Abstract
Structurally modified virus particles can be obtained from the rod-shaped or filamentous virions of plant viruses and bacteriophages by thermal or chemical treatment. They have recently attracted attention of the researchers as promising biogenic platforms for the development of new biotechnologies. This review presents data on preparation, structure, and properties of the structurally modified virus particles. In addition, their biosafety for animals is considered, as well as the areas of application of such particles in biomedicine. A separate section is devoted to one of the most relevant and promising areas for the use of structurally modified plant viruses – design of vaccine candidates based on them.
Keywords: structurally modified viruses, spherical particles, plant viruses, bacteriophages, vaccines, biotechnology, biomedicine
INTRODUCTION
In the last decade, the structurally modified particles derived from plant viruses or bacteriophages have become an attractive protein platform for various applications in biotechnology and biomedicine [1-8]. Such particles are produced by thermal or chemical treatment of virions of rod-shaped and filamentous viruses [9-12].
Studies of the structural rearrangement of virions, properties of the resulting particles, and their interaction with mammalian cells/organisms are important research tasks. This is of fundamental importance and may also facilitate creation of new biotechnological platforms for antigen presentation, drug delivery, and biomarker production.
Structural transition from “rods” to “spheres” for the tobacco mosaic virus (TMV) (family Virgaviridae, genus Tobamovirus) was first described in 1956 by Hart [13]. However, the detailed study of the process occurred much later at the Department of Virology, Faculty of Biology, Moscow State University [1, 10]. These studies served as an impetus for scientific experiments and biomedical developments in Russia and other countries [2-4, 7, 14-22]. In particular, American researchers used the structurally modified TMV particles to develop contrast agents for magnetic resonance imaging (MRI) [2], as well as to deliver drugs to tumor tissues during chemotherapy [3].
Most of the results related to the structurally modified viruses have been demonstrated for TMV. Nevertheless, the possibility of structural modification has also been shown for other plant viruses [11, 12, 23] and for bacteriophages [9, 24].
The conditions for structural modification may differ for the viruses with same morphology, therefore, the study of structural modification of virions provides new fundamental knowledge about the structure and stability of viruses.
This review is devoted to the analysis of the accumulated data on the structure, properties, safety, and practical application of the structurally modified plant and bacterial viruses.
STRUCTURALLY MODIFIED PLANT VIRUSES
Structurally modified tobacco mosaic virus particles. Characteristics and properties. Tobacco mosaic virus is a rod-shaped virus, 300 nm long and 18 nm in diameter. TMV has a capsid consisting of 2130 identical coat protein (CP) subunits, which are assembled around the viral single-stranded RNA. In this way, a helical structure with 2-nm diameter central cavity is formed [25]. TMV occupies a unique place in the history of virology and is one of the most studied viruses. TMV research has largely determined fundamental ideas of the modern molecular virology. The accumulated knowledge has made it possible to develop new platforms based on TMV for application in medicine and biotechnology [26].
In 1956, Hart [13] used electron microscopy to study a heat-treated TMV. He reported that 10 second incubation at 80-98°C resulted in swelling of TMV particles at one or both ends, followed by transformation into “ball particles”. The volume of these particles was comparable to the volume of the initial viral “rods”. These studies were not continued and, one might say, were “forgotten” until 2011. In that year, the article by Atabekov et al. [10] was published in which such particles were again obtained and studied using modern experimental methods and approaches. This publication gave rise to a whole series of studies devoted to the particles obtained by thermal rearrangement of TMV. In these works, formation conditions, physicochemical properties, and structure of these particles were studied in detail. These structures have been called “spherical particles” (SPs) [1, 10, 27-31]. The conducted studies have shown that it is possible to obtain TMV SPs of a given size by thermal treatment of the native virus particles. The structurally modified TMV particles were studied using various methods (electron microscopy, nanoparticle tracking analysis, dynamic light scattering). These methods complemented each other, which made it possible to thoroughly describe the produced SPs. Structural transition of the TMV virions into SPs occurs through intermediate forms. In the first stage, structures with swelling on one or both ends are formed, which are further transformed into spherical particles. TMV SPs are highly stable and uniform in shape. They do not form aggregates and do not change shape and size when stored for at least 6 months. The same has been observed for the SPs undergoing sedimentation by centrifugation at 10,000g, reheating to a temperature of 98°C and cooling, repeated freezing to –20°C followed by thawing [10]. A number of studies have demonstrated the possibility of obtaining TMV SPs in preparative amounts [19, 32]. An important feature of the TMV SPs is that their size depends on the concentration of the original virus in the sample, which makes it possible to obtain SPs of a given diameter [10, 32] (Fig. 1).
Fig. 1.
Structurally modified particles generated by thermally-induced rearrangement of TMV virions. a) TMV, transmission electron microscopy, contrasting with 2% uranyl acetate, scale bar 200 nm; b) TMV SPs, transmission electron microscopy, scale bar 2 µm. Images were obtained at the Department of Virology, Faculty of Biology, Lomonosov Moscow State University.
Unlike TMV virions, TMV SPs do not contain RNA, and the protein isolated from them cannot be assembled into a regular structure. This indicates that thermal denaturation is irreversible in this case [10]. The TMV SPs protein was studied by the methods of circular dichroism, Raman spectroscopy, and fluorescence spectroscopy. In terms of structural characteristics, it turned out to be significantly different from the CP in the native TMV. Rearrangement of the TMV virions into SPs is accompanied by the increase in particle density. At the same time, there is a transition of CP subunits into a structure with a low content of α-helices and significant proportion of β-structures. The appearance of cross-β-structures is evidenced, in particular, by the strong reaction of TMV SPs with thioflavin T [28]. Using the tritium planigraphy technique, composition of amino acids on the surface of SPs was compared with the TMV virions. It was found that the surface of SPs is much more hydrophobic due to the fact that the SPs are assembled from the thermally denatured protein subunits. Thus, under conditions of thermal denaturation, CP subunits of TMV acquire a specific conformation favorable for the assembly of stable SPs. The amino acid composition of the surface of SPs differs significantly from that of TMV virions [31].
