Abstract
This experiment compared the effects of 2 chronic heat stress (HS) models, constant (coHS), and cyclic (cyHS), on broiler performance, carcass characteristics, and meat quality. A total of 720 male chicks from a Cobb 500 line were placed in 12 environmentally controlled chambers divided into 2 pens of 30 birds. Before the experimental HS models were applied, chamber temperatures were gradually decreased from 32°C at placement to 24°C on d 20. From 20 to 41 d, 4 chambers were set to 35°C (coHS), and 4 chambers were set to 35°C for 12 h and 24°C for the next 12 h (cyHS). Four thermoneutral chambers were maintained at 24°C with half of the birds pair-fed to equalize feed intake (FI) with coHS birds (TN-coPF) and half fed ad-libitum (TN-al). From 20 to 41 d, FI and BW gain (BWG) of cyHS, coHS and TN-coPF birds were decreased (P < 0.001), whereas feed conversion ratio (FCR) was increased (P < 0.001) for coHS and TN-coPF birds compared with TN-al birds. The overall BWG and FCR of coHS birds were lower (P < 0.001) than TN-coPF birds. Both HS models reduced (P < 0.001) carcass weight, pectoralis major yield, total breast meat yield, and increased (P < 0.001) wing yield relative to TN-al birds, with each of these measurements more impacted by coHS than by cyHS. Pair-fed birds had lower (P < 0.001) fat pad and a higher total breast meat yield than coHS birds. They also had the lowest (P < 0.001) pectoralis major ultimate pH and yellowness, and these parameters were lower (P < 0.001) for coHS birds than for TN-al birds. Both HS models reduced (P < 0.001) the incidence of woody breast and white striping. Thus, these data indicate that the detrimental effects of HS cannot be entirely explained by reduced FI and that HS per se affects metabolic pathways associated with muscle and lipid accretion in broilers.
Key words: heat stress, feed intake, performance, carcass characteristic, broiler
INTRODUCTION
Poultry production faces several important environmental challenges in meeting the increasing global demand for animal protein. By 2050, predictions estimate the animal based food demand will rise by nearly 70% (Searchinger et al., 2019), and meanwhile, climate change is a major concern for livestock production in the context of global warming. Temperate zones where most of the industrialized farming systems are found may lose 25% of their animal production due to global warming, and this scenario may be worse for some regions of Asia and Africa where extensive farming systems are more abundant (Nardone et al., 2010). By definition, heat stress (HS) is a common environmental stressor that occurs when the amount of heat produced by an animal surpasses the animal's capacity to dissipate the heat to its surrounding environment (Lara and Rostagno, 2013). This imbalance between heat production and body heat loss occurs when the environmental temperature rises above the upper critical temperature of the thermoneutral zone (Bernabucci et al., 2010; Lara and Rostagno, 2013).
Birds are particularly sensitive to heat because their capacity for heat loss is limited by feathering and the lack of sweat glands. Furthermore, the genetic selection of high performing birds over several decades has resulted in birds with elevated metabolic rates, making them more sensitive to hot temperatures (Lara and Rostagno, 2013). When environmental temperature rises above the thermoneutral zone, birds decrease their feed intake and physical activity to reduce heat production and increase panting and water consumption to dissipate heat (McFarlane et al., 1989; Mahmoud et al., 2015). However, at certain temperature thresholds, birds can no longer control their body temperature, leading to welfare problems and detrimental effects on performance, carcass characteristics, and meat quality ensue. From an economical perspective, HS has been estimated to cause total annual economic losses of $128 to $165 million to the US poultry industry (St-Pierre et al., 2003). However, due to a lack of more recent economic evaluations, these numbers are probably currently underestimated due to the growth of the poultry industry over the last decades and worsening of global warming predictions. Furthermore, it is likely that the consequences of HS are even more severe in tropical countries (Pawar et al., 2016).
Numerous studies have investigated the detrimental effect of HS on performance, most often under constant hot temperatures to provide marked responses to model HS (Alleman and Leclercq, 1997; Temim et al., 2000; Lu et al., 2007; Rosa et al., 2007). More recently, several publications have utilized daily cyclical HS to mimic the diurnal pattern observed in temperate countries during summer. These models consistently demonstrate a degradation of body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) when compared with birds in thermoneutral conditions (Zhang et al., 2012; De Souza et al., 2016; Awad et al., 2018), with constant HS having a greater effect than cyclic HS. Also, the magnitude of performance reductions observed with different HS models depends on several parameters including the temperature, relative humidity, length, and cyclicality of the heat period, as well as the age of the birds at the beginning of the stress period. Degradations in carcass composition and meat quality have also commonly been observed under cyclic and constant HS (Baziz et al., 1996; Song and King, 2015; Zeferino et al., 2016; De Antonio et al., 2017; Roushdy et al., 2018).
A reduction in feed intake (FI) in attempt to limit heat production is the main factor explaining degraded performance in response to HS (Syafwan et al., 2011). However, other physiological responses need to be considered, as pair-feeding birds under thermoneutral conditions to equalize FI with those subjected to HS treatments indicates that reduced FI alone does not fully account for the reduction in growth performance associated with HS (De Souza et al., 2016). Further investigations comparing both chronic constant and cyclical HS models within the same experiment for market-age birds reared in floor pens are required. As such, the objective of this experiment was to compare 2 chronic HS models (constant and cyclic) to assess their influence on performance, carcass characteristics, and meat quality of broilers reared to 42 d, and to determine the direct effect of constant HS on these parameters independent of decreased FI.
MATERIAL AND METHODS
Animals and Experimental Design
All animal care and procedures were approved by the Institutional Animal Care and Use Committee at the University of Arkansas (protocol #20020).
Seven hundred and twenty male chicks from a Cobb 500 female breeder line were obtained from the Cobb hatchery (Fayetteville, AR). Upon arrival, chicks were selected to ensure that the weight of each group of 30 chicks fell within 3% of the expected group weight based on a preliminary weight of approximately one-half of the population. Birds were allocated to 12 environmentally controlled chambers divided into 2 pens. Each chamber measured 2.44 × 3.66 m and was divided by wire paneling into 2 pens of 4.47 m2 that each housed 30 chicks. Each pen within the chambers was equipped with hanging pan feeders, nipple waters, and concrete floors covered with fresh pine shavings. Before experimental HS models were applied, ambient temperatures of all the chambers were gradually decreased from 32°C at placement to 24°C on d 20.
