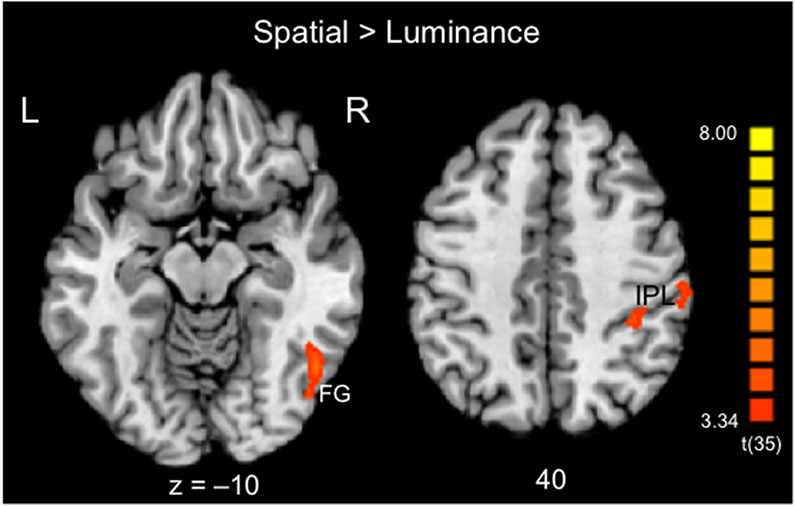
Whole-brain GLM contrasts were conducted to identify areas that showed stronger activation for the Spatial condition compared to the Luminance condition (Spatial > Luminance) (Table 1 and Figure 3). In this group analysis, children showed an activation peak in the right hemisphere in the fusiform gyrus (FG) and two peaks in the right hemisphere in the inferior parietal lobule (IPL).2
Table 1.
Activation clusters from the whole-brain analysis of all participants, Spatial > Luminance.
| Location description | BA | Peak Tal cords. |
Center of gravity |
Peak t- value |
Average t-value |
Average p-value |
Cluster extent (mm3) |
|---|---|---|---|---|---|---|---|
| Fusiform gyrus | 37 | 47, −59, −9 | 46, −61, −7 | 5.62 | 3.98 | 0.000448 | 1085 |
| Inferior parietal lobe | 40 | 35, −38, 39 | 36, −36, 40 | 4.60 | 3.84 | 0.000557 | 271 |
| Inferior parietal lobe | 40 | 53, −29, 43 | 54, −29, 42 | 4.72 | 3.89 | 0.000512 | 425 |
Figure 3.
Group-level activation map across all child participants: Areas displaying significantly stronger activation for the contrast of Spatial > Luminance for the line bisection task. Activation was observed in the right hemisphere in the fusiform gyrus (FG, BA 37) and in the inferior parietal lobule (IPL, BA 40) in two clusters. Activation maps are overlaid on the Colin27 brain template transformed into Talairach space and thresholded at p < .001 single-voxel threshold combined with a k < 0.05 cluster-size threshold. Activation details can be found in Table 1.
We next sought to examine this response profile in greater depth. To investigate the response activation profile within an ROI, the average percent signal change across all voxels in the ROI was extracted separately for each child participant and condition (thresholded at p < .001 single-voxel threshold combined with a k < 0.05 cluster-size threshold). This was done separately for the left and the right hemispheres to evaluate potential differences in activation. Multiple linear regressions were calculated to predict activation (percent signal change) based on age, accuracy, and RT for behavioral performance in the scanner. Separate linear regressions were calculated for the left hemisphere and for the right, in light of the possibility that activation may be significantly related to age or performance in one hemisphere but not the other.
For the anatomically-defined parietal ROI, a significant regression equation was found for the Spatial condition in the right hemisphere, F(3, 32) = 2.93, p = .043, with an R2 of .22, with age emerging as the only significant predictor (p = .017; Table 2). Nonsignificant regression equations were found for the Spatial condition in the left hemisphere (F(3, 32) = 1.89, p = .15, R2 of .15). Regression equations were not significant for the Luminance condition for either the left (F(3, 32) = 0.53, p = .67, R2 of .05) or the right (F(3, 32) = 0.19, p = .91, R2 of .02) hemispheres. (For parallel analyses using the adult-defined functional ROI, see Supporting Information.) Overall, these findings indicate an increase and strengthening of activation associated with the Spatial condition in the right hemisphere only, as children age.
Table 2.
Summary of the linear regression equation for variables predicting activation (percent signal change) for the Spatial condition in the parietal anatomically-defined ROI in the right hemisphere. Age emerged as the only significant predictor of activation.
| Variable | B | SE B | β | p |
|---|---|---|---|---|
| Age | 0.07 | 0.03 | 0.40 | 0.017* |
| Accuracy | <0.001 | <0.001 | 0.26 | 0.12 |
| RT | 0.129 | 1.06 | 0.02 | 0.90 |
p < .05.
Laterality indices
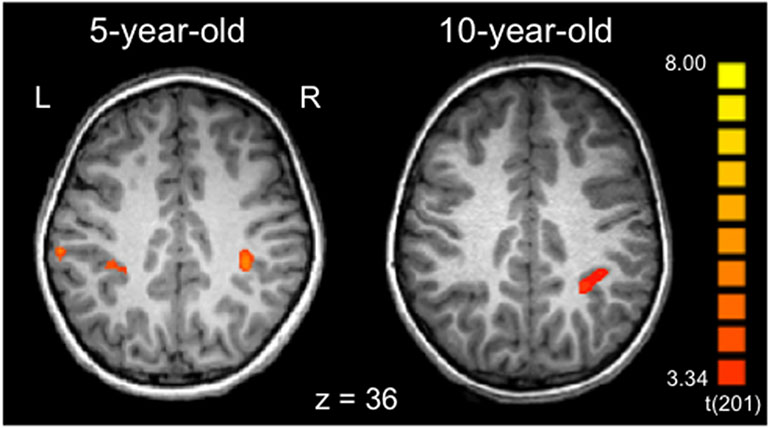
We next investigated whether the profile of lateralization for the line bisection task changes with age. As a first step, we carried out whole-brain analyses for individual child participants, examining children’s activation for the Spatial > Luminance contrast. Inspection of the individual participant maps revealed that younger participants showed activation in the right hemisphere parietal areas described above as well as in the homotopic areas of the left hemisphere. Figure 4 shows an example of this pattern, comparing a 5 year-old to a 10 year-old (see Supporting Information Figure S4 for additional examples). These observations suggest that the youngest children show a bilateral pattern of activation which changes to a right-lateralized (adult-like) pattern over age.
Figure 4.