As a result of thermal rearrangement, TMV SPs acquire properties different from those of virions. In particular, they have unique adsorption capabilities. Protein molecules of various sizes and amino acid profile (including antigens of human pathogens) can be adsorbed on the surface of TMV SPs. Moreover, SPs can adsorb polymers and native virus particles of small size and spherical shape [1, 4, 7, 27, 29, 30, 33-35]. The process of obtaining complexes of SPs with target agents is extremely simple: it involves short incubation (10-15 min), during which the molecules or virions bind to the surface of SPs via non-covalent bonds. In a number of studies, our group demonstrated another feature of TMV SPs – increase in the immune response to antigens of various nature and molecular weight adsorbed on the surface of SPs [1, 4, 7, 34].
All these properties made it possible to consider the possibilities of using TMV SPs as a biogenic protein platform and to develop approaches for their practical application.
Application of TMV SPs in the development of vaccines. Modern vaccinology is associated with the use of recombinant bacterial and viral antigens. Search for novel safe adjuvants is an important task. They are needed to improve immunogenicity of the antigens, reduce the dose of active vaccine substances and cost of its production. Adjuvants not only enhance immune response and its duration, but can also influence the type of response (humoral and/or cellular). Adjuvants stimulate immune response to different antigens with different efficacy: for example, adjuvants based on aluminum compounds are ineffective in some cases [36-39].
TMV SPs certainly have potential for vaccine development. They were used to form complexes with recombinant antigens of coronavirus [7], rubella virus [4], rotavirus [40], anthrax [35], and avian influenza virus [41] (Table 1).
Table 1.
Prototypes of TMV SPs based vaccine candidates
| Vaccine candidate | Valence | Antigen | References |
|---|---|---|---|
| Rubella virus | monovalent | epitope of E1 protein | [4] |
| Avian influenza virus | polyvalent | epitopes of HA and M2 proteins | [41] |
| Rabies virus | monovalent | inactivated virion | [42] |
| Rotavirus | monovalent | epitope of VP6 protein | [40] |
| Puumala virus (hantavirus) | monovalent | inactivated virion | [38] |
| Coronavirus SARS-CoV-2 | polyvalent | RBD domain, conserved fragments of the S2 subunit | [7] |
| Bacillus anthracis bacterium | monovalent | protective antigen – PA | [35] |
The resulting compositions of TMV SPs in complex with antigens (vaccine candidates) are at various stages of development. In all cases, the possibility of adsorption of recombinant antigens and antigenic specificity of the proteins in the compositions of SPs has already been shown by methods of fluorescence microscopy, immunoelectron and/or immunofluorescence microscopy. In the recently published article, TMV SPs were used as a platform (depot) and adjuvant in the development of the vaccine candidate against COVID-19. It has been shown that the TMV SPs significantly enhance immunogenicity (total IgG titers) of the recombinant antigens and contribute to the stimulation of a balanced Th1/Th2 immune response. When immunizing Syrian hamsters, the candidate vaccine induced production of the antibodies that neutralize SARS-CoV-2, suggesting that this approach is promising [7]. The adjuvant properties of TMV SPs have also been shown in other studies. One example is a rubella vaccine candidate. It is a composition consisting of the antigen (tetraepitope A of glycoprotein E1) in complex with TMV SPs. This complex provides a significant increase in antibody titer (~10-fold) compared to immunization with only antigen itself or a mixture of antigen with aluminum hydroxide adjuvant [4]. Currently, preclinical trials of the rubella vaccine candidate based on the TMV SPs have been successfully completed.
When immunizing animals, SPs significantly enhance humoral immune response to the inactivated Puumala virus vaccine and have adjuvant activity in terms of the production of cytokines IL-12 and IFN-γ [38]. In addition, it has been shown that the SPs enhance protective properties of the widely used non-adjuvanted inactivated rabies vaccine produced in Russia – Rabikan. The result was increase in the protective activity of the Rabikan vaccine when used together with TMV SPs, comparable to the effect of incomplete Freund’s adjuvant [42] (Table 1).
A number of studies have shown that when animals are immunized with the vaccine candidates based on TMV SPs, most of the antibodies are produced against the target antigen, and not against the protein particle used as an adjuvant [4, 7].
Another application of TMV SPs in the vaccine development is stabilization of antigens during storage. It is known that proteins – components of drugs – can be subjected to spontaneous degradation during production and storage. Our study has shown the possibility of stabilizing the protective antigen, which is the main antigen of the anthrax pathogen, by adsorbing it on the surface of TMV SPs. The recombinant protective antigen (rPA) is the main component of almost all currently developed vaccines. Instability of this protein is mainly associated with the presence of proteolytic sites, as well as with spontaneous deamidation of asparagine residues, leading to degradation of rPA. The rate of deamidation is greatly increased when aluminum hydroxide is used as an adjuvant, which considerably reduces protective properties of the vaccines during storage and clinical applications [43, 44]. Our studies have shown that a good way to achieve high storage stability is to introduce amino acid substitutions for the sites responsible for protein destabilization into rPA and then adsorb it on the surface of TMV SPs [8, 35].
Application of TMV SPs as carriers for functionally active molecules and in antitumor therapy. Methods for covalent binding of the functionally active molecules to TMV SPs, as well as methods for encapsulation of the molecules during thermal rearrangement of SPs have been developed in a number of studies.
The study by Dr. N. F. Steinmetz research group showed that the TMV SPs could be effectively used for bioconjugation via the functional side chains of amino acid residues of lysine (amino group), aspartic and glutamic acids (carboxyl group), and cysteine (thiol group) on their surface [3]. Chemical modification of TMV SPs expands the possibilities of their use in the formation of complexes with the functionally active compounds for various biomedical applications. In particular, the possibility of TMV SPs conjugation was shown in an experiment in which the carboxyl groups of their glutamic acids were covalently bound to the chemotherapeutic drug doxorubicin. In the same study, the authors tested the possibility of non-covalent encapsulation of doxorubicin by simply adding it to the TMV virions during thermal rearrangement. Using two breast cancer cell lines, both approaches demonstrated effective drug delivery (cell uptake) and cancer cell killing.
