Abstract
X-linked agammaglobulinaemia (XLA) is a primary immunodeficiency (PID) resulting from a defect in the B cell development. It has conventionally been thought that T cells play a major role in the development and function of the B cell compartment. However, it has also been shown that B cells and T cells undergo bidirectional interactions and B cells also influence the structure and function of the T cell compartment. Patients with XLA offer a unique opportunity to understand the effect of absent B cells on the T cell compartment. In this review, we provide an update on abnormalities in the T cell compartment in patients with XLA. Studies have shown impaired memory T cells, follicular helper T cells, T regulatory cells and T helper 17 in patients with XLA. In addition, these patients have also been reported to have abnormal delayed cell-mediated immune responses and vaccine-specific T cell-mediated immune responses; defective T helper cell polarization and impaired T cell receptor diversity. At present, the clinical significance of these T cell abnormalities has not been studied in detail. However, these abnormalities may result in an increased risk of viral infections, autoimmunity, autoinflammation and possibly chronic lung disease. Abnormal response to SARS-Cov2 vaccine in patients with XLA and prolonged persistence of SARS-Cov2 virus in the respiratory tract of these patients may be related to abnormalities in the T cell compartment.
Keywords: X-linked agammaglobulinaemia (XLA), Primary Immunodeficiency (PID), Severe acute respiratory syndrome coronavirus 2 (SARS-Cov2), Bruton tyrosine kinase (BTK), T cell receptor (TCR), T follicular helper cells (TFH)
Introduction
X-linked agammaglobulinaemia (XLA) is one of the primary immunodeficiency (PID) that manifests due to a defect in the humoral arm of the immune system [1]. XLA was first identified in 1952 by Colonel Ogden Bruton in an 8-year-old boy who had recurrent episodes of pneumococcal sepsis and absent gamma globulins. In 1993, the associated molecular defect in the Bruton tyrosine kinase (BTK) gene was identified [2]. BTK gene is located on the long arm of the X chromosome (Xq21.3-Xq22) [3]. Monogenic defect in BTK results in complete arrest in B cell at the pre-B cell stage of differentiation in the bone marrow [4].
Recurrent infections caused by encapsulated organisms are the hallmark clinical manifestation in patients with XLA [1]. Patients with XLA have a marked decrease in B cell numbers in peripheral blood, absent B cell-dependent lymphoid tissue and undetectable levels of serum immunoglobulins, and these patients fail to generate specific antibody responses [3].
Btk protein is required for the sustained calcium signalling in response to B cell receptor (BCR) engagement and regulates downstream transcriptional events required for B lineage growth and survival [5]. Btk belongs to the family of non-receptor tyrosine kinases that catalyse the transfer of phosphate group from ATP to tyrosine residues of a protein. It consists of 5 structural domains, i.e. at the N terminus pleckstrin homology domain (PH), Tec homology domain (TH), Src homology 3 (SH3) domain, SH2 domain and the catalytic kinase (SH1) domain [4]. Various mutations such as point mutations, insertions or deletions in each of these domains have been reported to result in XLA. These mutations often lead to reduce Btk mRNA transcript and subsequently altered protein expression and kinase activity. Btk protein is selectively expressed in haematopoietic cell lineages. In non-haematopoietic cell lineages, Btk is mainly associated with other non-receptor tyrosine kinases [4]. Additionally, expression of Btk is found in erythroid precursors, myeloid cells, mast cells and megakaryocytes. Mutations in Btk other than B cells have not been associated with clinically significant consequences [6]. The role of btk has also been described in dendritic cells (DCs) which play a pivotal role in generating the innate immune response by recognizing pathogens associated molecular patterns (PAMPs) through toll-like receptors (TLRs). XLA patients with defective btk have been reported to have impaired TLR signalling and reduced production of TNF-α, thereby increasing the susceptibility to severe bacterial and viral infections among these patients [7].
Btk protein is expressed from the CD34+ pro-B cell stage till the mature B cell stage [4]. However, it has been shown to be selectively downregulated in plasma cells and T lymphocytes [8].
It has been reported that T cells have profound effects on the function of B cells at various stages [9] [10]. It has also been suggested that the absence of B cells in circulation and lymphoid tissues may affect the composition and function of T cells [11]. Moreover, several clinical manifestations in patients with XLA such as predisposition to develop viral infections, autoimmune and inflammatory complications may be related to a more generalized immune defect that may not be limited to the B cell compartment [12]. Patients and animal models of XLA, therefore, offer a unique opportunity to investigate the effect of absent B cells on the T cell compartment [13]. In this review, we provide an update on T cell abnormalities reported in patients with XLA.
B Cell-T Cell Bidirectional Interaction
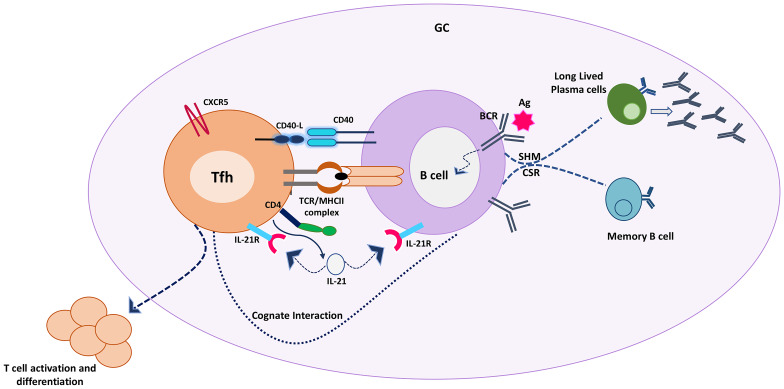
The immune system is capable of recognizing, interpreting and responding to various stimuli. The adaptive immune system has a unique property of developing a memory response following first exposure to a stimulus and can identify this stimulus again on re-exposure. This protective immune memory response is because of humoral and cellular components of the adaptive immune system provided by B and T cells respectively [14, 15]. B and T cells undergo cognate bidirectional interactions that leads to the generation of immune memory (Fig. 1).