Individual participant activation patterns (contrast: Spatial > Luminance). The 5-year-old shows bilateral inferior parietal activation (BA 40), while the 10-year-old shows activation in the same region but only in the right hemisphere. Activation maps are overlaid on the individual’s MPRAGE, transformed into Talairach space, and thresholded at p < .001 single-voxel threshold combined with a k < 0.05 cluster-size threshold. See Supporting Information Figure S4 for additional examples of individual children within the 5-10 year age range.
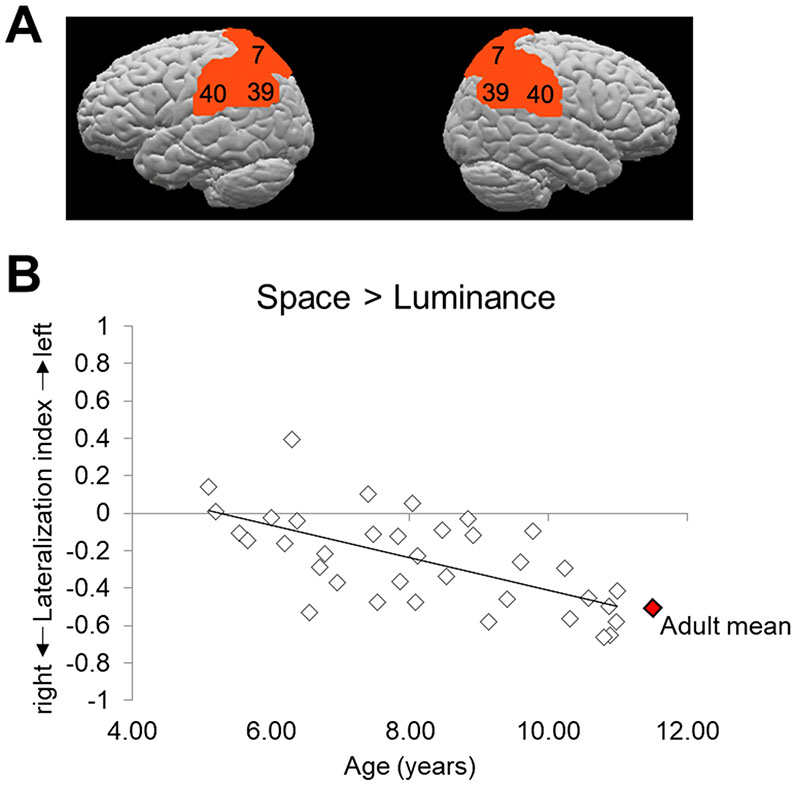
To quantify this developmental trend, laterality indices (LIs) were computed for individual participants (see Methods). We examined LI within the anatomically-defined bilateral posterior parietal ROI that comprised BAs 7, 40, and 39 (see Figure 5A). Figure 5B shows the LIs for individual children over age for the contrast of Spatial > Luminance. Overall, group activation was right-lateralized, with a mean LI of −.25 (SE = .04). However, as can be seen in the figure, the LI for individual children changes quite systematically over age. By the time children reach 10–12 years of age, they have LIs that resemble those of adults. To illustrate this point, Figure 5B includes the mean LI (−.48) from adults who performed this same task (Seydell-Greenwald et al., 2019). Pearson product-moment correlation coefficients (two-tailed) were computed to examine the relationships between LI and age as well as LI and behavioral performance (accuracy and RT). There was a significant negative correlation between LI and age, r = −.63, p < .001, with a developmental change for line bisection from bilateral activation in the youngest children to increasingly right-lateralized activation over age. LI was not significantly correlated with either accuracy or RT for the Spatial condition (accuracy: r = −.09, p = .60; RT: r = −.18, p = .30). Thus children who performed better or faster on the task were not more likely to show right-lateralized activation patterns. One final remaining question concerns the principal changes that drive this observed increase in lateralization as children age. This is investigated in the next section.
Figure 5.
Lateralization analysis. A) The anatomically-defined bilateral posterior parietal ROI encompassing Brodmann areas 7, 40, and 39 that was used to analyze lateralization of function in children for the line bisection task. B) Lateralization index values for individual child participants for the contrast Spatial > Luminance. The linear trendline of the data is shown. Participants show bilateral activation (i.e., LIs around zero) at younger ages and increasingly more right-lateralized activation (i.e., more negative LIs) over age. For illustration purposes, the adult mean LI for this same task (Seydell-Greenwald et al., 2019) is shown in red. This illustrates that by the time children are 10–12 years of age, they have reached adult-like patterns of lateralization on the line bisection task.
ROI analyses of activation within each hemisphere: Changes with age
To investigate whether changes in lateralization were driven by increases or “building up” of activation in the right hemisphere, decreases or “pruning back” of activation in the left hemisphere, or both (Cantlon et al., 2011), we considered, separately for the left and right hemisphere ROIs, Spatial activation and Luminance activation relative to baseline. To do this, the average percent signal change across all voxels in each ROI was extracted separately for each child participant and condition. Here, we report findings for the anatomical parietal ROI. A repeated-measures ANCOVA with Spatial activation (percent signal change over baseline, Figure 6) as the dependent variable and hemisphere as the within-subjects factor revealed a significant main effect of hemisphere (F(1,34) = 4.40, p = 0.04), and most critically, a significant age by hemisphere interaction (F(1,34) = 10.45, p = 0.003). For Luminance activation (Figure 7), there was not a significant main effect of hemisphere (F(1,34) = 0.14, p = 0.91), nor was there a significant age by hemisphere interaction (F(1,34) = 0.01, p = 0.94). To follow up on this, we conducted Pearson product-moment correlation coefficients (two-tailed) to explore the relationship between age and activation. We found that Spatial activation significantly increases with age in the right hemisphere (r = .35, p = .03), while remaining relatively flat over age in the left hemisphere (r = −.26, p = .14). In contrast, Luminance activation was not correlated with age in either the left (r = .02, p = .92) or the right (r = .04, p = .84) hemisphere. These findings illustrate a pattern of hemispheric specialization that occurs via a “building up” of activation specific to the Spatial condition in the right hemisphere. In sum, these collective results indicate a strong pattern of bilateral to right-lateralized spatial activation as children age.
Figure 6.
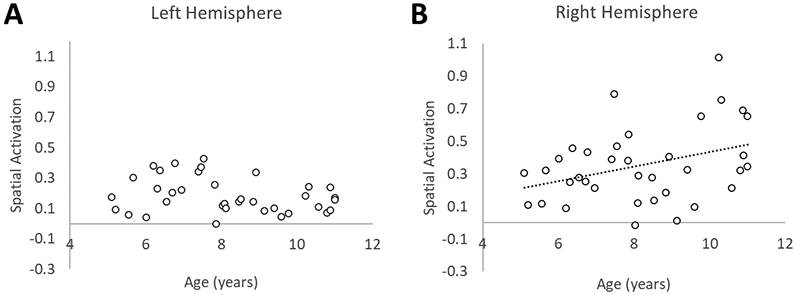
Anatomically-defined ROI: Developmental changes in Spatial activation over age. Activation associated with the Spatial condition significantly increases with age in the right hemisphere (B), but not in the left hemisphere (A). (B) includes the best fit linear trendline of the data.