On d 20, bird numbers were equalized to 25 per pen before application of the experimental treatments and to ensure that each group weight of 25 birds was similar (within 3% of the overall average). From 20 to 41 d, 3 different environmental conditions and 1 pair-feeding treatment formed a total of 4 treatments: thermoneutral birds fed ad-libitum (TN-al); cyclic HS (cyHS) birds fed ad libitum in 4 cyclic HS chambers for which the temperature was maintained at 35°C for 12 h daily (from 7:30 to 19:30) and reduced to 24°C each night; constant HS (coHS) birds fed ad libitum in 4 chambers for which the temperature was set to and maintained at 35°C; and a group kept in thermoneutral chambers and pair-fed to equalize feed intake to that of coHS bird (TN-coPF). These birds were fed one time per day with the same amount of feed consumed by coHS birds over the last 24 hours. Moreover, for the first week of the challenge only, as will be discussed below, an additional amount equal to the expected relative daily increase in FI was distributed to the TN-coPF birds according to the following formula: , with FId-2, d-1, d being the FI of coHS birds measured on day d-2, d-1, and d.
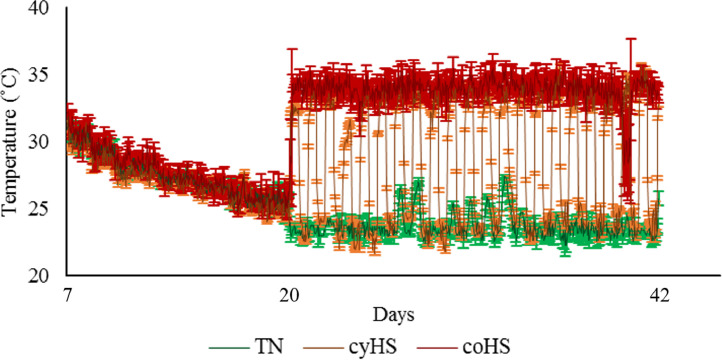
Each chamber was equipped with a Thermochron temperature logger (iButton, DS1922L, Embedded Data Systems, KY) to record environmental temperature hourly (Figure 1). As described previously (Rajaei-Sharifabadi et al., 2017), two birds per pen were randomly selected and equipped with the same temperature logger for continuous monitoring of core body temperature, but malfunctioning of the loggers prevented data recovery for most birds. Water was provided ad libitum throughout the experiment and a diet based on corn and soybean meal was formulated and fed in 3 phases: starter from d 0 to 13, grower from d 14 to 27, and finisher from d 28 to 42 (Table 1). The photoperiod was set at 23L:1D from placement to 7 d, 16L:8D from 8 to 28 d, and 18L:6D from 29 d until the end of the trial. Light intensity was set at 27 lux from 1 to 7 d, 16 lux from 8 to 14 d, and 6 lux from 15 d to the end of the experiment.
Figure 1.
Average chamber temperature recorded during the experiment. TN: Chambers with a continuous 24°C from d20. cyHS: Chambers with a cyclic high temperature (8 h at 35˚C and 12 h at 24˚C) from d20. coHS: Chambers with a continuous 35°C from d20.
Table 1.
Composition of starter, grower, and finisher diets.
| Item, % as-fed | Starter (0 to 13 d) | Grower (14 to 27 d) | Finisher (28 to 42 d) |
|---|---|---|---|
| Corn | 58.74 | 62.11 | 65.56 |
| Soybean meal (47.5%) | 37.53 | 33.98 | 29.75 |
| Poultry Fat | 0.50 | 1.00 | 1.59 |
| Limestone | 1.10 | 1.08 | 1.03 |
| Dicalcium phosphate | 0.78 | 0.62 | 0.43 |
| Salt | 0.40 | 0.41 | 0.41 |
| DL-methionine1 | 0.30 | 0.27 | 0.23 |
| L-lysine HCl | 0.13 | 0.11 | 0.09 |
| L-threonine | 0.10 | 0.04 | 0.02 |
| Choline chloride (60%) | 0.11 | 0.08 | 0.07 |
| Vitamin and mineral premix2 | 0.25 | 0.25 | 0.25 |
| Enzyme blend3 | 0.01 | 0.01 | 0.01 |
| Coccidiostat4 | 0.05 | 0.05 | 0.05 |
| Titanium Dioxide | 0.00 | 0.00 | 0.50 |
| Calculated nutrient composition5 | |||
| AMEn (kcal/kg) | 2,991 | 3,057 | 3,120 |
| Crude Protein | 22.81 | 21.28 | 19.45 |
| Digestible Lys | 1.22 | 1.12 | 1.00 |
| Digestible TSAA | 0.92 | 0.85 | 0.78 |
| Digestible Thr | 0.83 | 0.73 | 0.65 |
| Digestible Arg | 1.41 | 1.31 | 1.18 |
| Digestible Ile | 0.89 | 0.83 | 0.76 |
| Digestible Val | 0.98 | 0.92 | 0.85 |
| Total Ca | 0.90 | 0.84 | 0.76 |
| Total P | 0.57 | 0.53 | 0.47 |
| Available P | 0.45 | 0.42 | 0.38 |
| DEB6 (mEq) | 268 | 250 | 229 |
| Analyzed nutrient composition5 | |||
| Crude Protein | 25.70 | 23.33 | 19.99 |
| Total Ca | 0.80 | 0.80 | 0.62 |
| Total P | 0.56 | 0.51 | 0.45 |
HMTBA-Ca salt (Adisseo France S.A.S., Antony France).
