Abstract
Background
Altitude sickness (AS), which is caused by rapid exposure to low amounts of oxygen at high elevations, poses a great threat to humans working and traveling in these conditions. Acute mountain sickness includes high-altitude pulmonary edema and high-altitude cerebral edema. Acetazolamide (AZ) is often used to treat pulmonary edema caused by hypoxia. Additionally, the medicinal plant Rhodiola rosea L. (Rh) is often used to prevent AS in the Qinghai-Tibet plateau. However, the mechanisms of action of Rh and AZ in the treatment of AS remain unclear. To date, no research has been conducted to determine whether their combined use has better efficacy in the treatment and prevention of AS than their separate use.
Methods
We used the method of network pharmacology to analyze the mechanisms of Rh and AZ in combination in the prevention and treatment of AS, and also verified our results.
Results
The hypoxia-inducible factor (HIF)-1 signaling pathway, which is related to hypoxia, and other pathways related to pulmonary hypertension, became more enriched after the combined use of the 2 drugs. Additionally, Rh and AZ regulated most nodes in the AS network. Further, compared to their separate use, the combined use of Rh and AZ further downregulated the gene expression of HIF-1α and improved hemodynamics in rats, and thus helped the body to reduce its sensitivity to hypoxic environments and pulmonary artery pressure.
Conclusions
This study provides evidence supporting the combined use of AZ and Rh in the treatment of AS.
Keywords: Altitude sickness (AS), acetazolamide (AZ), Rhodiola, high altitude
Introduction
Altitude sickness (AS) refers to the negative health effect of high altitude caused by rapid exposure to low amounts of oxygen at high elevations (i.e., elevations >2,500 m); however, susceptible individuals can also get sick at low altitudes (1). The partial pressure of oxygen is lower at high altitudes. In general, hypoxia triggers a physiological regulation that helps the body adapt to low oxygen conditions (2). Sometimes, the body responds abnormally. These abnormal reactions can include dizziness, headache, vomiting, weakness, and sleep disorders (3,4). Acute mountain sickness can progress to high-altitude pulmonary edema or high-altitude cerebral edema (5). Chronic AS may occur following long-term exposure to a high altitude (6,7).
Various treatment methods are used to treat AS-induced symptoms. These methods are mainly divided to non-pharmacological interventions and pharmacological interventions. Non-pharmacological interventions are mainly to relieve symptoms of altitude sickness by inhaling oxygen or lowering the altitude of the patient. However, this approach is often limited due to the lack of oxygen facilities or transportation in the wilderness. Currently, pharmacological interventions is often used to prevent or alleviate the symptoms of AS. Dexamethasone and acetazolamide (AZ) are the two main drugs used to treat AS-induced symptoms. Dexamethasone can block the inflammation of alveolar hypoxia at several sites in the inflammatory cascade (8). However, it has side effects of hormonal drugs and is not suitable for long-term use. AZ is a carbonic anhydrase inhibitor that is effective in preventing AS (9-12). However, its mechanism of action remains controversial. Preliminary study has shown that AZ can cause alkaluria and metabolic acidosis by inhibiting carbonic enzymes (13). This physiological change requires the body to compensate through respiratory alkalosis via hyperventilation. Ultimately, AZ improves ventilation in response to hypoxic stimuli at high altitudes (14,15). However, other study suggests that AZ causes pulmonary vasodilation and is not associated with carbonic anhydrase inhibition (16). Given such contradictory results, the mechanism of action of AZ remains unclear. Additionally, the medicinal plant Rhodiola rosea L. (Rh) is widely used to prevent or treat AS in traditional Chinese medicine (17-19). Its mechanism of action has been studied; however, it is not yet completely understood. Further, the combined use of AZ and Rh in the prevention and treatment of AS has not been examined.
Here, we analyzed the mechanisms of action of AZ and Rh in the treatment of AS both separately and in combination by the method of network of pharmacology. Network pharmacology utilizes principles of systems biology and network analysis to interpret the mechanism of drugs in a complex disease, which is aligned with the theoretical significance of the herbal formula. This method is more suitable for analyzing complex diseases, such as AS-induced symptoms. Moreover, we validated key genes and pathways from network pharmacology analysis in the rat model. Our study not only provides evidence for their combined use, but also hopefully overcomes the side effects of hormonal drugs. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2111/rc).
Methods
Animals
The study was approved by the Medical Science Research Ethics Committee of Qinghai University School of Medicine (No. 2021-40), and animal handling and care procedures were conducted in accordance with institutional guidelines for the care and use of animals. All efforts were made to minimize the pain and suffering of the animals and to minimize the number of animals used. A protocol was prepared before the study without registration.
