Abstract
Purified CO dehydrogenase (CODH) from Clostridium thermoaceticum catalyzed the transformation of 2,4,6-trinitrotoluene (TNT). The intermediates and reduced products of TNT transformation were separated and appear to be identical to the compounds formed by C. acetobutylicum, namely, 2-hydroxylamino-4,6-dinitrotoluene (2HA46DNT), 4-hydroxylamino-2,6-dinitrotoluene (4HA26DNT), 2,4-dihydroxylamino-6-nitrotoluene (24DHANT), and the Bamberger rearrangement product of 2,4-dihydroxylamino-6-nitrotoluene. In the presence of saturating CO, CODH catalyzed the conversion of TNT to two monohydroxylamino derivatives (2HA46DNT and 4HA26DNT), with 4HA26DNT as the dominant isomer. These derivatives were then converted to 24DHANT, which slowly converted to the Bamberger rearrangement product. Apparent Km and kcat values of TNT reduction were 165 ± 43 μM for TNT and 400 ± 94 s−1, respectively. Cyanide, an inhibitor for the CO/CO2 oxidation/reduction activity of CODH, inhibited the TNT degradation activity of CODH.
2,4,6-Trinitrotoluene (TNT) is a chemical explosive that is a common contaminant of soils and groundwater at numerous Department of Defense facilities. For several decades, research has been conducted to develop ecologically sound means of remediating sites contaminated with this toxic compound. Studies investigating the potential for bioremediation (i.e., the use of microorganisms to metabolize hazardous wastes) have demonstrated that many aerobic and anaerobic microorganisms are capable of catalyzing the reduction of aryl nitro groups associated with TNT (3, 6, 8–10, 16), forming often uncharacterized products.
Aerobic microorganisms contain nitroreductases that catalyze such reductions. An aryl nitro reductase purified from Neurospora crassa reduces several nitrobenzenes, 3,5-dinitrobenzoic acid, and TNT (22). In the presence of NADPH, enzymes in the crude extract of Pseudomonas sp. strain CBS3 catalyzed the reduction of p-nitrobenzoate and TNT (16). An NADH- or NADPH-dependent nitro reductase from Enterobacter cloacae also catalyzes the reduction of TNT (3).
Some anaerobes reduce nitro groups to amines through a mechanism involving nitroso and hydroxyl-amino intermediates, while others, such as Clostridium acetobutylicum, reduce the nitro groups of TNT to hydroxyl-amino-nitrotoluenes without further reduction to the corresponding amines (6–8). The enzymes and/or proteins involved in these transformations have not been identified (9, 10), although ferredoxins, hydrogenases, CO dehydrogenases (CODHs), pyruvate-ferredoxin oxidoreductases, and sulfite reductases have all been implicated. For example, hydrogenase from Clostridium pasteurianum (in the presence of H2) and partially purified CODH from Clostridium thermoaceticum (in the presence of CO), reduced 2,4-diamino-6-nitrotoluene to 2,4-diamino-6-hydroxylaminotoluene when ferredoxin was included in the reaction mixture (10). Reduction also occurred with reduced ferredoxin or methyl viologen in the absence of enzymes, although the rate was slower by orders of magnitude (10). These results suggested that hydrogenase and CODH may act to reduce nitroaromatic compounds by reduction of ferredoxin or methyl viologen. This premise is congruent with the ability of numerous nonspecific agents (H2 on Pt, Zn, and sulfide) to reduce aryl nitro groups. The direct action of these enzymes on TNT, which has multiple nitro groups, has not been described previously.
CODHs from homoacetogens such as Clostridium thermoaceticum also catalyze the synthesis of acetyl-coenzyme A (from CO, a methyl group, and coenzyme A [CoA]) (13). The CO/CO2 redox activity occurs at a novel Ni-X-Fe4S4 cluster known as the C cluster (1, 5), while acetyl-CoA synthesis occurs at a different Ni-Y-Fe4S4 cluster known as the A cluster (14, 20, 21). X and Y are unidentified ligands that bridge the Ni and Fe4S4 components of these distinct clusters.
The primary purpose of studies reported here was to determine whether CODH directly catalyzes TNT reduction or serves only to transfer electrons to other species that reduce TNT nonspecifically. In this report, we show that CODH from C. thermoaceticum catalyzes the reduction of TNT in the presence of CO and that it does so in the absence of ferredoxins or viologens. The reductase activity saturated at increasing TNT concentrations, allowing Km and kcat determinations, and was inhibited by cyanide ion (a known inhibitor for the CO/CO2 redox activity of the enzyme). These results were used to propose the preliminary catalytic mechanism for the CODH-catalyzed reduction of TNT.