Supplied the following per kg of diet: vitamin A, 6,173 IU; vitamin D3, 4,409 ICU; vitamin E, 44 IU; vitamin B12, 0.01 mg; menadione, 1.20 mg; riboflavin, 5.29 mg; d-pantothenic acid, 7.94 mg; thiamine, 1.23 mg; niacin, 30.86 mg; pyridoxine, 2.20 mg; folic acid, 0.71 mg; biotin, 0.07 mg; manganese, 24 mg; zinc, 14.4 mg; selenium, 0.04 mg; copper, 0.68 mg; iodine, 0.47 mg.
Rovabio AdvancePhy T (Adisseo France S.A.S., Antony France).
BioCox60 (Huvepharma, INC., USA).
Values reported as percentages unless noted otherwise.
DEB: Dietary electrolyte balance.
Performance Measurements and Carcass Characteristics
Feed intake and body weights (BW) were measured at 0, 20, 27, 34, and 41 d posthatch and mortality was recorded daily. On d 42 birds were processed at the University of Arkansas Pilot Processing Plant (Fayetteville, AR) following an overnight feed withdrawal for 8 h. The weight of each processed bird was individually measured before they were subjected to electrical stunning (11 V, 11 mA for 11 s) and exsanguination via a jugular vein cut. After scalding at 53.8°C, feathers were removed with a commercial inline defeatherer (Foodcraft Model 3; Baker international, MI). Necks, heads, and feet were removed from each bird. Carcasses were then mechanically eviscerated. Carcass and abdominal fat weights were recorded before placing carcasses in ice water for a 4 h chill. Chilled carcasses were weighed, and pectoralis major (P. major) and minor (P. minor) muscles, wings, and leg quarters were removed and weighed. Total breast meat (TBM) was calculated as the sum of the P. major and P. minor weights, and the yield of each part was determined by division of the part weight by the individual back dock live weight. The P. major from each bird was placed on an aluminum tray, covered with plastic wrap and stored at 4°C until 24 h postmortem.
Meat Quality
P. major fillets were immediately scored for woody breast (WB) and white striping (WS) on a visual scale from 0 to 3 and an increment of 0.5 by a trained individual (Kuttappan et al., 2012b; Tijare et al., 2016; Kuttappan et al., 2016). To simplify data representation, WB scores were categorized as normal (0–0.5), mild (1–1.5), or severe (2.0–3.0). Similarly, WS scores were categorized as normal, faint, or apparent.
At 24 h postmortem, P. major color was measured according to the L* a* b* scale using a Minolta colorimeter (CR-400; Konica Minolta Sensing Inc., Sakai Osaka, Japan; size 102 (W) × 217 (H) × 63 (D) mm) with illuminant D65 and a 2.54-cm aperture. Three readings were performed on the ventral side of the right P. major and averaged to obtain the color result. Pectoralis major ultimate pH (pHu) was measured with a temperature-compensating pH meter (Testo 205; Testo Inc., West Chester, PA) inserted into the cranial region of the right P. major lobe with an average of 3 measurements per each sample (Orlowski et al., 2018).
Statistical Analysis
The experimental unit was the pen within chambers assigned to environmental conditions in a completely randomized design. Data for the 2 pens within the chambers assigned to the coHS and cyHS treatments were averaged and treated as 1 pen since the treatment was applied to the entire chamber. Thus, while each treatment had a total of 4 replicates, the number of individuals per replicate depended on the environmental condition, with data for birds under HS conditions (cyHS and coHS) consisting of an average of 2 pens in the respective chambers (50 birds total) and data for birds under thermoneutral conditions (TN-al and TN-coPF) representing 1 pen of 25 birds.
Growth performance (FI, BWG, FCR, and morality), carcass characteristics (P. major, P. minor, wings, leg quarters, TBM weights and yields), and meat quality (P. major pHu and color) were subjected to a one-way ANOVA and means were separated using a Tukey's HSD test. Mean differences were considered statistically significant when P < 0.05 and all analyses were performed using R (RStudio 1.3.1093).
RESULTS
Effect of Two Chronic HS Models and Pair-Feeding on Bird Performance
From 0 to 20 d, all birds were reared under the same environmental conditions and received the same diet. Diet analyses indicated that the CP content of the soybean meal may have been higher than estimated, resulting in higher analyzed dietary CP than expected, especially during the starter phase before experimental treatments were applied (Table 1). No differences (P > 0.05) among each set of 4 chambers were observed on BW, BWG, FI, FCR, and mortality during this period, and subsequent removal of some birds from each chamber before the beginning of the experimental phase allowed each treatment to have average bird weights within 1.3% of the grand mean weight for all treatments.
The live broiler performance data measured during the 3 cumulative periods from 20 to 27 d, 20 to 34 d, and 20 to 41 d are presented in Table 2. During the first week of the challenge (d 20 to 27), birds in the coHS and TN-coPF treatments had the lowest (P < 0.001) FI, while birds in the TN-al had the highest FI, and FI of cyHS birds was intermediate. Compared with the TN-al group, BW and BWG were reduced (P < 0.001) by cyHS, further reduced by coHS, and BWG of the TN-coPF group was intermediate to the cyHS and coHS groups. Cyclic HS and TN-al had the lowest (P < 0.001) FCR, coHS birds had the highest FCR, and FCR of the TN-coPF treatment was intermediate to these groups.
Table 2.