For this study, 48 Sprague-Dawley (SD) rats were purchased from Beijing Vital River Laboratory Animal Technology Co. (Beijing, China) and housed under standard light, temperature, and humidity conditions (12:12 h light/dark cycle, 21±1 ℃, 55%±5% humidity). The rats had free access to drinking water and laboratory rodent chow.
Network pharmacology analysis
Target information was collected using literature mining and reverse docking. In the mining literature, all the available target information on AZ and the chemical constituents of Rh was collected from the PubChem database and the Comparative Toxicogenomic Database (CTD, http://www.ctdbase.org). Only the active targets from the PubChem and interacted genes from CTD were selected from the research results. In reverse docking, PharmMapper (20,21) was used to search for possible targets for the compounds. Only the top 10 targets from all the predicted targets of each compound were selected as potential targets.
An interaction network was constructed for each protein by use of the STRING database which is an integration of known and predicted protein interactions (22). Cytoscape software (Version 3.8.4; http://www.cytoscape.org/) and the Network Analyzer plugin (Version 1.0, http://med.bioinf.mpiinf.mpg.de/netanalyzer/) were used to visualize the network and calculate the basic network parameters (23). The size of the nodes corresponds to the node degree. Other analyses were performed based on the STRING analysis modules.
RT-PCR
The healthy male SD rats (weighing 300±30 g) were randomly divided into the following four groups (n=6 rats/group): (I) the AZ group; (II) the Rh group; (III) the AZ + Rh group; and (IV) the control group. The rats in each of the 4 groups were treated with AZ [100 mg/kg, intragastrically (i.g.)], Rh (30 mg/kg, i.g.), AZ + Rh (100 mg/kg AZ and 30 mg/kg Rh, i.g.), and saline, respectively. The doses were selected according to previous research (24-26). All the rats were then subjected to hypoxic conditions with 15% O2 ventilation for 0 and 5 h, respectively. Afterward, the rats were anesthetized using pentobarbitone sodium [50 mg/kg, intraperitoneal (i.p.)], and the lung tissue was removed, quickly frozen in liquid nitrogen, and ground. Total ribonucleic acid (RNA) was extracted using a TRIzol RNA kit and analyzed using reverse transcription-polymerase chain reaction (RT-PCR). The following primers were used: F5'-CCA GAT TCA AGA TCA GCC AGC A-3' and R5'-GCT GTC CAC ATC AAA GCG TAC TCA-3'.
Hemodynamics test
The experimental procedure was based on our previous methods (27). Briefly, healthy male SD rats (weighing 300±30 g) were randomly divided into the following 4 groups (n=6 rats per group): (I) the AZ group; (II) the Rh group; (III) the AZ + Rh group; and (IV) the control group. The rats in each of the 4 groups were treated with AZ (100 mg/kg, i.g.), Rh (30 mg/kg, i.g.), AZ + Rh (100 mg/kg AZ and 30 mg/kg Rh, i.g.), and saline for 2 h, respectively. The animals were then anesthetized using pentobarbitone sodium at a dose of 50 mg/kg. To maintain body temperature at 37±0.5 ℃, a heating pad was placed underneath each rat. The tidal volume (6–7 mL·kg−1) was adjusted according to each animal’s body weight and other physiological parameters. The respiratory rate was maintained at 70 breaths per minute. To keep the lungs inflated, we maintained the positive end-expiratory pressure at 2.5 cmH2O. We connected the intravascular cannula to the pressure transducer and then to the biosignal acquisition system of Power Lab (ML206; AD Instruments, Castle Hill, NSW, Australia) aligned to the level of the right atrium and calibrated prior to use.
Next, 4 different diameters of polyethylene catheters were inserted into the left common carotid artery, the right external jugular vein, the right ventricle, and the left atrium to measure mean arterial pressure (MAP), central venous pressure (CVP), pulmonary artery pressure (PAP), and left atrial pressure (LAP), respectively. After opening the chest cavity, a pulsed Doppler flow probe was placed under the ascending aorta to measure the ascending aortic blood flow (ABF). Mechanical ventilation was maintained via tracheal intubation and measurement airway pressure (Paw).
The experimental indicators of all groups were measured for 5 min under normoxic condition and continuously recorded for another 5 min under the hypoxic condition with 15% oxygen (O2) ventilation. Catheters were connected to the Power Lab biological signal acquisition system for continuous real-time hemodynamic monitoring and the collation of sampling records.
Preparation of Rh extracts
The roots of Rh were crushed and extracted with 90% ethanol under reflux 3 times, and the ethanol was then evaporated to obtain a crude extract for subsequent experiments. A 30 mg/mL aqueous solution was used for the administration.
