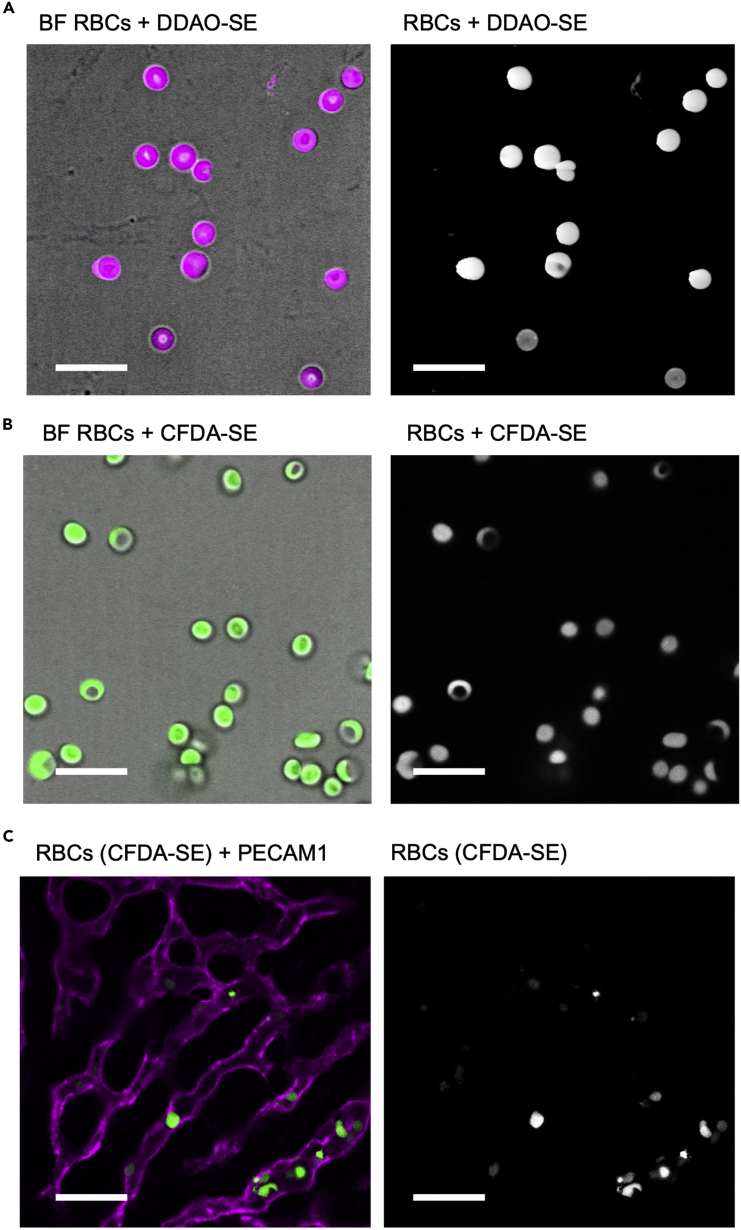
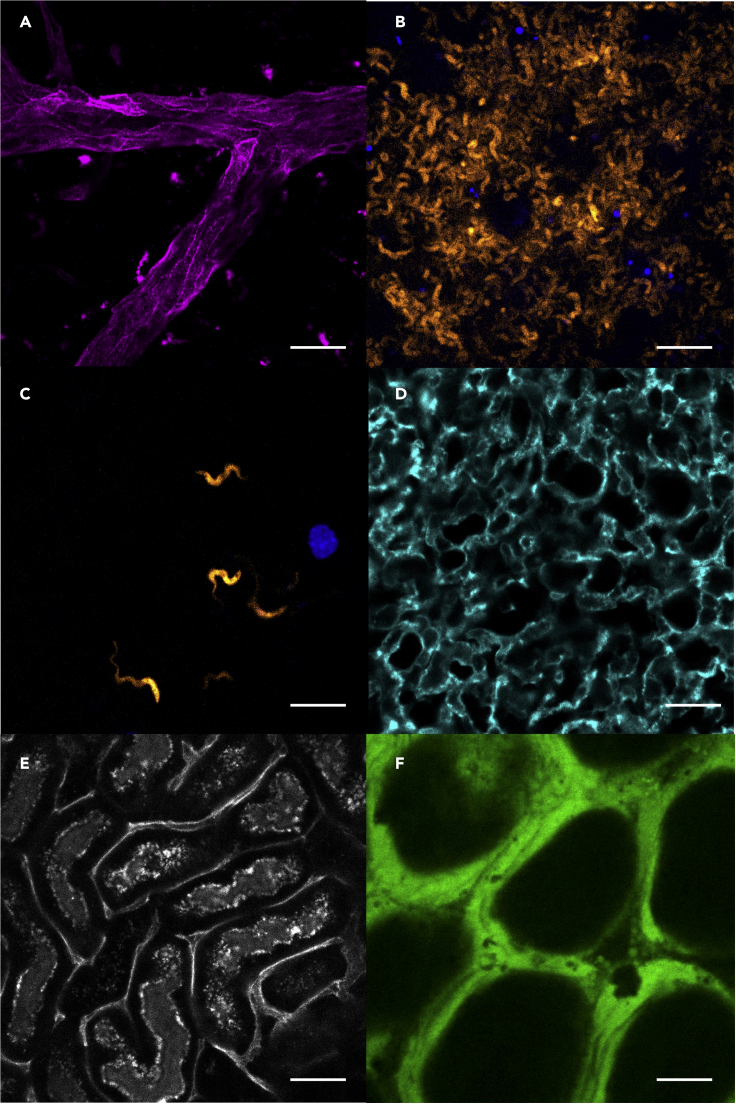
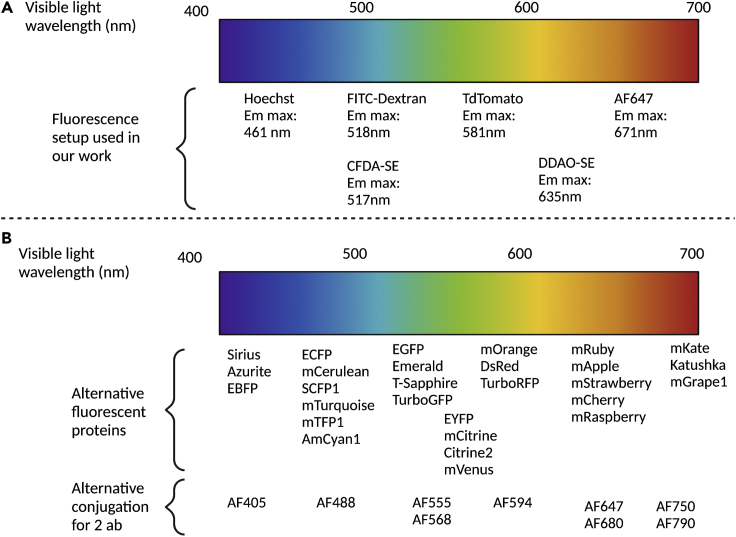
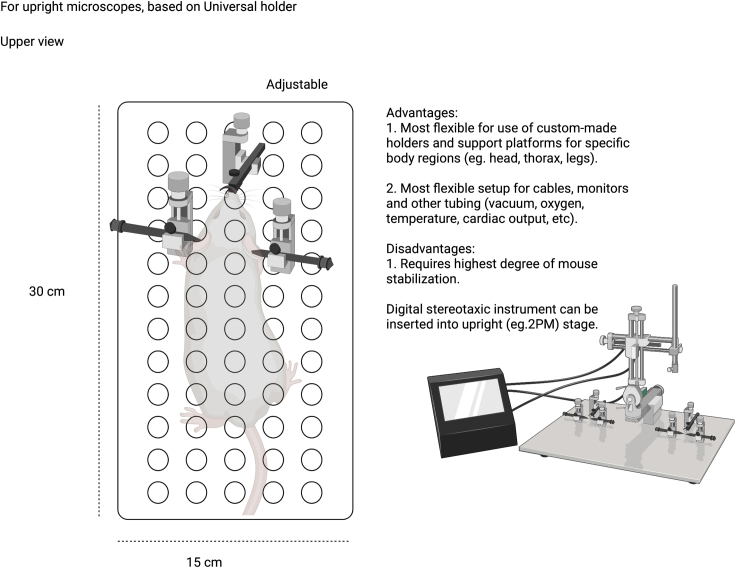
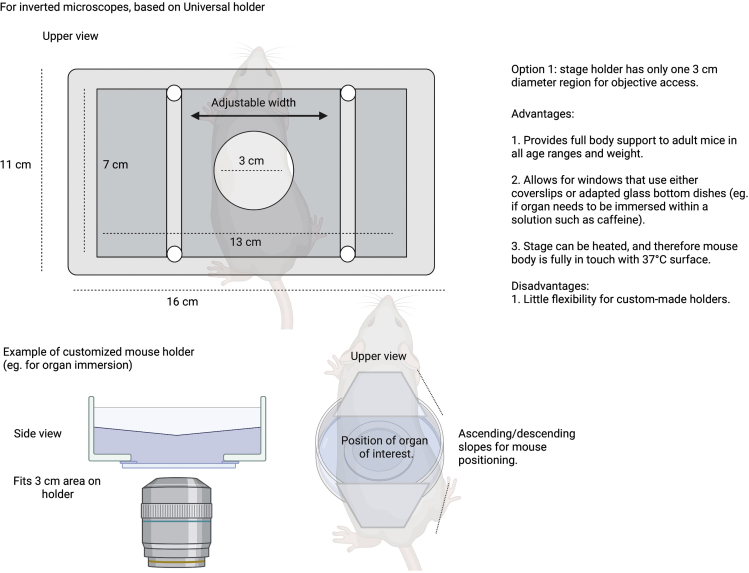
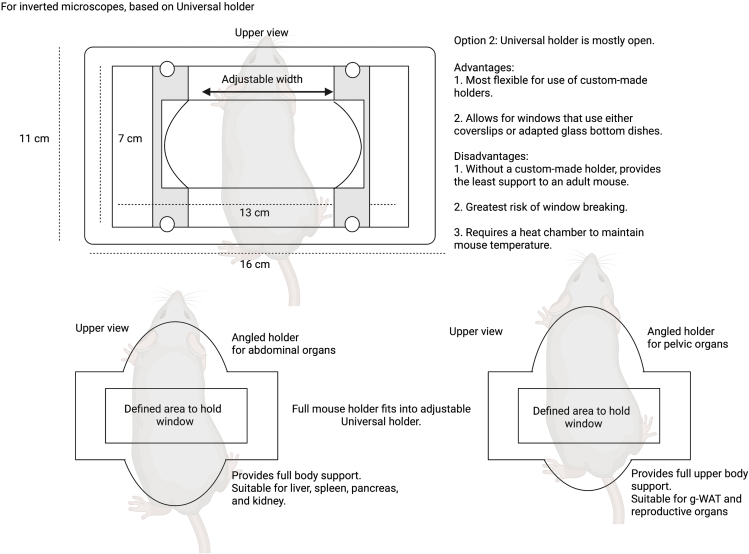
Summary
Intravital microscopy (IVM) involves surgical procedures to expose the internal organs of live anesthetized animals to visualize fluorescently labeled components in situ, in vivo at subcellular resolution. Here, we provide an IVM protocol for time-lapse imaging of dynamic Trypanosoma brucei-host interactions in ten mammalian organs and in systemic circulation. We describe intraperitoneal or intradermal injection of mice with T.brucei. We then detail surgical procedures to prepare ten organs for IVM, followed by imaging of host-T. brucei interactions.
For complete details on the use and execution of this protocol, please refer to De Niz et al. (2021).
Subject areas: Cell Biology, Microbiology, Microscopy, Model Organisms
Graphical abstract
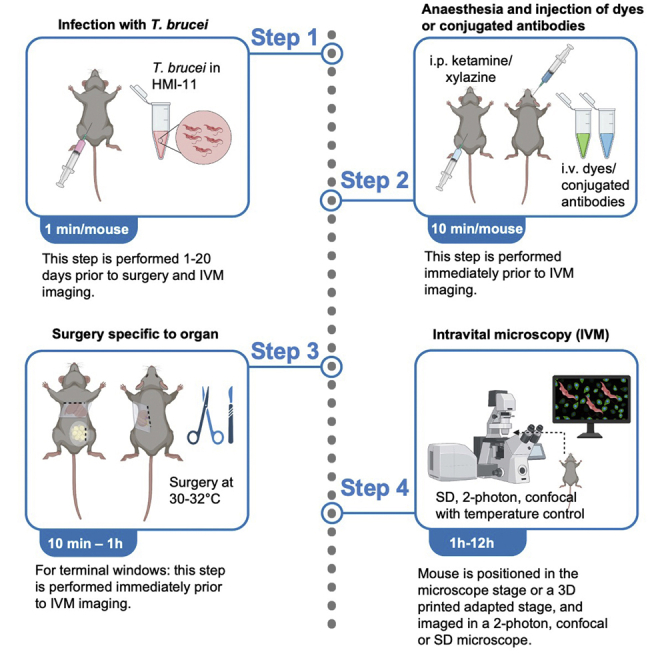
Highlights
-
•
An intravital microscopy protocol to study T. brucei in vivo in rodents
-
•
Surgical procedures to prepare 10 rodent organs for intravital microscopy
-
•
In vivo imaging of host-T. brucei interactions in ten rodent organs and blood vasculature
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Intravital microscopy (IVM) involves surgical procedures to expose the internal organs of live anesthetized animals to visualize fluorescently labeled components in situ, in vivo at subcellular resolution. Here, we provide an IVM protocol for time-lapse imaging of dynamic Trypanosoma brucei-host interactions in ten mammalian organs and in systemic circulation. We describe intraperitoneal or intradermal injection of mice with T.brucei. We then detail surgical procedures to prepare ten organs for IVM, followed by imaging of host-T. brucei interactions.
Before you begin
The protocol below describes the specific steps for performing intravital microscopy in C57BL/6 mice infected with Trypanosoma brucei, and injected with FITC-Dextran, Hoechst and conjugated antibodies against various vascular receptors. However, this protocol can be used for other mice and other fluorescent markers.
Institutional permissions
-
1.
Obtain necessary animal licenses for project and personnel prior to starting animal experimentation. C57BL/6J mice (6–10 weeks old, 20–27 g) were used in this study. All animal experiments were approved by the Animal Ethics Committee of the Institute of Molecular Medicine (IMM), University of Lisbon. Mice were housed under a 12 h light/dark cycle and provided with food and water ad libitum.
Preparation of surgical instruments
-
2.
Sterilize the surgical instruments. This can be done by autoclave/steam sterilization, or by chemical sterilization using 0.1% benzalkonium chloride for 10 min. For surgeries of consecutive animals to implant terminal windows, sterilize with 70% ethanol.
-
3.
The protocol below describes the specific steps for imaging T. brucei within 10 organs and blood flow, but can be adapted to other Trypanosoma species, and other organs.
Note: Although chronic windows can be surgically implanted for each organ, in this protocol we describe protocols for terminal windows only.
Generate 3D-printed stages and adapt as needed
Timing: days to weeks including design, printing, and testing
-
4.Special mouse holders for microscope stages were designed using specialized software. In this case we used TopSolid, a CAD/CAM (computer-aided design/computer-aided manufacturing) system.
-
a.For each design, simulate 3D dimensions (including height, width, length, and orientation relative to the organ that each specific stage will help visualize).
-
a.
-
5.
Use 3D printer to generate the mouse holders.
-
6.