Cumulative live performance of broilers from 20 to 27 d, 20 to 34 d, and 20 to 41 d reared under different environmental conditions and feed regimens.
| Period | Parameter3 | Treatment1 |
SEM2 | P-values | |||
|---|---|---|---|---|---|---|---|
| TN-al | cyHS | coHS | TN-coPF | ||||
| 20 d BW, kg | 0.975 | 0.968 | 0.968 | 0.965 | 0.015 | 0.788 | |
| 20 to 27 d | 27 d BW, kg | 1.693a | 1.588b | 1.361d | 1.467c | 0.035 | <0.001 |
| BWG, kg | 0.717a | 0.620b | 0.390d | 0.501c | 0.027 | <0.001 | |
| FI, kg | 1.039a | 0.931b | 0.760c | 0.880b | 0.030 | <0.001 | |
| FCR | 1.448c | 1.503c | 1.956a | 1.761b | 0.043 | <0.001 | |
| Mortality, % | 2.00 | 0.00 | 0.00 | 0.99 | 1.288 | 0.140 | |
| 20 to 34 d | 34 d BW, kg | 2.492a | 2.215b | 1.633d | 1.795c | 0.065 | <0.001 |
| BWG, kg | 1.516a | 1.248b | 0.663d | 0.829c | 0.058 | <0.001 | |
| FI, kg | 2.314a | 2.409a | 1.477c | 1.619b | 0.056 | <0.001 | |
| FCR | 1.492c | 1.936b | 2.270a | 2.016ab | 0.112 | <0.001 | |
| Mortality, % | 2.67 | 0.00 | 1.50 | 2.51 | 2.552 | 0.557 | |
| 20 to 41 d | 41 d BW, kg | 3.312a | 2.841b | 1.748d | 2.040c | 0.099 | <0.001 |
| BWG, kg | 2.336a | 1.873b | 0.777d | 1.074c | 0.094 | <0.001 | |
| FI, kg | 3.781a | 3.178b | 2.069c | 2.223c | 0.109 | <0.001 | |
| FCR | 1.559c | 1.710c | 2.806a | 2.185b | 0.169 | <0.001 | |
| Mortality, % | 2.67 | 0.00 | 3.00 | 8.14 | 3.726 | 0.082 | |
TN-al: Birds reared under continuous 24°C and ad libitum feeding. cyHS: Birds reared under cyclic high temperature (8 h at 35˚C and 12 h at 24˚C) and ad libitum feeding. coHS: Birds reared under continuous 35°C and ad libitum feeding. TN-coPF: Birds reared under continuous 24°C and pair-fed to the coHS treatment.
SEM: pooled standard error of the mean.
BW: Body weight; BWG: Body weight gain; FI: Feed intake; FCR: Feed conversion ratio.
Means within row without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's multiple comparison test.
During the 20 to 34 d cumulative period, the FI of cyHS birds was similar to that of TN-al birds, reduced (P < 0.001) in the TN-coPF group, and reduced to a greater extent with coHS. As observed from 20 to 27 d, BW and BWG were reduced (P < 0.001) by cyHS relative to the TN-al group, further reduced by coHS, with the TN-coPF group being intermediate to the cyHS and coHS groups. The FCR was lowest (P < 0.001) for the TN-al treatment, highest for the coHS treatment, and intermediate for the cyHS and TN-coPF treatments.
During the total cumulative period from 20 to 41 d, compared with TN-al birds, the reduction in FI (P < 0.001) was greatest and similar in the coHS and TN-coPF groups, while FI of cyHS bird was intermediate. Compared with the TN-al group, BW and BWG were reduced (P < 0.001) by cyHS, further reduced by coHS, with the TN-coPF group being intermediate to the cyHS and coHS groups. The lowest (P < 0.001) FCR was observed for TN-al and cyHS groups, the highest observed for coHS birds, with TN-coPF birds having an intermediate FCR. No differences (P > 0.05) in mortality were observed between treatments during any individual period or the total cumulative period.
Effect of Two Chronic HS Models and Pair-Feeding on Carcass Characteristics and Part Weights
The hot and chilled carcass, hot fat pad weight and yields of processed birds are presented in Table 3. Hot and chilled carcass weights were the highest (P < 0.001) for TN-al birds, intermediate for cyHS birds, and lowest for coHS and TN-coPF birds. The hot and chilled carcass yield of TN-coPF birds was decreased compared to the other treatments, and coHS birds had a higher chilled carcass yield than TN-al birds. Compared to TN-al condition, hot fat pad weights were more drastically reduced (P < 0.001) under coHS than with cyHS, and TN-coPF had an even lower abdominal fat weight than coHS birds. Hot fat pad yield was the highest (P < 0.001) with coHS birds, intermediate with TN-al and cyHS birds, and the lowest for TN-coPF birds.
Table 3.
Carcass characteristics and part weights and yields of broilers reared under different environmental conditions and feed regimens from 20 to 41 d and processed at 42 d.
| Treatment1 |
SEM2 | P-values | |||||
|---|---|---|---|---|---|---|---|
| Parameter | TN-al | cyHS | coHS | TN-coPF | |||
| Hot Carcass | Weight, kg | 2.565a | 2.143b | 1.365c | 1.512c | 0.0909 | <0.001 |
| Yield3, % | 74.9a | 75.1a | 75.1a | 72.5b | 0.3005 | <0.001 | |
| Hot Fat Pad | Weight, kg | 0.0349a | 0.0283b | 0.0213c | 0.0099d | 0.0015 | <0.001 |
| Yield, % | 1.02b | 1.00b | 1.24a | 0.52c | 0.0678 | <0.001 | |
| Chilled Carcass | Weight, kg | 2.617a | 2.189b | 1.414c | 1.555c | 0.0897 | <0.001 |
| Yield, % | 76.4b | 77.1ab | 77.9a | 74.5c | 0.0049 | <0.001 | |
| P. major | Weight, kg | 0.715a | 0.552b | 0.312d | 0.380c | 0.0313 | <0.001 |
| Yield, % | 20.89a | 19.30b | 17.03c | 18.19bc | 0.5680 | <0.001 | |
| P. minor | Weight, kg | 0.134a | 0.113b | 0.068d | 0.084c | 0.0042 | <0.001 |
| Yield, % | 3.91a | 3.95a | 3.71b | 4.02a | 0.0740 | <0.001 | |
| TBM4 | Weight, kg | 0.849a | 0.665b | 0.379d | 0.464c | 0.0352 | <0.001 |
| Yield, % | 24.80a | 23.25b | 20.75c | 22.21b | 0.5940 | <0.001 | |
| Wings | Weight, kg | 0.259a | 0.228b | 0.161c | 0.174c | 0.0090 | <0.001 |
| Yield, % | 7.56d | 8.00c | 8.91a | 8.36b | 0.1030 | <0.001 | |
| Leg quarters | Weight, kg | 0.800a | 0.709b | 0.457c | 0.487c | 0.0291 | <0.001 |
| Yield, % | 23.36b | 24.90a | 24.67a | 23.34b | 0.4480 | <0.001 | |
TN-al: Birds reared under continuous 24°C and ad libitum feeding. cyHS: Birds reared under cyclic high temperature (8 h at 35˚C and 12 h at 24˚C) and ad libitum feeding. coHS: Birds reared under continuous 35°C and ad libitum feeding. TN-coPF: Birds reared under continuous 24°C and pair-fed to the coHS treatment.