Fig. 1.
T-B cell cognate interaction in the germinal centre leading to activation and differentiation of T cells along with the generation of long-lived plasma and memory B cells. Activated B cells present the peptide antigen via MHC class II molecule to cognate T cells that recognize the MHC peptide complex through TCR. CD40/CD40L costimulation along with the release of cytokines enables the differentiation of both B and T cells. B cells undergo class switch recombination and somatic hypermutation to produce long-lived antibody-secreting plasma cells and memory cells. T cells become activated as effectors or differentiate into memory T cells to sustain the cell-mediated immune response. Tfh follicular helper T cells, GC germinal centre B cells, CD40L cluster of differentiation 40 ligand, CD40 cluster of differentiation 40 receptor, TCR T cell receptor, MHC II major histocompatibility class II, CD4 cluster of differentiation 4, CXCR5 C-X-C chemokine receptor type 5, IL21 interleukin 21, IL21R interleukin 21 receptor, BCR B cell receptor, CSR class switching and recombination, SHM somatic hypermutation
T cells play a vital role in generating and shaping antigen-specific B-cell responses through the formation of a germinal centre (GC) in the secondary lymphoid organs where B cells experience isotype switching and affinity maturation. The GC reaction is predominantly regulated by follicular helper T cells (TFH). TFH cells are CD4+ helper T cells that express B cell homing receptor CXCR5 on their surface. The first encounter that results in cross-linking between TFH and their cognate follicular B cells takes place at the follicular borders in the spleen or interfollicular region in lymph nodes. These interactions trigger the formation and maintenance of germinal centres through CD40 ligand (CD40L, CD154)-CD40 interaction expressed on the surface of T and B cells respectively. This interaction is indispensable for effective B cell polarization into long-lived plasma cells or memory cells [16].
B cells also play several important roles in providing sequential inputs for maintaining T cell immunity [17]. B cells act as key antigen-presenting cells (APCs) due to their peculiar ability to uptake antigens through surface immunoglobulins. As a result, B cells play a fundamental role in the TFH cell differentiation by providing sustained antigenic stimulus. In the absence of B cells, TFH cell precursors fail to achieve the mature phenotype [16]. This suggests that both B and T cells complement each other in generating a mature GC reaction.
The antigen-presenting property of B cells may also modulate the generation and maintenance of effective memory T cell responses. T cells recognize antigens by engaging their T cell receptors (TCRs) with the antigenic peptide bound to the major histocompatibility complex (MHC) present on the surface of APCs [17].
B cells may also influence the cytokine pattern of T helper cells. T cells release various types of cytokines like interferon-γ or interleukin-4 (IL-4), IL-5 and IL-13 that polarize T cell response towards Th1 or Th2 respectively. Polarization of T cell is, therefore, determined by B cells that influence cytokine pattern [18].
As a close interaction exists between B and T cells which is necessary for the generation of memory cells and their activation, a deficit in either arm of the immune system will affect the memory responses [19].
The effect of absent B cells on T cells has been studied in various experimental animal models. However, these studies are difficult to replicate in humans. Therefore, XLA provides a disguised potential opportunity to study the effect of the absence of B cells on the number and function of various T cell subsets [13].
T Cell Abnormalities in Patients with XLA
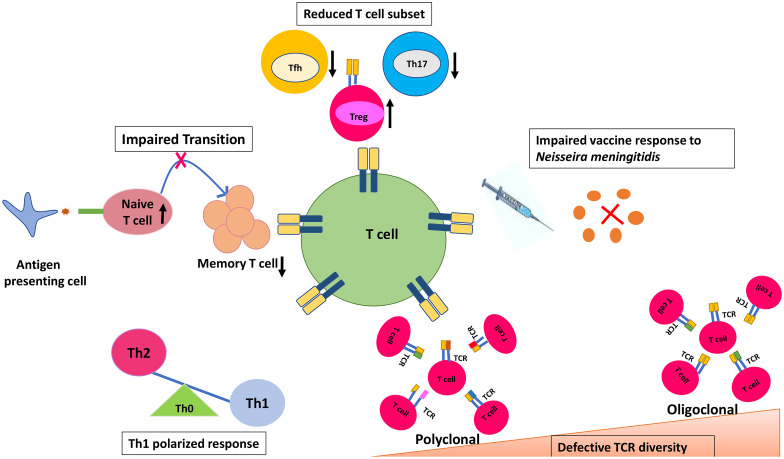
There are conflicting results from various studies that have assessed the T-cell-mediated immune responses in patients with XLA [20, 21]. While some studies have reported that T cells may generate specific responses to foreign and self-antigens in the absence of B cells, others have reported that B cells act as an indispensable component in generating and maintaining T cell-specific immune responses [22–24]. A few studies have shown the impact of absent B cells on the memory T cell compartment, TFH compartment and the development of TCR structure [17, 25, 26] (Fig. 2).
Fig. 2.
T cell abnormalities reported in patients with X-Linked agammaglobulinaemia. Th0 naïve T helper, Th1 type 1 T helper, Th2 type 2 T helper, TFH T follicular helper cells, Th17 T helper 17, Treg regulatory T cell
Naïve and Memory T Helper Cell Abnormalities in Patients with XLA
T cell memory generation in patients with XLA is subject to controversy. Available data suggest that neither B cells nor antigen persistence is essential for maintaining either the CD4+ or CD8+ T-cell memory, as both cell populations have a long life span [13, 22, 27].
On the other hand, data from various studies suggests that B cells are essential for the maintenance of the T cell memory pool especially the CD4+ T cells. Few studies have proposed that memory CD4+ lymphocytes need chronic antigenic stimulus from follicular dendritic cells (FDCs) [24]. These FDCs form the immune complexes containing antigens on their surface. These immune complexes serve as an antigenic reservoir for chronic T cell stimulation. B cells contribute to this process by producing antibodies that form immune complexes and thereby providing a conducive environment that is essential for the entrapment of antigens and T cell stimulation [15, 28].