MATERIALS AND METHODS
Materials.
TNT was purchased from Chem Service (West Chester, Pa.). [U-ring-14C]TNT was obtained from Chemsyn Science (Lenexa, Kans.). 2-Hydroxylamino-4,6-dinitrotoluene, 4-hydroxylamino-2,6-dinitrotoluene, and 2,4-dihydroxylamino-6-nitrotoluene were synthesized from biotransformation of TNT using C. acetobutylicum and characterized by mass spectroscopy, 1H nuclear magnetic resonance, and infrared spectroscopy (6). Solvents of high-pressure liquid chromatography (HPLC)-grade methanol and 2-propanol were obtained from Fisher Scientific. Perchloric acid was purchased from Mallinckrodt (St. Louis, Mo.). Potassium cyanide (Baker) was prepared in 50 mM NaOH solutions.
Purification of CODH.
CO dehydrogenase was isolated and purified anaerobically as described earlier (11, 17). The final purity was >90% based on the visual estimate of a sodium dodecyl sulfate-polyacrylamide gel. Ferredoxin was cleanly separated from CODH during the gradient DEAE step of the purification, as it remains bound beyond the highest salt concentration used (0.4 M NaCl) (12). Moreover, the electron paramagnetic resonances characteristic of eight-iron ferredoxins were not observed in the spectra of dithionite-reduced samples. All experiments involving the enzyme were performed anaerobically either in a Vacuum/Atmospheres Ar-Atmosphere glove box or a Forma Scientific glove box (90:10 [vol/vol] N2 and H2, respectively). After purification, the enzyme was passed through a Sephadex G25 column equilibrated in 20 mM Tris (pH 8.0) and 10 mM dithiothreitol.
Analysis of TNT and other nitroaromatic compounds.
TNT and its metabolites were fractionated and quantified by using a Waters (Milford, Mass.) HPLC apparatus with diode array UV-visible (UV-VIS) detection and the Millenium Chromatography manager. Spectra were continuously acquired at between 200 and 400 nm, and chromatograms were extracted at 230 nm for quantification. Analytes were separated on a reversed-phase Waters Nova-Pak C8 column (3.9 by 150 mm) at room temperature with an isocratic mobile-phase mixture of 82% water and 18% 2-propanol at 1 ml/min. This system has been well established for separation of hydroxylamino derivatives of TNT reduction compared to related aminated forms (6–8, 19). Peak identification was based on the comparison of elution times and UV spectra with authentic standards.
TNT transformation assay.
CODH (1.8 to 110 μg) was injected into septum-sealed vials containing 2 ml of 50 mM Tris-HCl (pH 7.9) and various concentrations of TNT (41 to 210 μM) at room temperature (∼27°C) under 1 atm of CO. At increasing times after adding CODH, 100-μl aliquots were removed, mixed with 10 μl of 70% perchloric acid to quench the reaction, and analyzed by HPLC.
Purification of [U-ring-14C]TNT.
A solution of [U-ring-14C]TNT in methanol (106 dpm/ml) was purified by silica column chromatography (ethyl acetate hexane, 1:6 [vol/vol]). An aliquot containing the purified [U-ring-14C]TNT was dried by evaporation, and the residue was dissolved in methanol to obtain a stock solution (105 dpm/ml) of [U-ring-14C]TNT. The purity, as evaluated by HPLC fractionation (using the HPLC method), was 98.6%. The stock (20 μl) was mixed with unlabeled TNT (10 ml of a 10-mg/ml concentration) also in methanol to prepare secondary stock solutions.
Kinetics of TNT transformation.
The secondary stock solution (10 μl) was used in the TNT degradation assays described above. Certain processes occurred more rapidly than others, and so to probe both slow and rapid processes different amounts of CODH were used. For assays quenched at times less than 20 min (probing the rapid processes), 1.8 μg of CODH was employed, while 110 μg was used for assays quenched at later times (probing the slow processes). Reaction rates were assumed to be proportional to CODH concentration; thus, the results of the two sets of experiments were normalized to the CODH concentrations used. The concentration profiles of HPLC-detectable intermediates and products were monitored (between 200 and 400 nm) as a function of reaction time. Aliquots of HPLC fractions (both peaks and background regions) were added directly to 5 ml of scintillation cocktail (Ready Gel; Beckman, Fullerton, Calif.), which were then counted (Beckman LS 3801) for 10 min to quantify the 14C in each fraction.