In another study by the same group of scientists, the properties of TMV virions and TMV SPs in stimulating an antitumor response were compared. B16F10 melanoma cells, a highly aggressive and poorly immunogenic tumor model, widely used to study various drugs in immunotherapy, were used for this propose [22]. Previously, antitumor activity of various plant viruses, from icosahedral cowpea mosaic virus (CPMV) to filamentous potato virus X (PVX) and papaya mosaic virus [45-47] was shown using this model. An impressive result was shown in the experimental mouse model with induced melanoma: intratumoral administration of a suspension of TMV or TMV SPs led to the decrease in the rate of tumor growth and increase in the survival time of animals. However, analysis of these TMV-based drugs showed lower efficacy in the experiments compared to CPMV. The authors note that the TMV SPs with a too large diameter (about 250 nm) were used in the work. They suggest that even more effective protection can be achieved by using particles with smaller diameter [22].
Wu et al. [20] used β-cyclodextrin (β-CD) to chemically modify the surface of TMV virions. Upon thermal rearrangement of such particles, SPs with an average diameter of 88 nm were formed. SPs modified with β-CD were able to form a complex with adamantane (a chemical compound and its analogs used in treatment of various diseases) [20]. Previously, the same authors studied the possibility of assembling such complexes based on the native TMV virions, modified by β-CD. In addition to adamantane, these complexes could include folic acid, rhodamine B, doxorubicin, or polyethylene glycol [48]. Based on these data, the scientists suggested that the described approach for the assembly of supramolecular complexes is promising.
Some other studies have demonstrated the possibility of immobilization of gold and silver nanoparticles on the surface of TMV SPs [21, 49]. These results may have potential applications, for example, in the development of biosensors, as well as in antitumor and antibacterial therapy. Unfortunately, there are no new studies published by these scientific teams. Promising results on immobilization of the metal ions on the surface of TMV SPs were obtained by Dr. N. F. Steinmetz research group. The scientists modified inner surface of the TMV virions with gadolinium (III) (Gd) chelate complexes, widely used in clinical practice as contrast agents for MRI. The complexes of TMV SPs-Gd (diameter 170 nm) provided higher relaxation time compared to the free chelate complexes of Gd or TMV-Gd. The relaxation time was comparable to such highly contrasting agents for MRI as synthetic dendrimers conjugated with a Gd chelate complex [2, 14]. To prolong the action of the complexes in the body, the scientists coated the TMV SPs–Gd complexes (75 nm in diameter) with the biologically inert and stable silica (silicon dioxide). Mineralization of TMV SPs–Gd led to an almost threefold increase in the relaxation time. In addition, such complexes were more rapidly absorbed by macrophages and were protected from antibody recognition. The authors concluded that the silica-coated SP TMV–Gd can be used in MRI diagnostics of diseases associated with inflammatory processes [18].
A large arsenal of approaches, previously tested and studied using virions of various plant viruses, is used to study possible applications of TMV SPs in biotechnology and medicine. These particles are able to enter cells, and are also capable of chemical bioconjugation and non-covalent encapsulation of various therapeutically significant compounds. Due to these properties, TMV SPs could be an attractive platform for the delivery of drugs or contrast agents and could find their application in medicine. The principal possible areas of application of TMV SPs are shown in Fig. 2.
Fig. 2.
Possible areas of application of TMV SPs.
Structurally modified particles from plant viruses with different morphology. Currently, TMV is represented in biotechnological and biomedical research more than other phytoviruses. However, some other plant viruses with morphology/structure different from TMV can be structurally modified by physical impact. The structurally modified spherical particles were obtained by thermal treatment of virions of plant viruses belonging to various taxonomic groups. These include the rod-shaped virions of a representative of the genus of tobamoviruses – Dolichos enation mosaic virus (DEMV) (Sunn-hemp mosaic virus), hordeiviruses – barley stripe mosaic virus (BSMV) [23]; filamentous virions of potyviruses – potato virus A (PVA) [50] and potexviruses; alternanthera mosaic virus (AltMV) [12] and potato virus X (PVX) [11]. It should be noted that the attempts of temperature-induced rearrangement of the virions of plant viruses with icosahedral symmetry type were unsuccessful. In particular, heat treatment of spherical cauliflower mosaic virus (CaMV) and bean mild mosaic virus (BMMV) virions did not result in structural modification, and no changes in virion morphology and size was detected [23].
Thermally-induced rearrangement of virions of other rod-shaped or filamentous plant viruses into spherical particles occurs in the same way as in TMV: in two stages through formation of intermediate forms. The complete structural rearrangement of BSMV, DEMV, and AltMV virions occurs at 94°C, and of PVX – at 90°C (Table 2).
Table 2.
Possibility of thermally-induced rearrangement of plant viruses and properties of SPs obtained from them
| TMV | DEMV | BSMV | AltMV | PVX | PVA | BMMV | CaMV | |
|---|---|---|---|---|---|---|---|---|
| Family, Genus | Virgaviridae, Tobamovirus | Virgaviridae, Tobamovirus | Virgaviridae, Hordeivirus | Alphaflexiviridae, Potexvirus | Alphaflexiviridae, Potexvirus | Potyviridae, Potyvirus | Tombusviridae | Caulimoviridae, Caulimovirus |
| Virion morphology | rod-shaped | rod-shaped | rod-shaped | filamentous | filamentous | filamentous | icosahedral | icosahedral |
| Genome | RNA | RNA | RNA | RNA | RNA | RNA | RNA | DNA |
| Transformation into SPs | yes | yes | yes | yes | yes | yes | no | no |
| Structural transition temperature,°C | 94 | 94 | 94 | 94 | 90 | 60* | – | – |
| Size dependency on concentration | yes | yes | no | yes | yes | n/a | – | – |
| Presence of cross-β-structures in SPs | yes | n/a | n/a | yes | yes | yes | – | – |
| Change in the surface amino acid composition | yes | n/a | n/a | yes | n/a | n/a | – | – |
| Adsorption properties | yes | n/a | n/a | yes | yes | n/a | – | – |
Note. n/a – not assessed.
* Conditions for PVA SPs formation differ from the conditions for obtaining SPs from other plant viruses.