SEM: pooled standard error of the mean.
Yields calculated relative to live body weight taken immediately prior to processing.
TBM: Total breast meat = P. major + P. minor.
Means within row without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's multiple comparison test.
Compared with the TN-al group, weights of P. major, P. minor and TBM were reduced (P < 0.001) by cyHS, further reduced by coHS, and the TN-coPF group was intermediate to the cyHS and coHS groups. Wing and leg quarter weights followed the same trend (P < 0.001) except that there was no difference between coHS and TN-coPF birds. For yields of these parts, coHS birds had a greater (P < 0.001) reduction of the P. major yield compared to cyHS birds, while TN-coPF yields were not different than coHS and cyHS birds. Constant HS birds had the lowest (P < 0.001) P. minor yield, which was similar among other groups. The TBM yield was the highest (P < 0.001) under the TN-al treatment, the lowest under coHS, and intermediate under cyHS and TN-coPF conditions. On the other hand, leg quarter yield was increased (P < 0.001) by the two HS models compared to TN-al and TN-coPF conditions. The wing yield was the lowest (P < 0.001) with TN-al birds and the highest with coHS birds. Pair fed and cyHS birds had a lower wing yield than coHS birds, with a reduction more important in cyHS birds.
Effect of Two Chronic HS Models and Pair-Feeding on Meat Quality
No difference (P > 0.05) was observed in the incidence of severe WB and apparent WS between the treatments (Table 4). The incidence of mild WB was highest (P < 0.001) for TN-al, intermediate for cyHS, and lowest and not different between coHS and TN-coPF. An inverse relationship was observed for the incidence of normal WB (P < 0.001). The incidence of faint WS was highest (P < 0.001) and not different for TN-al and cyHS, intermediate for coHS, and lowest for TN-coPF, with an inverse response observed for the incidence of normal WS (P < 0.001).
Table 4.
Pectoralis major muscle myopathy distribution of broilers reared under different environmental conditions and feed regimens from 20 to 41 d and processed at 42 d.
| Parameter | Score | Treatment1 |
SEM2 | P-values | |||
|---|---|---|---|---|---|---|---|
| TN-al | cyHS | coHS | TN-coPF | ||||
| Woody Breast3 (%) | Normal | 28.81c | 69.67b | 87.54a | 100.00a | 7.1071 | <0.001 |
| Mild | 67.74a | 30.33b | 12.46c | 0.00c | 7.6967 | <0.001 | |
| Severe | 3.45 | 0.00 | 0.00 | 0.00 | 1.9956 | 0.073 | |
| White Striping4 (%) | Normal | 1.67c | 2.13c | 32.67b | 83.99a | 4.8876 | <0.001 |
| Faint | 93.33a | 94.70a | 67.33b | 16.01c | 6.0797 | <0.001 | |
| Apparent | 5.00 | 3.17 | 0.00 | 0.00 | 3.7671 | 0.215 | |
TN-al: Birds reared under continuous 24°C and ad libitum feeding. cyHS: Birds reared under cyclic high temperature (8 h at 35˚C and 12 h at 24˚C) and ad libitum feeding. coHS: Birds reared under continuous 35°C and ad libitum feeding. TN-coPF: Birds reared under continuous 24°C and pair-fed to the coHS treatment.
SEM: pooled standard error of the mean.
P. major fillets were considered normal, mild, or severe for woody breast if the fillet was flexible throughout, stiff in cranial region, or if stiff in the cranial and caudal regions, respectively.
P. major fillets were considered normal, faint, or apparent for white striping if they displayed no visible stripes, stripes less than 1 mm, or stripes larger than 1 mm, respectively.
Means within row without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's multiple comparison test.
As presented in Table 5, compared to TN-al condition, the P. major pHu was not affected by cyHS or coHS conditions, whereas TN-coPF birds had a lower (P < 0.001) pHu than all other groups. Concerning the P. major color measurements, L* was the highest (P < 0.001) for the coHS treatment, but not different among the other groups. Values for a* did not differ (P > 0.05) among treatments, whereas P. major b* values were decreased (P < 0.001) with coHS birds and to a greater extent in TN-coPF birds.
Table 5.
Pectoralis major pHu, L*, a*, and b* of broilers reared under different environmental conditions and feed regimens from 20 to 41 d and processed at 42 d.
| Parameter3 | Treatment1 |
SEM2 | P-values | |||
|---|---|---|---|---|---|---|
| TN-al | cyHS | coHS | TN-coPF | |||
| pHu | 5.89ab | 5.94a | 5.85b | 5.75c | 0.039 | <0.001 |
| L* | 56.5b | 56.0b | 59.1a | 55.0b | 0.858 | <0.001 |
| a* | 2.74 | 2.43 | 2.58 | 2.64 | 0.233 | 0.324 |
| b* | 9.65a | 8.84ab | 8.77b | 7.07c | 0.403 | <0.001 |
TN-al: Birds reared under continuous 24°C and ad libitum feeding. cyHS: Birds reared under cyclic high temperature (8 h at 35˚C and 12 h at 24˚C) and ad libitum feeding. coHS: Birds reared under continuous 35°C and ad libitum feeding. TN-coPF: Birds reared under continuous 24°C and pair-fed to the coHS treatment.
SEM: pooled standard error of the mean.
pHu: ultimate pH; L*: lightness; a*: redness; b*: yellowness.
Means within row without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's multiple comparison test.