RESULTS
Reduction of TNT catalyzed by CODH.
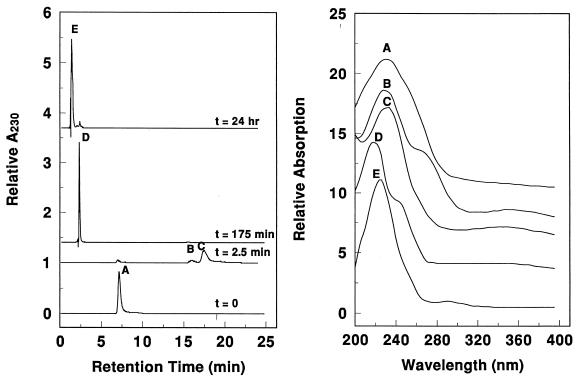
Experiments were conducted to determine whether purified CODH catalyzed the CO-dependent reduction of TNT in the absence of electron transfer mediators such as methyl viologen or ferredoxins. In these studies, catalytic amounts of CODH were added to buffered solutions containing TNT in the presence of 1 atm of CO. Samples were taken periodically and analyzed by HPLC. The resulting chromatograms and spectra are shown in Fig. 1. Prior to adding CODH, the only species detected was TNT (peak A in the t = 0 chromatogram). After 2.5 min, virtually all TNT was consumed and two other species appeared (peaks B and C in the t = 2.5 min chromatogram). The combined concentration of these species increased at roughly the same rate as the TNT declined (not shown). The UV-VIS spectra of B and C (Fig. 1, right panel) and their HPLC retention times matched those of 2-hydroxylamino-4,6-dinitrotoluene (2HA46DNT) and 4-hydroxylamino-2,6-dinitrotoluene (4HA26DNT) respectively, and we tentatively identify the products as those previously characterized. At a longer reaction time (Fig. 1, t = 175 mm), these monohydroxylamino derivatives were absent, and species D was present at its highest concentration. The HPLC retention time and UV-VIS spectrum of D were identical to 2,4-dihydroxylamino-6-nitrotoluene (24DHANT). At even longer times (Fig. 1, t = 24 h), 24DHANT converted to more polar species (labeled E). The retention time and UV-VIS spectrum of E matched the final product of TNT transformation by a C. acetobutylicum extract, which was reported previously (7). This product, either 4-amino-6-hydroxylamino-3-methyl-2-nitrophenol or 6-amino-4-hydroxylamino-3-methyl-2-nitrophenol, results from a catalyzed Bamberger rearrangement of 24DHANT (7).
FIG. 1.
HPLC chromatogram (left panel) and UV-VIS spectra (right panel) of CODH-dependent TNT degradation products. CODH (3.5 μg) was injected into a vial containing 440 μM TNT and 1 atm of CO at room temperature. Aliquots (100 μl) were analyzed by HPLC at t = 0, 0.042, 2.9, and 24 h after injection and then monitored at 230 nm. UV-VIS spectra were obtained for peak fractions, designated A to E. Acid was not used to quench the reaction.
Similar experiments were repeated in the absence of either CO or CODH. TNT was not reduced by CO in the absence of CODH nor by CODH in the absence of CO. Moreover, the species B, C, D, and E were stable for at least 1 day under anaerobic conditions in the absence of CO or CODH. We conclude that CODH catalyzes the CO-dependent reduction of TNT to 2HA46DNT and 4HA26DNT and catalyzes the reduction of these compounds to 24DHANT.
Kinetics of the first step in the CODH-catalyzed TNT transformation.
The CODH-catalyzed rate of TNT degradation to 2HA46DNT and 4HA26DNT at various concentrations of TNT is shown in Fig. 2 (left panel). Inverse rates in the linear range (the first 60 s) were plotted as a function of inverse substrate concentration in Fig. 2 (right panel). CODH-catalyzed degradation of TNT exhibited saturation kinetics, indicating that TNT bound to one of the two active sites of CODH. The apparent Km for CODH for TNT was 165 ± 43 μM and the kcat was 400 ± 94 s−1 under the conditions of the experiment.
FIG. 2.