Data analyzing and statistical analysis
The data were analyzed using SPSS (version 27.0; SPSS, Inc., Chicago, IL, USA). The values are shown as the mean ± standard deviation. Comparisons of the means among different groups (≥3 groups) were determined using an analysis of variance. The independent sample t-test was used to evaluate the significance of the difference between two groups; the statistical significance was set at P<0.05.
Results
Network pharmacology
The ingredients of Rh were derived from a review, and 52 compounds were selected (28) as detailed in the supplementary material (see Figure S1). The target information about the 52 constituents of Rh and AZ was obtained by data mining and reverse docking. After removing any duplicates and non-Homo sapiens targets, 1400 Homo-sapiens targets were selected for the ingredients of Rh, and 113 Homo sapiens targets were selected for the ingredients of AZ (see Table S1).
The target-target networks of Rh and AZ were built by inputting the 1,400 targets of Rh and 113 targets of AZ into the STRING database. Additionally, an integrated network was established by importing all the targets. The topological properties of the 3 target networks are shown in Table 1. The degree of nodes increased after integration, indicating that many targets are shared between Rh and AZ. Further, after 63 nodes from the AZ-related target network were added to the Rh-related target network, the number of edges increased by more than 2,000 edges, suggesting that most of the nodes in the AZ-related target network are relevant to the Rh-related target network. This correlation suggests that their combined use is synergistic in the treatment of certain diseases.
Table 1. The topological properties of the Rh- and AZ-related target networks.
| Name | Rh | AZ | Combined |
|---|---|---|---|
| Number of nodes | 1,085 | 63 | 1,124 |
| Number of edges | 26,338 | 300 | 28,358 |
| Average node degree | 48.5 | 9.52 | 50.5 |
| Average local clustering coefficient | 0.423 | 0.555 | 0.425 |
| Expected number of edges | 14,861 | 89 | 16,263 |
| PPI enrichment P value | <1.0e-16 | <1.0e-16 | <1.0e-16 |
Rh, Rhodiola; AZ, acetazolamide; PPI, protein-protein interaction.
Herbal medicine usually exhibits diverse pharmacological activities and regulates multiple cellular pathways; thus, studying Rh- and AZ-related cellular pathways is helpful in analyzing the mechanism of treating AS. In this study, 210 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were identified for Rh, 135 of which were found to be highly enriched (false discovery rate <E-05) (see Table S2). Additionally, we identified 13 enriched pathways for AZ and 139 enriched pathways in the combined target network. Among these pathways, both Rh and AZ regulated the hypoxia-inducible factor 1 α (HIF-1α) signaling pathway, which is related to hypoxia (29).
Further, after integrating the Rh- and AZ-regulated targets, the HIF-1α signaling pathway became more enriched. The false discovery rate dropped from 1.28×E-20 in the AZ-enriched pathways and 1.37×E-17 in the Rh-enriched pathways to 1.45×E-23. In total, 39 Rh-related targets and 16 AZ-related targets were mapped to this pathway. However, after combining all the targets of Rh and AZ, the observed genes in this pathway increased to 48. This means that Rh and AZ not only share targets in this pathway but also have different targets. This further shows that they can regulate this pathway together. In Figure 1, the genes marked by a purple box are regulated by Rh, the genes marked by a black box are regulated by AZ, and the genes marked by a red box are regulated by both Rh and AZ. After integration, the number of targets regulated by Rh and AZ in this pathway increased.
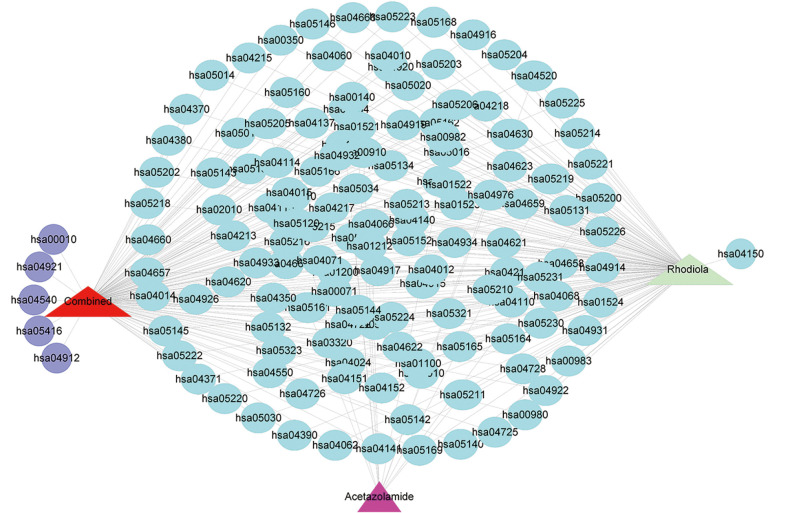
Figure 1.