Test mouse holders, preferably using a dummy mouse, to determine fit in the various microscope stages, and reach of the objectives.
Generate fluorescently tagged Trypanosoma brucei lines to facilitate imaging in vivo
Timing: variable depending on the parasite line needed
-
7.
Acquire existing reporter parasite lines, or de novo generate fluorescently tagged Trypanosoma lines as required, that aid in the visualization of the parasites in vivo.
Note: Choose a bright and photostable fluorescent reporter that does not overlap with the dyes/fluorophores you will use for imaging other host/parasite elements.
Note: In the work we present here, we used GFP (Zimmermann et al., 2017) and TdTomato (Calvo-Alvarez et al., 2018).
Alternatives: Multiple other reporter lines have been generated (Dean et al., 2015) that could potentially be tested in vivo.
Optimize dye concentration/antibody titration and imaging settings to use in vivo
Timing: 1 day to 1 week
Note: For intravenous injection via the tail vein, a heating lamp or warm water must be used to ensure vasodilation prior to injection.
-
8.Determine dye concentrations optimal for experimentation and optimize intravenous injection.
-
a.Begin by intravenously injecting relevant dyes (e.g., Hoechst, FITC-Dextran, or any other dye of choice). Concentrations and image parameters should be confirmed in published work and tested for potential effects on parasite and host parameters, per dye of choice.
-
b.In our work, we used Hoechst at a concentration of 40 μg/kg mouse body weight. We used 70 kDa FITC-Dextran at 500 mg/kg mouse body weight. Mice used in our work weighted 25–30 g.
-
a.
-
9.Determine antibody fluorophore most suitable for experimentation and optimize intravenous injection.
-
a.Determine the fluorophore that is most suitable/stable for the required observations (e.g., AF488, AF594, AF647, etc.).
-
b.Determine the concentration required and the dynamics of the antibody in vivo (e.g., saturation point, time required for visualization, specificity, background, etc.).
-
c.In our work, we used all antibodies at 20–30 μg per mouse (25 g body weight), and determined that the fluorophore with highest stability and less problems of autofluorescence or bleaching was A647.
-
a.
-
10.Determine microscope type and microscope settings most suitable for the biological question.
-
a.Microscope types include 2-photon, confocal, of spinning disc confocal microscopes.
-
b.Settings include exposure time, imaging speed, most suitable objective, number of frames to acquire per organ, etc.
-
c.Determine best setup to image multiple fluorescent markers simultaneously.
-
d.In our work, we used a Zeiss Cell Observer SD and/or a 3i Marianas SDC and/or an AiryScan Zeiss LSM880 and/or a Zeiss LSM710 and/or SP8 Multiphoton microscope. We describe specific parameters in sections below.
-
a.
Note: Settings might vary across microscopes, and should be defined in advance depending on the question answered and each specific microscope settings and requirements.
Optimize surgeries in dummy tissues/mice, or euthanized mice prior to use in vivo
Timing: Varied depending on surgical experience and skill
CRITICAL: Consult with the attending veterinarian for specific surgical training and familiarization with mouse anatomy before conducting any in vivo procedure. Become familiar with cauterization and suturing procedures prior to performing surgical methods in vivo. Become familiar with the characteristics and use of anesthetic and analgesic medication prior to performing surgical methods in vivo.
-
11.
Become familiar with the basic surgical tools (Figure 1) and the surgical procedure to expose, handle, and image each organ of interest. In subsequent sections we give details of each surgery performed in our work.
-
12.
It is suggested to practice surgical sutures in advance. In our work we used available kits (see key resources table) to test various suture types.
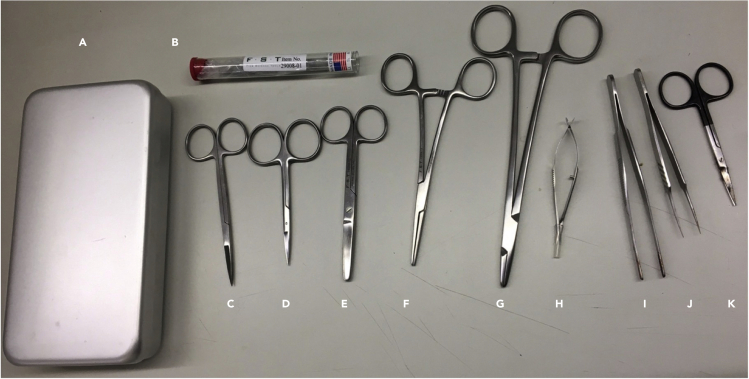
Figure 1.
Basic surgical equipment for intravital microscopy
(A–K) (A) Instrument case, (B) sharpening stone square, (C) fine scissors sharp, (D) fine scissors straight large loops 10 cm, (E) scissors straight blunt 12 cm, (F) MayoHegar needle holder, (G) crile hemostat straight, (H) Vannas spring scissors straight 3 mm cutting edge, (I) standard pattern forceps straight 13 cm, (J) super grip forceps straight, (K) fine scissors toughcut straight 9 cm.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| Mouse (mus musculus): C57BL/6, males/females, 6–10 weeks | Charles River | Strain code: 027 |
| Mouse (mus musculus):: CD36−/−, males/females, 6–10 weeks | The Jackson Laboratory | Stock code: 019006 |
| Experimental models: Cell lines | ||
| T. brucei TdTom/Ty1/RFLuc | Calvo-Alvarez et al., 2018 | https://pubmed.ncbi.nlm.nih.gov/29339220/ |
| T. brucei PAD1::GFP | Zimmermann et al., 2017 | https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1006324 |
| Chemicals, peptides, and recombinant proteins | ||
| Rompun 2% (xylazine) (16 mg/kg) | Bayer | NA |
| Imalgene 1000 (ketamine) (120 mg/kg) | Merial | NA |
| Buprex or Budale (Buprenorphine 0.3 mg/mL) (100 μL / 15–30 g every 8–12 h) | Richter pharma | NA |
| Carboxfluorescein diacetate succinimidyl ester (CFDA-SE) – 20 μM | Invitrogen | Cat# V12883 |
| 7-hydroxy-9H-(1,3-dichloro-9,9-dimethylacridine-2-one) succinimidyl ester (DDAO-SE)- 10 μM | Invitrogen | Cat# C34564 |
| 70 kDa FITC-Dextran (dilution: 500 mg/kg) | Sigma -ldrich | CAS 60842-46-8 |
| Hoechst 33342 (dilution: 40 μg/kg) | Sigma-Aldrich | CAS 875756-97-1 |
| HMI-11 culture media | See Table 1 | See Table 1 |
| IMDM with 25 mM HEPES and L-Glutamine | Invitrogen | Cat# E15-819 |
| Heat inactivated Fetal Bovine Serum | PAA | Cat# A15-104 |
| Hypoxanthine | Thermo Fisher Scientific | Cat# A11481-06 |
| Bathocuproinedisulfonic acid | Sigma-Aldrich | Cat# 146625-5G |
| L-cysteine | Sigma-Aldrich | CAS 52-90-4 |
| Pyruvic acid | Sigma-Aldrich | CAS 127-17-3 |
| 2-mercaptoethanol | Sigma-Aldrich | CAS 60-24-2 |
| RPMI-1640 media | Thermo Fisher Scientific | Cat #11875101 |
| Phosphate Buffered Saline | Thermo Fisher Scientific | Cat# 20012027 |
| Heparin sodium | Sigma-Aldrich | CAS 9041-08-1 |
| Antibodies | ||
| Alexa Fluor 647 anti-mouse CD36 clone HM36. Isotype Armenian Hamster IgG 0.5 mg/mL (20 μg/ 30 g body weight) | BioLegend | Cat# 102610 |
| Alexa Fluor 647 rat anti-mouse CD62P (P-selectin) clone RB40.34 0.2 mg/mL (20 μg/ 30 g body weight) | BD Pharmingen | Cat# 563674 |
| Alexa Fluor 647 anti-mouse CD102 (ICAM2) clone 3C4 (MIC2/4). Isotype Rat IgG2a,k (20 μg/ 30 g body weight) | BioLegend | Cat# 105612 |
| Alexa Fluor 647 anti-mouse CD54 (ICAM1) clone YN1/1.7.4. Isotype Rat IgG2b,k (20 μg/ 30 g body weight) | BioLegend | Cat# 116114 |
| Alexa Fluor 647 anti-mouse CD31 (PECAM1) clone 390. Isotype Rat IgG2a,k (20 μg/ 30 g body weight) | BioLegend | Cat# 102416 |
| Mouse CD106/VCAM-1 FITC conjugate (50 μg/ 30 g body weight) | Invitrogen | Cat# RMCD10601 |
| Purified Rat Anti-mouse CD102 (20 μg/ 30 g body weight) | BD Pharmingen | Cat#553326 |
| Blocking antibody RB40.34 against CD62-P (20 μg/ 30 g body weight) | BD Biosciences | Cat# 553742 |
| Blocking antibody P2H3 against CD62-E (20 μg/ 30 g body weight) | eBioscience | Cat# 14-0627-82 |
| Blocking antibody CBR 1C2/2 against CD102 (20 μg/ 30 g body weight) | eBioscience | Cat# BMS109 |
| Blocking antibody YN1/1.7.4 against CD45 (20 μg/ 30 g body weight) | eBioscience | Cat#16-0541-85 |
| Blocking antibody against CD31 (20 μg/ 30 g body weight) | Abcam | Cat#ab32457 |
| Blocking antibody against CD36 (185-1G2) (20 μg/ 30 g body weight) | Thermo Fisher Scientific | Cat# MAS-14112 |
| Blocking antibody Mouse IgG1 K isotype control (P3.6.2.8.1) (20 μg/ 30 g body weight) | eBioscience, Thermo Fisher Scientific | Cat# 16-4714-82 |
| Blocking antibody Mouse IgG2 K isotype control (eBM2a) (20 μg/ 30 g body weight) | eBioscience, Thermo Fisher Scientific | Cat# 14-4724-82 |
| Critical commercial assays | ||
| AlexaFluor 647 Conjugation Kit | Abcam | Cat# ab269823 |
| Software and algorithms | ||
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| SlideBook 6.0.22 | Intelligent Imaging Innovations | https://www.intelligent-imaging.com/slidebook |
| ZEN 2.6 (blue edition) | Carl Zeiss Imaging | https://www.zeiss.com/microscopy/int/products/microscope-software/zen.html |
| GraphPad Prism 9 | GraphPad Software Inc | https://www.graphpad.com/scientific-software/prism/ |
| TopSolid | TopSolid | https://www.topsolid.com/products/topsoliddesign.htm |
| Imaris 9.7 | Oxford Instruments | https://imaris.oxinst.com/versions/9-7 |
| BioRender | BioRender | https://biorender.com |
| Other | ||
| Dumoxel 3C Tweezers | Electron Microscopy Services | Cat# 0103-3C-PO |
| Dumoxel Style1 Tweezers | Electron Microscopy Services | Cat# 0103-1-PO |
| S&T JF5TC SuperGrip Forceps Straight | Interfocus | Cat# 00632-11 |
| Standard Pattern Forceps Straight 13 cm | Interfocus | Cat# 11000-13 |
| Crile Hemostat Straight | Interfocus | Cat# 13004-14 |
| Sharpening Stone Square | Interfocus | Cat# 29008-01 |
| Fine Scissors Straight Large Loops 10 cm | Interfocus | Cat# 14040-10 |
| Fine Scissors ToughCut Straight 9 cm | Interfocus | Cat# 14058-09 |
| Vannas Spring Scissors Straight 3 mm Cutting Edge | Interfocus | Cat# 15000-00 |
| Scissors Straight BluntBlunt 12 cm | Interfocus | Cat# 14000-12 |
| MayoHegar Needle Holder | Interfocus | Cat# 12004-18 |
| Moria Instrument Case | Interfocus | Cat# 20311-21 |
| Surgical scalpel | Securimed | Cat# 2185 |
| Surgical glue | 3M VetbondTM/MC tissue adhesive | Cat# 1469SB |
| Surgical tape | 3 M | Cat# 1530-0 |
| Cotton swabs and cotton balls | NA | NA |
| Various surgical sutures (kit) | Pinnacle Medics | NA |
| 8-0 Polypropylene surgical sutures | Johnson & Johnson | Cat# 8740H |
| 6-0 Polyglactin surgical sutures | Johnson & Johnson | Cat# D5890 |
| 4-0 Silk surgical sutures | Johnson & Johnson | Cat# K881H |
| Cautery pen | AgnThos | 59016 |
| Surgical drill | BrainTree scientific | MD-1200 120V |
| Nitrile examination gloves | Ansell | NA |
| Ventilator | Ugo Basile | Cat# 28025 |
| Heating lamp | Welch Allyn | Cat# LS-150 |
| Rib retractor | Fine Science Tools | Cat# 17003-03 |
| Animal warmer | Softron | Cat# THC-31 |
| Endotracheal intubation tube | Generated in-house | Maruyama et al., 2021 |
| Insulin Needles | BD | Cat# 326769 |
| Rectangular coverglass (#1.5, 100 mm × 60 mm) | Menzel Gläser | NA |
| Circular coverglass (#1.5, 12 mm) | Menzel Gläser | NA |
| 3i Marianas SDC microscope | Intelligent Imaging Innovations | SN# 1648 |
| Zeiss Cell Observer SDC microscope | Carl Zeiss microimaging | SN# 3851001637 |
| Zeiss LSM 880 | Carl Zeiss microimaging | SN# 2811000267 |
| Zeiss LSM 710 | Carl Zeiss microimaging | SN# 2503000400 |
| Multiphoton Microscope Leica TCS SP8 MP | Leica Microsystems | https://www.leica-microsystems.com/products/confocal-microscopes/p/leica-tcs-sp8-mp/ |
Materials and equipment
HMI-11 medium
| Reagent | Final concentration | Amount |
|---|---|---|
| IMDM with 25 mM HEPES and L-Glutamine | 880 mL | |
| Heat inactivated Fetal Bovine Serum | 10% | 100 mL |
| Hypoxanthine (100×) | 1/100 | 10 mL |
| Bathocuproinedisulfonic acid (1000×) | 1/1000 | 1 mL |
| L-cysteine (100×, 18.2 mg/mL) | 1/100 | 10 mL |
| Pyruvic acid (98%) | 100 mg/L | 69.2 μL |
| 2-mercaptoethanol (100 mM) | 1/500 | 2 mL |
| Total | 1 L |
Store at 4°C. Stocks of FBS should be kept at −20°C. Upon thawing and addition of FBS to HMI-11 media, use within 4 weeks.
-
•
For preparation of 100× stocks of hypoxanthine, dissolve 0.4 g of NaOH in 100 mL of dH2O, then add 1.36 g hypoxanthine and dissolve using magnet. Filter, and store in 5 mL aliquots at −20°C.
-
•
For preparation of 1000× stocks of bathocuproinedisulfonic acid (BA), dissolve 0.56 g of BA in 20 mL of dH2O. Filter and store at 25°C.
-
•
For preparation of 100× stocks of L-cysteine, dissolve 1.82 g of L-cysteine in 100 mL of dH2O, then filter and store in 5 mL aliquots at −20°C.
-
•
For preparation of 2-mercaptoethanol, dilute 1:143 in milli-Q water (for a 100 mM concentration). Do 10 mL stocks, filter, and store at 4°C.
Step-by-step method details
In this paper, we will describe how to visualize fluorescent parasites in organs of anesthetized mice. We will describe the detailed steps for 10 organs independently. Later on, we provide optional protocols for a) blocking receptors, b) quantifying the mean fluorescence intensity of labeled vasculature, c) fluorescently labeling and visualizing red blood cells, and d) restricting blood flow. Readers can refer to the point of relevance to own research questions.