Figure 7.
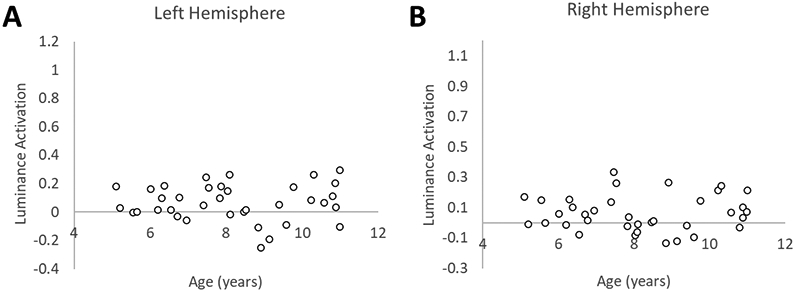
Anatomically-defined ROI: Developmental changes in Luminance activation over age. Activation associated with the Luminance condition does not show a relationship to age in either the left (A) or the right hemisphere (B).
Discussion
In the present study we asked whether certain dominant patterns of hemispheric lateralization shown among adults are prominent from early development onward, or whether lateralization patterns change significantly over time. The answer to this question is important for our understanding of how lateralization patterns come to be the way they are in adulthood and whether there are general principles of lateralization development that span different types of cognitive functions. Our work was inspired by recent studies on the development of hemispheric lateralization for language, which shows that although language (specifically, syntax and sentential semantics) tends overwhelmingly to be left-lateralized in healthy adults, its lateralization profile undergoes significant developmental change between the ages of 4 and 13 years, starting out more bilateral and becoming strongly left-lateralized by late childhood/early adolescence (Berl et al., 2014; Olulade et al., 2020). These findings raise the question of whether this same developmental pattern is also observed in the realm of spatial functions, which classically have been assumed to be right-lateralized. We tested this question using fMRI to examine brain activation in children between the ages of 5 and 11 years, using the line bisection/Landmark task, which is known to robustly and reliably activate the right inferior and superior parietal lobes in healthy adults (Seydell-Greenwald et al., 2019; see Badzakova-Trajkov et al., 2016, for a review).
In the Spatial condition of our line bisection task, children judged whether the top or bottom of a segmented line was longer. In the control Luminance condition, they judged whether the top or bottom of the line was brighter. Because we aimed to investigate changes in lateralization over a broad developmental period and wanted to avoid the potential influence of task difficulty on activation patterns (Yeatman et al., 2010; Murphy & Garavan, 2004; Just et al., 1996), the “Line Game” was designed to be relatively easy to carry out for all ages. Participants showed high accuracy in behavioral performance and no relationship between level of performance (either accuracy or RT) and any aspect of the activation patterns we observed. The crucial question, however, concerned the lateralization profile for this task over age and specifically whether the adult pattern of right lateralization emerges at the earliest ages tested, or whether there is a developmental change culminating in right lateralization. The results showed the latter, with the youngest children (5–6 years of age) showing bilateral patterns of activation (LIs close to 0) and the oldest (10 years of age) showing right-lateralized patterns of activation (negative LIs) close to those of adults (found in Seydell-Greenwald et al., 2019). Regression and correlational analyses within each hemisphere showed consistent and significant increases with age in activation for the Spatial condition in the right hemisphere, but not in the left hemisphere. Age was not a significant predictor for activation for the Luminance condition in either hemisphere.3 This pattern of increasing right-hemisphere activation with age for line bisection is similar to those found for other visual-spatial tasks among children between the ages of 7 and 20 (Lidzba et al., 2013; Lidzba et al., 2006; Everts et al., 2009).
Our findings show a parallel with the development of lateralization for language, as found by Berl et al. (2014) and Olulade et al. (2020). In both studies, the youngest children (5–7 years old) showed bilateral activation in a language comprehension task requiring that they indicate whether sentences defining a familiar noun were true. Language activation occurred in the typical regions of the left hemisphere known to support language in adults as well as in the homotopic regions of the right hemisphere. This bilateral activation pattern can help explain the finding that children who have sustained perinatal strokes to the left hemisphere can develop language, with high levels of performance in adolescence on a range of tasks (Newport et al., 2017; in preparation), and no differences between those who have sustained left vs. right strokes earlier in life (Stiles et al., 2013). The most telling result is that children who have sustained perinatal damage to the left hemisphere show fully developed language as adolescents and young adults, indicating that both right and left hemispheres are initially capable of supporting language function.
Consistent with these findings on language, the present results for the line bisection task demonstrate that homotopic regions of the left and right parietal lobes support spatial function in the young brain. This would suggest that, following a perinatal stroke affecting critical regions of the right hemisphere, the homologous regions of the left hemisphere should be capable of supporting the same spatial functions. The existing literature on perinatal stroke outcomes for spatial functions is somewhat limited but overall indicates few substantial differences in the spatial performance of children who have sustained perinatal strokes to the right vs. left hemisphere (Stiles et al., 2012; Ferrara et al., 2017), suggesting that the two hemispheres of the young brain may be equally capable of supporting a range of spatial functions.
Why and how does the lateralization pattern for the line bisection task (or any spatial task, for that matter) start out bilateral and become lateralized over development? First, one might think that using a vertical line as the main stimulus (rather than the traditional horizontal line) might result in fewer attentional shifts towards the left and right, which could lead to less activation of attentional networks that, historically, have been characterized as right-lateralized. However, studies on healthy adults have used both vertical and horizontal lines in versions of this task and have found no differences in lateralization (Fink et al., 2001). Furthermore, recent theories and extensive experimental support show that visual attentional networks are not predominantly right-lateralized as had been assumed, but rather are bilateral, with weighting between the hemispheric contributions subject to task and individual differences (Szczepanski & Kastner, 2013). Second, one might think that the increasing right lateralization over age could be due to the level of difficulty of the task: older children can carry out the task with only right-lateralized brain activation because it is significantly easier for them, whereas younger children require activation in both hemispheres (Schlaggar et al., 2002). However, as discussed in the Results section, we intentionally designed the task to be easy for all participants and found no correlations between any measure of activation and performance variables such as accuracy or reaction time.