Drug-KEGG pathway interaction (the triangles represent drugs; the circles represent pathways; light blue indicates the co-regulated pathways; purple indicates the newly added pathways). KEGG, Kyoto Encyclopedia of Genes and Genomes.
Additionally, after integrating the targets regulated by Rh and AZ, the enriched pathways underwent subtle changes. As Figure 1 shows, after integration, in addition to the shared pathways, the following 5 new purple pathways were added: glycolysis/gluconeogenesis (has00010), oxytocin signaling pathway (has04921), gap junction (has04540), viral myocarditis (has05416), and gonadotropin-releasing hormone (GnRH) signaling pathway (has04912).
The AS network was constructed by searching the CTD using the following keywords: “Altitude Sickness”, “Brain Edema”, “Hypoxia, Brain”, “Hypoxia”, “Hypoxia-Ischemia, Brain”, “Polycythemia”, “Pulmonary Arterial Hypertension”, and “Pulmonary edema of mountaineers”. The top 50 genes were found to be related to AS (see Table S3). Ultimately, 235 genes were selected to construct the AS network after removing the redundant data (see Figure 2A).
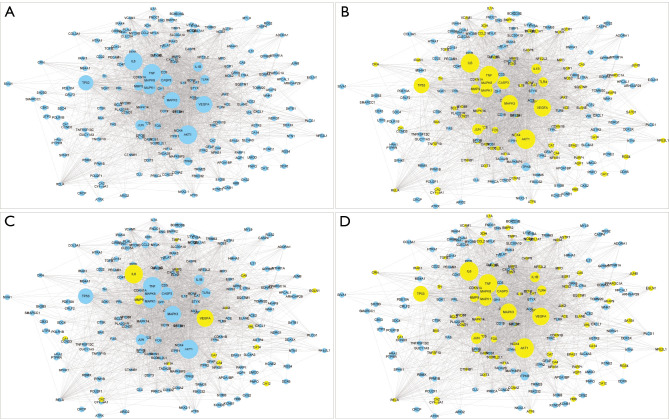
Figure 2.
Rh and AZ regulate AS networks. (A) AS network. (B) The Rh-related protein targets presented in the AS network. (C) The AZ-related protein targets presented in the AS network. (D) The Rh- and AZ-related protein targets presented in the AS network. Rh, Rhodiola; AZ, acetazolamide; AS, altitude sickness.
After mapping the Rh- and AZ-targeted proteins/genes into the AS network, we found that Rh modulated 86 proteins of AS (see Figure 2B), AZ modulated 16 proteins of AS (see Figure 2C), and together, Rh and AZ modulated 89 proteins of AS (see Figure 2D). Thus, Rh and AZ regulated most targets in the AS network. Further, excluding 3 different targets, Rh and AZ shared most of the targets. This evidence shows that when used in combination, Rh and AZ complement or enhance the regulation of high-altitude disease networks.
Rh and AZ reduced the expression of HIF-1α
The network pharmacology results showed that the HIF-1α signaling pathway was enriched by Rh and AZ. Additionally, the HIF-1α signaling pathway became more enriched after integration. We checked these results using RT-PCR. As Figure 3 shows, the expression of HIF-1α significantly increased under hypoxia (P<0.05). However, the use of Rh and AZ reversed this increase, and they were more effective when used in combination than when used separately.
Figure 3.
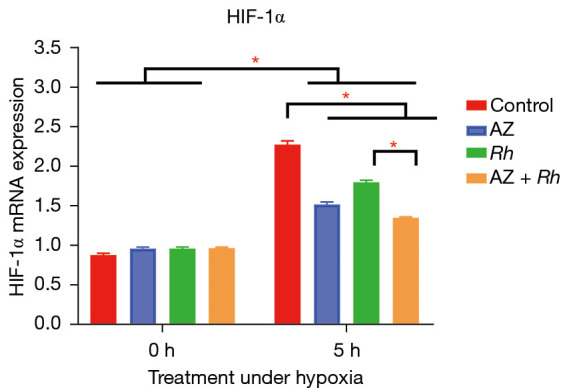
The expression of HIF-1α after treatment with Rh and AZ at normoxia and hypoxia. 0 h means the Rats were treated with normoxia and 5 h means the Rats were treated with hypoxia for 5 hours. The expression of HIF-1α was significantly increased under hypoxia. Rh and AZ inhibited this increase, and their combined use inhibited this increase more significant than alone use. *, P≤0.05. All data is presented mean ± SD. HIF, hypoxia-inducible factor; Rh, Rhodiola; AZ, acetazolamide.