Note: The protocols hereby presented are aimed at providing details that enable other researchers to address their own research questions (e.g., any fluorescent line/dye/conjugated antibody should be tested according to the researchers’ own aims), and readers are encouraged to use this methodology as a basis to addressing their own questions rather than as an exhaustive list of all the research possibilities this methodology opens.
(Optional step) Inject mice with antibodies against specific endothelial receptors you aim to block
Timing: 5 min
This step is optional, and it aims to block endothelial receptors (e.g., ICAM1, VCAM1, PECAM1, CD36). It takes place prior to mouse infection with T. brucei to ensure that receptors are not available for binding during the early stages of infection.
-
1.
Determine duration of blocking antibody lifetime in mice. This includes antibody affinity, and receptor turnover rate. This optimization step should be done for each receptor of interest according to the reader’s own research questions.
CRITICAL: Ensure you are achieving maximum blocking at the correct time windows. Depending on the time of infection of interest, this can involve a single or multiple injections.
-
2.
Dilute blocking antibodies to concentration desired. Calculate volume needed per mouse.
-
3.
Secure mice in adequate restrainer to allow for intravenous injection, and inject antibodies.
-
4.
In our protocol, we intravenously injected all antibodies at 20–30 μg per mouse (body weight of 25 g), daily, over a course of 6 days.
Infect mice with Trypanosoma brucei by intraperitoneal or intradermal injection
Timing: 24–25 h
The aim of this step is to establish an infection in C57BL/6J mice with T. brucei (Figure 2A).
-
5.
Thaw a stabilate of T. brucei blood trypomastigotes and place in HMI-11 culture media for a minimum of 24 h. (For comparison of cells immediately after thawing and 24 h after thawing see Figures 2B and 2C and Methods Videos S1 and S2).
Note: In our experience, quantification of live parasites can be over-estimated when using a stabilate, thus affecting the inoculum of live parasites, and readouts at least during the initial days of infection. We therefore recommend a recovery period for the parasites in culture.-
a.Centrifuge cells at 800 g for 1 min to remove glycerol from freezing solution.
-
b.Resuspend in 5–10 mL of HMI-11 media and place into a 75 mm2 flask at 37°C with 5% CO2.
Methods video S1. Imaging T. brucei viability, related to step 5Video shows T. brucei parasites from a glycerol stabilate immediately after thawing from storage at −80°C.Download video file (1,013.5KB, mp4)Methods video S2. Imaging T. brucei viability, related to step 5Video shows T. brucei parasites 24 h after thawing from storage at −80°C.Download video file (454.7KB, mp4) -
a.
-
6.After 24 h, observe T. brucei parasites under the microscope and assess their suitability for infection by checking their motility and general morphology.
 CRITICAL: Recover desired number of parasites by diluting or concentrating parasites as necessary. In this protocol, we injected 2 × 103 parasites per mouse.
CRITICAL: Recover desired number of parasites by diluting or concentrating parasites as necessary. In this protocol, we injected 2 × 103 parasites per mouse.-
a.Resuspend parasites in HMI-11 volume required to have 2 × 103 parasites per 200 μL per mouse (i.e., 104 parasites/mL).
-
a.
-
7.
Load an insulin syringe (29G) with 200 μL of HMI-11 + T. brucei, and inject C57BL/6 mice by intraperitoneal or intradermal injection.
Note: Depending on the time points desired for intravital microscopy (IVM) injection should be done at approximately the same time of day that each imaging session will take place. For instance if a time course of 20 days will be done, and each imaging session will begin at 2 pm, infection should be performed at 2 pm of day 0 (i.e., the day of infection).
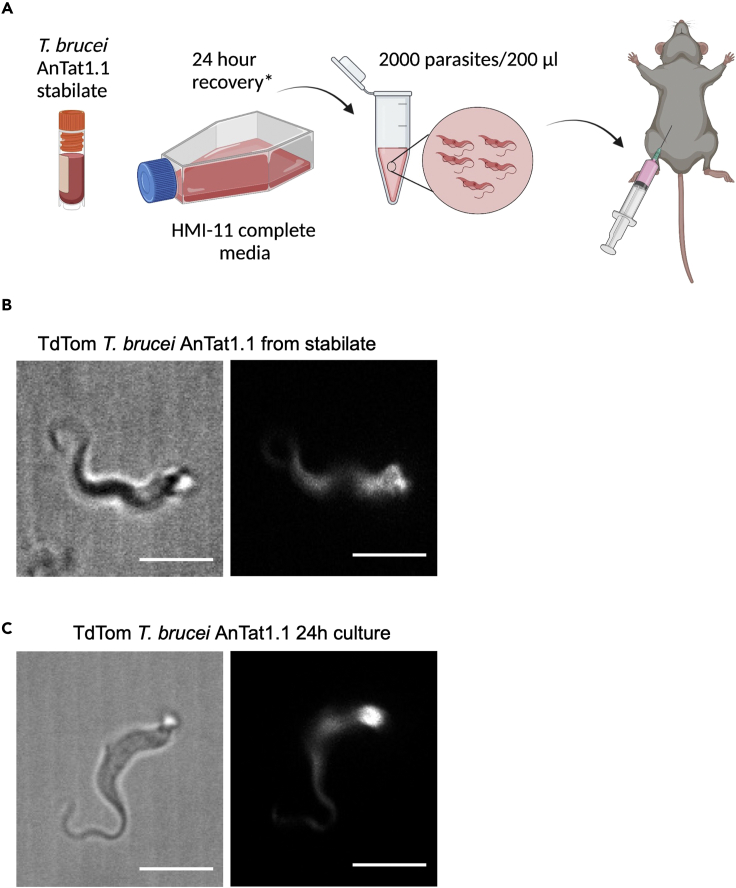
Figure 2.
Principle of infection of C57BL/6J mice with 2000 Trypanosoma brucei parasites
(A) Infection procedure: Begin by thawing a blood stabilate from liquid nitrogen or −80°C storage. Centrifuge the stabilate for 2 min at 800 g and remove all supernatant to remove glycerol. Resuspend in 10 mL of HMI-11 complete media and recover for 24 h at 37°C in 5% CO2. Count parasites using a suitable method (e.g., various automatic counting methods or manually using a Neubauer chamber), and dilute (or concentrate) to have 2000 parasites/200 μL volume. If various mice will be infected, calculate accordingly. Inject 200 μL intraperitoneally into C57BL/6J mice.
(B and C) How should T. brucei look in morphology? (B) shows T. brucei immediately after thawing a stabilate from liquid nitrogen or −80°C storage (left image shows bright field image; right image shows TdTomato fluorescence). (C) shows T. brucei after 24 h of culture in HMI11 media (left image shows bright field image; right image shows TdTomato fluorescence). Scale bar: 5 μm.
Anesthetize mice prior to surgical procedures and IVM
Timing: 5–10 min
-
8.Prepare ketamine/xylazine solution.
-
a.Add 0.8 mL of xylazine (20 mg/mL) to a sterile 15 mL Falcon tube.
-
b.Add 1.2 mL of ketamine (100 mg/mL) to the same tube.
-
c.Add 8 mL of pharmaceutical grade distilled water or sterile 0.9% NaCl to obtain 10 mL of anesthetic solution.
-
a.
Note: This solution can be stored for a month at 4°C for one week, and be used in multiple experiments.
-
9.
Weigh the mice before injection of anesthetic solution in order to administer a correct dose.
-
10.
Administer 100 μL of anesthetic mixture per 10 g of body weight (i.e., 120 mg/kg ketamine + 16 mg/kg xylazine). Administration is via intraperitoneal injection.
-
11.
Place the animal back in its cage until it is immobilized (confirm loss of upright reflex).
Note: Before reaching its full effect, the mixture of ketamine/xylazine can cause confusion and agitation in some mice. This may cause a tendency to jump. Mice should always be placed in a cage to avoid any injuries. In addition, anesthetized mice should never be left in a cage with awake mice for prolonged periods as anesthesia leaves them vulnerable to attacks by cage mates.
Note: In animals heavier than 30 g (usually older animals), as well as sick animals (regardless of weight) (i.e., beyond day 13 of infection with T. brucei at the dosage described in this protocol), this dosage of anesthetic needs to be adjusted. While additional volume (ie. with up to 50% additional dosage) might be needed to achieve full anesthetic effect, care should be taken to avoid overdosage. Additional drug administration can be done intraperitoneally. Alternatively, an inhalational anesthetic can be used (e.g., isoflurane). In animals that are overweight, anesthetics can have unpredictable effects due to the metabolism of the anesthetic – this is the focus of research in other areas. We therefore suggest care if working with animals above 30 g. In sick animals (overweight or not), the recommended dosage considering body weight only, can be lethal. We therefore suggest that in sick animals (overweight or not), care is taken, and a lower initial dose is used.
CRITICAL: Avoid stressing the animal while holding or manipulating it. Stress can alter the effects of the anesthetic and its duration. Additionally, the person responsible for experimentation must have proficiency in intraperitoneal injection to avoid causing any internal bleeding or damage that might compromise surgeries or IVM.
CRITICAL: Additional aliquots of anesthetic must be available in surgery and imaging sites, in order to ensure boosts of anesthesia when required, during the entire duration of the procedure. Usually, additional boosts are administered in hourly periods. Care should be taken to avoid overdosage. check pedal withdrawal reflex to monitor anesthetic depth.
Prepare the microscope at least 1 h prior to starting surgeries for IVM
Timing: 1 h
-
12.
Turn on microscope including all lasers to be used.
-
13.
Turn on incubation system to 27°C–30°C (if the microscope contains a temperature-controlled chamber). Otherwise, turn on heating mat to 27°C–30°C to ensure that the mouse does not suffer hypothermia once it is anesthetized and surgery has been performed.
-
14.
Prepare all settings in hardware (e.g., stage) and software (e.g., exposure time, acquisition rate, laser power, etc.) before starting surgeries to ensure that the equipment is ready to use immediately after the surgeries.
CRITICAL: Body temperature quickly drops in anesthetized mice. Heating should be ensured at all times during surgery. Moreover, hypothermia can affect the behavior of T. brucei both in exposed surfaces and inside a mouse.
Note: If more than one mouse will be imaged in the same day, it is recommended to perform the anesthesia and surgeries on each mouse immediately prior to imaging, rather than as a batch.
Perform surgeries for IVM
Timing: Variable (see specific timing for each organ)
Note: Care should be taken in each surgery, to avoid damaging the blood vasculature. Any damage to the vasculature may result in blood loss and reduced life span. In some cases, with a single surgical incision, more than one organ can be exposed (and imaged if desired). This is consistent with decreasing animal usage, while inducing little additional stress on any one animal.
CRITICAL: One or more organs can be exposed through a single incision. Regardless of the number of organs studied, great care should be taken during the imaging procedure, to ensure that the animal remains anesthetized the entire time. Constant monitoring of anesthetic effects should be done, and top-up/repeat doses provided at adequate intervals, or an inhalational anesthetic should be provided throughout the entire procedure. In long imaging procedures, great care should be taken to ensure that the organ remains hydrated, and that no physical alterations occur on the tissue (eg. bleeding, burning due to imaging, etc.).
Note: Windows can be chronic or terminal. We have recently published detailed methods and uses of intravital microscopy and existing surgical methods in the context of parasitology, as a mini-series collection ((De Niz et al., 2019b; 2019c; 2019a, 2020). In this work, we will focus on the description of terminal windows only. Terminal windows allow for visualization over several hours, but do not envisage recovery of the mouse after imaging. Terminal windows can be secured in place using sutures, vacuum, or surgical glue.
-
15.Before starting any of the surgeries described below, check for righting and pedal withdrawal reflexes.
-
a.To assess righting reflex, place the mouse in a dorsal position and determine its ability to return to the ventral position. Once the mouse is unable to return to the ventral position, proceed to check for the righting and pedal withdrawal reflex.
-
b.Pinch the metacarpal region of the hind foot either with the index and thumb, or with a blunt forceps (with care not to injure the mouse) (Methods Video S3). Check both hind feet and proceed to surgery ONLY when this reflex is absent in both feet.
-
a.
-
16.
Protect the mouse’s eyes from drying using specialized gel (e.g., Puralube® or Lacrilube®) for this purpose.
-
17.
For all surgeries described below, clean the mouse hair and skin with 70% ethanol at the site where you wish to make a surgical incision.
Note: Cleaning the site with ethanol will sterilize the region and will prevent hair from dispersing across the surgical site and the surgical material. If post-surgical recovery will take place, the hair must be removed to prevent any contamination.
Note: Before starting to image, observe under microscope to remove any potential hair. Hair is heavily auto-fluorescent and can block important imaging sites at the site of surgical incision. Any remaining individual hairs can be carefully removed using forceps or a moist cotton swab.
Video shows hind leg reflex during anesthesia. Surgery should only be performed once this reflex is absent.
Surgeries to expose the various organs of interest are detailed below. The order followed for the lists is based on the anatomical location (starting from cranial (i.e., the head), and ending in the caudal region (i.e., the pelvis)), rather than by the order of complexity. For order of complexity, we suggest observing details described in Figure 3, and summarized main challenges in Table 1.
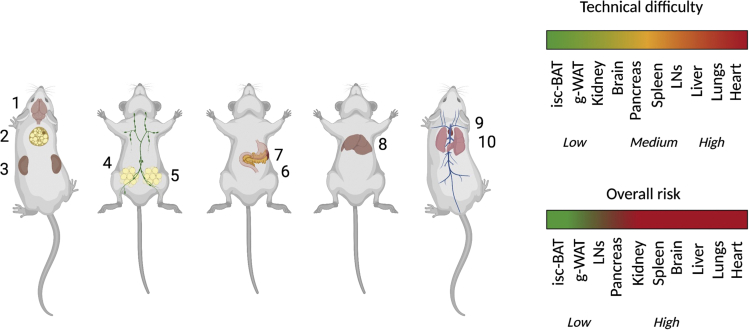
Figure 3.
Order of surgeries and IVM. Schematic showing the order we suggest to follow if multiple surgeries will be performed in the same animal
This is further explained in Table 1. Surgeries with low risk include the brain (unless a full craniotomy is performed) (1), the interscapular brown adipose tissue (isc-BAT) (2), the kidneys (3), the lymph node (4), and the gonadal white adipose tissue (5). Surgeries with medium risk include the spleen (6), pancreas (7) and liver (8). The surgeries with the highest risk include the heart (9) and the lungs (10). Created with BioRender.com.
Table 1.
Surgical challenges associated with each organ
| Surgery | Risk | Technical difficulty |
|---|---|---|
| Brain | Low if no craniotomy is required. High if craniotomy is required. |
Low if no craniotomy is required. Medium if craniotomy is required. |
| interscapular-Brown Adipose Tissue | Low: no major bleeding likely. No complex stabilization required. | Low: a simple incision will expose the organ. |
| Kidney | High risk of major bleeding and systemic compromise if surgery is unsuccessful. | Low: a simple incision will expose the organ and stabilization is easy to perform. |
| Gonadal-White Adipose Tissue | Low: if unsuccessful, no major systemic compromise exists. | Low: a simple incision will expose the organ. |
| Lymph node | Low: if unsuccessful, no risk of major systemic compromise exists. | Medium: it is a complicated surgery and requires high precision, specialized stages, and a high level dexterity. |
| Pancreas/Spleen | High risk of major bleeding and systemic compromise if surgery is unsuccessful. | High: a precise incision is required to avoid organ retraction and major bleeding. |
| Liver | High risk of major bleeding and systemic compromise if surgery is unsuccessful. | High: a precise incision is required for adequate organ stability and to avoid major bleeding. |
| Lungs/Heart | High: failure could result in respiratory or cardiac collapse, or poor local or systemic oxygenation. | High: thoracotomy, tracheotomy and intubation are complex procedures. |
Procedure for brain IVM
Timing: 10–30 min
-
18.