Crockard et al. reported an impaired memory T cell population with preserved naïve T cell population in patients with XLA when compared to healthy controls and patients with common variable immunodeficiency (CVID) [21]. In this study, the CD4+ T cells subset was further classified into CD4+CD45RA+ naïve T cells and CD4+CD45RO+ memory helper T cells. Further characterization defined helper T cells as helper inducers (CD4+CD29+, CD4+CD45RO+) that help B cells in the production of immunoglobulins and suppressor inducers (CD4+CD45RA+, CD4+Leu8+) that play a role in inducing suppressor cell activity [29]. CD29 is a marker which is expressed on previously activated T cells. A significant depletion of CD4+CD45RO+ and CD4+CD29+ T cells was seen in patients with XLA with preserved CD4+CD45RA+ and CD4+Leu8+ cells, thereby suggesting a reduced memory CD4+ T cell pool and defective transition from naïve to memory T cells [21]. The authors also suggested that these observations are unlikely to be because of age or the effect of immunoglobulin replacement therapy [21]. Failure of differentiation of naïve T cells in patients with XLA is supported by the fact that in the absence of B cells the bidirectional communication between T and B cells is impaired and as a result, T cell activation does not take place [19]. A large multicentre cohort of patients with XLA published from our own centre reported reduced absolute CD4+ T cell counts in 2 patients [30]. In addition, reversed CD4:CD8 ratio has also been reported in patients with XLA with chronic sinusitis [31].
The presence of underlying chronic respiratory disease in patients with XLA has been reported to significantly alter the T lymphocyte proportions [32]. Sharapova et al. also reported reduced memory T cells (CD4+CD45RO+) proportions with increased naïve T cell proportions (CD4+CD45RA+) in patients with XLA as compared to control [32]. However, a subset of patients with XLA who had the chronic respiratory disease was found to have higher memory CD4+ T cells proportions. Similarly, patients with XLA who had the chronic respiratory disease were found to have higher naïve and memory CD8+ T cell counts. This difference was, however, not seen in patients with XLA who had no chronic respiratory disease [32].
Memory T cells preferentially respond to antigens presented by B cells while naïve T cells become tolerant when exposed to antigens presented by B cells; this could be one reason that patients with XLA have downregulated memory subset [33].
Vaccine-Specific T Cell Responses in Patients with XLA
Paroli et al. reported in vitro hepatitis B envelope antigen (HBenv Ag)-specific CD4+ T-cell response in patients with XLA following vaccination against hepatitis B virus. The proliferative capacity of T cells that quantifies the expansion of circulating-specific memory T cells and frequency of T helper cell responses was measured. It was observed that peripheral blood mononuclear cells from both patients with XLA as well as control group showed equal proliferative capacities when stimulated with HBenvAg [13]. Moreover, the frequencies of HBenvAg-specific T helper cell response as recorded by enzyme-linked immunospot (ELISPOT) and intracellular IFN-γ and IL-4 production were similar in T cells of patients with the XLA and control group. These observations suggest that neither T-cell memory nor the Th1 and Th2 cell polarization is impaired by B-cell deficiency in humans. Furthermore, the impact of impaired pre-B cell differentiation in shaping the central (CD27+) and effector memory T (CD27−) cells was investigated through ELISPOT. It was observed that both populations were retained in patients with XLA 24 months after vaccination [34]. These results indicate that neither of the pool of memory T cells (effector or central memory) was affected because of absent B cells [13].
Similarly, Liu et al. studied the influenza vaccine-specific CD4+ and CD8+ T cell responses in patients with XLA after administration of inactivated trivalent influenza vaccine (TIV). In vitro frequencies of CD4+ and CD8+ T cell responses recorded before and after vaccination (at an interval of 1 week, 1 month, 3 months and 6 months) showed no difference between the control and XLA group. This study also evaluated the vaccine-specific memory CD8+ cytotoxic T lymphocytes (CTLs) induction in patients with XLA. Memory response to influenza virus vaccine (pre- and post-vaccination) showed similar trends in the control group and in patients with XLA. These results suggest that the absence of B cells may not impair the generation of virus-specific T cell immunity [35].
Plebani et al. reported preserved T cell functions in patients with XLA. They studied the in vitro proliferative capacity and cytokine production of T cells following stimulation with polyclonal T cell activators and unaffected memory T cell responses assessed after immunization with T cell-dependent tetanus toxoid antigen [20]. These studies suggest that T cell responses are not dependent on B cells, and therefore, vaccines targeting T cell-mediated response may be effectively used in patients with XLA.
On the other hand, Morales et al. reported the role of B cells in T cell memory generation and maintenance in patients with XLA with Neisseria meningitidis outer membrane vesicles (Nm OMVs) antigen [17]. It was observed that patients with XLA had impaired T cell memory response to Nm OMV antigen while the response to influenza antigen was preserved. Nm OMVs upregulate the expression of MHC class II, CD40 and CD86/80 on the surface of mucosa-associated B cells that serve as APCs in the induction of T cell memory response. The possible explanation for reduced meningococcal specific proliferative capacity of T cells may be due to the impaired CD40-CD40L interaction due to the absence of B cells that act as APCs. Authors have suggested that CD40-CD40 ligand interaction is indispensable for memory T cell response to Nm OMVs while memory T cell response to influenza antigen may not utilize this pathway. This is further substantiated by the observation that memory T cell response to Nm OMV antigen is also impaired in patients with X-linked Hyper IgM syndrome [17].
T Follicular Helper Cells in Patients with XLA
A recent study by Edwards et al. reported a significantly reduced number of follicular T helper cells (Tfh) that are critical for B cells in germinal centre reaction [36]. Similarly, Barbosa et al. reported low Tfh cells in 6 patients with congenital agammaglobulinaemia (2 with XLA) [25]. Low Tfh cells seen in patients with XLA are likely because of impaired GC formation. Sharapova et al. assessed Tfh cells in 11 patients with XLA. It was observed that patients with XLA without the chronic respiratory disease (7 patients) had significantly reduced CD4+CD45RO+CXCR5+ follicular memory T cells and CD4+CXCR5+PD1+ follicular helper T cells in comparison to healthy controls. These cells were found to be normal in patients with XLA with chronic respiratory disease [32].
