Abstract
Recipients after hematopoietic stem cell transplantation (HSCT) or chimeric antigen receptor T-cell (CAR-T) therapy are at increased risk for unfavorable outcomes after SARS-CoV-2 infection. The efficacy of COVID-19 vaccines remains undetermined in this vulnerable population, we therefore conducted a pooled analysis to evaluate the immune response after vaccination. A total of 46 studies were finally included, comprising 4757 HSCT and 174 CAR-T recipients. Our results indicated that HSCT and CAR-T recipients had an attenuated immune response to SARS-CoV-2 vaccination compared with healthy individuals, while time interval between transplant and vaccination, immunosuppressive therapy (IST) and lymphocyte counts at vaccination significantly affected the humoral response in HSCT recipients. In addition, seroconversion was significantly higher in patients with BCMA-based CAR-T than those with CD19-based CAR-T. Thus, an adapted vaccination strategy for HSCT and CAR-T recipients may be required, and further research on the effect of a booster dose of COVID-19 vaccine and the role of cellular response after vaccination is warranted.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-022-01300-9.
Keywords: Hematopoietic stem cell transplantation (HSCT), Chimeric antigen receptor T-cell (CAR-T) therapy, SARS-CoV-2 vaccination, Immune response
To the Editor,
The pandemic caused by SARS-CoV-2 has led to global mortality of over 6 million deaths and vaccination is the primary strategy to stop this public health emergency. Recipients after hematopoietic stem cell transplantation (HSCT) or chimeric antigen receptor T-cell (CAR-T) therapy are at increased risk for severe COVID-19 and unfavorable outcomes [1]. Previous studies showed blunted humoral responses to vaccination against SARS-CoV-2 among HSCT and CAR-T recipients [2–6]. With emerging evidence available, we performed a comprehensive meta-analysis to evaluate the immune responses to COVID-19 vaccines in recipients of HSCT and CAR-T (Additional file 1: Methods S1 and Additional file 1: Fig. S1).
Overall, 44 studies comprising 4757 HSCT patients [1182 of autologous HSCT (autoHSCT), 3495 of allogeneic HSCT (alloHSCT), 80 of autoHSCT or alloHSCT (mixed)] (Additional file 1: Table S1) and 12 studies comprising 174 CAR-T recipients were included (Additional file 1: Table S2). For HSCT, five studies investigated the response after partial vaccination, 38 studies evaluated the response after completed vaccination, and seven studies assessed the response after a third dose. As for CAR-T, 11 studies evaluated the response after completed vaccination, and one study assessed the response following a third dose.
The seropositive rates of the second and third dose were significantly higher than the first dose, while no significant difference in seroconversion between the second and third dose (Fig. 1A). The pooled humoral response rate was 81.4% following completed vaccination in HSCT patients (Additional file 1: Fig. S2), with response rates of 86.1% and 79.6% for autoHSCT and alloHSCT. The response rates after one and three vaccine doses were 40.8% and 78.6%, respectively (Additional file 1: Figs. S3–S4). Although pooled analysis could not be performed due to heterogeneity of data, significantly lower antibody titres were observed in HSCT patients compared with healthy controls (Additional file 1: Table S3).
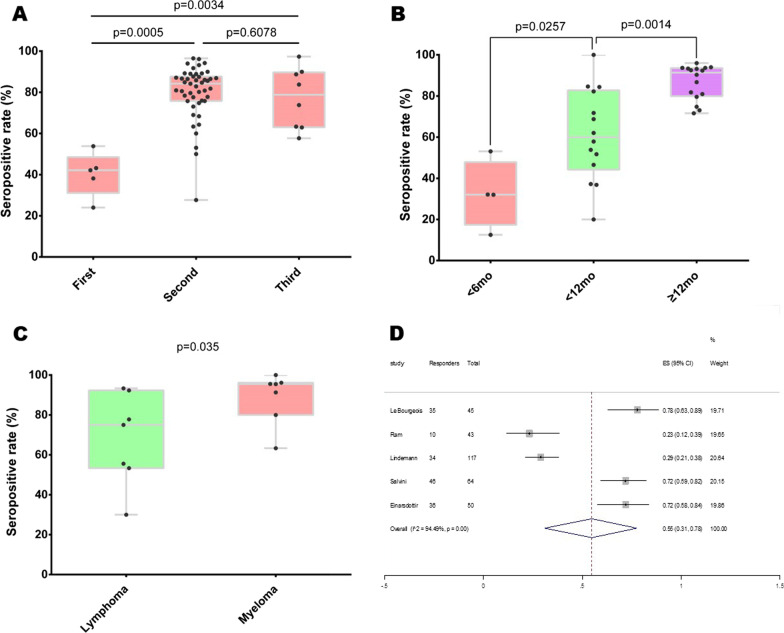
Fig. 1.