Explanations of a developmental change from bilateral activation towards lateralization in the line bisection task must be speculative at present, but one approach to consider possible mechanisms is to use parallel findings in other domains as a starting point. One such case is the well-known development of right lateralization in face perception. A number of studies have found that face processing is bilateral in early life (Dundas et al., 2014; Behrmann & Plaut, 2020; Lochy et al., 2019) and then becomes robustly right-lateralized over development, in conjunction with the onset of reading and the formation of the Visual Word Form Area (VWFA). Dundas et al. (2013) examined accuracy in children’s, adolescents’, and adults’ abilities to judge whether two faces or two words presented sequentially were identical or not. Stimuli were presented briefly to the right vs. left visual hemifield (hence processed primarily by the left vs. right hemisphere, respectively). Adults showed the expected hemispheric superiority to faces when presented in the left visual field (right hemisphere) and to words when presented in the right visual field (left hemisphere); children (ages 7–9) and adolescents (ages 11–13) showed only the right visual field superiority for words, with no left visual field superiority for faces. A measure of reading comprehension predicted the degree of right-hemisphere lateralization for faces, and the authors suggested that the process of learning to read leads to the development of left lateralization for words and, in turn, right lateralization for faces. Additional studies have shown that, indeed, the process of learning to read contributes to the establishment of the VWFA, its response to letters and words, and in turn, decreasing neural response to faces in these and neighboring areas (Cantlon et al. 2011; Dehaene et al., 2015). The results of this process are that letter and word recognition is established as a left-lateralized function, while perception of faces becomes right-lateralized. This mechanism for establishing lateralization (for faces) is thus entwined with specific experience, i.e., learning to read.
Following this possible mechanism whereby lateralization of function over development is associated with experience, one speculative hypothesis is that, in addition to involving spatial judgements, the line bisection task also involves judgments of relative magnitude, another function which undergoes some segregation over development and in conjunction with learning mathematics. The parietal areas in which we observed activation are engaged when processing spatial extent as well as temporal extent and numerosity, and are thought to represent a general-purpose system for magnitude estimation (Walsh, 2003; Hubbard et al., 2005; Dehaene & Brannon, 2011; Cantlon, 2009). Our line bisection task compared two conditions: the Spatial condition requires both magnitude estimation/comparison and localization, as participants must decide which end is ‘longer’ in addition to identifying whether that ‘longer’ end is on the top or bottom of the figure. The control Luminance condition requires participants to decide which end is ‘brighter’ as well as whether that end is on the top or bottom. Thus, both tasks require localization, but only the Spatial condition requires a magnitude comparison. Evidence suggests that tasks involving magnitude comparison activate right-lateralized parietal regions, including comparisons of physical sizes (Pinel et al., 2004) as well as approximate magnitudes (Piazza et al., 2006; Cantlon et al., 2006). For example, individuals who sustain damage to the right parietal regions in adulthood classically show biases towards the right region of space when carrying out the line bisection task, and these same errors are reflected in similar tasks for number (Zorzi et al., 2002). However, tasks involving exact number and/or symbolic number are thought to be more left-lateralized in areas such as the left intraparietal sulcus, medial frontal gyrus and left precentral gyrus (Venkatraman et al., 2005; Vogel et al., 2017). The greater engagement of left parietal areas for exact and/or symbolic number coincides with findings that experience with mathematics results in the establishment of a left-lateralized region adjacent to the VWFA, dubbed the “visual number form area (VNFA),” which responds selectively to numerical symbols (Dehaene, 2011; Price & Ansari, 2011; Shum, 2013; but also see Grotheer et al., 2016, for evidence of bilateral involvement for number forms). Thus an evolving division of labor for magnitude comparisons in the parietal regions, with right-lateralized regions activated for approximate estimates of relative magnitude and left-lateralized regions activated for exact computations and symbolic magnitudes, would be analogous to the changing activation patterns for faces as children learn to read and the VWFA becomes established (Dehaene, 2005). If the two different kinds of magnitude representations (approximate and non-symbolic; exact and symbolic) segregate at some point in development (Dehaene, 2005; Dehaene et al., 2003), this could lead to increasing right lateralization for the approximate magnitude judgments required in our version of the line bisection task.
This speculative proposal brings together the present findings with those on the developmental increase in right lateralization for faces, consequent on learning to read (Cantlon et al., 2011; Dehaene et al., 2015). It is especially interesting to note that the developmental timeline towards right lateralization of faces is roughly the same as that towards right lateralization for the line bisection task. This timeline is also similar to that observed for increasing left lateralization of language (Berl et al., 2014; Olulade et al., 2020). The pattern in all three cases is that of bilateral activation observed at approximately age 5, increasing lateralization with age, and adult levels of lateralization by around age 10. Although these cases may seem quite different, it is intriguing to consider whether the processes of hemispheric specialization across different cognitive domains have similar underlying mechanisms.
In conclusion, the present study uses an adapted version of the “Landmark Task” to investigate the lateralization profile in children. We find that younger children show bilateral involvement of parietal areas, which shifts to right-lateralized parietal activation as children age. These findings suggest new avenues of research to examine developmental trajectories of a wide range of functions, as we seek to discover whether lateralization over development shares common principles for different tasks. It is important to note that not all spatial tasks show the same profiles over development (e.g., visual-spatial construction, Ferrara et al., 2020). In future work it will thus be critical to consider the computational requirements of different spatial tasks and the ways in which these may engage the potential representational biases of the two hemispheres.
Supplementary Material
Research Highlights.
fMRI was used to examine neural activation associated with a line bisection task in children ages 5–11 years.
Older children showed right-lateralized activation in the same areas previously identified among adults.
Younger children showed activation in the same areas as older children and adults but also in homotopic regions of the left hemisphere.
This illustrates a developmental change in lateralization, with early bilateral representation in homotopic areas of the two hemispheres followed by a change towards right-lateralization as children grow into adulthood.
Acknowledgements:
This research was supported in part by a T32 Postdoctoral Research Fellowship through NIH (5T32 HD 046388) to KF; NIH grants K18 DC014558 and R01 DC 016902, American Heart Association grant 17GRNT33650054, and the Feldstein Veron Innovation Fund to ELN; by funds to BL as a George Bergeron Visiting Scholar; by the NIH-funded DC Intellectual and Developmental Disabilities Research Center (U54 HD090257); by NIH grant R21 HD 095273, NIH grant NCATS KL2 TR001432 (through the Georgetown and Howard Universities Center for Clinical and Translational Science), and by funds from the Center for Brain Plasticity and Recovery at Georgetown University and MedStar National Rehabilitation Hospital.
Footnotes
Conflict of Interest Statement: The authors certify that they do not have any financial, personal, or professional interest that raises an actual or potential conflict of interest pertaining to this research or this submission.