Kinetics of CODH-dependent TNT degradation. CODH (1.8 μg) was injected into a vial at room temperature containing 1 atm of CO and 41 (■), 62 (●), 83 (⧫), 109 (▾), and 210 (▴) μM TNT. (Left panel) Plot of TNT concentration versus time after adding CODH. (Right panel) Double-reciprocal plot using initial velocities (0 to 60 s) from the left-panel data. The line was curve fitted by linear regression with 95% confidence. The standard deviation values for the kinetic parameters were determined by using StatMost Software.
Time course of TNT transformation and product formation.
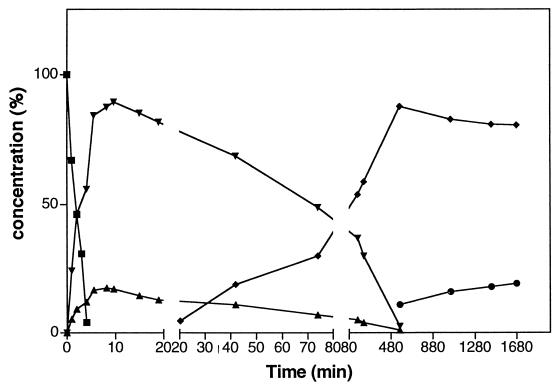
To monitor the pathway of CODH-catalyzed TNT transformation, 14C-labeled TNT was employed to determine the proportion of each intermediate at various times during the reaction. Results from samples removed at various times and monitored by HPLC are plotted in Fig. 3 as the percentage of the 14C (initially present as TNT) in various intermediates as a function of time after adding CODH. The enzyme quickly converted TNT to 4HA26DNT and 2HA46DNT, with about four times more 4HA26DNT produced than 2HA46DNT. These monohydroxylamino derivatives slowly converted to 24DHANT, which eventually converted to the Bamberger rearrangement product.
FIG. 3.
Temporal transformation of TNT by CODH. The initial compound TNT (■) was converted to 4HADNT (▾), 2HADNT (▴), 24-DHANT (⧫), and polar product resulting from Bamberger rearrangement (●) during the reaction with CODH in the presence of saturated CO. For reasons described in Materials and Methods, the enzyme amount (1.8 μg) used for the fast early phase of TNT transformation was 1/60th of that used for the latter, slower phase of TNT transformation. The reaction time is normalized for the same amount of enzyme (1.8 μg).
Inhibition of CODH-catalyzed transformation of TNT by cyanide.
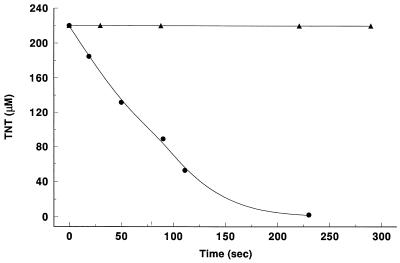
Cyanide is a potent inhibitor of the CO oxidation activity of CODH. Therefore, experiments were conducted to examine the impact of CN− on CODH-catalyzed transformation of TNT. As shown in Fig. 4, enzyme treated with CN− was inactive toward TNT, while the control sample rapidly transformed TNT. One peculiar (and poorly understood) property of CN−-inhibited enzyme is that it can be reactivated by incubation in an atm of CO for ca. 1 h (1). CN−-inhibited enzyme was reactivated to some extent (13%) toward TNT degradation after incubating 40 min under 1 atm of CO.
FIG. 4.
Analysis of inhibition of CODH-catalyzed TNT degradation. The degradation of TNT with enzyme treated with CN (▴) or left untreated (●) is shown. CODH (1.8 μg) was treated with 10 eq/αβ KCN for about 0.5 h and then assayed as described in the text in 2 ml of assay buffer (lacking cyanide) with 220 μM TNT and 1 atm of CO. An equivalent sample not treated with KCN was also assayed.