* Conditions for PVA SPs formation differ from the conditions for obtaining SPs from other plant viruses.
Differences in the temperatures and conditions of thermal rearrangement of the morphologically similar plant viruses (PVX and AltMV) indicate differences in the structure and stability of their virions. It was shown that AltMV SPs and PVX SPs do not contain RNA. Studies of the protein structure revealed differences in the secondary and tertiary structures of CP in SPs and in native virions [11, 12]. Similarly to the TMV SPs, structural transition of AltMV and PVX virions is accompanied by the appearance of a larger fraction of ß-structures in the protein. The adsorption properties of SPs and their ability to bind model antigens on their surface were analyzed. The obtained characteristics of PVX SPs and AltMV SPs indicate potential possibility of their use as platforms for presentation of the target antigens and formation of the functionally active complexes. Moreover, presence of the chemically reactive amino acids on the surface has been shown for AltMV SPs [12].
Studies of the structurally modified spherical particles obtained from various representatives of plant viruses should be continued. It is very likely that the properties and features that distinguish them from TMV SPs would provide additional information about the structure of virions, and would also be useful for creating biogenic platforms.
Safety of SPs application. Plant viruses are safe for mammals, they are not pathogenic for them and cannot replicate in human cells [51]. The possibility of biodegradation of the TMV SPs protein was studied in vitro: TMV SPs undergo complete proteolysis in the presence of proteinase K, while native virions remain resistant to the enzyme [27]. Biodistribution profile of the TMV SPs with a diameter of about 50 nm was studied in mice: SPs predominantly accumulated in the spleen and liver 4 h after injection and were cleared from circulation by macrophages. Twenty-four hours after administration, SPs were not found in animal tissues. This gives them an advantage over synthetic materials of non-biological origin, which can persist in tissues for a long period and whose biodegradation is accompanied with formation of by-products leading to the induction of oxidative stress, as well as apoptosis [52]. TMV SPs show good compatibility with blood (does not cause hemolysis or clotting) and tissues (no signs of inflammation, apoptosis, degeneration or necrosis) [15]. Further studies were carried out on three animal models (mice, rats, rabbits): introduction of the 300-nm diameter TMV SPs intramuscularly or intraperitoneally did not cause any toxic effects. The evaluation included preclinical studies of local tolerability after a single administration, as well as local and systemic effects after repeated administration of SPs, such as physiological, histological, and hematological changes. Reproductive toxicity has also been studied [53, 54]. Furthermore, safety of the SPs in the composition of the rubella vaccine candidate has been demonstrated in preclinical studies in three animal species (mice, rats, rabbits) [4].
Thus, TMV SPs are biologically compatible with animal cells, biodegradable, and safe.
STRUCTURALLY MODIFIED BACTERIOPHAGE PARTICLES AND THEIR APPLICATIONS
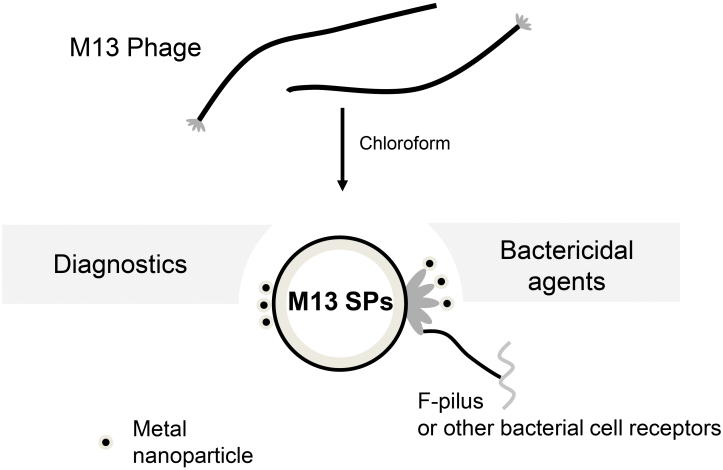
The possibility of producing structurally modified spherical particles was demonstrated for the bacteriophage M13 (family Inoviridae, genus Inovirus). M13 virions have a filamentous shape with a length of 880 nm and a diameter of 6.5 nm [55]. The virion of Inovirus M13 bacteriophage contains a single-stranded circular DNA (6407 nt), which is packaged within approximately 2700 copies of the p8 major coat protein [5, 24, 56]. The p8 consists of almost 100% α-helices [9]. The capsid also includes minor coat proteins: p3, p6, p5, p9 [5]. It is interesting to note that in this case, structural transition does not occur under the effect of high temperature, but upon short-term treatment of the suspension of phage particles with an equal volume of chloroform at 24°C [9]. The possibility of structural modification has also been demonstrated for other representatives of the Inovirus genus: for bacteriophages fd and f1 [24, 57]. It has been shown that treatment with chloroform results in the changes in hydrophobic interactions between the p8 protein subunits, and 2/3 of the viral DNA is released through a pore formed by five subunits of the p3 minor coat protein. Thus, treatment of the virions of filamentous bacteriophages with chloroform leads to formation of spherical particles with asymmetric arrangement of p8 and p3 proteins [5, 9, 57]. The size of spherical particles formed as a result of the structural rearrangement of M13 bacteriophage (M13 SPs), according to transmission electron microscopy, is about 39 nm and remains stable for 12 h when incubated on ice or at room temperature. However, with longer incubation, the size of M13 SPs becomes more variable and may increase [24]. With the structural modification of M13, as in the case of the TMV coat protein, a decrease in the content of α-helices in the p8 protein is observed [9]. Contrasting M13 SPs with phosphotungstic acid revealed the presence of cavity in these nanoparticles [24]. It should be noted that the TMV SPs, unlike M13 SPs, are not hollow [32]. Lowering the treatment temperature with chloroform to 2°C made it possible to observe formation of an intermediate form during the structural transition of filamentous virions into M13 SPs. The intermediate form is represented by a 250 nm long and 15 nm wide rod-shaped particle, which has a hollow central channel and broadening at one end, the increase of which subsequently leads to formation of the spherical particle [9].