Cell-Mediated Immunity in Patients with XLA
In addition to the decreased number of specific T cell subsets, a severe reduction in the cell-mediated immunity in terms of reduced inflammation indicative of poor delayed hypersensitivity response to common recall antigens has been reported in patients with XLA. Rozynska et al. observed that T cells from patients with XLA when cultured in vitro along with B cells and DCs of control subjects failed to provide help for the IgG and IgM production against pokeweed mitogen [37]. In vitro increased suppressive activity of T cells from patients with XLA towards normal T cells was observed [37]. Crockard et al. reported impaired in vivo cell-mediated immunity assessed using skin prick in patients with XLA when compared with healthy controls. Defective functional T cell responses in XLA represent the state of energy that arises due to the low number of CD4+CD45RO+ and CD4+CD29+ cells, thereby indicating an inability to respond to recall antigens [21, 38].
Various factors contribute to the responsiveness of T cells such as the type of antigens, cytokines or chemokine microenvironment and the type of APC involved [18]. Animal studies have shown that B cells are essential for the maintenance of long-term T cell memory as well as in shaping the T helper cell outcome [13]. Similar results have been reported from human studies [17].
Impaired Th1/Th2 Balance in Patients with XLA
B cells are efficient APCs and present antigens to primed T cells. In addition, B cells also influence T cell stimulation during the primary response. B cells are believed to influence the balance between Th1/Th2 cells by affecting the function of dendritic cells (DCs). DCs are an APC that sensitize the Th0 cell population to develop into Th1 and Th2 cell types in the presence of IL-12 and IL-10 respectively. B cells regulate the secretion of these cytokines from DCs. B cell-deficient animal models show increased production of IL-12 from DCs, thereby leading to a Th1-polarized response. B cells suppress the release of IL-12 from DCs and maintain a balance between Th1 and Th2 responses [39, 40]. Studies from btk-mutant murine models (with B-cell deficiency) have shown an inability to mount a response against extracellular pathogens due to predominant Th1-cell polarization [41]. Amedei et al. have reported similar observations in patients with XLA. It was shown that patients with XLA have skewed Th1 profile as compared to a nonpolarized Th0 profile with the ability to produce all cytokines in healthy individuals [42]. These studies reflect the role of B cells in providing signals to T cells that influence the outcome of T cell responses [40].
Th17 Cells, Treg Cells and Recent Thymic Emigrants (RTE) in Patients with XLA
Barbosa et al. reported 6 patients with congenital agammaglobulinaemia (4 patients with XLA) with reduced Th17 cells [25]. Sharapova et al. reported no significant difference in the absolute numbers of Treg cells and RTE in patients with XLA and healthy controls. However, it was observed that patients with XLA with the chronic respiratory disease had a significantly lower percentage of Treg cells and RTE [32]. In contrast, Shelyakin et al. have reported a reduced number of naïve regulatory T cells in patients with XLA [11]. Low Th17 cells with disseminated molluscum infection have been reported in a case with XLA [43]. Table 1 provides a review of studies that have reported T cell defects and T cell receptor abnormalities in patients with XLA.
Table 1.
Review of various studies that have reported T cell defects and T cell receptor abnormalities in patients with XLA
| Author, country, year [reference] | Number of patients | Aim of the study | Methodology used | Salient findings | Remarks |
|---|---|---|---|---|---|
| Sharapova et al. (Belarus and Russia), 2017 [32] | 22 | To examine the subpopulations of T cells in children with XLA with or without chronic respiratory disease | Flow cytometry and ELISA | In children with XLA with CRD, RTE and Tregs were reduced, while absolute lymphocyte counts of CD3+, CD8+, CD3+DR+ and CD4+CD45RO+ T cells were significantly increased. However, significantly reduced numbers of Tfh and CD4+CD45RO+ with higher counts of naïve T cells were detected in children with XLA without CRD | Chronic respiratory infections alter the T cell homeostasis in patients with congenital lack of B cells |
| Barbosa et al. (Portugal), 2011 [25] | 6 patients with congenital agammaglobulinaemia 31 patients with CVID, 30 healthy controls | To evaluate the contribution of B cells in maintaining the homeostasis of the circulating Th17 compartment | Flow cytometry and ELISA | Severely reduced circulating Th17 cells in congenital agammaglobulinaemia patients |
Findings are suggestive of a relationship between Th17-cell homeostasis and B-cell maturation exists Similar results in patients with XLA and CVID |
| Martini et al. (Multicentric), 2011 [3] | 13 adults and 3 children with XLA; 9 with CVI; 20 healthy control | To assess the T cell development in the absence of B cells | Flow cytometry | Reduced memory CD4+CD45RO+ memory cells and CD4+CD45RO+CXCR5+ circulating CXCR5+ memory T cells were observed | The B-T cell’s germinal centre interaction is essential for CD4 + T cells’ activation in Humans |
| Crockard et al. (Ireland), 1992 [21] | 9 XLA; 9 CVI; 18 Control | To evaluate the CD4 lymphocyte subset composition in patients with XLA and CVID | Flow cytometry and in vivo assessment of cell-mediated immunity | Significant reduction in CD4+CD45RO+ and CD4+CD29+ cells resulting in delayed cutaneous hypersensitivity in patients with XLA | Defective cell-mediated immunity in XLA patients may result from the deficiency of circulating memory T cells |
| Morales-Aza et al. (UK), 2009 [17] | 7 XLA, 2 XHIGM, 3 control | To assess the role of B cells in the maintenance T cell memory and generation of immunity to infection | Flow cytometry and cell culture proliferation assay | Absence of CD4 CD45RO immune memory and reduced proliferation of T cells to Neisseria meningitidis (Nm) antigens in subjects with XLA | B cells are required to generate and maintain T cell memory |
| Rozynska et al. (England), 1989 [37] | 6 XLA patients, 12 CVH | To check the contribution of accessory and T cell defects in acquired and inherited hypogammaglobulinaemia | Flow cytometry, ELISA, lymphocyte culture | Abnormal T helper function for immunoglobulin production (IgM and IgG) and excessive suppressor T cell activity in patients with XLA | Impaired dendritic cell help and T cell function may develop in patients with acquired and inherited hypogammaglobulinaemia and may expand the clinical phenotype |