Place the mouse in the ventral recumbency.
-
19.Preparation for surgical site (Figure 4 left panel). Perform a surgical incision on the mouse scalp to expose the region of interest in the skull and brain. For exposing the region of interest, the incision can extend throughout any region from the olfactory bulb to the posterior region of the head (Figure 4). Various options exist to proceed.
-
a.Option 1: Imaging can be performed through the skull, without any further surgical procedure (i.e., if a 2 PM is used).
-
b.Option 2: Skull thinning can be performed using a spherical surgical drill, in order to access deeper layers of the brain. The drill is run at 30,000 rpm, until the thickness of the remaining skull is 10–20 μm. This can be measured by second harmonic generation.
-
c.Option 3: A surgical incision to remove a piece of the skull can be performed using both a surgical drill and surgical instruments. This must be performed with great care in order to avoid brain damage in the form of swelling, oedema or vascular damage. This option requires the placement of a window to reduce brain swelling. This is only suitable for short term imaging (i.e., up to 2 h).
-
a.
-
20.Place window for imaging (Figure 4 middle panel). Before placing a window or imaging, verify that no hair is present on the area of interest. Once this is verified, various options exist to proceed.
-
a.Option 1: Depending on the type of surgery, a window may or may not be required. If not required (i.e., when the skull is intact or thinned), immersion oil can be used directly on the skull. Alternatively, the surgical site can be covered with a hydration solution or 1×PBS.
-
b.Option 2: A coverslip can be placed on the site of interest by adding surgical glue around the contour of the incision (shown on image by dotted lines).
-
c.Option 3: A coverslip can be secured on a holder attached to a vacuum pump, and placed on top of the surgical site of interest. This will serve both to keep the coverslip in place and keep a constant pressure on the brain to prevent quick oedema. Allow for lose adherence to the brain surface via 20–25 mmHg of reversible vacuum.
-
a.
-
21.
Place mouse on stage and image (Figure 4 right panel). Once the surgical incision is ready, place the mouse on the ventral recumbency. The mouse head must be immobilized for imaging. This can be achieved using a stereotaxic instrument that provides support to both ears and the front of the head e.g., via the teeth.
-
22.
Once the mouse is secure and ready for imaging, inject dyes or conjugated antibodies of interest.
CRITICAL: Care should be taken during the surgery to avoid injuring the blood and lymphatic vasculature.
-
23.
Use an upright microscope with at least a 63× objective, and position the objective at the site of the surgical incision.
-
24.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Video S4.
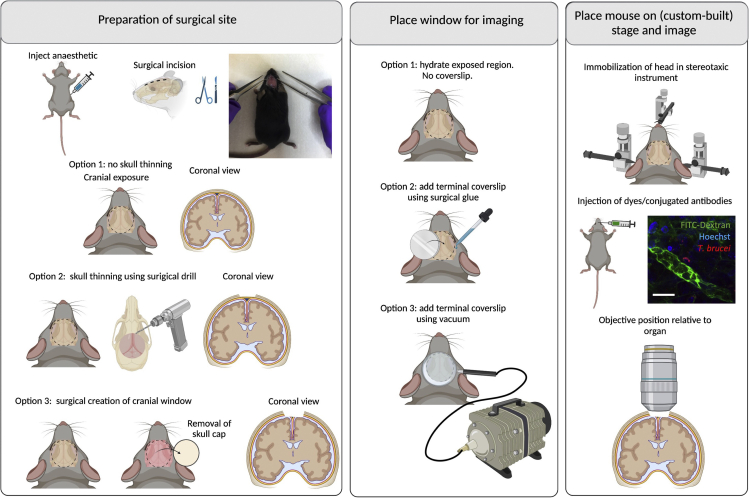
Figure 4.
Surgeries for implanting terminal windows in the mouse brain
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Three options exist for surgical preparation: a) Option 1: No skull thinning. Coronal view shows untouched cranium and meninges. b) Option 2: Skull thinning using surgical drill. Coronal view shows thinned cranium with untouched meninges. c) Option 3: Surgical creation of cranial window. This involves the removal of the skull cap, and imaging directly on the brain. Coronal view shows full view into the brain. Middle panel: Place window for imaging. Several options exist for imaging. Option 1: no coverslip is placed, and imaging is done directly on the hydrated exposed tissue. Option 2: A round coverslip is placed using surgical glue. Option 3: A round coverslip attached to a vacuum ring is placed on top of the incision. Right panel: Place mouse on stage and image. The head of the mouse must be secured/immobilized using a stereotaxic instrument. This includes securing at 3 main points: the teeth, and both ears. A support for the chin can be used to help immobilization. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. An upright microscope is best suited to image the brain. The objective reaches the brain from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (green) in the brain in an uninfected mouse.
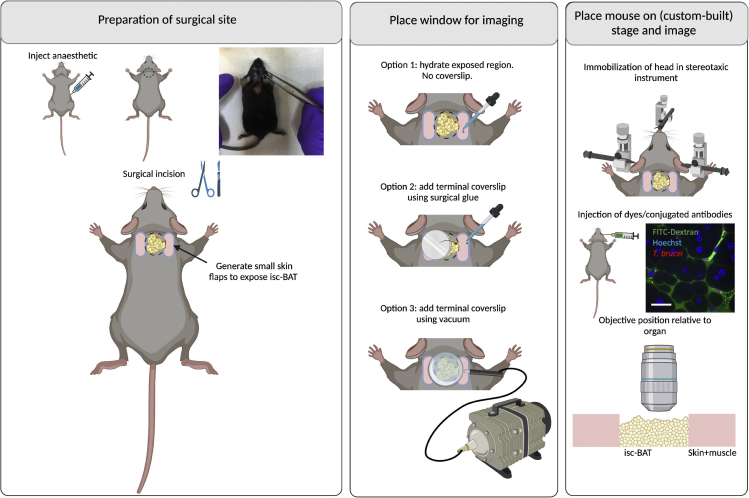
Procedure for interscapular brown adipose tissue (isc-BAT) IVM
Timing: 10–30 min
-
25.
Place the mouse in the ventral recumbency.
-
26.
Preparation for surgical site (Figure 5 left panel). To visualize the isc-BAT, perform a surgical incision immediately below the neck and skull, and slightly above the shoulder blades. For exposing the region of interest, the incision can extend throughout any region from the base of the neck to the region between the shoulder blades (Figure 5).
-
27.Place window for imaging (Figure 5 middle panel). Various options exist to proceed.
-
a.Option 1: Depending on the type of surgery, a window may or may not be required. If not required, immersion oil can be used directly on the isc-BAT. Alternatively, the surgical site can be covered with a hydration solution or 1×PBS.
-
b.Option 2: A coverslip can be placed on the site of interest by adding surgical glue around the contour of the incision (shown on image by dotted lines).
-
c.Option 3: A coverslip can be secured on a holder attached to a vacuum pump, and placed on top of the surgical site of interest. This will serve both to keep the coverslip in place and to stabilize the imaging region. Allow for lose adherence to the tissue surface via 20–25 mmHg of reversible vacuum.
-
a.
-
28.
Place mouse on stage and image (Figure 5 right panel). Once the surgical incision is ready, place the mouse in the ventral recumbency. The mouse head must be immobilized for imaging. This can be achieved using a stereotaxic instrument that provides support to both ears and the front of the head e.g., via the teeth.
-
29.
Once the mouse is secure and ready for imaging, inject dyes or conjugated antibodies of interest.
CRITICAL: Care should be taken during the surgery to avoid injuring the blood and lymphatic vasculature.
-
30.
Use an upright microscope with at least a 63× objective, and position the objective at the site of the surgical incision.
-
31.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Video S5.
Figure 5.
Surgeries for implanting terminal windows in the mouse interscapular brown adipose tissue (isc-BAT)
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Generate a small cut between the neck and the shoulder blades to find the interscapular brown adipose tissue. Middle panel: Place window for imaging. Several options exist for imaging. Option 1: no coverslip is placed, and imaging is done directly on the hydrated exposed tissue. Option 2: A round coverslip is placed using surgical glue. Option 3: A round coverslip attached to a vacuum ring is placed on top of the incision. Right panel: Place mouse on stage and image. The head of the mouse must be secured/immobilized using a stereotaxic instrument. This includes securing at 3 main points: the teeth, and both ears. A support for the chin can be used to help immobilization. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. An upright microscope is best suited to image the isc-BAT. The objective reaches the isc-BAT from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (white), Hoechst (blue), and TdTom AnTat1.1E T. brucei (red) in the isc-BAT at day 5 post-infection.
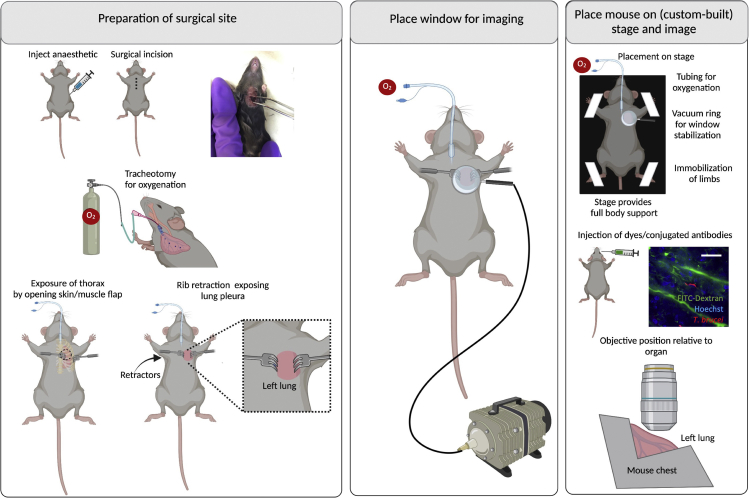
Procedure for lung IVM
Timing: 20–30 min
CRITICAL: Alteration to oxygenation will likely affect other organs, and may include alterations to host and pathogen parameters. Care should be taken to avoid alterations to oxygenation.
Note: The protocol used in our work (with slight modifications) is based on (Looney et al., 2011). Multiple protocols are available, alternative to the one described below (Fiole and Tournier, 2016). These protocols depend on whether mechanical stabilization is needed (i.e., if not needed, an easier strategy to image the lung could be to oversample the free-moving lung); whether ventilation alterations are acceptable, and whether ventilation is required (i.e., if it is not required, this allows the use of bronchus clamping or sequential apnea and gated imaging). For a better explanation helping to decide on these parameters for thoracic surgery and imaging, readers are referred to (Fiole and Tournier, 2016) exploring the advantages and disadvantages of each method.
-
32.
Place the mouse in the dorsal recumbency.
-
33.Preparation for surgical site (Figure 6 left panel). (Optional) Perform assisted ventilation using a nose cone (Rindfield and McBrian, 2012) or endotracheal intubation, and link to a ventilator.
-
a.Several protocols exist for endotracheal intubation. A recent example is described in detail in (Maruyama et al., 2021).
-
b.Intubate mice and initiate assisted ventilation.
-
a.
-
34.
Perform a thoracotomy – an incision of 0.5 cm–1 cm wide on the left side of the chest. This involves re-sectioning up to 3 ribs around the 5th rib, and stabilization using rib retractors.
-
35.
Place window for imaging (Figure 6 middle panel). Use a suction system linked to a ring that acts as a holder for a glass slide to create a vacuum. This leads to stabilization of the lung. Use a round coverslip (#1.5, 15–20 mm diameter) to make a window. Allow for lose adherence to the lung surface via 20–25 mmHg of reversible vacuum.
-
36.
Place mouse on stage and image (Figure 6 right panel). Place the mouse in dorsal recumbency. A custom-built stage must allow for full-body support of the mouse. All limbs and the tail should be immobilized with the aid of adhesive tape. Similarly, tubing for oxygenation and for vacuum generation should be considered in the setup that will be introduced to the microscope.
CRITICAL: Spread suction force over large vacuum area to prevent injuries on the lung.
-
37.
Use an inverted microscope with at least a 63× objective, and position the objective at the site of the optical window over the surgical incision.
-
38.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Video S6.
Figure 6.
Surgeries for implanting terminal windows in the mouse lungs
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Then perform a tracheotomy to Intubate mice for assisted ventilation. Follow by performing a thoracotomy with the aid of rib retractors. Middle panel: Place window for imaging. Use a round coverslip attached to a vacuum ring and place on top of the incision. Right panel: Place mouse on stage and image. A custom-built stage provides full body support to the mouse and allows space for tubing (used for vacuum, oxygenation and intravenous cannulation). Immobilize all limbs and tail. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. An upright microscope is best suited to image the lungs. The objective reaches the lungs from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (green), Hoechst (blue), and TdTom AnTat1.1E T. brucei (red) in the lungs at day 5 post-infection.
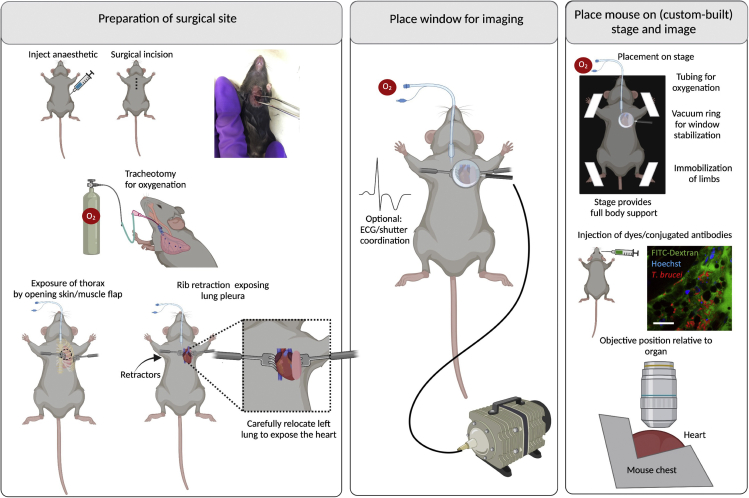
Procedure for heart IVM
Timing: 20–30 min
CRITICAL: Alteration to oxygenation will likely affect other organs, and may include alterations to host and pathogen parameters. Care should be taken to avoid alterations to oxygenation.
Note: The protocol used in our work (with slight modifications) is based on (Looney et al., 2011). The main challenge with heart surgery is that upon opening the thoracic chamber, lung collapse can occur. This can alter oxygenation to other organs, and drastically shorten the lifespan of the mouse. Although in this procedure the heart is imaged, several steps required for lung stabilization, are performed as described in steps 32–36.
-
39.
Place the mouse in the dorsal recumbency.
-
40.Preparation for surgical site (Figure 7 left panel). Perform assisted ventilation using endotracheal intubation, and link to a ventilator.
-
a.Intubate mice and initiate assisted ventilation.
-
a.
-
41.
Perform a thoracotomy – an incision of 0.5 cm–1 cm wide on the left side of the chest. This involves re-sectioning up to 3 ribs around the 5th rib, and stabilization using rib retractors. Carefully move the lung to the left, to display the heart instead.
-
42.