The possibility of structural modification of filamentous bacteriophages under the effect of chloroform was demonstrated in the early 1980s [9]. However, only in 2007 Olsen et al. [58] highlighted the possibility of using spheroids from filamentous bacteriophages of the Inoviridae family as components of biosensors for Salmonella typhimurium. For this purpose, filamentous bacteriophages with affinity to S. typhimurium were obtained by phage display. After treatment with chloroform, these phages turned into spherical particles. The resulting SPs were subsequently used to create an affinity monolayer of the biosensor. The work described above, as well as a number of publications on TMV SPs and demonstration of their biotechnological potential, arouse interest in SPs based on filamentous bacteriophages [1, 2, 10, 58]. A group of scientists from the University of California has published a number of papers on the production and application of M13 SPs, with affinity to gold [6, 55, 59]. The intermediate forms and the M13 SPs themselves were covered with gold, and in both cases ability of the particles to act as a photothermally-induced antibacterial agent was shown using the example of Escherichia coli cells. It is interesting that the size of the formed intermediate forms (161 ± 33 nm) and SPs (60 nm) carrying the gold-binding peptide in the p8 protein differed from the SPs and intermediate forms of the native M13. It should be noted that the minor capsid protein p3 in the composition of the intermediate forms and M13 SPs retains its ability to bind to E. coli receptors. Due to this, these particles can be considered as a targeted antibacterial agent capable of inducing photothermal lysis of the target bacterial cells. The authors of this study suggest that, by modifying the p3 protein, it is possible to expand the spectrum of bacteria with which the structurally modified M13-based particles coated with gold can bind [6]. In another work, the same scientific group simultaneously modified the p8 (modified with a gold-binding peptide) and p3 (modified with a zinc-binding peptide) proteins of bacteriophage M13. Subsequent treatment with chloroform led to formation of bifunctional M13 SPs. Next, Au and ZnS were synthesized on the surface of bifunctional M13 SPs, and thus the hybrid nanostructures asymmetrically coated with gold and zinc sulfide were obtained [5]. It was shown previously that the Au/ZnS nanoparticles can be considered as regenerable catalysts for photocatalytic reactions [60].
Taking into account the above results, it can be assumed that SPs obtained by treating filamentous bacteriophages of the Inoviridae family can be used for the development of bactericidal agents, biosensors for diagnostics, and also as a scaffold for the design of hybrid nanostructures (Fig. 3).
Fig. 3.
Possible areas of application of M13 SPs.
CONCLUSIONS
Half a century after the discovery of structural rearrangement of the rod-shaped TMV virions into SPs via thermal treatment, the intensive development of biomedicine aroused interest in a more detailed study of this phenomenon. This has led to numerous publications on the properties and application of the structurally modified viral particles. Studying conditions and features of structural modification of the morphologically similar virus particles could provide important fundamental knowledge about organization, structure, and stability of virions. Most of the studies devoted to characterization and search for potential areas of application of the structurally modified plant virus particles were carried out with TMV SPs. The unique adsorption properties of TMV SPs and their effective adjuvant properties, as well as wide possibilities for surface modification of TMV SPs, make them a promising platform for various biotechnologies. They are biocompatible, safe and biodegradable, which is a clear advantage for possible use of SPs in medicine. TMV SPs have antitumor activity, could become a platform/adjuvant for developing vaccine candidates against viral and bacterial infections, and could also find application in diagnostics and microelectronics.
However, studies on the possibility of application of the structurally modified viral particles should not be limited only to TMV SPs. It is possible that similar particles obtained from the virions of other plant viruses could be used to obtain biogenic platforms with new properties that are different from the TMV SPs. The ease of isolation and purification of plant viruses, high potential for scaling up their commercial production, and safety for humans and animals are important advantages of plant virus SPs over other biopolymers and synthetic nanomaterials.
In addition to the structurally modified particles of plant viruses, attention should be paid to the works on generating SPs from filamentous bacteriophages. A number of areas of their possible application have been suggested. Thus, SPs based on M13 are of particular interest as photothermal bactericidal agents and as a scaffold/carrier for formation of complexes with various metal compounds. Efficient methods have already been reported for obtaining bacteriophage M13 in quantities necessary for practical use and with high degree of purity [61]. This also makes it possible to consider it as a promising object for future developments.
It should be noted that at present the range of practical applications of the structurally modified viral particles correlates with the approaches proposed earlier for virions and virus-like particles of plant viruses [62-64]. The studies of the native virions of plant viruses and bacteriophages played a significant role in the work with the structurally modified particles. It may be expected that, due to their unique characteristics, these particles could also find application in new areas of biotechnology and medicine.
The presented data allow, in our opinion, to make an unambiguous conclusion that the study of structurally modified viral particles is a very promising area of research. The results of such studies may be of practical importance for modern virology and biotechnology.
Acknowledgments
The study was carried out within the framework of scientific direction of the Interdisciplinary Scientific and Educational School of Moscow University “Molecular Technologies of Living Systems and Synthetic Biology”.
Abbreviations
- AltMV
alternanthera mosaic virus
- Gd
Gadolinium (III) ions
- CP
coat protein
- MRI
magnetic resonance imaging
- PVX
potato virus X
- SP
spherical structurally modified particles
- TMV
tobacco mosaic virus
Contributions
O. V. Karpova and N. A. Nikitin – concept of the review article; O. A. Kondakova, E. A. Evtushenko, O. A. Baranov, and N. A. Nikitin – collection and analysis of literature, writing the text; E. A. Evtushenko, N. A. Nikitin, and O. V. Karpova – editing the text, O. A. Baranov and N. A. Nikitin – visualization.
Funding
The work was financially supported by the Russian Science Foundation (grant no. 18-14-00044).