DISCUSSION
Our results show that in the absence of electron transfer mediators, the purified CODH catalyzes the reductive degradation of TNT. TNT is rapidly reduced to the initial intermediates 4-hydroxylamino-2,6-dinitrotoluene (∼80%) and 2-hydroxylamino-4,6-dinitrotoluene (∼20%). CODH then catalyzes the reductive transformation of these monohydroxylamino derivatives, at a slower rate than the initial reduction of TNT, to 2,4-dihydroxylamino-6-nitrotoluene. The final product of CODH transformation is a Bamberger rearrangement product. Moreover, CODH-catalyzed TNT transformation exhibited saturation kinetics, indicating that TNT binds and is reduced at one of the two active sites on the enzyme. In a previous report, partially purified CODH reduced 2,4-diamino-6-nitrotoluene (DANT) to 2,4-diamino-6-hydroxylaminotoluene in the presence of methyl viologen and CO (10). The catalytic rate of CODH-catalyzed DANT reduction reported in that study was estimated to be about 8 s−1. However, it was not determined whether the reduction was catalyzed by CODH or if methyl viologen was responsible for the reduction and CODH simply served to reduce methyl viologen. Since the reduction of DANT also occurred in the presence of dithionite-reduced methyl viologen (or benzyl viologen or ferredoxin) and in the absence of CODH, the reduction was concluded to be nonspecific and probably mediated by any redox enzyme capable of reducing viologens or ferredoxins (10). In contrast, our results indicate a catalytic rate of 400 s−1 for TNT transformation and suggest that TNT binds to an active site of CODH. Furthermore, CODH was capable of reducing aryl nitro groups in the absence of auxiliary electron carriers.
The fact that cyanide inhibits the reaction and that adding CO restores activity indicates a CO activated site is required for TNT reduction and suggests that TNT may bind to the active site for CO-CO2 redox catalysis, namely, the C cluster. Given the electronic similarities of CO2 and an aryl nitro group (R-NO2) and the proposed mechanism for the two-electron reduction of CO2 by CODH (2, 15), it is reasonable to suggest a related mechanism for the four-electron reduction of TNT. This mechanism would involve a nucleophilic attack and two-electron reduction of R-NO2 by the fully reduced (Cred2) state of the enzyme, followed by two protonations and loss of water. The resulting nitroso intermediate (RNO) would be similarly attacked and reduced by another fully reduced enzyme, and two more protonations would yield the product RNHOH. Further studies are required to test the validity of this mechanism.
The reaction intermediates and products of TNT reported here appear to be identical to those found in the transformation of TNT by C. acetobutylicum (7, 8), suggesting that this organism may contain CODH or an enzyme exhibiting a similar mechanism. A CODH has been reported in C. pasteurianum (18), and elements of CODH have been found in a BLAST search of the genome of C. acetobutylicum ATCC 824. However, CODH may not be responsible for TNT degradation in C. acetobutylicum since transformation was stimulated by H2 (8). Further comparison of TNT transformation in these two different clostridia would give insights into the enzymatic mechanisms of TNT transformation in anaerobic bacteria. Information gained from this study will help identify optimal conditions and point out potential limitations or opportunities of particular enzymatic systems for the degradation of explosives.
ACKNOWLEDGMENTS
This work was supported by a grant from the DSWA project 01-97-1-0020 and by the Advanced Technology Program of Texas (FICE code 010366, project 020).
REFERENCES
- 1.Anderson M E, Lindahl P A. Organization of clusters and internal electron pathways in CO dehydrogenase from Clostridium thermoaceticum: relevance to the mechanism of catalysis and cyanide inhibition. Biochemistry. 1994;33:8702–8711. doi: 10.1021/bi00195a011. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M E, Lindahl P A. Spectroscopic states of the CO oxidation/CO2 reduction active site of carbon monoxide dehydrogenase and mechanistic implications. Biochemistry. 1996;35:8371–8380. doi: 10.1021/bi952902w. [DOI] [PubMed] [Google Scholar]