The immune response to SARS-CoV-2 vaccines in HSCT recipients. A Boxplots of seropositive rates (%) after the first, second and third dose of vaccination; B boxplots of seropositive rates (%) according to the interval between transplant and vaccination (< 6 months, between 6 and 12 months and ≥ 12 months); C boxplots of seropositive rates (%) in autoHSCT recipients according to underlying diseases (lymphoma or myeloma); D pooled analysis of T-cell response rate based on IFN-γ ELISPOT assay after vaccination. In boxplots, each point indicates a study cohort where data were available. Pairwise comparisons are based on the nonparametric Mann–Whitney U independent-samples test
Our results indicated response rate significantly increased with the time interval from HSCT to vaccination: 38.2% within 6 months, 62.3% between 6 and 12 months, and 87.9% after 12 months (Fig. 1B and Additional file 1: Fig. S5). Among autoHSCT recipients, stratified analysis by underlying diseases demonstrated myeloma patients had a marginal increased seroconversion rate compared to lymphoma patients (P = 0.035, Fig. 1C).
In addition, immunosuppressive therapy (IST: OR = 5.86, 95% CI: 3.74–9.18, P < 10−5; Additional file 1: Fig. S6) and lymphopenia (lymphocyte counts < 1G/L among alloHSCT: OR = 4.44, 95% CI: 2.56–7.70, P < 10–5; Additional file 1: Fig. S7) at vaccination were significantly associated with seronegative response. And neither the status of graft-versus-host disease (GVHD) (Additional file 1: Fig. S8) nor age (Additional file 1: Fig. S9) was significantly associated with seroconversion after vaccination. Furthermore, T-cell response rate based on IFN-γ ELISPOT assay was 55% in HSCT patients (Fig. 1D).
As for CAR-T recipients, the combined serological response rate after SARS-CoV-2 vaccination was 35.9% (Fig. 2A). When stratified by different constructs, seroconversion was significantly higher in patients with BCMA-based CAR-T than those with CD19-based CAR-T (Fig. 2B).
Fig. 2.
The serological response to SARS-CoV-2 vaccines in CAR-T recipients. A Pooled analysis of serological response rate after vaccination; B boxplots of seropositive rates (%) according to CAR-T constructs (CD19 and BCMA). Each point indicates a study cohort where data were available. Pairwise comparisons are based on the nonparametric Mann–Whitney U independent-samples test
Our analysis demonstrated suboptimal immune responses to SARS-CoV-2 vaccination in patients after HSCT and CAR-T, especially for CD19-based CAR-T recipients. Although we found no significant difference between the second and third dose, the boost vaccination against SARS-CoV-2 was identified to improve humoral response in HSCT patients initially seronegative following the second dose [7, 8]. Moreover, antibody levels were reported to significantly increase after the third dose, counteracting the waning immunity after completed vaccination [9, 10]. Of note, new evidence illustrated a majority of alloHSCT patients without GVHD produced neutralizing antibody against Delta and Omicron variants after a third dose [11], underscoring the benefits of a booster dose. Additionally, we found HSCT patients vaccinated after recent transplantation, on IST or with lymphopenia were at higher risk of insufficient responses to COVID-19 vaccines, indicating the importance of immune recovery status for SARS-CoV-2 vaccination.