As a check to ensure that different levels of smoothing did not impact our results, data were also analyzed using an 8 mm FWHM Gaussian kernel (which is the level of spatial smoothing used to analyze adult data in Seydell-Greenwald et al., 2019). Findings were comparable and not qualitatively different for 3 mm and 8 mm smoothing.
In addition to this activation, Seydell-Greenwald et al. (2019) previously found that adults also showed activation in the superior parietal lobe (SPL, BA 7) for the contrast of Spatial > Luminance. As a group, children showed activation in the same area; however, this was only evident at a more lenient threshold (p < 0.003). This may suggest that reliance on these areas is common in adults, but not as consistent in children.
Interestingly, this illustrates a pattern of cortical specialization that differs from what has been found for responses to faces, words, and objects in the visual cortex, where the development of category-specific responses is driven by decreases in responses to nonpreferred categories (Cantlon et al., 2011). This demonstrates that hemispheric specialization for different cognitive or visual abilities can occur over the course of development via either a “building up” or “pruning back” of neural responses.
Data availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
References
- Badzakova-Trajkov G, Häberling S, Roberts RP, Corballis MC (2010). Cerebral asymmetries: Complementary and independent processes. PLoS One, 5(3), e9682. doi: 10.1371/journal.pone.0009682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badzakova-Trajkov G, Corballis MC, & Häberling S (2016). Complementarity or independence of hemispheric specializations? Neuropsychologia, 93, 386–393. [DOI] [PubMed] [Google Scholar]
- Behrmann M, & Plaut DC (2020). Hemispheric organization for visual object recognition: A theoretical account and empirical evidence. Perception. doi: 10.1177/0301006619899049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl MM, Mayo J, Parks EN, Rosenberger LR, VanMeter J, Bernstein R, Vaidya CJ, Gaillard WD (2014). Regional differences in the developmental trajectory of lateralization of the language network. Human Brain Mapping, 35, 270–284. doi: 10.1002/hbm.22179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiach E, & Luzzatti C (1978). Unilateral neglect of representational space. Cortex, 14, 129–133. [DOI] [PubMed] [Google Scholar]
- Bogen JE, & Gazzaniga MS (1965). Cerebral commissurotomy in man: Minor hemisphere dominance for certain visuospatial functions. Journal of Neurosurgery, 23, 394–399. doi: 10.3171/jns.1965.23.4.0394 [DOI] [Google Scholar]
- Bradshaw JL, Nettleton NC, Wilson LE, & Bradshaw CS (1987). Line bisection by left-handed preschoolers: A phenomenon of symmetrical neglect. Brain and Cognition, 6(4), 377–385. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Spataro JA, Harris M, Nettleton NC, & Bradshaw J (1988). Crossing the midline by four to eight year old children. Neuropsychologia, 26(2), 221–235. [DOI] [PubMed] [Google Scholar]
- Broca P (1861). Remarks on the seat of the faculty of articulated language, following an observation of aphemia (loss of speech). Bulletin de la Société Anatomique, 6, 330–357. [Google Scholar]
- Cai Q, Van der Haegen L, & Brysbaert M (2013). Complementary hemispheric specialization for language production and visuospatial attention. Proceedings of the National Academy of Sciences of the United States of America, 110, E322–E330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, Platt ML, & Brannon EM (2009). Beyond the number domain. Trends in Cognitive Sciences, 13(2), 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, Pinel P, Dehaene S, & Pelphrey KA (2011). Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cerebral Cortex, 21(1), 191–199. doi: 10.1093/cercor/bhq078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA (2006). Functional imaging of numerical processing in adults and 4-y-old children. PLoS Biology, 4(5), e125. doi: 10.1371/journal.pbio.0040125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Keller TA, Eddy W, & Thulborn K (1999). Graded functional activation in the visuospatial system with the amount of task demand. Journal of Cognitive Neuroscience, 11, 9–24. [DOI] [PubMed] [Google Scholar]
- Cavézian C, Valadao D, Hurwitz M, Saoud M, & Danckert J (2012). Finding center: Ocular and fMRI investigations of bisection and landmark task performance. Brain Research, 1437, 89–103. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Pinel P, Dehaene S, Pelphrey KA (2011). Cortical representations of symbols, objects, and faces, are pruned back during early childhood. Cerebral Cortex, 21, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokron S, & De Agostini M (1995). Reading habits and line bisection: A developmental approach. Cognitive Brain Research, 3(1), 51–58. [DOI] [PubMed] [Google Scholar]
- Çiçek M, Deouell LY, Knight RT (2009). Brain activity during landmark and line bisection tasks. Frontiers in Human Neuroscience, 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thompson WL, Anderson AK, Brookheimer SY, Rosen BR, Belliveau JW (1996). Changes in cortical activity during mental rotation: A mapping study using functional magnetic resonance imaging. Brain, 119, 89–100. [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2011). Spatial neglect and attention networks. Annual Review of Neuroscience, 34, 569–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE, 1993. A PET study of visuospatial attention. Journal of Neuroscience, 13, 1202–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV, 1997. In: Davison AC, Hinkley DV (Eds.), Bootstrap Methods and their Application, 1st Ed. Cambridge Univ. Press, Cambridge. [Google Scholar]
- Dehaene S, & Brannon E (Eds.). (2011). Space, time and number in the brain: Searching for the foundations of mathematical thought. Elsevier Academic Press. [Google Scholar]
- Dehaene S, Cohen L, Morais J, Kolinsky R. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat Rev Neurosci. 2015. Apr;16(4):234–44. doi: 10.1038/nrn3924. Epub 2015 Mar 18. PMID: 25783611. [DOI] [PubMed] [Google Scholar]
- Dehaene S (2005) Evolution of human cortical circuits for reading and arithmetic: The neuronal recycling hypothesis. In: Dehaene S, Duhamel J-R, Hauser MD, Rizzolatti G, editors. From monkey brain to human brain. A Fyssen Foundation symposium. MIT Press, pp. 133–157. [Google Scholar]
- Dehaene S (1992). Varieties of numerical abilities. Cognition, 44, 1–42, doi: 10.1016/0010-0277(92)90049-N [DOI] [PubMed] [Google Scholar]