| Bateman et al. (UK), 2012 [61] | 58 CVID, 14 IgA-deficient patients, 15 IgG subclass with IgA-deficient patients and 9 XLA patients, 48 healthy controls and 31 disease-matched control | To check T cell subpopulation composition in various antibody deficiencies | Flow cytometry |
Significant reduction of CD4 + effector T cells, increased numbers of CD4 + naive T cells and RTE Significantly reduced numbers of putative follicular T cells in the XLA group as compared to healthy controls |
T cell subsets are altered in XLA |
| Amedei et al. (Italy), 2001 [42] | XLA 31; control 31 | To check the balance of the T helper cell compartment in patients with XLA | Flow cytometry and ELISA | Predominant Th1 response seen in patients with XLA | In the absence of B cells, T helper cells show skewed TH1 response to the mitogenic or antigenic stimulus |
| Edwards et al. (Australia), 2019 [36] | Total patients with PAD: 62 (9 XLA); control 59 | To identify the immune cell markers associated with non-infectious complications in patients with predominantly antibody deficiencies | Flow cytometry | Tfh 17 cells were specifically reduced in patients with XLA | |
| Ramesh et al. (USA), 2015 [57] | 15 XLA; 18 control | To examine TCR structure in absence of B cells | NGS | Significantly different V gene segment usage, and the varied V–J combinations between XLA and normal controls | Molecular structure of TCR is altered in the absence of B cells |
| Rawat et al. (India), 2021 [30] | XLA: 145 | To assess the clinical and molecular profile of XLA patients | Retrospective data collection | Reduced absolute CD4 + T cell counts in 2 patients with XLA | - |
XLA X-linked agammaglobulinaemia, CRD chronic respiratory disease, PAD primary antibody deficiency, RTE recent thymic emigrants, CVI common variable immunodeficiency, CVID common variable immunodeficiency, CVH common variable hypogammaglobulinaemia, ELISA enzyme-linked immunosorbent assay, TFH T follicular helper cells, Tregs T regulatory cells, TCR T cell receptor
Clinical Implications of Various T Cell Abnormalities in Patients with XLA
Patients with XLA often encounter recurrent bacterial infections. However, enteroviral infections are a major cause of mortality in these patients [44]. As btk deficiency hampers the memory T cell compartment and alters the function of DCs, it might be a possible reason for increased susceptibility to viral infections in patients with XLA. Even though patients with XLA have been reported to have low Th17 cells, these patients are not predisposed to develop candida infections. It has been hypothesized that other pathways for the production of IL-17 such as myeloid cells, NK cells, and γδ T cells are intact in patients with XLA [25]. We reported a patient with XLA with disseminated molluscum contagiosum infection who was also found to have low T helper 17 cells [43]. Impaired Treg cell populations may be a possible reason for the increased risk of autoimmune complications.
One study has reported a difference in proportions of Treg cells, Tfh cells, CD4+ and CD8+ naïve and memory T cells between patients with XLA with and without chronic lung disease [32]. Whether these cellular abnormalities are leading to chronic lung disease or these cellular abnormalities are developing as a results of chronic lung disease remains contentious at this point of time.
New immunization approaches targeting B cells APCs and DCs for effective immune response should be taken into consideration in patients with XLA [17].
T Cell Abnormalities in Patients with Acquired Loss of B Cells
Similar to patients with XLA who have congenital absence of B cells, patients who have acquired absence of B cells (such as patients with various autoimmune diseases and malignancies who have been treated with anti CD20 therapy (rituximab) may also develop a spectrum of T cell abnormalities [45]. Me´let et al. reported T cell abnormalities in patients with rheumatoid arthritis who were treated with rituximab. Flow cytometry analysis of the T cell population in this study revealed depletion of CD3+, CD4+ and CD8+ T cells by 35%, 37% and 24% respectively. The effect was more profound in CD4 + T cells with a severe reduction to 5.8% in some of the patients while the effect was equally prominent in both naïve and memory T cell compartment. It was also observed that T cell abnormalities result in decreased responsiveness to rituximab treatment and susceptibility to opportunistic infections. It has been hypothesized that this indirect effect on the T cell compartment results from B cell depletion as B cells act as APCs and stimulate the CD4+ T cell proliferation through DCs. B cells also provide chemokine and cytokines that play a role in cell migration and retention. Improvement in T cell subsets has been reported to be associated with B cell recovery after a few months, indicating that B cells are important in shaping the T cell compartment [45]. Similar to this study, several other authors have also reported quantitative alterations in the T cell compartment following treatment with rituximab [46, 47]. Besides these studies, various authors have reported a qualitative reduction in T cell activation markers such as CD40L, CD69 or HLA–DR in patients with systemic lupus erythematous following treatment with rituximab. Reduction in the number of costimulatory molecule expressing B cells resulted in reduced expression of costimulatory molecules in T cells [48].
Thus, abnormalities in the T cell compartment have been reported in patients with acquired loss of B cells following the use of rituximab [48]. However, it must be emphasized that these results may be difficult to compare with congenital absence of B cells as is seen in patients with XLA because of the following reasons:
In patients with XLA, because of the absence of B cells from the early stages of development of the immune system and as B cells are also important for several developmental stages of T cells, it is possible that the effect on the T cell compartment is more profound in patients with XLA as compared to patients with low B cells following use of B cell-depleting therapies [48].
T cell abnormalities in patients with XLA are likely to persist for life while T cell abnormalities following the use of B cell depletion therapies may normalize later [49].
B cell-depleting therapies are used in patients with autoimmune diseases and malignancies. These diseases may itself be associated with a spectrum of T cell abnormalities [50].