Place window for imaging (Figure 7 middle panel). Use a suction system linked to a ring that acts as a holder for a glass slide to create a vacuum. This leads to stabilization of the thorax, and allows a direct partial immobilization of the heart. Use a round coverslip (#1.5, 15–20 mm diameter) to make a window. Allow for lose adherence to the heart surface via 20–25 mmHg of reversible vacuum.
-
43.
Place mouse on stage and image (Figure 7 right panel). Place the mouse in dorsal recumbency. A custom-built stage must allow for full-body support of the mouse. All limbs and the tail should be immobilized with the aid of adhesive tape. Similarly, tubing for oxygenation and for vacuum generation should be considered in the setup that will be introduced to the microscope.
Optional: Important for heart imaging will be heartbeat-induced artifacts. Image acquisition and image processing methods exist to address this motion.
CRITICAL: Spread suction force over large vacuum area to prevent injuries on the heart.
-
44.
Use an inverted microscope with at least a 63× objective, and position the objective at the site of the optical window over the surgical incision.
-
45.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Videos S7 and S8.
Figure 7.
Surgeries for implanting terminal windows in the mouse heart
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Then perform a tracheotomy to intubate mice for assisted ventilation. Follow by performing a thoracotomy with the aid of rib retractors, and carefully move the left lung sideways to expose the heart. Middle panel: Place window for imaging. Use a round coverslip attached to a vacuum ring and place on top of the incision. Right panel: Place mouse on stage and image. A custom-built stage provides full body support to the mouse and allows space for tubing (used for vacuum, oxygenation and intravenous cannulation). Immobilize all limbs and tail. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. An upright microscope is best suited to image the heart. The objective reaches the heart from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of A647-ICAM2 (white), in the heart at day 3 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), in the heart at day 3 post-infection. Parasites are visible by dark contrast.
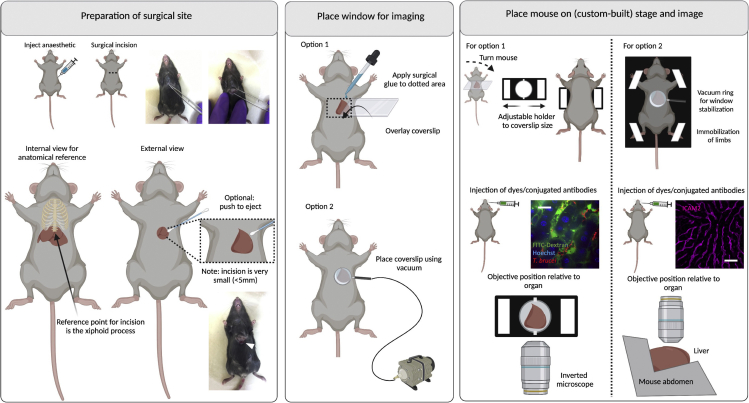
Procedure for liver IVM
Timing: 10–20 min
Note: The protocol used in this work is based on a previously published protocol, also established in parasitology by (Thiberge et al., 2007).
-
46.
Place the mouse in dorsal recumbency.
-
47.Preparation for surgical site (Figure 8 left panel). Locate the xiphoid process (caudal thoracic limit reference) and perform a small horizontal surgical incision immediately below it.
-
a.Begin by using the forceps to lift the skin away from the liver.
-
b.Make a small cut on the skin. Once the abdominal muscles become visible, make a small horizontal cut to reach the liver. Liver lobes will be identifiable by their coloration (brownish/red).
-
a.
Note: Although cutting large vessels in the skin and muscle while making the incision is unlikely, you should have a cauterizer ready to solve any major bleeding coming from these tissues.
CRITICAL: The incision on the skin and muscle must be small (i.e., no more than 5 mm in width) in order to prevent major alterations to the cardiac and respiratory output. Care should be taken to make small cuts to approach the liver, as the liver lobes are very close to the skin surface at this anatomical point.
CRITICAL: The liver should not be directly handled with the surgical instruments. Handling with the forceps is likely to either collapse the immediate vasculature or cause major perforations and bleeding.
-
48.
To expose the liver lobe, extend the incision carefully as needed. Then, push the left liver lobe through the incision with the aid of moist cotton swabs.
-
49.
Moisten the liver with saline solution (0.9% NaCl) or 1×PBS.
-
50.Place window for imaging (Figure 8 middle panel).
-
a.Option 1: For a terminal window, add surgical glue above and below the surgical incision, and place a rectangular coverslip on top of the organ.
-
b.Option 2: Alternatively, use a suction system linked to a ring that acts as a holder for a glass slide to create a vacuum. This leads to stabilization of the abdomen, and allows a direct partial immobilization of the liver. Use a round coverslip (#1.5, 15–20 mm diameter) to make a window. Allow for lose adherence to the liver surface via 20–25 mmHg of reversible vacuum.
-
a.
CRITICAL: Do not press the coverslip very hard against the liver. This poses the risk of interrupting blood flow or damaging the tissue. Any damage can affect host and parasite parameters.
-
51.
Place mouse on stage and image (Figure 8 right panel). Place the mouse on ventral recumbency (window option 1) or dorsal recumbency (window option 2), using a suitable microscope stage. While custom-built stages can be used, a conventional stage with adjustable sections suitable for imaging glass slides and coverslips can be used. Care should be taken, however, that key points in the rest of the body are well supported (e.g., head at the anterior end, and legs and tail at the posterior end).
-
52.
For window option 1, use an inverted microscope with a 63× or 100× oil immersion objectives, and position the objective below the window on the surgical incision. For window option 2, use an upright microscope with a 63× or 100× oil immersion objectives, and position the objective above the window on the surgical incision.
-
53.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Videos S9 and S10.
Figure 8.
Surgeries for implanting terminal windows in the mouse liver
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Find the xiphoid process (at the bottom of the ribs) to locate the site for incision. Make a small incision (up to 5 mm) and with the aid of cotton buds, eject either the middle or part of the left liver lobe. Middle panel: Place window for imaging. Two options exist for imaging the liver either using an inverted microscope (option 1) or an upright microscope (option 2). Option 1: Use a rectangular coverslip and secure on the mouse using surgical glue. Option 2: Use a round coverslip attached to a vacuum ring and place on top of the incision. Right panel: Place mouse on stage and image. Option 1: For imaging in an inverted microscope, an adjustable holder can be used on the microscope stage. The mouse is turned to display the ventral side (and the incision), and the coverslip attached to the mouse is secured on the holder. Note: This setup will likely be sufficient for a mouse of 6–10 weeks old. Younger mice will likely need support for the head and lower body. Older mice will likely bend the coverslip, risking that it breaks within the microscope. For older mice, option 2 is therefore recommended. The objective reaches the liver from the bottom.Option 2: A custom-built stage provides full body support to the mouse and allows space for tubing (used for vacuum, and intravenous cannulation). Immobilize all limbs and tail. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. The objective reaches the liver from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of A647-ICAM2 (magenta), 70 kDa FITC-Dextran (white), and TdTom AnTat1.1E T. brucei (red) in the liver at day 3 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and Hoechst (blue) in the liver in an uninfected mouse.
Procedure for spleen IVM
Timing: 10–20 min
-
54.
Place the mouse in right lateral recumbency.
-
55.Preparation for surgical site (Figure 9 left panel).
-
a.Begin by using the forceps to lift the skin away from the spleen.
-
b.Make a small cut on the skin only, and retract the skin to generate a small field of view.
-
c.Make sure you can visualize the underlying spleen.Note: Although cutting large vessels in the skin while making the incision is unlikely, you should have a cauterizer ready to solve any major bleeding coming from these tissues.
-
d.Once the underlying muscle is exposed, locate a large ascending vessel, and perform a small diagonal incision in its vicinity but with the utmost care to avoid it.
 CRITICAL: The incision on the skin and muscle must be small (i.e., no more than 5 mm in width) in order to prevent major bleeding and stabilize the organ better. However, in an infected animal, splenomegaly will require that this incision is larger than in an uninfected mouse. This enlargement of the incision should be done with great care to avoid important vasculature in the vicinity.
CRITICAL: The incision on the skin and muscle must be small (i.e., no more than 5 mm in width) in order to prevent major bleeding and stabilize the organ better. However, in an infected animal, splenomegaly will require that this incision is larger than in an uninfected mouse. This enlargement of the incision should be done with great care to avoid important vasculature in the vicinity. CRITICAL: The spleen should be handled with great care. Avoid using any sharp forceps to handle it, as this might create bleeding that can be life-threatening. Blunt forceps can be used, however, great care should be taken not to perform dents on the organ, nor collapse the underlying vasculature of the organ.
CRITICAL: The spleen should be handled with great care. Avoid using any sharp forceps to handle it, as this might create bleeding that can be life-threatening. Blunt forceps can be used, however, great care should be taken not to perform dents on the organ, nor collapse the underlying vasculature of the organ.
-
a.
-
56.
Place window for imaging (Figure 9 middle panel). The pancreas is located in the medial side of the spleen, and is more resistant to handling with blunt forceps. The pancreas can be used as an anchor point to pull the medial side of the spleen through the surgical incision.
CRITICAL: Great care should be taken not to pull the spleen with much force, nor create significant tension during exposure (at risk of rupturing the arteries in the middle and posterior of the organ).
CRITICAL: Expose the pancreas and up to 1/3 of the spleen, and make sure enough tissue is exposed to prevent retraction into the body.
CRITICAL: Care should be taken during the surgery to avoid injuring the blood and lymphatic vasculature.
-
57.Once it is clear that retraction of the organ into the body does not occur, hydrate the organ with saline solution (0.9% NaCl) or 1×PBS.
-
a.Option 1: For a terminal window, add surgical glue above and below the surgical incision, and place a coverslip on top of the organ.
-
b.Option 2: Alternatively, use a suction system linked to a ring that acts as a holder for a glass slide to create a vacuum. This leads to stabilization of the organ and surrounding tissues, and allows a direct partial immobilization of the spleen. Use a round coverslip (#1.5, 15–20 mm diameter) to make a window. Allow for lose adherence to the spleen surface via 20–25 mmHg of reversible vacuum.
-
a.
CRITICAL: Do not press the coverslip very hard against the spleen as this can cause vascular collapse. Any damage can affect host and parasite parameters.
-
58.
Place mouse on stage and image (Figure 9 right panel). Place the mouse in (window option 1) left lateral recumbency, or (window option 2) right lateral recumbency, on a suitable microscope stage.
-
59.
For window option 1, use an inverted microscope with a 63× or 100× oil immersion objectives, and position the objective below the window on the surgical incision. For window option 2, use an upright microscopy with a 63× or 100× oil immersion objectives, and position the objective above the window on the surgical incision.
-
60.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Videos S11, S12, and S13.
Figure 9.
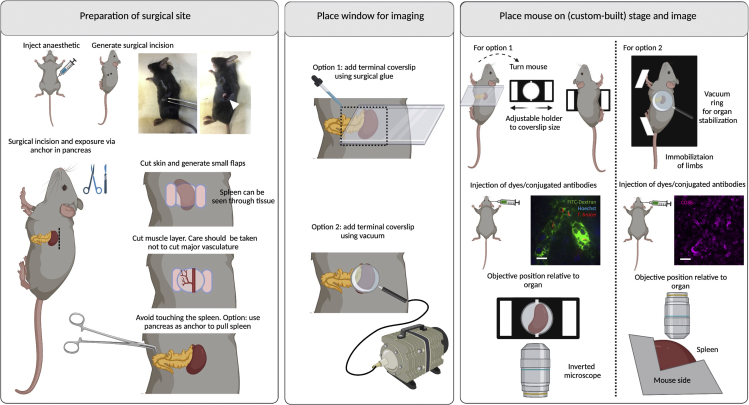
Surgeries for implanting terminal windows in the mouse spleen
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Make a small incision (up to 5 mm) and carefully expose the spleen. In infected mice the spleen is more fragile and therefore we recommend using the pancreas as an anchor point to carefully pull the spleen. Middle panel: Place window for imaging. Two options exist for imaging the spleen either using an inverted microscope (option 1) or an upright microscope (option 2). Option 1: Use a rectangular coverslip and secure on the mouse using surgical glue. Option 2: Use a round coverslip attached to a vacuum ring and place on top of the incision. Right panel: Place mouse on stage and image. Option 1: For imaging in an inverted microscope, an adjustable holder can be used on the microscope stage. The mouse is turned laterally to display the left side (and the incision), and the coverslip attached to the mouse is secured on the holder. Note: This setup will likely be sufficient for a mouse of 6–10 weeks old. Younger mice will likely need support for the head and lower body. Older mice will likely bend the coverslip, risking that it breaks within the microscope. For older mice, option 2 is therefore recommended. The objective reaches the spleen from the bottom.Option 2: A custom-built stage provides full body support to the mouse and allows space for tubing (used for vacuum, and intravenous cannulation). Immobilize all limbs and tail. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. The objective reaches the spleen from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and TdTom AnTat1.1E T. brucei (red) in the spleen at day 3 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and TdTom AnTat1.1E T. brucei (red) in the spleen at day 5 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and TdTom AnTat1.1E T. brucei (red) in the spleen at day 2 post-infection.
Procedure for pancreas IVM
Timing: 10–20 min
-
61.
Place the mouse in right lateral recumbency.
-
62.Preparation for surgical site (Figure 10 left panel).
-
a.Begin by using the forceps to lift the skin away from the pancreas.
-
b.Make a small cut on the skin only, and retract the skin to generate a small field of view.
-
c.Make sure you can visualize the underlying pancreas.Note: Although cutting large vessels in the skin while making the incision is unlikely, you should have a cauterizer ready to solve any major bleeding coming from these tissues.
-
d.Once the underlying muscle is exposed, locate a large ascending vessel, and perform a small diagonal incision in its vicinity but with the utmost care to avoid it.
 CRITICAL: The incision on the skin and muscle must be small (i.e., no more than 5 mm in width) in order to prevent major bleeding and stabilize the organ better.
CRITICAL: The incision on the skin and muscle must be small (i.e., no more than 5 mm in width) in order to prevent major bleeding and stabilize the organ better.
-
a.
-
63.
Place window for imaging (Figure 10 middle panel). The pancreas is located in the medial side of the spleen, and is relatively resistant to handling with blunt forceps.
CRITICAL: Expose the pancreas, and make sure enough tissue is exposed to prevent retraction into the body.
CRITICAL: Care should be taken during the surgery to avoid injuring the blood and lymphatic vasculature.
-
64.Once it is clear retraction of the organ into the body does not occur, hydrate the organ with saline solution (0.9% NaCl) or 1×PBS.
-
a.Option 1: For a terminal window, add surgical glue above and below the surgical incision, and place a coverslip on top of the organ.
-
b.Option 2: Alternatively, use a suction system linked to a ring that acts as a holder for a glass slide to create a vacuum. This leads to stabilization of the organ and surrounding tissues, and allows a direct partial immobilization of the pancreas. Use a round coverslip (#1.5, 15–20 mm diameter) to make a window. Allow for lose adherence to the pancreas surface via 20–25 mmHg of reversible vacuum.
-
a.
-
65.
Place mouse on stage and image (Figure 10 right panel). Place the mouse in left lateral recumbency (window option 1), or right lateral recumbency (window option 2) on a suitable microscope stage.
-
66.
For window option 1, use an inverted microscope with a 63× or 100× oil immersion objectives, and position the objective below the window on the surgical incision. For window option 2, use an upright microscopy with a 63× or 100× oil immersion objectives, and position the objective above the window on the surgical incision.
-
67.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Videos S14 and S15.