- Dehaene S (2011) The number sense: how the mind creates mathematics (Oxford UP, Oxford: ), Ed 2. [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L (2003). Three parietal circuits for number processing. Cognitive Neuropsychology, 20, 487–506. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, & Hertz-Pannier L (2002). Functional neuroimaging of speech perception in infants. Science, 298, 2013–2015. [DOI] [PubMed] [Google Scholar]
- Dellatolas G, Coutin T, & De Agostini M (1996). Bisection and perception of horizontal lines in normal children. Cortex, 32(4), 705–715. [DOI] [PubMed] [Google Scholar]
- Desrocher ME, Smith ML, & Taylor MJ (1995). Stimulus and sex differences in performance of mental rotation: Evidence from event-related potentials. Brain and Cognition, 28, 14–38. doi: 10.1006/brcg.1995.1031 [DOI] [PubMed] [Google Scholar]
- Dundas EM, Plaut DC, & Behrmann M (2014). An ERP investigation of the co-development of hemispheric lateralization of face and word recognition. Neuropsychologia, 61C, 315–323. doi: 10.1016/j.neuropsychologia.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundas E, Plaut D, & Behrmann M (2013). The joint development of hemispheric lateralization for words and faces. Journal of Experimental Psychology: General American Psychological Association, 142(2), 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Lidzba K, Hauser T-K, & Wilke M (2011). Assessing language and visuospatial functions with one task: A “dual use” approach to performing fMRI in children. NeuroImage, 58, 923–929. doi: 10.1016/j.neuroimage.2011.06.048 [DOI] [PubMed] [Google Scholar]
- Eckert MA, Hu D, Eliez S, Bellugi U, Galaburda A, Korenberg J, Mills D, Reiss AL. Evidence for superior parietal impairment in Williams syndrome. Neurology. 2005;64:152–153. [DOI] [PubMed] [Google Scholar]
- Everts R, Lidzba K, Wilke M, Kiefer C, Mordasini M, Schroth G,…Steinlin M (2009). Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Human Brain Mapping, 30, 473–483. doi: 10.1002/hbm.20523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failla CV, Sheppard DM, & Bradshaw JL (2003). Age and responding-hand related changes in performance of neurologically normal subjects on the line-bisection and chimeric-faces tasks. Brain and Cognition, 52(3), 353–363. [DOI] [PubMed] [Google Scholar]
- Ferrara K, Seydell-Greenwald A, Landau B & Newport EL (2017, November). Visual-spatial ability after perinatal stroke: Common deficits in patients with right- and left-hemisphere lesions. Poster presented at the annual meeting of the American Society of Neurorehabilitation, Baltimore, MD. [Google Scholar]
- Ferro JM, Martins IP, & Távora L (1984). Neglect in children. Annals of Neurology, 15(3), 281–284. doi: 10.1002/ana.410150314. [DOI] [PubMed] [Google Scholar]
- Fierro B, Brighina F, Oliveri M, Piazza A, La Bua V, Buffa D, & Bisiach E (2000). Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport, 11, 1519–1521. [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Shah NJ, Weiss PH, Halligan PW, Grosse-Ruyken M, Ziemons K, Zilles K, Freund HJ (2000). Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology, 54, 1324–1331. [DOI] [PubMed] [Google Scholar]
- Flöel A, Buyx A, Breitenstein C, Lohmann H, & Knecht S (2005). Hemispheric lateralization of spatial attention in right- and left-hemispheric language dominance. Behavioural Brain Research, 158, 269–275. [DOI] [PubMed] [Google Scholar]
- Ferrara K, Seydell-Greenwald A, Chambers CE, Newport EL, & Landau B (2020). Development of bilateral parietal activation for complex visual-spatial function: Evidence from a visual-spatial construction task. Developmental Science, e13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, McCourt ME, Javitt DC (2003). Right hemisphere control of visuospatial attention: line-bisection judgments evaluated with high-density electrical mapping and source analysis. NeuroImage, 19, 710–726. [DOI] [PubMed] [Google Scholar]
- Gerrits R, Verhelst H, Vingerhoets G (2020). Mirrored brain organization: Statistical anomaly or reversal of hemispheric functional segregation bias? Proceedings of the National Academy of Sciences of the United States of America, 117(25), 14057–14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotheer M, Ambrus GG, & Kovács G (2016) Causal evidence of the involvement of the number form area in the visual detection of numbers and letters. Neuroimage 132, 314319, doi: 10.1016/j.neuroimage.2016.02.069 [DOI] [PubMed] [Google Scholar]
- Hausmann M, Waldie KE, & Corballis MC (2003). Developmental changes in line bisection: A result of callosal maturation? Neuropsychology, 17(1), 155–160. [PubMed] [Google Scholar]
- Hecaen H, Penfield W, Bertrand C, & Malmo R (1956). The syndrome of apractognosia due to lesions of the minor cerebral hemisphere. AMA Archives of Neurology & Psychiatry, 75(4), 400–434. doi: 10.1001/archneurpsyc.1956.02330220064007 [DOI] [PubMed] [Google Scholar]
- Heilman KM & van den Abell. (1980). Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology, 30, 327–330. [DOI] [PubMed] [Google Scholar]
- Hesterberg T, Moore DS, Monaghan S, Clipson A, & Epstein R. (2005). Bootstrap Methods and Permutation Tests, In: Moore DS, McCabe GP (Eds.), 5th Ed. Introduction to the Practice of Statistics, vol. 14. WH Freeman and Co, pp. 1–70. [Google Scholar]
- Hoyos PM, Kim NY, Cheng D, Finkelston D, & Kastner S (2020). Development of spatial biases in school-aged children. Developmental Science, e13053. [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Piazza M, Pinel P, & Dehaene S (2005). Interactions between number and space in parietal cortex. Nature Reviews Neuroscience, 6, 435–448. doi: 10.1038/nrn1684 [DOI] [PubMed] [Google Scholar]
- Hugdahl K & Westerhausen R (2010). The Two Halves of the Brain. MIT Press, Cambridge, MA. [Google Scholar]
- Ivry RB, & Robertson LC (1998). The two sides of perception. Cambridge, MA: MIT Press. [Google Scholar]
- Jansen A, Flöel A, Menke R, Kanowski M, & Knecht S (2005). Dominance for language and spatial processing: Limited capacity of a single hemisphere. NeuroReport,> 16, 1017–1021. [DOI] [PubMed] [Google Scholar]
- Janssen A, Pauls T (2003). How do bootstrap and permutation tests work? Annals of Statistics, 31, 768–806. [Google Scholar]
- Johnston C & Shapiro E (1986). Hemi-inattention resulting from left hemisphere brain damage during infancy. Cortex, 22, 279–287. [DOI] [PubMed] [Google Scholar]
- Jordan K, Wüstenberg T, Heinze H-J, Peters M, Jäncke L (2002). Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia, 40(13), 2397– 2408. doi: 10.1016/S0028-3932(02)00076-3 [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, & Thulborn KR (1996). Brain activation modulated by sentence comprehension. Science, 274(5284),114–116. doi: 10.1126/science.274.5284.114 [DOI] [PubMed] [Google Scholar]