DISCUSSION
As expected, both coHS and cyHS models impaired BW, BWG, FI, and FCR, with a greater impact resulting from constant versus cyclic HS exposure. Furthermore, markedly reduced performance (67% reduction in BWG and 80% increase in FCR) during the cumulative challenge period indicates that birds were quite stressed when maintained at 35°C continuously. Performance was also negatively affected by cyHS (20% reduction in BWG and 10% increase in FCR), but to a lesser extent than with coHS. With both models, reduced performance was mainly caused by the decrease in FI induced by hot temperatures; however, the TN-coPF birds presented better performance than the coHS birds, indicating that HS per se directly contributes to decreased performance. Similarly, De Souza et al. (2016) noted that the reduced growth under cyHS is more related to decreased FI than directly to HS, while coHS led to greater metabolic impacts.
During the 2 first weeks of challenge, TN-coPF birds received the same amount of feed consumed by coHS birds plus an amount equal to the expected relative daily increase in feed intake of those same birds. Because the expected daily increases in FI of coHS birds did not occur, this led to a higher FI of TN-coPF birds compared to coHS birds. This was corrected during the third week of the challenge, with no difference in FI observed between the coHS and TN-coPF for the total cumulative challenge period. Comparison of the performance of these pair-fed birds with the coHS birds indicates that, in this experiment, approximately 81% of the degradation of performance under constant high temperature was caused by decreased FI, with the remaining 19% directly associated with the physiologic changes induced by the elevation of temperature. These relative reductions are in the general range of values reported in other studies (Geraert et al., 1996; Bonnet et al., 1997; Faria Filho et al., 2007; De Souza et al., 2016; Habashy et al., 2017). Several physiological changes are responsible for impaired performance following HS per se such as oxidative stress, inflammation, and compromised intestinal integrity. From a metabolic standpoint, reduced protein turnover and increased fat deposition have been observed with HS birds compared to pair-fed birds (Geraert et al., 1996; Temim et al., 2000; Faria Filho et al., 2007; De Souza et al., 2016).
Compared with ad libitum fed birds reared under thermoneutral conditions, increased chilled carcass yield under coHS, as observed in the current study, has been previously reported, as has decreased carcass yield of pair-fed birds (Baziz et al., 1996; Lu et al., 2007; Rosa et al., 2007; Zeferino et al., 2016). Under HS condition, the reduction of relative organ weights (heart, liver) and reduced feathering to improve heat losses could partially explain this increase in carcass yield (Geraert et al., 1993; Zeferino et al., 2016). Concerning TN-coPF birds, reduced carcass yield could partially be explained by increased mobilization of fat as an energy source (Zhan et al., 2007). This hypothesis is supported by the large reduction in abdominal fat deposition of pair-fed birds compared with birds in TN-al condition. Conversely, coHS increased abdominal fat deposition, which agrees with previous studies (Zeferino et al., 2016). Moreover, increased intramuscular fat deposition in birds subjected to coHS observed by others (Zhang et al., 2012) reveals an increase in the amount of energy retained as fat (Geraert et al., 1996; Faria Filho et al., 2007). However, cyHS did not affect abdominal fat yield in the current experiment, which is in agreement with Orlowski et al. (2020) and Greene et al. (2021) and supports the notion that metabolism alterations depend on the HS model.
Whereas an increased proportion of energy appeared to be stored as lipid under coHS, P. major, and P. minor yields were decreased. This trend was also observed under cyHS, but to a lower extent. Broilers have been selected to have high P. major yield for decades, and marked decreases in BWG observed under HS should logically result in decreased weights of this highest selective trait (Orlowski et al., 2020). Similarly, less muscle protein deposition (Temim et al., 2000; Zhang et al., 2012) and less energy retention as protein (Geraert et al., 1996; Faria Filho et al., 2007) following HS has been observed in several studies. Furthermore, leg quarter and wing yields are increased under HS, which can be partially explained by the decrease in P. major yield, resulting in increased relative yield of other carcass parts. Additionally, leg quarters are comprised of oxidative fibers whereas P. major muscle is comprised of glycolytic fibers that are more dependent on glycogen stores, and these are depleted following decreased FI in response to HS (Temim et al., 2000; Zeferino et al., 2016). In the current study, parts yield reductions responded similarly in restricted fed birds as in coHS birds, indicating that the observed effect of HS on carcass part yield is mainly associated with decreased FI. However, leg quarter yield was not increased in TN-coPF birds compared to TN-al birds.
Woody breast and WS are major concerns for the meat industry as they are associated with a decrease in meat quality. White striping is characterized by white striations parallel to muscle, while WB results in a tougher consistency of breast fillets (Kuttappan et al., 2016). Compared to the TN-al treatment, the incidence of WB was reduced to a greater extent by coHS than by cyHS, and WB was completely absent in P. major muscles from broilers in the TN-coPF group. White striping was not decreased by cyHS, but was reduced with coHS, and more importantly with TN-coPF. Orlowski et al. (2020) and Greene et al. (2021) observed similar results under cyHS, but Orlowski also reported a reduction of WS under cyclic HS. The incidence of both WB and WS has been related to rapid growth and high P. major muscle yields (Griffin et al., 2018). Thus, the marked reduction in FI and BWG of coHS and TN-coPF birds could explain the low occurrence of muscle myopathies in those treatments. However, TN-coPF birds presented a lower incidence of these conditions than coHS, despite having higher weight gain and P. major yield. This may have been related to behavioral differences, as WB has been associated with a resting behavior (Norring et al., 2019) that is often displayed by HS birds in an attempt to minimize the heat production through reduced physical activity (Mack et al., 2013).
In our experiment, the pHu was only decreased under coHS condition and not cyHS. Zhang et al. (2012) observed decreased pHu in response to both coHS and cyHS, whereas other studies have not shown differences in pHu in response to cyHS (Orlowski et al., 2020; Greene et al., 2021) or coHS (Lu et al., 2007, 2017). As observed here, Lu et al. (2007, 2017) and Zeferino et al. (2016) also found that the P. major from coHS birds had higher pHu than TN-coPF birds. Lowered pHu during HS could be explained by greater conversion of pyruvate into lactate during chronic HS (Song and King, 2015; Lu et al., 2017). The increased lightness of P. major under constant HS has been observed in previous studies, but responses in redness and yellowness to HS vary in the literature (Lu et al., 2007; Zhang et al., 2012; Zeferino et al., 2016). The lower b* values observed under coHS and TN-coPF conditions could be associated with the less severe WS scores observed in those treatments (Kuttappan et al., 2013a). The higher yellowness observed with severe WS has been associated with higher fat content (Kuttappan et al., 2012a, 2013b,c; Petracci et al., 2014), but given that restricted fed birds are expected to have low intramuscular fat content, this should not be the case for chronic HS birds (Lu et al., 2017).