- 3.Bryant C, DeLuca M. Purification and characterization of an oxygen-insensitive NAD(P)H nitroreductase from Enterobacter cloacae. J Biol Chem. 1991;266:4119–4125. [PubMed] [Google Scholar]
- 4.DeRose V J, Telser J, Anderson M E, Lindahl P A, Hoffman B M. A multinuclear ENDOR study of the C-cluster in CO dehydrogenase from Clostridium thermoaceticum: evidence for HxO and histidine coordination to the [Fe4S4] center. J Am Chem Soc. 1998;120:8767–8776. [Google Scholar]
- 5.Hu X, Spangler N J, Anderson M E, Xia J, Ludden P W, Lindahl P A, Münck E. Nature of the C-cluster in Ni-containing carbon monoxide dehydrogenase. J Am Chem Soc. 1996;118:830–845. [Google Scholar]
- 6.Hughes J, Wang C Y, Bhadra R, Richardson A, Bennett G, Rudolph F. Reduction of 2,4,6-trinitrotoluene by Clostridium acetobutylicum through hydroxylamino-nitrotoluene intermediates. Environ Toxicol Chem. 1998;17:343–348. [Google Scholar]
- 7.Hughes J, Wang C, Yesland K, Richardson A, Bhadra R, Bennett G, Rudolph F. Bamberger rearrangement during TNT metabolism by Clostridium acetobutylicum. Environ Sci Technol. 1998;32:494–500. [Google Scholar]
- 8.Khan T A, Bhadra R, Hughes J. Anaerobic transformation of 2,4,6-TNT and related nitroaromatic compounds by Clostridium acetobutylicum. J Ind Microbiol Biotechnol. 1997;18:198–203. [Google Scholar]
- 9.McCormick N G, Feeherry F E, Levinson H S. Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl Environ Microbiol. 1976;31:949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preuss A, Fimpel J, Diekert G. Anaerobic transformation of 2,4,6-trinitrotoluene (TNT) Arch Microbiol. 1993;159:345–353. doi: 10.1007/BF00290917. [DOI] [PubMed] [Google Scholar]
- 11.Ragsdale S W. Enzymology of the acetyl-CoA pathway of CO2 fixation. CRC Crit Rev Biochem Mol Biol. 1991;26:261–300. doi: 10.3109/10409239109114070. [DOI] [PubMed] [Google Scholar]
- 12.Ragsdale S W, Baur J R, Lindahl P A. The amino acid sequence and redox properties of the 8-iron ferredoxin II from Clostridium thermoaceticum. FASEB J. 1991;5:A470–A470. [Google Scholar]
- 13.Ragsdale S W, Kumar M. Nickel-containing carbon monoxide dehydrogenase/acetyl-CoA synthase. Chem Rev. 1996;96:2515–2539. doi: 10.1021/cr950058+. [DOI] [PubMed] [Google Scholar]
- 14.Russell W K, Stülhandske M V, Xia J, Scott R A, Lindahl P A. Spectroscopic, redox, and structural characterization of the Ni-labile and nonlabile forms of the acetyl-CoA synthase active site of carbon monoxide dehydrogenase. J Am Chem Soc. 1998;120:7502–7510. [Google Scholar]
- 15.Seravalli J, Kumar M, Lu W-P, Ragsdale S W. Mechanism of carbon monoxide oxidation by the carbon monoxide dehydrogenase/acetyl-CoA synthase from Clostridium thermoaceticum: kinetic characterization of the intermediates. Biochemistry. 1997;36:11241–11251. doi: 10.1021/bi970590m. [DOI] [PubMed] [Google Scholar]
- 16.Schackmann A, Müller R. Reduction of nitroaromatic compounds by different Pseudomonas species under aerobic conditions. Appl Microbiol Biotechnol. 1991;34:809–813. [Google Scholar]
- 17.Shin W, Anderson M E, Lindahl P A. Heterogenous nickel environments in carbon monoxide dehydrogenase from Clostridium thermoaceticum. J Am Chem Soc. 1993;115:5522–5526. [Google Scholar]
- 18.Thauer R K, Fuchs G, Käufer B, Schnitker U. Carbon monoxide oxidation in cell-free extracts of Clostridium pasteurianum. Eur J Biochem. 1974;45:343–349. doi: 10.1111/j.1432-1033.1974.tb03559.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Hughes J B. Derivatization and separation of 2,4,6-trinitrotoluene metabolic products. Biotechnol Tech. 1998;12:839–842. [Google Scholar]
- 20.Xia J, Lindahl P A. Assembly of an exchange-coupled [Ni:Fe4S4] cluster in the α subunit of carbon monoxide dehydrogenase from Clostridium thermoaceticum with spectroscopic properties and CO-binding ability mimicking those of the acetyl-CoA synthase active site. J Am Chem Soc. 1996;118:483–483. [Google Scholar]
- 21.Xia J, Hu Z, Popescu C V, Lindahl P A, Münck E. Mössbauer and EPR study of the Ni-activated α-subunit of carbon monoxide dehydrogenase from Clostridium thermoaceticum. J Am Chem Soc. 1997;119:8301–8312. [Google Scholar]
- 22.Zucker M, Nason A. Nitroaryl reductase from Neurospora crassa. Methods Enzymol. 1955;2:406–411. [Google Scholar]