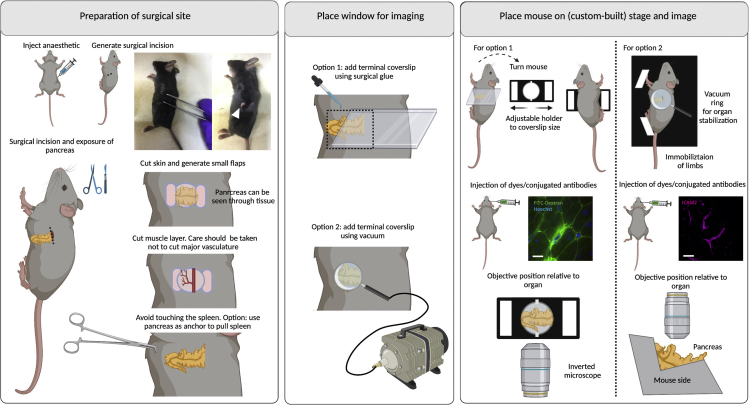
Figure 10.
Surgeries for implanting terminal windows in the mouse pancreas
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Make a small incision (up to 5 mm) and carefully expose the pancreas. Middle panel: Place window for imaging. Two options exist for imaging the pancreas either using an inverted microscope (option 1) or an upright microscope (option 2). Option 1: Use a rectangular coverslip and secure on the mouse using surgical glue. Option 2: Use a round coverslip attached to a vacuum ring and place on top of the incision. Right panel: Place mouse on stage and image. Option 1: For imaging in an inverted microscope, an adjustable holder can be used on the microscope stage. The mouse is turned laterally to display the left side (and the incision), and the coverslip attached to the mouse is secured on the holder. Note: This setup will likely be sufficient for a mouse of 6–10 weeks old. Younger mice will likely need support for the head and lower body. Older mice will likely bend the coverslip, risking that it breaks within the microscope. For older mice, option 2 is therefore recommended. The objective reaches the spleen from the bottom. Option 2: A custom-built stage provides full body support to the mouse and allows space for tubing (used for vacuum, and intravenous cannulation). Immobilize all limbs and tail. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. The objective reaches the spleen from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and TdTom AnTat1.1E T. brucei (red) in the pancreas at day 2 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), Hoechst (blue), and TdTom AnTat1.1E T. brucei (red) in the pancreas at day 5 post-infection.
Procedure for kidney IVM
Timing: 10–20 min
-
68.
Place the mouse in ventral recumbency.
-
69.Preparation for surgical site (Figure 11 left panel).
-
a.Begin by using the forceps to lift the skin away from the kidney.
-
b.Make a small cut on the skin and retract it to generate a small field of view.
-
c.Make sure you can visualize the underlying kidney under the muscle layer – it will be clear due to the dark red colur.
-
a.
Note: Although cutting large vessels in the skin while making the incision is unlikely, you should have a cauterizer ready to solve any major bleeding coming from these tissues.
CRITICAL: The incision on the skin and muscle must be small (i.e., no more than 5 mm in width) in order to prevent major bleeding and stabilize the organ better. Any enlargement of the incision should be done with great care to avoid blood vasculature in the vicinity.
-
70.
Place window for imaging (Figure 11 middle panel). The kidney is resistant to handling with blunt forceps. Do not use sharp tools to handle it. Gently retract the surgical incision and expose the dorsal side of the organ.
CRITICAL: Great care should be taken not to pull the kidney with much force, nor create significant tension during exposure (at risk of rupturing blood vessels of the organ).
-
71.Once it is clear that retraction of the kidney into the body does not occur, hydrate the organ with saline solution (0.9% NaCl), or 1×PBS.
-
a.Option 1: For a terminal window, add surgical glue above and below the surgical incision, and place a coverslip on top of the organ.
-
b.Option 2: Alternatively, use a suction system linked to a ring that acts as a holder for a glass slide to create a vacuum. This leads to stabilization of the organ and surrounding tissues, and allows a direct partial immobilization of the kidney. Use a round coverslip (#1.5, 15–20 mm diameter) to make a window. Allow for lose adherence to the kidney surface via 20–25 mmHg of reversible vacuum.
-
a.
-
72.
Place mouse on stage and image (Figure 11 right panel). Place the mouse in left lateral recumbency (window option 1), or dorsal recumbency (window option 2) on a suitable microscope stage.
-
73.
For window option 1, use an inverted microscope with a 63× or 100× oil immersion objectives, and position the objective below the window on the surgical incision. For window option 2, use an upright microscopy with a 63× or 100× oil immersion objectives, and position the objective above the window on the surgical incision.
-
74.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Video S16.
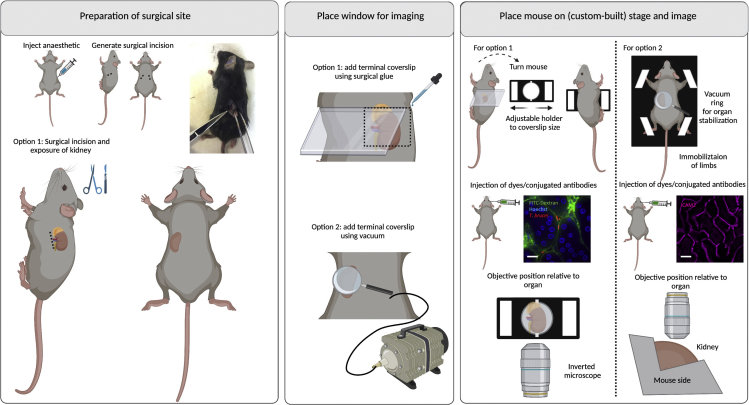
Figure 11.
Surgeries for implanting terminal windows in the mouse kidney
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Make a small incision (up to 5 mm) and carefully expose the kidney. Middle panel: Place window for imaging. Two options exist for imaging the kidney either using an inverted microscope (option 1) or an upright microscope (option 2). Option 1: Use a rectangular coverslip and secure on the mouse using surgical glue. Option 2: Use a round coverslip attached to a vacuum ring and place on top of the incision. Right panel: Place mouse on stage and image. Option 1: For imaging in an inverted microscope, an adjustable holder can be used on the microscope stage. The mouse is turned laterally to display the left or right side (and the incision), and the coverslip attached to the mouse is secured on the holder. Note: This setup will likely be sufficient for a mouse of 6–10 weeks old. Younger mice will likely need support for the head and lower body. Older mice will likely bend the coverslip, risking that it breaks within the microscope. For older mice, option 2 is therefore recommended. The objective reaches the kidney from the bottom. Option 2: A custom-built stage provides full body support to the mouse and allows space for tubing (used for vacuum, and intravenous cannulation). Immobilize all limbs and tail. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. The objective reaches the kidney from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), Hoechst (blue), and TdTom AnTat1.1E T. brucei (red) in the kidney at day 4 post-infection.
Procedure for lymph node IVM
Timing: 20–30 min
Note: This protocol requires the generation of suitable stages/holders for positioning the mouse within the microscope. This protocol is based on a published method by (Liou et al., 2012). The method described below is adapted to the popliteal lymph node, but other lymph nodes are accessible to visualization too, and the protocol should be adapted accordingly.
-
75.
Place the mouse in the ventral recumbency.
-
76.
Preparation for surgical site (Figure 12 left panel). Use the forceps to lift the skin away from the mid-calf, and cut vertically up to the upper thigh.
-
77.
Generate skin flaps by making two horizontal skin incisions at the top of the vertical incision line. Separate the skin as needed, to expose the lymph node.
-
78.
Separate the lymph node from the surrounding adipose and muscle tissues.
CRITICAL: Care should be taken during the surgery to avoid injuring the blood and lymphatic vasculature.
-
79.
Hydrate the lymph node with warm saline solution (0.9% NaCl) or 1×PBS.
-
80.
Place window for imaging (Figure 12 middle panel). Place a glass coverslip on the tissue and secure using either surgical glue or via suction using vacuum, and place the mouse in the microscope stage.
-
81.
Place mouse on stage and image (Figure 12 right panel). Use an upright microscope with a 63× or 100× oil immersion objectives, and position the objective below the window on the surgical incision.
-
82.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Video S17.
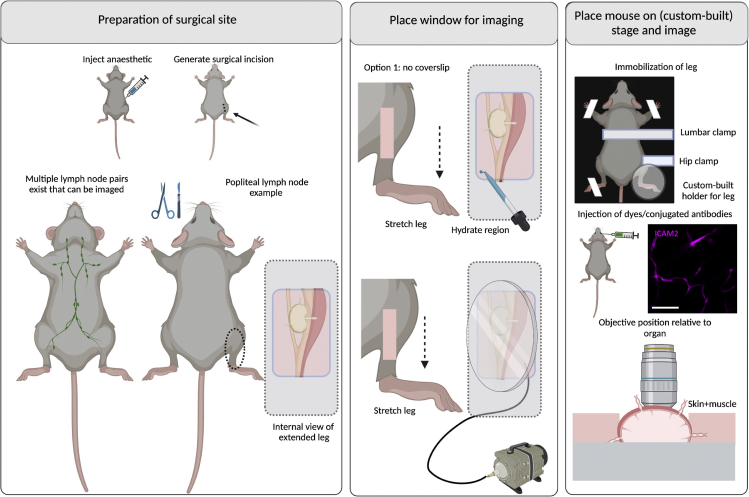
Figure 12.
Surgeries for implanting terminal windows in the mouse popliteal lymph node
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Make a small incision on one of the lower limbs (up to 2 mm) and carefully move the subcutaneous adipose tissues and muscles to expose the popliteal lymph node. Middle panel: Place window for imaging. Two options exist for imaging the popliteal lymph node either with or without a coverslip. Option 1: Hydrate the site of incision and image directly. Option 2: Use a round coverslip attached to a vacuum ring and place on top of the incision. Right panel: Place mouse on stage and image. A custom-built stage provides full body support to the mouse and allows space for tubing (used for vacuum, and intravenous cannulation). Stretch the leg fully and immobilize all limbs and tail. For stabilization of the leg, a lumbar and hip clamp must be used. Depending on the microscope, an additional custom-built support for the leg might be required. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. The objective reaches the lymph node from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow) and Hoechst (blue) in the lymph nodes in uninfected mice.
Procedure for gonadal white adipose tissue IVM
Timing: 5–10 min.This protocol is also suitable to image the reproductive organs of both male and female mice
-
83.
Place the mouse in dorsal recumbency.
-
84.
Preparation for surgical site (Figure 13 left panel). Locate the site of interest, i.e., in the pelvis, on either lateral location, at the level of the upper thigh and before the knee.
-
85.
Use the forceps to lift the skin away from the pelvis, and cut diagonally and downwards to expose the underlying muscle. Locate the gonadal adipose tissue, and make a small incision in the muscle and peritoneum, to expose the gonadal adipose tissue.
CRITICAL: Care should be taken during the surgery to avoid injuring the vasculature in the vicinity, and to enter the region of the hind leg adipose tissue and muscle. The surgical incision must remain within the confines of the pelvis and must be very small in order to stabilize the organ adequately.
-
86.
Through the incision in the peritoneum, place the forceps inside the pelvis and with care, withdraw the full gonadal adipose tissue.
CRITICAL: Care should be taken not to extend the adipose tissue too much in order to avoid damage to the vasculature and tissue integrity.
-
87.
Hydrate the tissue with 1×PBS or saline solution (0.9% NaCl).
-
88.Place window for imaging (Figure 13 middle panel).
-
a.Option 1: For a terminal window, add surgical glue above and below the surgical incision, and place a coverslip on top of the organ.
-
b.Option 2: Alternatively, use a suction system linked to a ring that acts as a holder for a glass slide to create a vacuum. This leads to stabilization of the organ and surrounding tissues, and allows a direct partial immobilization of the g-WAT. Use a round coverslip (#1.5, 15–20 mm diameter) to make a window. Allow for lose adherence to the g-WAT surface via 20–25 mmHg of reversible vacuum.
-
a.
-
89.
Place mouse on stage and image (Figure 13 right panel). For window option 1, use an inverted microscope with a 63× or 100× oil immersion objectives, place the mouse on the stage in the ventral recumbency, and position the objective below the window on the surgical incision. For window option 2, place the mouse in dorsal recumbency and use an upright microscopy with a 63× or 100× oil immersion objectives, and position the objective above the window on the surgical incision.
-
90.
Confirm that no significant vascular damage, or blood flow alterations occurred, and acquire images as needed (see steps 98–103). See Methods Videos S18 and S19.
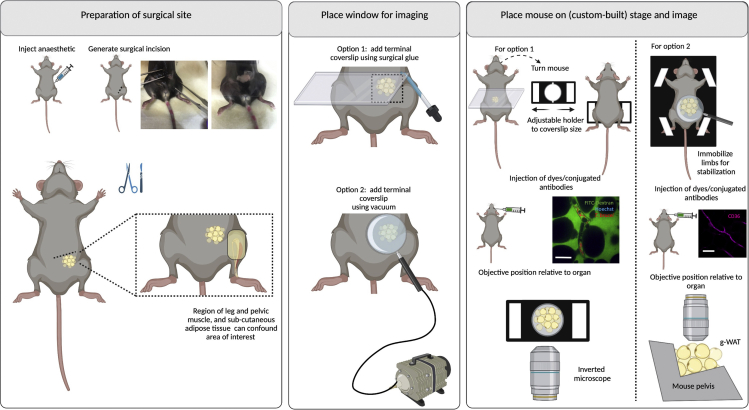
Figure 13.
Surgeries for implanting terminal windows in the mouse gonadal white adipose tissue (g-WAT)
Left panel: Preparation of surgical site. Begin by injecting anesthetic intraperitoneally. Diagram and picture show site of incision. Make a small incision in the pelvis, lateral to the area of the knee (up to 5 mm) and carefully expose the g-WAT. Middle panel: Place window for imaging. Two options exist for imaging the g-WAT either using an inverted microscope (option 1) or an upright microscope (option 2). Option 1: Use a rectangular coverslip and secure on the mouse using surgical glue. Option 2: Use a round coverslip attached to a vacuum ring and place on top of the incision. Right panel: Place mouse on stage and image. Option 1: For imaging in an inverted microscope, an adjustable holder can be used on the microscope stage. The mouse is turned to display the ventral side (and the incision), and the coverslip attached to the mouse is secured on the holder. Note: This setup will likely be sufficient for a mouse of 6–10 weeks old. Younger mice will likely need support for the head and lower body. Older mice will likely bend the coverslip, risking that it breaks within the microscope. For older mice, option 2 is therefore recommended. The objective reaches the g-WAT from the bottom. Option 2: A custom-built stage provides full body support to the mouse and allows space for tubing (used for vacuum, and intravenous cannulation). Immobilize all limbs and tail. Depending on the experimental conditions, dyes can be added intravenously either before or after securing the mouse on the stage. The objective reaches the g-WAT from the top. Scale bar: 20 μm. Created with BioRender.com.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow) and Hoechst (blue) in the g-WAT at day 4 post-infection. Parasites are visible by dark contrast.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (green) and TdTom AnTat1.1E T. brucei (white) in the g-WAT at day 4 post-infection.
(Optional) prepare and inject RBC markers to visualize distribution across vasculature
Timing: 1 h and 15 min
The main aim of this step is to use labeled RBCs as markers for cell distribution across the vasculature, which can be used to compare relative parasite distribution.
-
91.
Following the surgery and window implantation in the tissue of choice, inject fluorescently labeled RBCs by intravenous injection. In our protocol we compared the distribution of labeled RBCs upon injection through retro-orbital plexus, or tail vein, and saw no differences in perfusion time or distribution.