T Cell Dysfunction in Patients with XLA: Evidence from Studies on SARS-CoV2 Infection and Vaccination
Patients with XLA are also predisposed to develop viral infections in addition to bacterial infections. It has also been reported that SARS-CoV2 infection in patients with XLA may be associated with prolonged viral persistence and delayed recovery even though the CD8+ T cell responses were found to be normal in these patients [51]. Hence, the persistence of the virus in the respiratory tract for a prolonged duration could be related to an abnormal antibody response. However, it has also been shown that T cell responses to the COVID-19 vaccine are impaired in a few patients with XLA [52]. Salinas et al. evaluated response to the SARS-CoV-2 vaccine in primary antibody deficiency patients (49 CVID patients and 6 XLA patients). It was observed that 5/6 patients produced IFN-γ against the spike-specific antigen indicating intact T cell response [53]. However, larger studies are needed on this subject to draw more specific conclusions.
T Cell Receptor (TCR) Structure in Patients with XLA
The structure of TCR depends on the usage of V (variable), D (diversity) and J (joining) gene segments and by recombinational events that give rise to an enormous degree of diversity. Insertions or deletions of n and p nucleotides eventually give rise to non-productive or productive sequences complementarity determining region 3 (CDR3) loop of TCR that forms the core of the antigen-binding site. The rearrangement of antigen receptor is central to the development of adaptive immune responses. The length and sequence heterogeneity of this CDR3 play a pivotal role as peptide-binding residues in TCR [54].
The diversity in the structural framework of the T-cell receptor (TCR) is mainly attributed to combinatorial and junctional diversity generated during the process of gene rearrangement [55]. Studies from murine models indicate that B cells do regulate the generation of TCR diversity [56]. B cells with MHC class II molecule on their surface are involved in the development of T cell diversity [26]. Alterations in the TCR development due to absent B cells in patients with XLA have been demonstrated through high-throughput sequencing studies.
In patients with XLA, defects in TCR due to few nucleotide deletions and insertions in V, D and J gene segments in both the productive as well as non-productive sequences lead to a high degree of TCR clonal sharing. The functionality of TCR is also reported to be affected as individuals with XLA have been reported to have fewer charged amino acid sequences, one of the determining factors for the functional TCRs and greater surface hydrophobicity in the CDR3 loop [26].
Similarly, Shelyakin et al. reported reduced TCR receptor diversity in naïve CD4+ T cells in 10 young patients with XLA [11]. The pattern of TCR repertoire observed among these patients was similar to the TCR repertoire seen in the healthy elderly cohort suggesting that patients with XLA develop early thymic involution. Further evaluation of the TCR repertoire of naïve T regulatory cells in patients with XLA revealed increased repertoire convergence and decreased diversity due to few distinct nucleotide sequences in the CDR3 region. In addition, shorter length and few nucleotides insertion in the TCR-CDR3 region in patients with XLA exhibited a limited capacity for conformational changes and narrowed antigen specificities of naïve CD4+ cells [11]. This alteration in TCR was found to be more profound in the naïve Treg subset. In addition to altered TCR profile, naïve Tregs of patients with XLA also exhibited altered transcriptome profile. There was upregulation of several genes (such as TNFRSF13B, IL1b, CXCL4, CCL20 and S100A11) in naïve Tregs that usually do not show such high expression in these cells. Alteration in the TCR receptor profile and transcriptome of the naïve Tregs in patients with XLA may be responsible for inflammatory complications [11].
The exact clinical implication of constricted TCR repertoire in patients with XLA is unclear. Diminished junctional diversity and lack of highly modified clones have often been reported in several PIDs such as severe combined immune deficiency, MHC class II defects and common variable immunodeficiency (CVID). This leads to the inability of the immune system to expand T cell clones to full capacity following stimulation with antigens [57]. This may predispose an individual with low TCR diversity to develop infections.
TCR signalling regulates effective immunity and immune tolerance by controlling the development of T cells and peripheral T cell responses. Aberrations in TCR may result in altered TCR signalling and may also impair the central tolerance through an altered negative selection of autoreactive TCRs. These autoreactive TCRs may give rise to immune dysregulations that may present as autoimmune and inflammatory complications [58, 59].
Similar observations have also been reported in patients with acquired loss of B cells following the use of rituximab. Papalexandri et al. reported restricted T cell repertoire post-rituximab administration in patients who underwent allogenic haematopoietic cell transplantation for chronic graft versus host disease (GvHD) and Epstein-Barr virus (EBV) reactivation. Upon profiling of TCR beta gene repertoire (TRB), it was found that 3 TRBV (V β repertoire) genes constituted approximately half of the total repertoire, thereby indicating remarkable skewing. Clonal sharing of TRBV gene segments impairs the diversity and may give rise to oligoclonal rather than polyclonal TCR. A restricted TCR diversity was reported to be associated with EBV and cytomegalovirus (CMV) reactivation and chronic GvHD in patients with particular germ line encoded T cell clones [60].
As mentioned above, it may not be possible to compare the results of TCR alterations following the use of B cell depletion therapy from patients with XLA. However, results of the study on restricted TCR repertoire following acquired depletion of B cells suggest that the defect could be more profound in patients with XLA.
Conclusion
To conclude, B cells also play an important role in the development and function of the T cell compartment. A spectrum of T cell abnormalities has been reported in patients with XLA. These may vary from the mild reduction in Tfh cells to markedly decrease Th17 cells, Treg cells, memory T cells and an altered TCR repertoire. At present, the clinical significance of these T cell abnormalities has not been studied in detail. However, these abnormalities may result in an increased risk of viral infections, autoimmunity, autoinflammation and possibly chronic lung disease.