- Karnath HO (1988). Deficits of attention in acute and recovered visual hemi-neglect. Neuropsychologia, 26(1), 27–43. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Gitelman DR & Albert NM (1998). Neural systems that encode categorical versus coordinate spatial relations: PET investigations. Psychobiology, 26, 333–347. [Google Scholar]
- Kosslyn SM, Koenig O, Barrett A, Cave CB, Tang J, & Gabrieli JD (1989). Evidence for two types of spatial representations: hemispheric specialization for categorical and coordinate relations. Journal of Experimental Psychology, 15, 723–735. doi: 10.1037/0096-1523.15.4.723 [DOI] [PubMed] [Google Scholar]
- Kucian K, von Aster M, Loenneker T, Dietrich T, Mast FW, & Martin E (2007). Brain activation during mental rotation in school children and adults. Journal of Neural Transmission, 114, 675–686. doi: 10.1007/s00702-006-0604-5 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS & Baker CI (2009). Circular analysis in systems neuroscience – the dangers of double dipping. Nature Neuroscience, 12(5), 535–540. doi: 10.1038/nn.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenneberg E (1967). Biological Foundations of Language. New York, NY: Wiley. [Google Scholar]
- Levy J (1977). The mammalian brain and the adaptive advantage of cerebral asymmetry. Annals of the New York Academy of Sciences, 299(1), 264–272. doi: 10.1111/j.1749-6632.1977.tb41913.x [DOI] [PubMed] [Google Scholar]
- Lidzba K, Ebner K, Hauser TK, & Wilke M (2013). Complex visual search in children and adolescents: Effects of age and performance on fMRI activation. PLOS ONE, 8(12), e85168. doi: 10.1371/journal.pone.0085168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidzba K, Staudt M, Wilke M, Grodd W, & Krägeloh-Mann I, (2006). Lesion-induced right-hemispheric language and organization of nonverbal functions. Neuroreport, 26, 929–933. doi: 10.1097/01.wnr.0000221841.12632.d6 [DOI] [PubMed] [Google Scholar]
- Lochy A, de Heering A, & Rossion B (2019). The non-linear development of the right hemispheric specialization for human face perception. Neuropsychologia, 126, 10–19. doi: 10.1016/j.neuropsychologia.2017.06.029 [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1981). A cortical network for directed attention and unilateral neglect. Annals of Neurology, 10, 309–325. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen RK, Morris CA, Berman KF. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–631. [DOI] [PubMed] [Google Scholar]
- McFie J, Piercy MF, & Zangwill OL (1950). Visual-spatial agnosia associated with lesions of the right cerebral hemisphere. Brain, 73, 167–190. doi: 10.1093/brain/73.2.167 [DOI] [PubMed] [Google Scholar]
- Moore DS, McCabe GP, Duckworth WM, & Sclove II (2002). In: Moore DS, McCabe GP, Duckworth WM, Sclove II (Eds.), 1st ed. The Practice of Business Statistics: Using Data for Decisions, vol. 18. WH Freeman and Co, pp. 1–73. [Google Scholar]
- Murphy K, & Garavan H (2004). Artifactual fMRI group and condition differences driven by performance confounds. NeuroImage, 21(1), 219–228. doi: 10.1016/j.neuroimage.2003.09.016 [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Herting MM, Maxwell EC, Bruno R, Fair D (2013). Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain and Cognition, 82(1), 58–68. doi: 10.1016/j.bandc.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport EL, Landau B, Seydell-Greenwald A, Turkeltaub PE, Chambers CE, Dromerick AW, Carpenter J, Berl MM, & Gaillard WD (2017). Revisiting Lenneberg’s hypotheses about early developmental plasticity: Language organization after left-hemisphere perinatal stroke. Biolinguistics, 11, 407–421. [PMC free article] [PubMed] [Google Scholar]
- Olulade OA, Seydell-Greenwald A, Chambers CE, Turkeltaub PE, Dromerick AW, Berl MM, Gaillard WD, & Newport EL (2020). The neural basis of language development: Changes in lateralization over age. Proceedings of the National Academy of Sciences of the United States of America. doi: 10.1073/pnas.1905590117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro K, Nuerk H-C, & Brugger P (2018). Visuospatial biases in preschool children: Evidence from line bisection in three-dimensional space. Journal of experimental child psychology, 173, 16–27. [DOI] [PubMed] [Google Scholar]
- Paquier P, Van Mourik M, Van Dongen H, Catsman-Berrevoets C, Creten W, Stronks D (1999). Clinical utility of the judgment of line orientation test and facial recognition test in children with acquired cerebral lesions. Journal of Child Neurology, 14, 243–248. [DOI] [PubMed] [Google Scholar]
- Peronnet F, & Farah MJ (1989). Mental rotation: An event-related potential study with a validated mental rotation task. Brain and Cognition, 9(2), 279–288. doi: 10.1016/0278-2626(89)90037-7 [DOI] [PubMed] [Google Scholar]
- Piazza M, Mechelli A, Price CJ, & Butterworth B (2006). Exact and approximate judgements of visual and auditory numerosity: An fMRI study. Brain Research, 1106(1), 177–188. doi: 10.1016/j.brainres.2006.05.104. [DOI] [PubMed] [Google Scholar]
- Pinel P, Piazza M, Le Bihan D, Dehaene S (2004). Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments, Neuron, 41(6), 983–993, doi: 10.1016/S0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- Poeppel D (2003). The analysis of speech in different temporal integration windows: Cerebral lateralization as ‘asymmetric sampling in time’. Speech Communication, 41, 245–255. [Google Scholar]
- Price GR & Ansari D (2011). Symbol processing in the left angular gyrus: Evidence from passive perception of digits. Neuroimage, 57, 1205–1211, doi: 10.1016/j.neuroimage.2011.05.035 [DOI] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Anwander A, Spada D, Baldoli C, Poloniato A, Lohmann G, Friederici AD (2011). Neural language networks at birth. Proceedings of the National Academy of Sciences of the United States of America, 108, 16056–16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitto LA, Berens MS, Kovelman I, Dubins MH, Jasinska K, Shalinsky M (2012). The “perceptual wedge hypothesis” as the basis for bilingual babies’ phonetic processing advantage: New insights from fNIRS brain imaging. Brain and Language, 121, 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, Reynolds MF, Kwon H, Galaburda A. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A (1941). Psychological examination of traumatic encephalopathy. Archives De Psychologie, 28, 286–340. doi: 10.1080/13854049308401883 [DOI] [Google Scholar]
- Rosch RE, Bishop DVM, & Badcock NA (2012). Lateralized visual attention is unrelated to language lateralization and not influenced by task difficulty – a functional transcranial Dopper study. Neuropsychologia, 50, 810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W, Ugurbil K, Georgopoulos A, & Kim SG (1997). Time-resolved fMRI of mental rotation. Neuroreport, 8(17), 3697–3702. doi: 10.1097/00001756-199712010-0000 [DOI] [PubMed] [Google Scholar]