-
92.To prepare labeled RBCs, we adapted a previously published protocol (Theron et al., 2010).
-
a.Remove 10–20 μL of blood from the tail vein of an unlabeled, uninfected mouse and collect in RPMI 1640, in tubes treated with heparin to prevent coagulation.
-
b.Count the required number of RBCs to label, using a Neubauer chamber or other method that enables quantification of RBC concentration.
-
c.Centrifuge cells at 800 g for 5 min, and resuspend the pellet in 20 μM CFDA-SE or 10 μM DDAO-SE, and incubate for 1 h at 37°C.
-
d.Wash the suspension with complete RPMI 1640 medium twice.
-
e.Resuspend in a final volume of 200 μL of 1× PBS, and inject the full volume into an infected mouse ready for IVM imaging. Figure 14 shows labeled RBCs before and after injection into mice.
-
a.
Note: The dye should be chosen based on the compatibility with other fluorescent reporters/markers used in the same assay. For instance, if antibodies conjugated to A647 are used to label the vasculature, then CFDA-SE is preferable. Conversely, if FITC-Dextran is a requirement, DDAO-SE would be a more suitable dye to use in this context for RBC labeling. It is possible, however, to use several combinations, although care should be taken to ensure that the various markers can be clearly visualized.
Figure 14.
Exogenous labeling of red blood cells for IVM visualization
Red blood cells labeled with (A) DDAO-SE and (B) CFDA-SE before and (C) after injection into mice as seen in the liver vasculature. Labeled cells were used as markers for cell distribution and blood flow. Scale bars: 5 μm (A and B); 30 μm (C).
(Optional) Inject dyes and conjugated antibodies to visualize receptors of interest (or other markers of interest)
Timing: 1 min
The main aim of this step is to visualize the vascular endothelium, host and parasite nuclei, vascular permeability and extravascular parenchyma within the mouse body.
-
93.
Following the surgery and window implantation in the tissue of choice, inject conjugated antibodies for the vascular receptor of interest (or other cellular or subcellular markers of interest).
-
94.
In our work, we performed intraocular and tail vein injections, and demonstrated that perfusion time and labeling is similar regardless of the injection site.
-
95.
In our work, we resuspended the desired concentration (per mouse) of Hoechst, 70 kDa FITC-Dextran and A647-conjugated antibodies in 100–200 μL 1× PBS, and injected the full volume into the mouse.
-
96.
Determine perfusion kinetics and begin imaging at a suitable time post-injection.
Note: Hoechst labeling occurs immediately and is stable for several hours after injection. FITC-Dextran is a marker of vascular permeability and will diffuse across injured endothelium over time. If such kinetics are important for the research question, imaging should begin immediately after injection of the dye.
(Optional) restricting blood flow to image parasite behavior within the confines of the vascular endothelium
Timing: 1–10 min
The main aim of this step is to visualize T. brucei behavior within the vascular endothelium, without blood flow.
-
97.
To visualize parasite behavior without blood flow, it is possible to block the blood flow within a specific vessel by surrounding the vessel or capillary of interest with sutures and tightening the sutures to completely or partially block blood flow.
Note: While surgical clamps exist, if they are used, they should be specific for small animal surgeries. Larger clamps (eg. used for human surgeries) have the risk of resulting in severed vasculature or unsuccessful occlusion.
Imaging and finalizing the IVM procedure
Timing: Variable depending on imaging time required, mouse survival, surgery success. Generally, minimum of 30 min, maximum 8 h
-
98.
After successfully performing the surgery and labeling steps of interest, position the mouse in the stage, and image as required, depending on the research question.
-
99.
For maximum tissue coverage and quantification purposes, we recommend imaging at least 30 fields of view per tissue per mouse. To diminish possible quantification biases, we recommend these fields of view should be contiguous to one another, and a method for surveillance should be established in advance.
-
100.
For time lapse imaging, suitable total time and time intervals should be established, that enable answering the question of interest.
CRITICAL: During imaging, the mouse will be alive and anesthetized. Care should be taken to ensure that the mouse remains fully anesthetized for the whole duration of the process. Care should be also taken to provide cardiac resuscitation in some instances.
-
101.
For all terminal windows, if more than one surgery will be performed, as each respective imaging is finished, the surgical incision can be carefully closed using stitches.
-
102.
For all terminal windows, once all surgeries are finished, euthanize the mouse using an anesthetic overdose and/or cervical dislocation while the mouse remains anesthetized.
-
103.
Place mice cadavers in freezer or dispose according to institute regulations.
Expected outcomes
The expected outcomes of this protocol are:
-
•
To establish a T. brucei infection in rodent models with fluorescent reporter lines. If reporter lines are not available, alternatives include exogenous labeling with suitable dyes prior to injection into mice or parasite identification using Hoechst and FITC-Dextran as to visualize the nuclei and kinetoplasts and provide contrast with the tissue parenchyma respectively. For any particular dye of choice, it should be established whether and how this affects host and parasite viability.
-
•
To image T. brucei or other Trypanosoma species within the organs and vasculature of anesthetized living mice by carefully surgically exposing those organs through terminal windows. Fluorescent markers, either through the genetic modification of T. brucei, or the injection of fluorescent dyes or conjugated antibodies allows labeling of structures of interest, and host-pathogen interactions. Intravital images and videos can be used for image analysis of multiple parameters of interest including parasite location, behavior and cellular features, as well as host-specific changes and host-parasite interactions throughout infection. In our work (De Niz et al., 2021), we used these tools to analyze T. brucei interactions with the host vasculature, including multiple receptors present in endothelial cells. images of various organs, acquired using intravital microscopy, are shown in Figure 15 and Methods Videos S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15, S16, S17, S18, and S19. However, multiple other questions can be addressed using these methods, and although we did not explore them in detail in this protocol, genetically modified mice with specific fluorescent reporters can be included in the range of questions to address.
Figure 15.
Trypanosoma brucei interactions with the host vasculature, including multiple receptors present in endothelial cells
(A–F) Images show (A) ICAM1-AF647 labeled vasculature in the brain, (B) T. brucei-TdTomato tagged parasites and Hoechst labeling in the pancreas, (C) T. brucei-TdTomato tagged parasites in the heart, (D) CD36-AF647 labeled vasculature in the lungs, (E) CD31-AF647 labeled vasculature in the kidney, (F) FITC-Dextran labeled extravascular niche in the g-WAT. Scale bar 15 μm.
Limitations
Each surgery poses a risk of significant damage. Adequate training and skill in performing the surgical incisions will ensure that these risks are reduced to the minimum. Possible alterations can arise from the surgeries and the imaging sessions themselves. These include, for instance, immune cell recruitment to the site of injury, or to the site of imaging. The action of imaging (and the physical parameters altered through it) could affect important parameters. If relevant to the biological question addressed, careful controls should be introduced. Accidental damaging of the vasculature during surgery will likely result in bleeding into the parenchyma of organs, which can be a confounding factor if scoring intra/extravascular location. It is important to avoid this, and to note whether free erythrocytes are present in the organ parenchyma in significant quantities, suggesting that a vessel could have been damaged.
Intravital microscopy (as performed in the protocols we describe here) allows visualization only to a certain depth in the organ of interest. This depends on the microscope used, and the anatomy of the organ itself. This limitation in depth should always be taken into account when making conclusions about the data acquired. The protocols here described, however, can be used in combination with others that allow whole-organ analysis of the parameters of interest, as some regions of the organ cannot be easily reached using the methods here described. As for all microscopy, adequate contrast is necessary to visualize the factors of interest (i.e., cells, tissues, or subcellular structures). Therefore choosing markers with strong fluorescence that withstand the imaging procedure (including, potentially, long time-lapse imaging) is essential. Autofluorescence has been extensively reported in various organs. Relevant to point 4 discussed above, choosing suitable fluorescent proteins and dyes is essential.
The number of parameters that can be simultaneously imaged will be determined by the fluorophores available, the acquisition speed of the equipment used, and the phenomenon observed. In our work, this was limited to 5 different fluorescent reporters used simultaneously, but alternative options exist, some of which are shown (Figure 16).
Figure 16.
Possible combinations of fluorescent proteins, dyes, and fluorophore conjugations for antibodies
(A) Combinations used in our T. brucei -host interactions research. Hoechst, with an emission max. of 461 nm, was used to label nucleic acids from host and parasites. FITC-Dextran, with an emission max. of 518 nm was used to label the intra- and extravascular compartments of the host and provide contrast to visualize cells in all compartments. CFDA-SE, and DDAO-SE with an emission max. of 517 nm and 635 nm respectively, were used to label exogenous RBCs and use them as markers across the infected host vasculature. TdTomato with an emission max. of 581 nm was used as the fluorescent reporter for T. brucei. Most fluorescently conjugated antibodies to identify vascular endothelium receptors (i.e., ICAM1, ICAM2, VCAM1, CD36 and PECAM1) used the fluorophore AF647 with emission max. of 671 nm.
(B) Several other combinations of fluorescent proteins, fluorescent dyes and fluorophores for antibody conjugation are possible. Created with BioRender.com.
Troubleshooting
Problem 1
3D printed stages do not fit correctly in the microscope stages or do not correctly hold the organ of interest (“before you begin”, steps 4–6).
Potential solution
It is normal that 3D printed stages need to be ‘fine-tuned’ in order to provide the best access to the organ imaged. Although accurate measurements and design should be aimed for from the beginning, give enough time to fine-tune small details once the stage is tested in the desired microscope. General schematics are provided in Figures 17, 18, and 19.
Figure 17.
General sketch of holder for upright microscopes
It consists of a base of a defined length and width (e.g., 30 cm × 15 cm), to which custom-made platforms for body support, and stereotaxic devices for head support, can be attached. Externally, tubing and cabling (i.e., for anesthesia, oxygen, vacuum, temperature control, cardiac monitoring, etc.) can be organized and attached on custom-made supports. This is most suitable for brain, isc-BAT, lungs, heart, kidneys, and lymph nodes, although can be adapted for other organs. A mouse schematic has been inserted for reference. Created with BioRender.com.
Figure 18.
General sketch of holder for inverted microscopes (option 1)
This holder allows flexibility of use with glass-bottom dishes (suitable in case organs need to be immersed in solution e.g., to control peristalsis), as well as rectangular windows. The main advantage of this type of holder is that it is ‘closed’. Its many surfaces provide adequate support to an adult mouse, without the risk of the pressure being concentrated on the window and the objective. Multiple custom-made holders are compatible with this prototype, including the adapted “glass bottom dish” which incorporates open segments that accommodate the mouse body, and low-angled inclinations for better organ positioning during imaging. This is most suitable for liver, spleen, pancreas, placenta, g-WAT, and kidneys. A mouse schematic has been inserted for reference. Created with BioRender.com.
Figure 19.
General sketch of holder for inverted microscopes (option 2)
This stage holder allows flexibility of use with multiple custom-made mouse holders. However, its ‘open’ surfaces can be problematic for imaging both, young mice, and old (heavy) mice. For young mice, the lower and upper body might not reach the solid surfaces (resulting in the mouse’s body being mostly without support other than the window. This is problematic both, for imaging stability and the integrity of the window, and for mouse stability during imaging. For old/heavy mice, although the upper and lower surfaces will provide body support, this might not be enough to ensure window integrity. Therefore, examples of custom-made mouse holders include those with added support for the mouse body (e.g., for abdominal or pelvic). This is most suitable for liver, spleen, pancreas, placenta, g-WAT, and kidneys. A mouse schematic has been inserted for reference. Created with BioRender.com.
Problem 2
Fluorescent reporter for parasite is not stable or not optimal for IVM (“before you begin”, step 7).
Potential solution
In our experience with T. brucei but also other parasites, a strong fluorescent reporter is desirable. This was achieved either by a choice of a stable protein such as TdTomato or GFP, or by modulation expression of the fluorescent protein through a strong promoter (e.g., cytosolic expression of mCherry in Plasmodium parasites was strong under an Hsp70 promoter, but not strong under an ef1a promoter).
Problem 3
Conjugated antibody injected leads to over- or under-saturation of the site of interest (“before you begin”, step 9).
Potential solution
Microscope settings can be modified according to the antibody to be evaluated, however they must remain unchanged if a comparison of fluorescence intensity will be made across time. In such case, care should be taken to decide an optimal concentration to use throughout an entire time course of infection (if this is the question of interest), to avoid over- or under-saturation at any point of interest. This will hinder image analysis. To prevent this problem, we suggest that during the optimization period, the same antibody is tested in an uninfected animal, an infected animal with an early infection, and an infected animal with late infection.
Problem 4
Blocking antibodies only partially block the receptors of interest (“step-by-step method details”, steps 1–4).
Potential solution
A careful assessment of the blocking efficiency of the antibodies used should be performed to determine the correct dosage and timing (i.e., in several doses or a single dose). Moreover, the literature should be surveyed to determine the best clones/types of antibody to use. Despite this, it is possible that several brands and/or clones of antibody must be used to find the most adequate one. Care should be taken with some antibodies to prevent detrimental consequences in the mice (for instance, some blocking antibodies against components of the endothelial junctions may result in bleeding and vascular damage). In such case a careful search in the literature and preliminary testing should be done to determine the optimal dosage and administration specifications.
Problem 5
Infection with T. brucei is unsuccessful (“step-by-step method details”, steps 5–7).
Potential solution
Infections may fail due to various reasons. In our experience, the most common reasons might include a) incorrect parasite administration to the mouse (i.e., route or injection technique), b) incorrect generation of stabilates, c) incorrect thawing and/or dilution of stabilates, d) that parasites directly from stabilates might not all be viable and therefore the calculation of parasite concentration for infection is overestimated. To solve problem 5a, we suggest adequate training for injecting via various administration routes. Potential solutions to problems 5b–5d include thawing parasite vials and placing in HMI-11 culture for 24 h before administering into mice. In this way, viability and motility can be more accurately assessed.
Problem 6
Anesthetic dosage is insufficient or inefficient (“step-by-step method details”, steps 8–11).
Potential solution
In our experience, problems with anesthetics might be due to various reasons including a) that the anesthetic solution is not fresh. This can be easily solved by preparing a fresh solution, usually every week. b) the mice are old and/or overweight. In mice that are overweight, administration of the anesthetic solution (even when accurately calculated for the corresponding body weight) might not result in deep sleep. This is influenced by the metabolism being altered by the excess of adipose tissue. While increasing the dosage in a stepwise manner is possible, in our experience this can result in overdose. We suggest using controlled isoflurane to complement the effect of the ketamine/xylazine solution. C) the mice are at late stages of T. brucei infection. In our experience, great care should be taken when anesthetizing mice that are at late stages of infection. We recommend diminishing the original recommended dose by 25%, and increasing as necessary in incremental steps until full anesthesia is reached. Alternatively, controlled isoflurane administration can be used, to complement the effect of the ketamine/xylazine solution. Infection can also affect the time between anesthetic administration, and great care should be taken to monitor ananesthetic effects in these mice. In our work, this starts being a complication to consider, from day 9 post-infection onwards.
Problem 7
Exposed organ/tissue becomes dry (“step-by-step method details”, steps 15–90, various steps).
Potential solution
An adequately placed window and lubricant to keep the organ moist is necessary to avoid desiccation. If the organ becomes dry, it is possible to re-lubricate between acquisition time points or at determined intervals. In some cases it is more convenient to introduce tubing above the tissue to enable constant flow of lubricant in the form of warm PBS, saline solution (0.9% NaCl) or other suitable lubricants depending on the organ.