Declarations
Ethical Approval and Informed Consent
Not eligible.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mattsson PT, Vihinen M, Smith CIE. X-linked agammaglobulinemia (XLA): A genetic tyrosine kinase (Btk) disease. BioEssays. 1996;18:825–834. doi: 10.1002/bies.950181009. [DOI] [PubMed] [Google Scholar]
- 2.Suri D, Rawat A, Singh S. X-linked agammaglobulinemia. Indian J Pediatr. 2016;83:331–337. doi: 10.1007/s12098-015-2024-8. [DOI] [PubMed] [Google Scholar]
- 3.Martini H, Enright V, Perro M, et al. Importance of B cell co-stimulation in CD4+ T cell differentiation: X-linked agammaglobulinaemia, a human model. Clin Exp Immunol. 2011;164:381–387. doi: 10.1111/j.1365-2249.2011.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawlings DJ. Bruton’s tyrosine kinase controls a sustained calcium signal essential for B lineage development and function. Clin Immunol. 1999;91:243–253. doi: 10.1006/clim.1999.4732. [DOI] [PubMed] [Google Scholar]
- 5.Conley ME. Early defects in B cell development: current opinion in allergy and clinical immunology. 2002;2:517–522. doi: 10.1097/00130832-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Futatani T, Miyawaki T, Tsukada S, et al. Deficient expression of Bruton’s tyrosine kinase in monocytes from X-linked agammaglobulinemia as evaluated by a flow cytometric analysis and its clinical application to carrier detection. Blood. 1998;91:595–602. doi: 10.1182/blood.V91.2.595. [DOI] [PubMed] [Google Scholar]
- 7.Taneichi H, Kanegane H, Mohamed Sira M, et al. Toll-like receptor signaling is impaired in dendritic cells from patients with X-linked agammaglobulinemia. Clin Immunol. 2008;126:148–154. doi: 10.1016/j.clim.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Smith CI, Baskin B, Humire-Greiff P, et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J Immunol. 1994;152:557–565. [PubMed] [Google Scholar]
- 9.Parker DC. T Cell-Dependent B Cell Activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 10.Crotty S. Follicular Helper CD4 T Cells (T FH ) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 11.Shelyakin PV, Lupyr KR, Egorov ES, et al. Naïve regulatory T cell subset is altered in X-linked agammaglobulinemia. Front Immunol. 2021 doi: 10.3389/fimmu.2021.697307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Trujillo VP, Scalchunes C, Cunningham-Rundles C, et al. Autoimmunity and inflammation in X-linked agammaglobulinemia. J Clin Immunol. 2014;34:627–632. doi: 10.1007/s10875-014-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paroli M, Accapezzato D, Francavilla V, et al. Long-lasting memory-resting and memory-effector CD4+T cells in human X-linked agammaglobulinemia. Blood. 2002;99:2131–2137. doi: 10.1182/blood.V99.6.2131. [DOI] [PubMed] [Google Scholar]
- 14.Germain RN. MHC-dependent antigen processing and peptide presentation: Providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 16.Petersone L, Edner NM, Ovcinnikovs V, et al. T cell/B cell collaboration and autoimmunity: an intimate relationship. Front Immunol. 2018;9:1941. doi: 10.3389/fimmu.2018.01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales-Aza B, Glennie SJ, Garcez TP, et al. Impaired maintenance of naturally acquired T-cell memory to the meningococcus in patients with B-cell immunodeficiency. Blood. 2009;113:4206–4212. doi: 10.1182/blood-2008-08-171587. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 19.Vitetta ES, Berton MT, Burger C, et al. Memory B and T Cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- 20.Plebani A, Fischer MB, Meini A, et al. T cell activity and cytokine production in X-linked agammaglobulinemia: implications for vaccination strategies. Int Arch Allergy Immunol. 1997;114:90–93. doi: 10.1159/000237649. [DOI] [PubMed] [Google Scholar]
- 21.Crockard AD, Boyd NA, McNeill TA, McCluskey DR. CD4 lymphocyte subset abnormalities associated with impaired delayed cutaneous hypersensitivity reactions in patients with X-linked agammaglobulinaemia. Clin Exp Immunol. 1992;88:29–34. doi: 10.1111/j.1365-2249.1992.tb03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Rosa F, Matzinger P. Long-lasting CD8 T cell memory in the absence of CD4 T cells or B cells. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 24.van Essen D, Dullforce P, Brocker T, Gray D. Cellular interactions involved in Th cell memory. J Immunol. 2000;165:3640–3646. doi: 10.4049/jimmunol.165.7.3640. [DOI] [PubMed] [Google Scholar]
- 25.Barbosa RR, Silva SP, Silva SL, et al. Primary B-cell deficiencies reveal a link between human IL-17-producing CD4 T-cell homeostasis and B-cell differentiation. PLoS ONE. 2011;6:e22848. doi: 10.1371/journal.pone.0022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramesh M, Simchoni N, Hamm D, Cunningham-Rundles C. High-throughput sequencing reveals an altered T cell repertoire in X-linked agammaglobulinemia. Clin Immunol. 2015;161:190–196. doi: 10.1016/j.clim.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murali-Krishna K, Lau LL, Sambhara S, et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 28.Zinkernagel RM, Bachmann MF, Kündig TM, et al. On immunological memory. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 29.Kansas GS, Wood GS, Engleman EG. Maturational and functional diversity of human B lymphocytes delineated with anti-Leu-8. J Immunol. 1985;134:3003–3006. [PubMed] [Google Scholar]
- 30.Rawat A, Jindal AK, Suri D, et al. Clinical and genetic profile of X-linked agammaglobulinemia: a multicenter experience from India. Front Immunol. 2021 doi: 10.3389/fimmu.2020.612323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pituch-Noworolska A, Zwonarz K, Błaut-Szlósarczyk A, et al. T lymphocytes and NK cells in X-linked agammaglobulinemia. Przegl Lek. 2013;70:1048–1050. [PubMed] [Google Scholar]
- 32.Sharapova SO, Pashchenko OE, Guryanova IE, et al. Recent thymic emigrants, T regulatory cells, and BAFF level in children with X-linked agammaglobulinaemia in association with chronic respiratory disease. Allergol Immunopathol. 2018;46:58–66. doi: 10.1016/j.aller.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs E, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 34.Scognamiglio P, Accapezzato D, Casciaro MA, et al. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J Immunol. 1999;162:6681–6689. [PubMed] [Google Scholar]