Ethics declarations
The authors declare no conflicts of interest in the financial or other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Karpova O., Nikitin N., Chirkov S., Trifonova E., Sheveleva A., et al. Immunogenic compositions assembled from tobacco mosaic virus-generated spherical particle platforms and foreign antigens. J. Gen. Virol. 2012;93:400–407. doi: 10.1099/vir.0.036293-0. [DOI] [PubMed] [Google Scholar]
- 2.Bruckman M. A., Hern S., Jiang K., Flask C., Yu X., et al. Tobacco mosaic virus and spheres as supramolecular high-relaxivity MRI contrast agents. J. Mat. Chem. B. 2013;1:1482–1490. doi: 10.1039/C3TB00461A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruckman M. A., Czapar A. E., VanMeter A., Randolph L. N., Steinmetz N. F. Tobacco mosaic virus-based protein nanoparticles and nanorods for chemotherapy delivery targeting breast cancer. J. Control Rel. 2016;231:103–113. doi: 10.1016/j.jconrel.2016.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trifonova E. A., Zenin V. A., Nikitin N. A., Yurkova M. S., Ryabchevskaya E. M., et al. Study of rubella candidate vaccine based on a structurally modified plant virus. Antiviral Res. 2017;144:27–33. doi: 10.1016/j.antiviral.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Plank J. M., Alibay Z., Ngo-Duc T. T., Lai M., Mayes E. A. S., et al. Bifunctional M13 bacteriophage nanospheroids for the synthesis of hybrid noncentrosymmetric nanoparticles. ACS Appl. Nano Mater. 2020;3:10668–10677. doi: 10.1021/acsanm.0c01876. [DOI] [Google Scholar]
- 6.Ngo-Duc T. T., Alibay Z., Plank J. M., Cheeney J. E., Haberer E. D. Gold-decorated M13 I-forms and S-forms for targeted photothermal lysis of bacteria. ACS Appl. Mater. Interfaces. 2020;12:126–134. doi: 10.1021/acsami.9b15682. [DOI] [PubMed] [Google Scholar]
- 7.Kovalenko A. O., Ryabchevskaya E. M., Evtushenko E. A., Manukhova T. I., Kondakova O. A., et al. Vaccine candidate against COVID-19 based on structurally modified plant virus as an adjuvant. Front. Microbiol. 2022;13:1–15. doi: 10.3389/fmicb.2022.845316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryabchevskaya E. M., Granovskiy D. L., Evtushenko E. A., Ivanov P. A., Kondakova O. A., et al. Designing stable bacillus anthracis antigens with a view to recombinant anthrax vaccine development. Pharmaceutics. 2022;14:806. doi: 10.3390/pharmaceutics14040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manning M., Chrysogelos S., Griffith J. Mechanism of coliphage M13 contraction: intermediate structures trapped at low temperatures. J. Virol. 1981;40:912–919. doi: 10.1128/JVI.40.3.912-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atabekov J., Nikitin N., Arkhipenko M., Chirkov S., Karpova O. Thermal transition of native tobacco mosaic virus and RNA-free viral proteins into spherical nanoparticles. J. Gen. Virol. 2011;92:453–456. doi: 10.1099/vir.0.024356-0. [DOI] [PubMed] [Google Scholar]
- 11.Nikitin N., Ksenofontov A., Trifonova E., Arkhipenko M., Petrova E., et al. Thermal conversion of filamentous potato virus X into spherical particles with different properties from virions. FEBS Lett. 2016;590:1543–1551. doi: 10.1002/1873-3468.12184. [DOI] [PubMed] [Google Scholar]
- 12.Manukhova T. I., Evtushenko E. A., Ksenofontov A. L., Arutyunyan A. M., Kovalenko A. O., et al. Thermal remodelling of Alternanthera mosaic virus virions and virus-like particles into protein spherical particles. PLoS One. 2021;16:e0255378. doi: 10.1371/journal.pone.0255378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart R. G. Morphological changes accompanying thermal denaturation of tobacco mosaic virus. Biochim. Biophys. Acta. 1956;20:388–389. doi: 10.1016/0006-3002(56)90301-8. [DOI] [PubMed] [Google Scholar]
- 14.Bruckman M. A., Yu X., Steinmetz N. F. Engineering Gd-loaded nanoparticles to enhance MRI sensitivity via T(1) shortening. Nanotechnology. 2013;24:462001. doi: 10.1088/0957-4484/24/46/462001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruckman M. A., Randolph L. N., VanMeter A., Hern S., Shoffstall A. J., Taurog R. E., et al. Biodistribution, pharmacokinetics, and blood compatibility of native and PEGylated tobacco mosaic virus nano-rods and -spheres in mice. Virology. 2014;449:163–173. doi: 10.1016/j.virol.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruckman M. A., Steinmetz N. F. Chemical modification of the inner and outer surfaces of Tobacco Mosaic Virus (TMV) Methods Mol. Biol. 2014;1108:173–185. doi: 10.1007/978-1-62703-751-8_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruckman M. A., Jiang K., Simpson E. J., Randolph L. N., Luyt L. G., et al. Dual-modal magnetic resonance and fluorescence imaging of atherosclerotic plaques in vivo using VCAM-1 targeted tobacco mosaic virus. Nano Lett. 2014;14:1551–1558. doi: 10.1021/nl404816m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruckman M. A., Randolph L. N., Gulati N. M., Stewart P. L., et al. Silica-coated Gd(DOTA)-loaded protein nanoparticles enable magnetic resonance imaging of macrophages. J. Mater. Chem. B. 2015;3:7503–7510. doi: 10.1039/C5TB01014D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruckman M. A., VanMeter A., Steinmetz N. F. Nanomanufacturing of Tobacco Mosaic virus-based spherical biomaterials using a continuous flow method. ACS Biomater. Sci. Eng. 2015;1:13–18. doi: 10.1021/ab500059s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y., Zhao X., Hu J., Lin Y., Wang Q. Preparation and characterization of functional Tobacco Mosaic virus spherical nanoparticles. Chinese J. Appl. Chem. 2017;34:379–384. doi: 10.11944/j.issn.1000-0518.2017.04.160302. [DOI] [Google Scholar]