Problem 8
Organ is strongly autofluorescent in the same wavelengths as specific fluorescent markers (“step-by-step method details”, steps 15–90, various sub-steps).
Potential solution
Most autofluorescence in all organs was within a range of wavelengths between 480 and 590 nm. Our solution for this problem was either finding strongly fluorescent proteins for our various proteins of interest, or choosing fluorescent proteins within wavelengths 405–480 and 590–700 nm (e.g., Hoechst and AF647).
Problems 9–18 refer to surgical incisions of various options.
Problem 9
General IVM.
Too many hairs cause autofluorescence and block regions of interest. (“step-by-step method details”, steps 15–90, various sub-steps).
Potential solution
While in our work we did not shave the mice for terminal windows, but rather cleaned the area with 70% EtOH, and then removed most remaining hair at the incision site with the help of cotton swabs, if the presence of hair is a significant problem, the mice can be shaved and/or treated with depilatory cream prior to the surgery. Alternatively, nude mice can be used.
Problem 10
IVM of the brain.
Unsuccessful drilling of the cranium results either in not enough penetration depth or in penetration of the brain tissue and bleeding (“step-by-step method details”, steps 19–20).
Potential solution
While surgical proficiency comes with practice, it is possible to gain some insight into the structure of the cranium practicing drilling in a ‘phantom’ (a structure resembling a cranium), or on the cranium of a euthanized mouse.
Problem 11
IVM of the brain.
Stabilization of the head is incomplete or partially successful resulting in motion artifacts while imaging (“step-by-step method details”, step 21).
Potential solution
Correctly secure the mice using the stereotactic equipment. In our experience, tight securing of the teeth and both ears is essential for an adequate position for imaging.
Problem 12
IVM of the lungs.
Lungs collapse when a surgical incision is generated in the thorax (“step-by-step method details”, steps 33–34).
Potential solution
This is a common problem if a thoracotomy is unsuccessful. Significant practice should be done to quickly and accurately generate the thoracic window while preventing a lung collapse. The vacuum should be adequate: too little pressure will result in inadequate immobilization, while too much pressure will result in bleeding and/or lung collapse. This surgery is one of the most complicated and it requires practice.
Problem 13
IVM of the lungs and heart.
Immobilization is not complete and too much movement from respiration and heartbeat impedes imaging (“step-by-step method details”, steps 33–36 and 40–43).
Potential solution
Adequate surgical incisions and immobilization via vacuum will be key in preventing motion artifacts. If this is not possible, other alternatives for this surgery exist, which are discussed in the methods of this work. Sometimes, the heartbeat and respiration can be controlled with anesthetics additional to ketamine/xylazine as well as muscle relaxants. This can be explored if alternative surgical methods are not compatible with the research question.
Problem 14
IVM of the liver.
Immobilization is not complete and too much movement from respiration and heartbeat impedes imaging (“step-by-step method details”, steps 47–49).
Potential solution
This is a common problem for liver surgeries, as the anatomical location of the liver makes it highly susceptible to respiration and cardiac-induced motion. The main solution we have found for this problem is the careful surgical incision under the xiphoid process, which should be very small (no more than 5 mm). Larger incisions will likely be associated with motion problems. Sometimes, the heartbeat and respiration can be controlled with anesthetics additional to ketamine/xylazine as well as muscle relaxants.
Problem 15
IVM of the liver.
Retraction of the liver lobe back into the body, preventing imaging (“step-by-step method details”, step 48).
Potential solution
This is a common problem for liver surgeries. To avoid this problem, we suggest a significantly large portion of the liver lobe is exposed before placing the window. Before positioning the mouse in the microscope stage, and after placing the window, make sure that the liver lobe has not retracted. Be careful when handling the mouse inside the microscope to prevent this problem.
Problem 16
IVM of the spleen.
Accidentally performing an incision in the spleen leading to bleeding (“step-by-step method details”, steps 54–57).
Potential solution
This mistake is often lethal to the mouse, as an incision on the spleen itself will cause massive blood loss. In our experience, depending on the size of the incision, this might be difficult to cauterize, or if it is cauterized it could result in significant changes to the vascular parameters of the organ. Our suggestion is to never handle the spleen directly, but to do so indirectly via the neighboring organs and tissues. In particular, spleens of mice infected with T. brucei are highly fragile and most direct handling will result in bleeding. This should be avoided completely.
Problem 17
IVM of the spleen.
Accidentally collapsing the spleen vasculature due to too much pressure either with the coverslip or with the objective. This results in altered blood flow (“step-by-step method details”, steps 54–57).
Potential solution
Great care should be taken not to cause vascular collapse due to increased pressure on the spleen while handing it for imaging. To avoid this collapse, care should be taken during the surgery and window placement, not to pressure the organ. Also, only a small portion of the organ should be exposed at any one time, to prevent this problem.
Problem 18
IVM of the spleen and pancreas.
Retraction of the spleen and pancreas back into the body after surgical exposure (“step-by-step method details”, steps 54–57).
Potential solution
This is a common problem of the splenic/pancreatic window surgery. Careful exposure and window implantation should be done to prevent retraction of these organs. Before handling the mouse inside the microscope, make sure that the organs have not retracted into the body. This can be solved with a combination of adequate positioning, surgical glue positioning, and the amount of organ exposed for imaging.
Problem 19
IVM of the gonadal adipose tissue.
Drying of the tissue (“step-by-step method details”, steps 84–88).
Potential solution
This is a common complication associated with surgery in this tissue. Great care should be taken to keep the organ hydrated throughout imaging.
Problem 20
IVM of the gonadal adipose tissue.
Finding the g-WAT after performing the incision can be challenging (“step-by-step method details”, step 84).
Potential solution
Practice this surgery and become familiar with the anatomical location of the leg and the organs in the pelvis to distinguish carefully between them. The incision to expose this organ should be relatively small (no more than 5 mm).
Problem 21
The coverslip used for the window breaks while positioning the mouse inside the microscope or stage (“step-by-step method details”, steps 21–23 (brain), steps 28–30 (isc-BAT), steps 36–37 (lungs), steps 43–44 (heart), steps 51–52 (liver), steps 58–59 (spleen), steps 65–66 (pancreas), steps 72–73 (kidney), step 81 (lymph nodes), step 89 (g-WAT).
Potential solution
As the coverslips used for imaging are relatively thin, this is not an uncommon problem. We recommend that when rectangular coverslips are used, they do not exceed measurements of 60 × 20 mm, and that the microscope holder is appropriately tightened around it. Lose fitting could result in the mouse becoming displaced. Very tight fitting could result in the coverslip breaking. Moreover, the coverslip should be centrally positioned on the mouse to distribute the pressure coming from the mouse’s body weight, equally. The microscope objective will help in stabilizing the mouse body for imaging.
Problem 22
Surgical glue falling on the objective (“step-by-step method details”, step 23 (brain), step 30 (isc-BAT), step 37 (lungs), step 44 (heart), step 52 (liver), step 59 (spleen), step 66 (pancreas), step 73 (kidney), step 81 (lymph nodes), step 89 (g-WAT).
Potential solution
This accident can happen, and great care should be taken to avoid it. As the surgical glue is used to secure the windows in place, it sometimes lies close to the border of the window. Wait at least 5 min before placing the mouse into the microscope so that the surgical glue can solidify.
Problem 23
Surgical glue falling on the exposed organ (“step-by-step method details”, step 27b (isc-BAT), step 50a (liver), step 57a (spleen), step 64a (pancreas), step 71a (kidney), step 80 (lymph nodes), step 88a (g-WAT).
Potential solution
This accident can happen, and great care should be taken to avoid it. As the surgical glue is used to secure the windows in place, it sometimes can enter the organ. Prevent this by using small amounts of glue on the mouse body. It is important to note that contact of the glue with the exposed organ can alter the parameters measured.
Problem 24
Laser power is too high and damages organ (“step-by-step method details”, steps 98–100).
Potential solution
This problem can occur and should be avoided by carefully monitoring the tissue while imaging, for any signs of damage (such as burns). If using fluorescent reporters, signs of damage might begin as photobleaching, and develop into structural changes in the tissue.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Mariana De Niz (mariana.deniz@medicina.ulisboa.pt).
Materials availability
This study did not generate unique reagents.
Acknowledgments
This work was supported by LT000047/2019-L (HFSP) and ALTF 1048-2016 (EMBO) to M.D.N. L.M.F. is an investigator CEEC of the Fundação para a Ciência e a Tecnologia (CEECIND/03322/2018), and the laboratory is funded by ERC (FatTryp, ref. 771714). We are grateful to Jose Rino, Sara Silva Pereira (iMM, Lisbon), and Daniel Costa (attending veterinarian at iMM rodent facility, Lisbon) for their helpful input and comments for this manuscript. We thank Brice Rotureau (Institut Pasteur, Paris) for providing the triple reporter (TY1-TdTomato-FLuc) AnTat1.1E T. brucei parasite line used in this protocol, and Fabien Guegan (iMM, Lisbon) for generating the PAD1:GFP line used in this protocol. We acknowledge the Rodent and Bioimaging facilities of the Instituto de Medicina Molecular.
Author contributions
M.D.N. performed all surgeries and designed and optimized this protocol. M.D.N. wrote this manuscript. L.M.F. supervised this study.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101450.
Data and code availability
This study did not generate any unique data sets or code. Please see (De Niz et al., 2021) for data sets generated using this protocol, relevant to the original publication.
References
- Dean S., Sunter J., Wheeler R.J., Hodkinson I., Gluenz E., Gull K. A toolkit enabling efficient, scalable and reproducible gene tagging in trypanosomatids. Open Biol. 2015;5:140197. doi: 10.1098/rsob.140197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiole D., Tournier J.-N. Intravital microscopy of the lung: minimizing invasiveness. J. Biophotonics. 2016;9:868–878. doi: 10.1002/jbio.201500246. [DOI] [PubMed] [Google Scholar]
- Liou H.L.R., Myers J.T., Barkauskas D.S., Huang A.Y. Intravital imaging of the mouse popliteal lymph node. J. Vis. Exp. 2012;8:3720. doi: 10.3791/3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney M.R., Thornton E.E., Sen D., Lamm W.J., Glenny R.W., Krummel M.F. Stabilized imaging of immune surveillance in the mouse lung. Nat. Methods. 2011;8:91–96. doi: 10.1038/nmeth.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Naemura K., Yoshihara K., Imanaka-Yoshida K., Kurihara H., Miyagawa-Tomita S. Surgical protocol for permanent ligation of the left anterior descending coronary artery in mice to generate a model of myocardial infarction. STAR Protoc. 2021;2:100775. doi: 10.1016/j.xpro.2021.100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Niz M., Meehan G.R., Tavares J. Intravital microscopy: imaging host–parasite interactions in lymphoid organs. Cell Microbiol. 2019;21:e13117. doi: 10.1111/cmi.13117. [DOI] [PubMed] [Google Scholar]
- De Niz M., Meehan G.R., Brancucci N.M.B., Marti M., Rotureau B., Figueiredo L.M., Frischknecht F. Intravital imaging of host-parasite interactions in skin and adipose tissues. Cell Microbiol. 2019;21:e13023. doi: 10.1111/cmi.13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Niz M., Nacer A., Frischknecht F. Intravital microscopy: imaging host-parasite interactions in the brain. Cell Microbiol. 2019;21:e13024. doi: 10.1111/cmi.13024. [DOI] [PubMed] [Google Scholar]
- De Niz M., Carvalho T., Penha-Gonçalves C., Agop-Nersesian C. Intravital imaging of host-parasite interactions in organs of the thoracic and abdominopelvic cavities. Cell Microbiol. 2020;22:e13201. doi: 10.1111/cmi.13201. [DOI] [PubMed] [Google Scholar]
- Calvo-Alvarez E., Cren-Travaillé C., Crouzols A., Rotureau B. A new chimeric triple reporter fusion protein as a tool for in vitro and in vivo multimodal imaging to monitor the development of African trypanosomes and Leishmania parasites. Infect Genet Evol. 2018 Sep;63:391–403. doi: 10.1016/j.meegid.2018.01.011. [DOI] [PubMed] [Google Scholar]
- De Niz M., Brás D., Ouarné M., Pedro M., Nascimento A.M., Henao Misikova L., Franco C.A., Figueiredo L.M. Organotypic endothelial adhesion molecules are key for Trypanosoma brucei tropism and virulence. Cell Rep. 2021;36:109741. doi: 10.1016/j.celrep.2021.109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindfield T., McBrian S. Assisted ventilation without endotracheal intubation in rats. J. Investig. Surg. 2012;25:197–199. doi: 10.3109/08941939.2011.616257. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7) doi: 10.1038/nmeth.2089. 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theron M., Hesketh R.L., Subramanian S., Rayner J.C. An adaptable two-color flow cytometric assay to quantitate the invasion of erythrocytes by Plasmodium falciparum parasites. Cytom. Part A. 2010;77:1067–1074. doi: 10.1002/cyto.a.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiberge S., Blazquez S., Baldacci P., Renaud O., Shorte S., Ménard R., Amino R. In vivo imaging of malaria parasites in the murine liver. Nat. Protoc. 2007;2:1811–1818. doi: 10.1038/nprot.2007.257. [DOI] [PubMed] [Google Scholar]
- Zimmermann H., Subota I., Batram C., Kramer S., Janzen C.J., Jones N.G., Engstler M. A quorum sensing-independent path to stumpy development in Trypanosoma brucei. PLoS Pathog. 2017;13:e1006324. doi: 10.1371/journal.ppat.1006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods video S1. Imaging T. brucei viability, related to step 5
Video shows T. brucei parasites from a glycerol stabilate immediately after thawing from storage at −80°C.
Video shows T. brucei parasites 24 h after thawing from storage at −80°C.
Video shows hind leg reflex during anesthesia. Surgery should only be performed once this reflex is absent.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (green) in the brain in an uninfected mouse.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (white), Hoechst (blue), and TdTom AnTat1.1E T. brucei (red) in the isc-BAT at day 5 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (green), Hoechst (blue), and TdTom AnTat1.1E T. brucei (red) in the lungs at day 5 post-infection.
Video shows the expected outcome of the injection of A647-ICAM2 (white), in the heart at day 3 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), in the heart at day 3 post-infection. Parasites are visible by dark contrast.
Video shows the expected outcome of the injection of A647-ICAM2 (magenta), 70 kDa FITC-Dextran (white), and TdTom AnTat1.1E T. brucei (red) in the liver at day 3 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and Hoechst (blue) in the liver in an uninfected mouse.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and TdTom AnTat1.1E T. brucei (red) in the spleen at day 3 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and TdTom AnTat1.1E T. brucei (red) in the spleen at day 5 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and TdTom AnTat1.1E T. brucei (red) in the spleen at day 2 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), and TdTom AnTat1.1E T. brucei (red) in the pancreas at day 2 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), Hoechst (blue), and TdTom AnTat1.1E T. brucei (red) in the pancreas at day 5 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow), Hoechst (blue), and TdTom AnTat1.1E T. brucei (red) in the kidney at day 4 post-infection.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow) and Hoechst (blue) in the lymph nodes in uninfected mice.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (yellow) and Hoechst (blue) in the g-WAT at day 4 post-infection. Parasites are visible by dark contrast.
Video shows the expected outcome of the injection of 70 kDa FITC-Dextran (green) and TdTom AnTat1.1E T. brucei (white) in the g-WAT at day 4 post-infection.
Data Availability Statement
This study did not generate any unique data sets or code. Please see (De Niz et al., 2021) for data sets generated using this protocol, relevant to the original publication.