In summary, the comparison of performance, carcass characteristics, and meat quality of birds reared under a thermoneutral, cyclic, or constant HS environment within the same experiment have confirmed that the response of birds to HS is largely dependent on the model used. Cooler nights during a diurnal HS seems to improve the ability of the bird to adapt or recover from cyclic HS. Additionally, impaired weight gain resulting from HS is predominantly caused by a decrease in FI, but the use of pair-fed birds confirmed that HS also has direct effects on the measurements reported in this study that are independent of FI. Further investigations on oxidative stress and metabolic changes elicited by these conditions are ongoing to better characterize bird responses to the different HS models tested herein.
Acknowledgments
Funding source
Adisseo France SAS and the University of Arkansas System Division of Agriculture.
ACKNOWLEDGMENTS
This research was supported by a research grant from Adisseo France SAS and with funding from the University of Arkansas System Division of Agriculture.
Disclosures
A. Preynat, P. Cozannet, and M. Briens are employees of Adisseo France SAS. Otherwise, there are no conflicts of interest to declare.
References
- Alleman F., Leclercq B. Effect of dietary protein and environmental temperature on growth performance and water consumption of male broiler chickens. Br. Poult. Sci. 1997;38:607–610. doi: 10.1080/00071669708418044. [DOI] [PubMed] [Google Scholar]
- Awad E.A., Idrus Z., Soleimani Farjam A., Bello A.U., Jahromi M.F. Growth performance, duodenal morphology and the caecal microbial population in female broiler chickens fed glycine-fortified low protein diets under heat stress conditions. Br. Poult. Sci. 2018;59:340–348. doi: 10.1080/00071668.2018.1440377. [DOI] [PubMed] [Google Scholar]
- Baziz H.A., Geraert P.A., Padilha J.C.F., Guillaumin S. Chronic heat exposure enhances fat deposition and modifies muscle and fat partition in broiler carcasses. Poult. Sci. 1996;75:505–513. doi: 10.3382/ps.0750505. [DOI] [PubMed] [Google Scholar]
- Bernabucci U., Lacetera N., Baumgard L.H., Rhoads R.P., Ronchi B., Nardone A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal. 2010;4:1167–1183. doi: 10.1017/S175173111000090X. [DOI] [PubMed] [Google Scholar]
- Bonnet S., Geraert P.A., Lessire M., Carré B., Guillaumin S. Effect of high ambient temperature on feed digestibility in broilers. Poult. Sci. 1997;76:857–863. doi: 10.1093/ps/76.6.857. [DOI] [PubMed] [Google Scholar]
- De Antonio J., Fernandez-Alarcon M.F., Lunedo R., Squassoni G.H., Ferraz A.L.J., Macari M., Furlan R.L., Furlan L.R. Chronic heat stress and feed restriction affects carcass composition and the expression of genes involved in the control of fat deposition in broilers. J. Agric. Sci. 2017;155:1487–1496. [Google Scholar]
- De Souza L.F.A., Espinha L.P., De Almeida E.A., Lunedo R., Furlan R.L., Macari M. How heat stress (continuous or cyclical) interferes with nutrient digestibility, energy and nitrogen balances and performance in broilers. Livest. Sci. 2016;192:39–43. [Google Scholar]
- Faria Filho D.E., Campos D.M.B., Torres K.A., Vieira B.S., Rosa P.S., Vaz A.M., Macari M., Furlan R.L. Protein levels for heat-exposed broilers: performance, nutrients digestibility, and energy and protein metabolism. Int. J. Poult. Sci. 2007;6:187–194. [Google Scholar]
- Geraert P.A., Guillaumin S., Leclercq B. Are genetically lean broilers more resistant to hot climate? Br. Poult. Sci. 1993;34:643–653. doi: 10.1080/00071669308417623. [DOI] [PubMed] [Google Scholar]
- Geraert P.A., Padilha J.C.F., Guillaumin S. Metabolic and endocrine changes induced by chronic heatexposure in broiler chickens: growth performance, body composition and energy retention. Br. J. Nutr. 1996;75:195–204. doi: 10.1079/bjn19960124. [DOI] [PubMed] [Google Scholar]
- Greene E.S., Cauble R., Kadhim H., De Almeida Mallmann B., Gu I., Lee S.O., Orlowski S., Dridi S. Protective effects of the phytogenic feed additive “comfort” on growth performance via modulation of hypothalamic feeding- and drinking-related neuropeptides in cyclic heat-stressed broilers. Domest. Anim. Endocrinol. 2021;74 doi: 10.1016/j.domaniend.2020.106487. [DOI] [PubMed] [Google Scholar]
- Griffin J.R., Moraes L., Wick M., Lilburn M.S. Onset of white striping and progression into wooden breast as defined by myopathic changes underlying Pectoralis major growth. Estimation of growth parameters as predictors for stage of myopathy progression. Avian Pathol. 2018;47:2–13. doi: 10.1080/03079457.2017.1356908. [DOI] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Adomako K., Attia Y.A., Rekaya R., Aggrey S.E. Effect of heat stress on amino acid digestibility and transporters in meat-type chickens. Poult. Sci. 2017;96:2312–2319. doi: 10.3382/ps/pex027. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.B., Apple J.K., Waldroup P.W., Owens C.M. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.B., Mauromoustakos A., McKee S.R., Emmert J.L., Meullenet J.F., Owens C.M. Estimation of factors associated with the occurrence of white striping in broiler breast fillets. Poult. Sci. 2013;92:811–819. doi: 10.3382/ps.2012-02506. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Huff G.R., Huff W.E., Hargis B.M., Apple J.K., Coon C., Owens C.M. Comparison of hematologic and serologic profiles of broiler birds with normal and severe degrees of white striping in breast fillets. Poult. Sci. 2013;92:339–345. doi: 10.3382/ps.2012-02647. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Lee Y.S., Erf G.F., Meullenet J.F.C., McKee S.R., Owens C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012;91:1240–1247. doi: 10.3382/ps.2011-01947. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.L., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., McKee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Lu Q., Wen J., Zhang H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult. Sci. 2007;86:1059–1064. doi: 10.1093/ps/86.6.1059. [DOI] [PubMed] [Google Scholar]