- 21.Rodríguez-Galvan A., Martinez-Loran E., Naveja J. J., Ornelas-Soto N., Basiuk V., et al. In situ metallization of thermally-treated tobacco mosaic virus using silver nanoparticles. J. Nanosci. Nanotechnol. 2017;17:4740–4747. doi: 10.1166/jnn.2017.13714. [DOI] [Google Scholar]
- 22.Murray A. A., Wang C., Fiering S., Steinmetz N. F. In situ vaccination with Cowpea vs Tobacco Mosaic Virus against melanoma. Mol. Pharm. 2018;15:3700–3716. doi: 10.1021/acs.molpharmaceut.8b00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trifonova E. A., Nikitin N. A., Arkhipenko M. V., Donchenko E. K., Atabekov J. G., et al. Comparative study of thermal remodeling of viruses with icosahedral and helical symmetry. Moscow Univ. Biol. Sci. Bull. 2017;4:179–183. doi: 10.3103/S0096392517040125. [DOI] [Google Scholar]
- 24.Griffith J., Manning M., Dunn K. Filamentous bacteriophage contract into hollow spherical particles upon exposure to a chloroform-water interface. Cell. 1981;23:747–753. doi: 10.1016/0092-8674(81)90438-4. [DOI] [PubMed] [Google Scholar]
- 25.Butler P. J. Self-assembly of tobacco mosaic virus: The role of an intermediate aggregate generating both specificity and speed. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:537–550. doi: 10.1098/rstb.1999.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lomonossoff G. P., Wege C. TMV particles: the journey from Fundamental studies to bionanotechnology applications. Adv. Virus. Res. 2018;102:149–176. doi: 10.1016/bs.aivir.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikitin N. A., Malinin A. S., Rakhnyanskaya A. A., Trifonova E. A., Karpova O. V., et al. Use of a polycation spacer for noncovalent immobilization of albumin on thermally modified virus particles. Polymer Sci. Ser. A. 2011;53:1026–1031. doi: 10.1134/S0965545X11110083. [DOI] [Google Scholar]
- 28.Dobrov E. N., Nikitin N. A., Trifonova E. A., Parshina E. Y., Makarov V. V., et al. β-structure of the coat protein subunits in spherical particles generated by tobacco mosaic virus thermal denaturation. J. Biomol. Struct. Dyn. 2014;32:701–708. doi: 10.1080/07391102.2013.788983. [DOI] [PubMed] [Google Scholar]
- 29.Nikitin N., Trifonova E., Karpova O., Atabekov J. Examination of biologically active nanocomplexes by nanoparticle tracking analysis. Microsc. Microanal. 2013;19:808–813. doi: 10.1017/S1431927613000597. [DOI] [PubMed] [Google Scholar]
- 30.Nikitin N. A., Malinin A. S., Trifonova E. A., Rakhnyanskaya A. A., Yaroslavov A. A., et al. Proteins immobilization on the surface of modified plant viral particles coated with hydrophobic polycations. J. Biomater. Sci. Polym. Ed. 2014;25:1743–1754. doi: 10.1080/09205063.2014.946877. [DOI] [PubMed] [Google Scholar]
- 31.Ksenofontov A. L., Fedorova N. V., Badun G. A., Serebryakova M. V., Nikitin N. A., et al. Surface characterization of the thermal remodeling helical plant virus. PLoS One. 2019;14:e0216905. doi: 10.1371/journal.pone.0216905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trifonova E. A., Nikitin N. A., Kirpichnikov M. P., Karpova O. V., Atabekov J. G. Obtaining and characterization of spherical particles – new biogenic platforms. Moscow Univ. Biol. Sci. Bull. 2015;70:194–197. doi: 10.3103/S0096392515040094. [DOI] [Google Scholar]
- 33.Trifonova E. A., Nikitin N. A., Gmyl A. P., Lazareva E. A., Karpova O. V., et al. Complexes assembled from TMV-derived spherical particles and entire virions of heterogeneous nature. J. Biomol. Struct. Dyn. 2014;32:1193–1201. doi: 10.1080/07391102.2013.816868. [DOI] [PubMed] [Google Scholar]
- 34.Evtushenko E. A., Ryabchevskaya E. M., Nikitin N. A., Atabekov J. G., Karpova O. V. Plant virus particles with various shapes as potential adjuvants. Sci. Rep. 2020;10:10365. doi: 10.1038/s41598-020-67023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryabchevskaya E. M., Evtushenko E. A., Granovskiy D. L., Ivanov P. A., Atabekov J. G., et al. Two approaches for the stabilization of Bacillus anthracis recombinant protective antigen. Hum. Vaccine Immunother. 2021;17:560–565. doi: 10.1080/21645515.2020.1772632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips B., Van Rompay K., Rodriguez-Nieves J., Lorin C., Koutsoukos M., et al. Adjuvant-dependent enhancement of HIV Env-specific antibody responses in infant rhesus macaques. J. Virol. 2018;92:e01051-18. doi: 10.1128/JVI.01051-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z. B., Xu J. Better adjuvants for better vaccines: Progress in adjuvant delivery systems, modifications, and adjuvant-antigen codelivery. Vaccines. 2020;8:128. doi: 10.3390/vaccines8010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurashova S. S., Ishmukhametov A. A., Dzagurova T. K., Egorova M. S., Balovneva M. V., et al. Various adjuvants effect on immunogenicity of Puumala Virus Vaccine. Front. Cell. Infect. Microbiol. 2020;10:545371. doi: 10.3389/fcimb.2020.545371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danielsson R., Eriksson H. Aluminium adjuvants in vaccines – a way to modulate the immune response. Semin. Cell Dev. Biolol. 2021;115:3–9. doi: 10.1016/j.semcdb.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Ryabchevskaya E. M., Evtushenko E. A., Arkhipenko M. V., Manukhova T. I., Donchenko E. K., et al. Novel approach for designing rotavirus vaccine candidate based on two plant viruses. Agric. Biol. 2020;55:1004–1017. doi: 10.15389/agrobiology.2020.5.1004eng. [DOI] [Google Scholar]
- 41.Kondakova O. A., Trifonova E. A., Arkhipenko M. V., Nikitin N. A., Atabekov J. G., et al. Development of avian influenza vaccine on the basis of structurally modified plant virus. Agric. Biol. 2017;52:731–738. doi: 10.15389/agrobiology.2017.4.731eng. [DOI] [Google Scholar]