Rh and AZ improved the hemodynamics of rats under hypoxic conditions
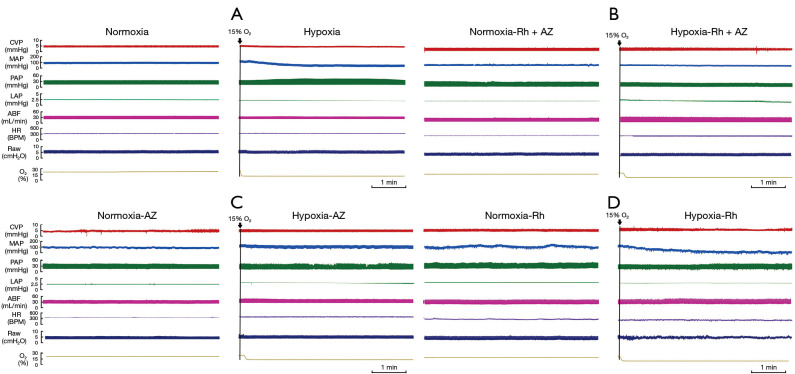
In addition to the HIF-1α signaling pathway, 5 new pathways were found to be enriched after integrating the Rh- and AZ-related networks. Among them, oxytocin and GnRH signaling pathways are related to blood pressure. These hypotheses were confirmed by monitoring the hemodynamics in rats. We monitored 7 indices, including CVP, MAP, PAP, LAP, ABF, heart rate (HR), and Paw, under normoxia or hypoxia after treatment with Rh and AZ. As Figure 4 and Table 2 shown, a hypoxic environment led to an increase in PAP (37.2±2.0 to 39.9±2.5 mmHg) and LAP (2.5±0.2 to 2.7±0.1 mmHg), and a decrease in MAP (97.8±4.1 to 92.3±3.9 mmHg) and ABF (32.1±1.3 to 27.7±1.2 mmHg) in rats. However, Rh and AZ suppressed this change. The inhibitory effect was more obvious after their combined use, especially in relation to PAP, LAP, and ABF.
Figure 4.
Hemodynamics of rats after treatment with Rh and AZ at normoxia and hypoxia. (A) Hemodynamic changes after normoxia and hypoxia treatment. (B) Hemodynamic changes after the combined treatment of Rh and AZ under the normoxia and hypoxia conditions. (C) Hemodynamic changes after treatment with AZ under the normoxia and hypoxia conditions. (D) Hemodynamic changes after treatment with Rh under the normoxia and hypoxia conditions. Rh, Rhodiola; AZ, acetazolamide.
Table 2. Results of the hemodynamic changes analysis in different groups of rats (n=6, mean ± SD).
| Indexes | O2 | Control | AZ | Rh | AZ + Rh | F | P |
|---|---|---|---|---|---|---|---|
| CVP (mmHg) | 20% O2 | 4.3±0.2 | 4.3±0.3 | 4.1±0.3 | 4.0±0.4 | 1.23 | 0.32 |
| 15% O2 | 4.4±0.2 | 4.2±0.4 | 4.2±0.2 | 3.9±0.2* | 4.25 | 0.02 | |
| MAP (mmHg) | 20% O2 | 97.8±4.1 | 96.1±3.2 | 96.7±3.4 | 96.4±3.6 | 0.30 | 0.82 |
| 15% O2 | 92.3±3.9# | 94.6±3.5 | 95.1±4.1 | 96.1±3.3 | 1.31 | 0.29 | |
| PAP (mmHg) | 20% O2 | 37.2±2.0 | 36.9±2.0 | 37.3±2.4 | 37.1±2.5 | 0.04 | 0.98 |
| 15% O2 | 39.9±2.5# | 37.8±2.5 | 37.9±0.35 | 37.3±0.03* | 2.91 | 0.05 | |
| LAP (mmHg) | 20% O2 | 2.5±0.2 | 2.4±0.1 | 2.5±0.3 | 2.3±0.2 | 1.43 | 0.26 |
| 15% O2 | 2.7±0.1# | 2.5±0.1* | 2.6±0.3 | 2.4±0.1* | 3.89 | 0.02 | |
| ABF (mL·min-1) | 20% O2 | 32.1±1.3 | 31.2±1.1 | 31.4±1.4 | 31.6±1.5 | 0.58 | 0.62 |
| 15% O2 | 27.7±1.2# | 30.1±1.2* | 29.8±1.2 | 30.7±1.3* | 6.72 | <0.01 | |
| Paw (cmH2O) | 20% O2 | 10.7±0.6 | 10.2±0.4 | 10.6±0.3 | 10.4±0.5 | 1.60 | 0.21 |
| 15% O2 | 11.0±0.5 | 10.6±0.3 | 11.2±0.5 | 10.9±0.6 | 1.19 | 0.33 | |
| HR (bpm) | 20% O2 | 331±20.8 | 312±18.6 | 325±20.5 | 309±19.8 | 1.93 | 0.15 |
| 15% O2 | 334±19.3 | 323±17.4 | 330±18.1 | 316±19.2 | 1.28 | 0.3 |
*, vs. Control, P≤0.05; #, vs. Control 20% O2, P<0.05. AZ, acetazolamide; Rh, Rhodiola; CVP, central venous pressure; MAP, mean arterial pressure; PAP, pulmonary artery pressure; LAP, left atrial pressure; ABF, ascending aortic blood flow; Paw, airway pressure; HR, heart rate.