- 35.Liu Y, Wu Y, Lam K-T, et al. Dendritic and T cell response to influenza is normal in the patients with X-linked agammaglobulinemia. J Clin Immunol. 2012;32:421–429. doi: 10.1007/s10875-011-9639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards ESJ, Bosco JJ, Aui PM, et al. Predominantly antibody-deficient patients with non-infectious complications have reduced naive B, Treg, Th17, and Tfh17 cells. Front Immunol. 2019;10:2593. doi: 10.3389/fimmu.2019.02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rozynska KE, Spickett GP, Millrain M, et al. Accessory and T cell defects in acquired and inherited hypogammaglobulinaemia. Clin Exp Immunol. 1989;78:1–6. [PMC free article] [PubMed] [Google Scholar]
- 38.Akbar AN, Salmon M, Janossy G. The synergy between naive and memory T cells during activation. Immunol Today. 1991;12:184–188. doi: 10.1016/0167-5699(91)90050-4. [DOI] [PubMed] [Google Scholar]
- 39.Moulin V, Andris F, Theilemans K, et al. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000 doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macaulay AE, DeKruyff RH, Umetsu DT (1998) Antigen-primed T cells from B cell-deficient JHD mice fail to provide B cell help. J Immunol (Baltimore, Md : 1950) 160:1694–700 [PubMed]
- 41.Langhorne J, Cross C, Seixas E, et al. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci USA. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amedei A, Romagnani C, Benagiano M, et al. Preferential Th1 profile of T helper cell responses in X-linked (Bruton’s) agammaglobulinemia. Eur J Immunol. 2001 doi: 10.1002/1521-4141(200106)31:6<1927::aid-immu1927>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 43.Banday AZ, Jindal AK, Arora K, Rawat A (2021) Extensive molluscum contagiosum in X-linked agammaglobulinemia. J Allergy Clin Immunol 9:985. 10.1016/j.jaip.2020.07.037 [DOI] [PubMed]
- 44.Kainulainen L, Vuorinen T, Rantakokko-Jalava K, et al. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. Journal of Allergy and Clinical Immunology. 2010;126:120–126. doi: 10.1016/j.jaci.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mélet J, Mulleman D, Goupille P, et al. Rituximab-induced T cell depletion in patients with rheumatoid arthritis: association with clinical response. Arthritis Rheum. 2013;65:2783–2790. doi: 10.1002/art.38107. [DOI] [PubMed] [Google Scholar]
- 46.Thurlings RM, Vos K, Wijbrandts CA, et al. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008;67:917–925. doi: 10.1136/ard.2007.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feuchtenberger M, Müller S, Roll P, et al. Frequency of regulatory T cells is not affected by transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. The Open Rheumatology Journal. 2008;2:81. doi: 10.2174/1874312900802010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwata S, Saito K, Tokunaga M, et al. Phenotypic changes of lymphocytes in patients with systemic lupus erythematosus who are in longterm remission after B cell depletion therapy with rituximab. J Rheumatol. 2011;38:633–641. doi: 10.3899/jrheum.100729. [DOI] [PubMed] [Google Scholar]
- 49.Mélet J, Mulleman D, Goupille P, et al. Rituximab-induced T cell depletion in patients with rheumatoid arthritis: association with clinical response: rituximab-induced T cell depletion and clinical response in RA. Arthritis Rheum. 2013;65:2783–2790. doi: 10.1002/art.38107. [DOI] [PubMed] [Google Scholar]
- 50.Chang C-M, Hsu Y-W, Wong HS-C, et al. Characterization of T-cell receptor repertoire in patients with rheumatoid arthritis receiving biologic therapies. Dis Markers. 2019;2019:2364943. doi: 10.1155/2019/2364943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckland MS, Galloway JB, Fhogartaigh CN, et al. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun. 2020;11:6385. doi: 10.1038/s41467-020-19761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ponsford MJ, Shillitoe BMJ, Humphreys IR, et al. COVID-19 and X-linked agammaglobulinemia (XLA) - insights from a monogenic antibody deficiency. Curr Opin Allergy Clin Immunol. 2021;21:525–534. doi: 10.1097/ACI.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 53.Salinas AF, Mortari EP, Terreri S, et al. SARS-CoV-2 vaccine induced atypical immune responses in antibody defects: everybody does their best. J Clin Immunol. 2021;41:1709–1722. doi: 10.1007/s10875-021-01133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dash P, Fiore-Gartland AJ, Hertz T, et al. Quantifiable predictive features define epitope specific T cell receptor repertoires. Nature. 2017;547:89–93. doi: 10.1038/nature22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.João C, Ogle BM, Gay-Rabinstein C, et al. B cell-dependent TCR diversification. J Immunol. 2004;172:4709–4716. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 57.Ramesh M, Hamm D, Simchoni N, Cunningham-Rundles C. Clonal and constricted T cell repertoire in common variable immune deficiency. Clin Immunol. 2017;178:1–9. doi: 10.1016/j.clim.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong GK, Heather JM, Barmettler S, Cobbold M. Immune dysregulation in immunodeficiency disorders: the role of T-cell receptor sequencing. J Autoimmun. 2017;80:1–9. doi: 10.1016/j.jaut.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Sogkas G, Atschekzei F, Adriawan IR, et al. Cellular and molecular mechanisms breaking immune tolerance in inborn errors of immunity. Cell Mol Immunol. 2021;18:1122–1140. doi: 10.1038/s41423-020-00626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papalexandri A, Karypidou M, Stalika E, et al. Skewing of the T-cell receptor repertoire in patients receiving rituximab after allogeneic hematopoietic cell transplantation: what lies beneath? Leuk Lymphoma. 2019;60:1685–1692. doi: 10.1080/10428194.2018.1543881. [DOI] [PubMed] [Google Scholar]
- 61.Bateman EA, Ayers L, Sadler R, Lucas M, Roberts C, Woods A, et al (2012) T cell phenotypes in patients with common variable immunodeficiency disorders: associations with clinical phenotypes in comparison with other groups with recurrent infections. Clin Exp Immunol 170(2):202-11 [DOI] [PMC free article] [PubMed]