- Saj A, Heiz J, Van Calster L, & Barisnikov K (2020). Visuospatial bias in line bisection in Williams syndrome. Journal of Intellectual Disability Research, 64(1), 57–61. [DOI] [PubMed] [Google Scholar]
- Seydell-Greenwald A, Ferrara K, Chambers CE, Newport EL, & Landau B (2017). Bilateral parietal activations for complex visual-spatial functions: Evidence from a visual-spatial construction task. Neuropsychologia, 106, 194–206. doi: 10.1016/j.neuropsychologia.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydell-Greenwald A, Pu SF, Ferrara K, Chambers CE, Newport EL, & Landau B (2019). Revisiting the landmark task as a tool for studying hemispheric specialization: What’s really right? Neuropsychologia, 127, 57–65. doi: 10.1016/j.neuropsychologia.2019.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydell-Greenwald A, Chambers CE, Ferrara K, & Newport EL (2020). What you say versus how you say it: Comparing sentence comprehension and emotional prosody processing using fMRI. Neuroimage, 209, 116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, & Ajax ET (1980). Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology, 30(5), 509–517. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE (2002). Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science., 296(5572):1476–9. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schuster V, Herholz P, Zimmermann KM, Westermann S, Frässle S, & Jansen A (2017). Comparison of fMRI paradigms assessing visuospatial processing: Robustness and reproducibility. PLoS One 12(10), e0186344. doi: 10.1371/journal.pone.0186344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DM, Bradshaw JL, & Mattingley JB (2002). Abnormal line bisection judgements in children with Tourette’s syndrome. Neuropsychologia, 40, 253–259. [DOI] [PubMed] [Google Scholar]
- Sheppard DM, Bradshaw JL, Mattingley JB, Lee P (1999). Effects of stimulant medication on the lateralisation of line bisection judgements of ADHD children. Journal of Neurology, Neurosurgery and Psychiatry, 66(1), 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum J, Hermes D, Foster BL, Dastjerdi M, Rangarajan V, Winawer J, Miller KJ, & Parvizi J (2013). A Brain Area for Visual Numerals. Journal of Neuroscience, 33(16) 6709–6715. doi: 10.1523/JNEUROSCI.4558-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry RW, Gazzaniga MS, & Bogen JE (1969). Interhemispheric relationships: The neocortical commissures; syndromes of hemisphere disconnection. In Vinken PJ and Bruyn GW (Eds.), Handbook of Clinical Neurology (pp. 273–290). New York, NY: John Wiley and Sons. [Google Scholar]
- Stiles J, Reilly JS, Levine SC, Trauner DA, Nass R (2012). Neural plasticity and cognitive development: Insights from children with perinatal brain injury. New York, NY: Oxford University Press. [Google Scholar]
- Szczepanski SM, Kastner S (2013). Shifting attentional priorities: control of spatial attention through hemispheric competition. Journal of Neuroscience, 33(12), 5411–5421. doi: 10.1523/JNEUROSCI.4089-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaris GA, Kim S-G, Strup JP, Andersen P, Ugurbil K, Georgopoulos AP (1996). Quantitative relations between parietal activation and performance in mental rotation. Neuroreport. 7, 773–776. [DOI] [PubMed] [Google Scholar]
- Teuber HL (1974). Why two brains? In Schmitt FO & Worden FG (Eds.), The Neurosciences: Third Study Program. Cambridge, MA: MIT Press. [Google Scholar]
- Tomasino B, Gremese M (2016). Eects of stimulus type and strategy on mental rotation network: An activation likelihood estimation meta-analysis. Frontiers in Human Neuroscience, 9, Article693. doi: 10.3389/fnhum.2015.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G (1998). Spatial hemineglect in humans. Trends in Cognitive Sciences, 2(3), 87–97. doi: 10.1016/S1364-6613(98)01145-0 [DOI] [PubMed] [Google Scholar]
- Vallar G & Perani D (1986). The anatomy of unilateral neglect after right-hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia, 24, 609–622. [DOI] [PubMed] [Google Scholar]
- Vallortigara G (2006). The evolutionary psychology of left and right: Costs and benefits of lateralization. Developmental Psychobiology, 48(6), 418–427. [DOI] [PubMed] [Google Scholar]
- van Vugt P, Fransen I, Creten W, Paquier P (2000). Line bisection performances by 650 normal children. Neuropsychologia, 38, 886–895. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Ansari D, Chee MW (2005). Neural correlates of symbolic and non-symbolic arithmetic. Neuropsychologia, 43(5), 744–753. doi: 10.1016/j.neuropsychologia.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Vogel SE, Haigh T, Sommerauer G, Spindler M, Brunner C, Lyons IM, Grabner RH (2017). Processing the order of symbolic numbers: A reliable and unique predictor of arithmetic fluency Journal of Numerical Cognition, 3, 288–308. [Google Scholar]
- Waberski TD, Gobbelé R, Lamberty K, Buchner H, Marshall JC, Fink GR (2008). Timing of visuo-spatial information processing: Electrical source imaging related to line bisection judgments. Neuropsychologia, 46, 1201–1210. [DOI] [PubMed] [Google Scholar]
- Walsh V (2003). A theory of magnitude: common cortical metrics of time, space and quantity. Trends in Cognitive Science, 7, 483–488. doi: 10.1016/j.tics.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Wernicke C (1874). Der aphasische Symptomenkomplex. Eine psychologische Studie auf anatomischer Basis. Cohn M & Weigert, Breslau. [Google Scholar]
- Wilke M & Lidzba K (2007). LI-toolbox: A new toolbox to assess lateralization in functional MR-data. Journal of Neuroscience Methods, 163, 128–136. [DOI] [PubMed] [Google Scholar]
- Wilke M & Schmithorst VJ (2006). A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. NeuroImage, 33, 522–530. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Ben-Shachar M, Glover GH, Feldman HM (2010). Individual differences in auditory sentence comprehension in children: An exploratory event-related functional magnetic resonance imaging investigation. Brain and Language, 114(2), 72–79. doi: 10.1016/j.bandl.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM (2008). Neuroimaging studies of mental rotation: A meta-analysis and review. Journal of Cognitive Neuroscience, 20, 1–19. doi: 10.1162/jocn.2008.20013 [DOI] [PubMed] [Google Scholar]
- Zatorre RJ & Belin P (2001). Spectral and temporal processing in human auditory cortex. Cerebral Cortex, 11(10), 946–953. doi: 10.1093/cercor/11.10.946 [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A (1992). Lateralization of phonetic and pitch discrimination in speech processing. Science, 256(5058), 846–849. doi: 10.1126/science.1589767. PMID: 1589767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.