Conclusions
Rh and AZ are the two main drugs used to prevent and treat AS. In clinical practice, Rh is more commonly used to prevent AS, while AZ is more commonly used to treat AS. However, there are only a few clinical reports on their combined use. The mechanisms of the treatment and prevention of AS are not clear. In this study, we analyzed the mechanisms involved in the treatment and prevention of AS using the network pharmacology method. We verified the results of the experiments and found that Rh and AZ can alleviate the symptoms of AS by regulating HIF-1α and hemodynamics. Further, these drugs are more effective when they are used in combination than when they are used separately.
HIF-1α, a subunit of HIF-1, is expressed inducible according to the oxygen content (30). It undergoes degradation through hydroxylation at specific prolyl residues and subsequent ubiquitination under normoxia (31). Conversely, under hypoxia, HIF-1α becomes stable after interacting with its coactivators, such as p300/CBP (32-34). Eventually, HIF-1α encodes proteins that increase O2 delivery and mediate adaptive responses to O2 deprivation (35). In the present study, we found that Rh and AZ not only enriched the HIF-1 signaling pathway, but also suppressed HIF-1α gene expression. These effects became more pronounced when Rh and AZ were used in combination. Thus, reducing the expression of HIF-1α reduced the sensitivity of rat lung tissue to a hypoxic environment.
Collectively, Rh and AZ enriched 5 new pathways after their combined use. Among them, the oxytocin signaling pathway is related to the cardiovascular system (36,37). Oxytocin could reduce the force and rate of heart contraction and increase vasodilatation by mediating the atrial natriuretic peptide-Cyclic GMP (ANP-cGMP), and Nitric oxide-Cyclic GMP (NO-cGMP) pathways (38-40), which may reduce symptoms such as pulmonary hypertension or hypertension caused by AS (41). Additionally, in the GnRH signaling pathway, GnRH activates its receptor (GnRHR) in the anterior pituitary and subsequently activate phospholipase C by coupling with Gq/11 proteins (42). Activated phospholipase C further activates the intracellular protein kinase C (PKC) pathway, which in turn leads to the efflux of intracellular calcium (43). Eventually, such changes induce the dilation of epithelial cells, resulting in vasodilation (44,45). Based on these observations, we deduced that the pathways regulated by Rh and AZ were related to cardiovascular disease. These results were supported by our hemodynamic tests of rats. Specifically, we found that Rh and AZ suppressed changes in PAP, LAP, MAP, and ABF under hypoxia. Pulmonary hypertension caused by hypoxia is the most common cause of AS. Whether used alone or in combination, Rh and AZ can alleviate this symptom, and their combined effects are more pronounced than they are alone.
Based on the results of our research, the combined use of Rh and AZ helped to prevent and treat AS. However, while our results provide preliminary evidence of the potential clinical applicability of Rh and AZ in the treatment of AS, further clinical trials need to be conducted.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 81560301), the Natural Science Foundation of Qinghai (grant No. 2022-ZJ-905), and Qinghai Province “High-End Innovative Talents and Thousand Talents Program” Leading Talent Project. The High-Altitude Medicine Research Center of Qinghai University provided the experimental platform.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Medical Science Research Ethics Committee of Qinghai University School of Medicine (No. 2021-40), and animal handling and care procedures were conducted in accordance with institutional guidelines for the care and use of animals. All efforts were made to minimize pain and suffering to the animals and to minimize the number of animals used.
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2111/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2111/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2111/coif). The authors have no conflicts of interest to declare.