Interestingly, seroconversion rate was significantly higher in patients with BCMA-based CAR-T compared to those with CD19-directed CAR-T. But due to sparse data, more studies are needed to validate our result and further investigate the impact of different CAR-T constructs on serological response after SARS-CoV-2 vaccination.
In summary, our study indicated that HSCT and CAR-T recipients developed impaired immune responses to COVID-19 vaccines. Thus, an adapted vaccination strategy for these patients may be required. Moreover, the effect of a booster dose and the role of cellular response after SARS-CoV-2 vaccination in HSCT and CAR-T recipients should be addressed in future research.
Supplementary Information
Additional file 1: Methods S1. Supplementary Figure S1. Flowchart of study selection. Supplementary Figure S2. Forest plots for the pooled analysis of serological response after completed vaccination in HSCT recipients. Supplementary Figure S3. Forest plots for the pooled analysis of serological response after one dose of vaccination in HSCT recipients. Supplementary Figure S4. Forest plots for the pooled analysis of serological response after three doses of vaccination in HSCT recipients. Supplementary Figure S5. Forest plots for the pooled analysis of serological response according to the interval between transplant and vaccination (<6 months, between 6-12 months and ≥12 months). Supplementary Figure S6. Forest plots for the association of immunosuppressive therapy and the risk of seronegative response after COVID-19 vaccination in HSCT recipients. Supplementary Figure S7. Forest plots for the association of lymphopenia and the risk of seronegative response after COVID-19 vaccination in alloHSCT recipients. Supplementary Figure S8. Forest plots for the association of the status of GVHD and the risk of seronegative response after COVID-19 vaccination in HSCT recipients. Supplementary Figure S9. Forest plots for the association of age and the risk of seronegative response after COVID-19 vaccination in HSCT recipients. Supplementary Table S1. Study characteristics for HSCT. Supplementary Table S2. Study characteristics for CAR-T. Supplemental Table S3. Anti-Spike (S) SARS-CoV-2 antibody titres in HSCT patients vs. healthy controls.
Acknowledgements
Not applicable.
Abbreviations
- HSCT
Hematopoietic stem cell transplantation
- CAR-T
Chimeric antigen receptor T-cell
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- COVID-19
Coronavirus disease 2019
- autoHSCT
Autologous HSCT
- alloHSCT
Allogeneic HSCT
- IFN-γ
Interferon-gamma
- ELISPOT
Enzyme-linked immunosorbent spot
- IST
Immunosuppressive therapy
- OR
Odds ratio
- CIs
Confidence intervals
- GVHD
Graft-versus-host disease
Author contributions
Concept and design: XW, KFT; acquisition and interpretation of data: LW, LS, KFT, XW; drafting of the manuscript: KFT, XW; critical revision of the manuscript: KFT, XW, LH. All authors read and approved the final manuscript.
Funding
This work was supported by grant from the Research Start-up Fund in Changning Maternity and Infant Health Hospital (2021Y-15).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ljungman P, de la Camara R, Mikulska M, Tridello G, Aguado B, Zahrani MA, Apperley J, Berceanu A, Bofarull RM, Calbacho M, Ciceri F, Lopez-Corral L, Crippa C, Fox ML, Grassi A, Jimenez MJ, Demir SK, Kwon M, Llamas CV, Lorenzo JLL, Mielke S, Orchard K, Porras RP, Vallisa D, Xhaard A, Knelange NS, Cedillo A, Kröger N, Piñana JL, Styczynski J. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35(10):2885–2894. doi: 10.1038/s41375-021-01302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhakal B, Abedin S, Fenske T, Chhabra S, Ledeboer N, Hari P, Hamadani M. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138(14):1278–1281. doi: 10.1182/blood.2021012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maneikis K, Šablauskas K, Ringelevičiūtė U, Vaitekėnaitė V, Čekauskienė R, Kryžauskaitė L, Naumovas D, Banys V, Pečeliūnas V, Beinortas T, Griškevičius L. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583–e592. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Y, Beyar-Katz O, Shefer G, Moshiashvili MM, Karni C, Gold R, Kay S, Glait-Santar C, Eshel R, Perry C, Avivi I, Apel A, Benyamini N, Shasha D, Ben-Ami R. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy—a single-center prospective cohort study. Transplant Cell Ther. 2021;27(9):788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redjoul R, Le Bouter A, Parinet V, Fourati S, Maury S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021;8(10):e681–e683. doi: 10.1016/S2352-3026(21)00274-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abid MA, Abid MB. SARS-CoV-2 vaccine response in CAR T-cell therapy recipients: a systematic review and preliminary observations. Hematol Oncol. 2022;40(2):287–291. doi: 10.1002/hon.2957. [DOI] [PubMed] [Google Scholar]
- 7.Abid MB, Rubin M, Ledeboer N, Szabo A, Longo W, Mohan M, Shah NN, Fenske TS, Abedin S, Runaas L, D'Souza A, Chhabra S, Dhakal B, Hamadani M. Efficacy of a third SARS-CoV-2 mRNA vaccine dose among hematopoietic cell transplantation, CAR T cell, and BiTE recipients. Cancer Cell. 2022;40(4):340–342. doi: 10.1016/j.ccell.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maillard A, Redjoul R, Klemencie M, Labussière Wallet H, Le Bourgeois A, D'Aveni M, Huynh A, Berceanu A, Marchand T, Chantepie S, Botella Garcia C, Loschi M, Joris M, Castilla-Llorente C, Thiebaut-Bertrand A, François S, Leclerc M, Chevallier P, Nguyen S. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. 2022;139(1):134–137. doi: 10.1182/blood.2021014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debie Y, Vandamme T, Goossens ME, van Dam PA, Peeters M. Antibody titres before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in patients with cancer. Eur J Cancer. 2022;163:177–179. doi: 10.1016/j.ejca.2021.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang A, Cicin-Sain C, Pasin C, Epp S, Audigé A, Müller NJ, Nilsson J, Bankova A, Wolfensberger N, Vilinovszki O, Nair G, Hockl P, Schanz U, Kouyos RD, Hasse B, Zinkernagel AS, Trkola A, Manz MG, Abela IA, Müller AMS. Antibody response to SARS-CoV-2 vaccination in patients following allogeneic hematopoietic cell transplantation. Transplant Cell Ther. 2022;28(4):214.e1–214.e11. doi: 10.1016/j.jtct.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canti L, Ariën KK, Desombere I, Humblet-Baron S, Pannus P, Heyndrickx L, Henry A, Servais S, Willems E, Ehx G, Goriely S, Seidel L, Michiels J, Willems B, Goossens ME, Beguin Y, Marchant A, Baron F. Antibody response against SARS-CoV-2 Delta and Omicron variants after third-dose BNT162b2 vaccination in allo-HCT recipients. Cancer Cell. 2022;40(4):335–337. doi: 10.1016/j.ccell.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Methods S1. Supplementary Figure S1. Flowchart of study selection. Supplementary Figure S2. Forest plots for the pooled analysis of serological response after completed vaccination in HSCT recipients. Supplementary Figure S3. Forest plots for the pooled analysis of serological response after one dose of vaccination in HSCT recipients. Supplementary Figure S4. Forest plots for the pooled analysis of serological response after three doses of vaccination in HSCT recipients. Supplementary Figure S5. Forest plots for the pooled analysis of serological response according to the interval between transplant and vaccination (<6 months, between 6-12 months and ≥12 months). Supplementary Figure S6. Forest plots for the association of immunosuppressive therapy and the risk of seronegative response after COVID-19 vaccination in HSCT recipients. Supplementary Figure S7. Forest plots for the association of lymphopenia and the risk of seronegative response after COVID-19 vaccination in alloHSCT recipients. Supplementary Figure S8. Forest plots for the association of the status of GVHD and the risk of seronegative response after COVID-19 vaccination in HSCT recipients. Supplementary Figure S9. Forest plots for the association of age and the risk of seronegative response after COVID-19 vaccination in HSCT recipients. Supplementary Table S1. Study characteristics for HSCT. Supplementary Table S2. Study characteristics for CAR-T. Supplemental Table S3. Anti-Spike (S) SARS-CoV-2 antibody titres in HSCT patients vs. healthy controls.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.