- Mack L.A., Felver-Gant J.N., Dennis R.L., Cheng H.W. Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens. Poult. Sci. 2013;92:285–294. doi: 10.3382/ps.2012-02589. [DOI] [PubMed] [Google Scholar]
- Mahmoud U.T., Abdel-Rahman M.A.M., Darwish M.H.A., Applegate T.J., Cheng H. Behavioral changes and feathering score in heat stressed broiler chickens fed diets containing different levels of propolis. Appl. Anim. Behav. Sci. 2015;166:98–105. [Google Scholar]
- McFarlane J.M., Curtis S.E., Simon J., Izquierdo O.A. Multiple concurrent stressors in chicks. Multiple Concurrent Stressors in Chicks. 1. Effect on weight gain, feed intake, and behavior. Poult. Sci. 1989;68:510–521. doi: 10.3382/ps.0680501. [DOI] [PubMed] [Google Scholar]
- Nardone A., Ronchi B., Lacetera N., Ranieri M.S., Bernabucci U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010;130:57–69. [Google Scholar]
- Norring M., Valros A., Valaja J., Sihvo H.K., Immonen K., Puolanne E. Wooden breast myopathy links with poorer gait in broiler chickens. Animal. 2019;13:1690–1695. doi: 10.1017/S1751731118003270. [DOI] [PubMed] [Google Scholar]
- Orlowski S.K., Cauble R., Tabler T., Hiltz J.Z., Greene E.S., Anthony N.B., Dridi S. Processing evaluation of random bred broiler populations and a common ancestor at 55 days under chronic heat stress conditions. Poult. Sci. 2020;99:3491–3500. doi: 10.1016/j.psj.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski S., Flees J., Greene E.S., Ashley D., Lee S.O., Yang F.L., Owens C.M., Kidd M., Anthony N., Dridi S. Effects of phytogenic additives on meat quality traits in broiler chickens. J. Anim. Sci. 2018;96:3757–3767. doi: 10.1093/jas/sky238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar S.S., Sajjanar B., Lonkar L.V., Kurade N.P., Kadam A.S., Nirmale A.V., Brahmane M.P., Bal S.K. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci. 2016;4:332–341. [Google Scholar]
- Petracci M., Mudalal S., Babini E., Cavani C. Effect of white striping on chemical composition and nutritional value of chicken breast meat. Ital. J. Anim. Sci. 2014;13:3138. [Google Scholar]
- Rajaei-Sharifabadi H., Greene E., Piekarski A., Falcon D., Ellestad L., Donoghue A., Bottje W., Porter T., Liang Y., Dridi S. Surface wetting strategy prevents acute heat exposure–induced alterations of hypothalamic stress– and metabolic-related genes in broiler chickens1. J. Anim. Sci. 2017;95:1132–1143. doi: 10.2527/jas.2016.1290. [DOI] [PubMed] [Google Scholar]
- Rosa P., De Faria Filho D.E., Dahlke F., Vieira B.S., Macari M., Furlan R.L. Performance and carcass characteristics of broiler chickens with different growth potential and submitted to heat stress. Braz. J. Poult. Sci. 2007;9:181–186. [Google Scholar]
- Roushdy E.M., Zaglool A.W., El-Tarabany M.S. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J. Therm. Biol. 2018;74:337–343. doi: 10.1016/j.jtherbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Searchinger, T., R. Waite, C. Hanson, J. Ranganathan, P. Dumas, E. Matthews, and C. Klirs. 2019. Creating a sustainable food future: a menu of solutions to feed nearly 10 billion people by 2050. Final report. WRI.
- Song D.J., King A.J. Effects of heat stress on broiler meat quality. Worlds Poult. Sci. J. 2015;71:701–709. [Google Scholar]
- St-Pierre N.R., Cobanov B., Schnitkey G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003;86:E52–E77. [Google Scholar]
- Syafwan S., Kwakkel R.P., Verstegen M.W.A. Heat stress and feeding strategies in meat-type chickens. Worlds Poult. Sci. J. 2011;67:653–674. [Google Scholar]
- Temim S., Chagneau A.-M., Peresson R., Tesseraud S. Chronic heat exposure alters protein turnover of three different skeletal muscles in finishing broiler chickens fed 20 or 25% protein diets. J. Nutr. 2000;130:813–819. doi: 10.1093/jn/130.4.813. [DOI] [PubMed] [Google Scholar]
- Tijare V.V., Yang F.L., Kuttappan V.A., Alvarado C.Z., Coon C.N., Owens C.M. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 2016;95:2167–2173. doi: 10.3382/ps/pew129. [DOI] [PubMed] [Google Scholar]
- Zeferino C.P., Komiyama C.M., Pelícia V.C., Fascina V.B., Aoyagi M.M., Coutinho L.L., Sartori J.R., Moura A.S.A.M.T. Carcass and meat quality traits of chickens fed diets concurrently supplemented with vitamins C and E under constant heat stress. Animal. 2016;10:163–171. doi: 10.1017/S1751731115001998. [DOI] [PubMed] [Google Scholar]
- Zhan X.A., Wang M., Ren H., Zhao R.Q., Li J.X., Tan Z.L. Effect of early feed restriction on metabolic programming and compensatory growth in broiler chickens. Poult. Sci. 2007;86:654–660. doi: 10.1093/ps/86.4.654. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]