(English Language Editor: L. Huleatt)
References
- 1.Luks AM, Swenson ER, Bärtsch P. Acute high-altitude sickness. Eur Respir Rev 2017;26:160096. 10.1183/16000617.0096-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Y, Costello JT, Williams TB, et al. A network physiology approach to oxygen saturation variability during normobaric hypoxia. Exp Physiol 2021;106:151-9. 10.1113/EP088755 [DOI] [PubMed] [Google Scholar]
- 3.Carod-Artal FJ. High-altitude headache and acute mountain sickness. Neurologia 2014;29:533-40. 10.1016/j.nrl.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 4.Marmura MJ, Hernandez PB. High-altitude headache. Curr Pain Headache Rep 2015;19:483. 10.1007/s11916-015-0483-2 [DOI] [PubMed] [Google Scholar]
- 5.Bärtsch P, Swenson ER, Paul A, et al. Hypoxic ventilatory response, ventilation, gas exchange, and fluid balance in acute mountain sickness. High Alt Med Biol 2002;3:361-76. 10.1089/15270290260512846 [DOI] [PubMed] [Google Scholar]
- 6.Jafarian S, Gorouhi F, Salimi S, et al. Sumatriptan for prevention of acute mountain sickness: randomized clinical trial. Ann Neurol 2007;62:273-7. 10.1002/ana.21162 [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Hypoxia and human disease-and the Journal of Molecular Medicine. J Mol Med (Berl) 2007;85:1293-4. 10.1007/s00109-007-0285-z [DOI] [PubMed] [Google Scholar]
- 8.Chao J, Viets Z, Donham P, et al. Dexamethasone blocks the systemic inflammation of alveolar hypoxia at several sites in the inflammatory cascade. Am J Physiol Heart Circ Physiol 2012;303:H168-H177. 10.1152/ajpheart.00106.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapia L, Irarrázaval S. Acetazolamide for the treatment of acute mountain sickness. Medwave 2019;19:e7737. 10.5867/medwave.2019.11.7736 [DOI] [PubMed] [Google Scholar]
- 10.Toussaint CM, Kenefick RW, Petrassi FA, et al. Altitude, Acute Mountain Sickness, and Acetazolamide: Recommendations for Rapid Ascent. High Alt Med Biol 2021;22:5-13. 10.1089/ham.2019.0123 [DOI] [PubMed] [Google Scholar]
- 11.Shimoda LA, Suresh K, Undem C, et al. Acetazolamide prevents hypoxia-induced reactive oxygen species generation and calcium release in pulmonary arterial smooth muscle. Pulm Circ 2021;11:20458940211049948. 10.1177/20458940211049948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao D, Wang Y, Zhang R, et al. Efficacy of acetazolamide for the prophylaxis of acute mountain sickness: A systematic review, meta-analysis, and trial sequential analysis of randomized clinical trials. Ann Thorac Med 2021;16:337-46. 10.4103/atm.atm_651_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdeen A, Sonoda H, Oshikawa S, et al. Acetazolamide enhances the release of urinary exosomal aquaporin-1. Nephrol Dial Transplant 2016;31:1623-32. 10.1093/ndt/gfw033 [DOI] [PubMed] [Google Scholar]
- 14.Leaf DE, Goldfarb DS. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol (1985) 2007;102:1313-22. 10.1152/japplphysiol.01572.2005 [DOI] [PubMed] [Google Scholar]
- 15.Swenson ER. Carbonic anhydrase inhibitors and high altitude illnesses. Subcell Biochem 2014;75:361-86. 10.1007/978-94-007-7359-2_18 [DOI] [PubMed] [Google Scholar]
- 16.Höhne C, Pickerodt PA, Francis RC, et al. Pulmonary vasodilation by acetazolamide during hypoxia is unrelated to carbonic anhydrase inhibition. Am J Physiol Lung Cell Mol Physiol 2007;292:L178-84. 10.1152/ajplung.00205.2006 [DOI] [PubMed] [Google Scholar]
- 17.Chiang HM, Chen HC, Wu CS, et al. Rhodiola plants: Chemistry and biological activity. J Food Drug Anal 2015;23:359-69. 10.1016/j.jfda.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SY, Li MH, Shi LS, et al. Rhodiola crenulata Extract Alleviates Hypoxic Pulmonary Edema in Rats. Evid Based Complement Alternat Med 2013;2013:718739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang S, Deng N, Zheng B, et al. Rhodiola extract promotes longevity and stress resistance of Caenorhabditis elegans via DAF-16 and SKN-1. Food Funct 2021;12:4471-83. 10.1039/D0FO02974B [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Ouyang S, Yu B, et al. PharmMapper server: a web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res 2010;38:W609-14. 10.1093/nar/gkq300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Shen Y, Wang S, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res 2017;45:W356-60. 10.1093/nar/gkx374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019;47:D607-13. 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13:2498-504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christou H, Michael Z, Spyropoulos F, et al. Carbonic anhydrase inhibition improves pulmonary artery reactivity and nitric oxide-mediated relaxation in sugen-hypoxia model of pulmonary hypertension. Am J Physiol Regul Integr Comp Physiol 2021;320:R835-R850. 10.1152/ajpregu.00362.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng G, Jia S, Li J, et al. Experimental Study of Preventive Effect of Salidroside on Mice with High Altitude Polycythemia. Journal of New Chinese Medicine 2016;48:304-6. [Google Scholar]