- 42.Nikitin N. A., Matveeva I. N., Trifonova E. A., Puhova N. M., Samuylenko A. Y., et al. Spherical particles derived from TMV virions enhance the protective properties of the rabies vaccine. Data Brief. 2018;21:742–745. doi: 10.1016/j.dib.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondakova O. A., Nikitin N. A., Evtushenko E. A., Ryabchevskaya E. M., Atabekov J. G., et al. Vaccines against anthrax based on recombinant protective antigen: Problems and solutions. Expert Rev. Vaccines. 2019;18:813–828. doi: 10.1080/14760584.2019.1643242. [DOI] [PubMed] [Google Scholar]
- 44.Kondakova O. A., Nikitin N. A., Evtushenko E. A., Granovskiy D. L., Atabekov J. G., et al. Anthrax: Life cycle, mechanisms of pathogenesis and prospects in the development of veterinary vaccines. Agric. Biol. 2021;56:415–433. doi: 10.15389/agrobiology.2021.3.415eng. [DOI] [Google Scholar]
- 45.Lizotte P. H., Wen A. M., Sheen M. R., Fields J., Rojanasopondist P., et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016;11:295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lebel M. È., Chartrand K., Tarrab E., Savard P., Leclerc D., et al. Potentiating cancer immunotherapy using papaya mosaic virus-derived nanoparticles. Nano Lett. 2016;16:1826–1832. doi: 10.1021/acs.nanolett.5b04877. [DOI] [PubMed] [Google Scholar]
- 47.Lee K. L., Murray A. A., Le D., Sheen M. R., Shukla S., et al. Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Lett. 2017;17:4019–4028. doi: 10.1021/acs.nanolett.7b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L., Zhao X., Lin Y., Huang Y., Wang Q. A supramolecular strategy to assemble multifunctional viral nanoparticles. Chem. Commun. 2013;49:9678–9680. doi: 10.1039/c3cc45559a. [DOI] [PubMed] [Google Scholar]
- 49.Shah, S., Shah, S., and Heddle, J. (2012) Wild-type tobacco mosaic virus (TMV) as a scaffold for gold nanoparticle fabrication, Proc. 6th Int. Conf. Gold Sci. Technbiol. Its Appl., 2P-072, Tokyo.
- 50.Ksenofontov A. L., Parshina E. Y., Fedorova N. V., Arutyunyan A. M., Rumvolt R., et al. Heating-induced transition of Potyvirus Potato Virus A coat protein into β-structure. J. Biomol. Struct. Dyn. 2016;34:250–258. doi: 10.1080/07391102.2015.1022604. [DOI] [PubMed] [Google Scholar]
- 51.Nikitin N. A., Trifonova E. A., Karpova O. V., Atabekov J. G. Biosafety of plant viruses for human and animals. Moscow Univ. Biol. Sci. Bull. 2016;71:128–134. doi: 10.3103/S0096392516030081. [DOI] [Google Scholar]
- 52.Nkanga C. I., Steinmetz N. F. The pharmacology of plant virus nanoparticles. Virology. 2021;556:39–61. doi: 10.1016/j.virol.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikitin N. A., Zenin V. A., Trifonova E. A., Ryabchevskaya E. M., Kondakova O. A., et al. Assessment of structurally modified plant virus as a novel adjuvant in toxicity studies. Regul. Toxicol. Pharmacol. 2018;97:127–133. doi: 10.1016/j.yrtph.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Nikitin N. A., Zenin V. A., Trifonova E. A., Ryabchevskaya E. M., Yurkova M. S., et al. Data in support of toxicity studies of structurally modified plant virus to safety assessment. Data Brief. 2018;21:1504–1507. doi: 10.1016/j.dib.2018.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ngo-Duc T. T., Zaman M. S., Moon C. H., Haberer E. D. Morphology manipulation of M13 bacteriophage template for nanostructure assembly. Proc. SPIE. 2014;9171:91710X. doi: 10.1117/12.2062417. [DOI] [Google Scholar]
- 56.Rakonjac J., Russel M., Khanum S., Brooke S. J., Rajič M. Filamentous phage: structure and biology. Adv. Exp. Med. Biol. 2017;1053:1–20. doi: 10.1007/978-3-319-72077-7_1. [DOI] [PubMed] [Google Scholar]
- 57.Lopez J., Webster R. E. Minor coat protein composition and location of the A protein in bacteriophage f1 spheroids and I-forms. J. Virol. 1982;42:1099–1107. doi: 10.1128/JVI.42.3.1099-1107.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olsen E. V., Sykora J., Sorokulova I., Neely W., Petrenko V., et al. Phage fusion proteins as bioselective receptors for piezoelectric sensors. ECS Transact. 2007;2:9–25. doi: 10.1149/1.2408983. [DOI] [Google Scholar]
- 59.Ngo-Duc T. T., Plank J. M., Chen G., Harrison R. E., Morikis D., et al. M13 bacteriophage spheroids as scaffolds for directed synthesis of spiky gold nanostructures. Nanoscale. 2018;10:13055–13063. doi: 10.1039/C8NR03229G. [DOI] [PubMed] [Google Scholar]
- 60.Madkour M., Sagheer F. A. Au/ZnS and Ag/ZnS nanoheterostructures as regenerated nanophotocatalysts for photocatalytic degradation of organic dyes. Opt. Mater. Express. 2017;7:158–169. doi: 10.1364/OME.7.000158. [DOI] [Google Scholar]
- 61.Passaretti P., Khan I., Dafforn T. R., Oppenheimer P. G. Improvements in the production of purified M13 bacteriophage bio-nanoparticle. Sci. Rep. 2020;10:18538. doi: 10.1038/s41598-020-75205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shukla S., Hu H., Cai H., Chan S. K., Boone C. E., et al. Plant viruses and bacteriophage-based reagents for diagnosis and therapy. Annu. Rev. Virol. 2020;7:559–587. doi: 10.1146/annurev-virology-010720-052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung Y. H., Cai H., Steinmetz N. F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Del. Rev. 2020;156:214–235. doi: 10.1016/j.addr.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balke I., Zeltins A. Use of plant viruses and virus-like particles for the creation of novel vaccines. Adv. Drug Del. Rev. 2019;145:119–129. doi: 10.1016/j.addr.2018.08.007. [DOI] [PubMed] [Google Scholar]