- 26.Wang Q, Wang L, Wang P, et al. Protective effect of rhodiogenin on myocardium in rats with myocardia ischemia-reperfusion injury. Chinese Traditional Patent Medicine 2021;43:3147-51. [Google Scholar]
- 27.Ji Q, Zhang Y, Zhang H, et al. Effects of β-adrenoceptor activation on haemodynamics during hypoxic stress in rats. Exp Physiol 2020;105:1660-8. 10.1113/EP088669 [DOI] [PubMed] [Google Scholar]
- 28.Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010;17:481-93. 10.1016/j.phymed.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 29.Fu X, Zhang F. Role of the HIF-1 signaling pathway in chronic obstructive pulmonary disease. Exp Ther Med 2018;16:4553-61. 10.3892/etm.2018.6785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, Zhong ZF, Wang SP, et al. HIF-1: structure, biology and natural modulators. Chin J Nat Med 2021;19:521-7. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi Y, Oguro A, Imaoka S. Feedback of hypoxia-inducible factor-1alpha (HIF-1alpha) transcriptional activity via redox factor-1 (Ref-1) induction by reactive oxygen species (ROS). Free Radic Res 2021;55:154-64. 10.1080/10715762.2020.1870685 [DOI] [PubMed] [Google Scholar]
- 32.Lee JW, Bae SH, Jeong JW, et al. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med 2004;36:1-12. 10.1038/emm.2004.1 [DOI] [PubMed] [Google Scholar]
- 33.Qin X, Chen H, Tu L, et al. Potent Inhibition of HIF1α and p300 Interaction by a Constrained Peptide Derived from CITED2. J Med Chem 2021;64:13693-703. 10.1021/acs.jmedchem.1c01043 [DOI] [PubMed] [Google Scholar]
- 34.Lanfranchi B, Rubia RF, Gassmann M, et al. Transcriptional regulation of HIF1α-mediated STAR expression in murine KK1 granulosa cell line involves cJUN, CREB and CBP-dependent pathways. Gen Comp Endocrinol 2022;315:113923. 10.1016/j.ygcen.2021.113923 [DOI] [PubMed] [Google Scholar]
- 35.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176-82. 10.1152/physiol.00001.2004 [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee O, Patil K, Sahu A, et al. An overview of the oxytocin-oxytocin receptor signaling network. J Cell Commun Signal 2016;10:355-60. 10.1007/s12079-016-0353-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iovino M, Messana T, Tortora A, et al. Oxytocin Signaling Pathway: From Cell Biology to Clinical Implications. Endocr Metab Immune Disord Drug Targets 2021;21:91-110. 10.2174/1871530320666200520093730 [DOI] [PubMed] [Google Scholar]
- 38.Jankowski M, Broderick TL, Gutkowska J. The Role of Oxytocin in Cardiovascular Protection. Front Psychol 2020;11:2139. 10.3389/fpsyg.2020.02139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szczepanska-Sadowska E, Wsol A, Cudnoch-Jedrzejewska A, et al. Complementary Role of Oxytocin and Vasopressin in Cardiovascular Regulation. Int J Mol Sci 2021;22:11465. 10.3390/ijms222111465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCook O, Denoix N, Radermacher P, et al. H2S and Oxytocin Systems in Early Life Stress and Cardiovascular Disease. J Clin Med 2021;10:3484. 10.3390/jcm10163484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broderick TL, Wang Y, Gutkowska J, et al. Downregulation of oxytocin receptors in right ventricle of rats with monocrotaline-induced pulmonary hypertension. Acta Physiol (Oxf) 2010;200:147-58. 10.1111/j.1748-1716.2010.02134.x [DOI] [PubMed] [Google Scholar]
- 42.Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol Cell Endocrinol 2018;463:131-41. 10.1016/j.mce.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maltsev AV, Evdokimovskii EV, Kokoz YM. Protein kinase C-mediated calcium signaling as the basis for cardiomyocyte plasticity. Arch Biochem Biophys 2021;701:108817. 10.1016/j.abb.2021.108817 [DOI] [PubMed] [Google Scholar]
- 44.Desaulniers AT, Cederberg RA, Lents CA, et al. Expression and Role of Gonadotropin-Releasing Hormone 2 and Its Receptor in Mammals. Front Endocrinol (Lausanne) 2017;8:269. 10.3389/fendo.2017.00269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muzorewa TT, Buerk DG, Jaron D, et al. Coordinated regulation of endothelial calcium signaling and shear stress-induced nitric oxide production by PKCβ and PKCη. Cell Signal 2021;87:110125. 10.1016/j.cellsig.2021.110125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as