Abstract
Exposure to low pH and organic acids in the bovine gastrointestinal tract may result in the induced acid resistance of Escherichia coli O157:H7 and other pathogens that may subsequently contaminate beef carcasses. The effect of acid adaptation of E. coli O157:H7 on the ability of acetic acid spray washing to reduce populations of this organism on beef carcass tissue was examined. Stationary-phase acid resistance and the ability to induce acid tolerance were determined for a collection of E. coli O157:H7 strains by testing the survival of acid-adapted and unadapted cells in HCl-acidified tryptic soy broth (pH 2.5). Three E. coli O157:H7 strains that were categorized as acid resistant (ATCC 43895) or acid sensitive (ATCC 43890) or that demonstrated inducible acid tolerance (ATCC 43889) were used in spray wash studies. Prerigor beef carcass surface tissue was inoculated with bovine feces containing either acid-adapted or unadapted E. coli O157:H7. The beef tissue was subjected to spray washing treatments with water or 2% acetic acid or left untreated. For strains ATCC 43895 and 43889, larger populations of acid-adapted cells than of unadapted cells remained on beef tissue following 2% acetic acid treatments and these differences remained throughout 14 days of 4°C storage. For both strains, numbers of acid-adapted cells remaining on tissue following 2% acetic acid treatments were similar to numbers of both acid-adapted and unadapted cells remaining on tissue following water treatments. For strain ATCC 43890, there was no difference between populations of acid-adapted and unadapted cells remaining on beef tissue immediately following 2% acetic acid treatments. These data indicate that adaptation to acidic conditions by E. coli O157:H7 can negatively influence the effectiveness of 2% acetic acid spray washing in reducing the numbers of this organism on carcasses.
The involvement of E. coli O157:H7 in food-borne illness outbreaks associated with the consumption of acidic foods such as apple cider, fermented sausage, yogurt, and mayonnaise (3, 9, 34) has drawn attention to the acid resistance properties of this pathogen, and many subsequent studies have demonstrated that this bacterium can survive in a variety of acidic foods (8, 25, 32, 33, 37, 39). In addition, other studies have shown that adaptation to acidic conditions can further improve the survival of E. coli O157:H7 in foods that are preserved by low pH and acids (30, 38). Leyer et al. (30) found that acid-adapted E. coli O157:H7 survived better than unadapted cells during sausage fermentation and exhibited enhanced survival in dry salami and apple cider. Tsai and Ingham (38) reported that adaptation to acid enhanced survival of E. coli O157:H7 in ketchup but not in mustard or pickle relish. In addition to promoting survival in low-pH foods, the development of acid resistance by E. coli O157:H7 may provide cross-protection against heat, salt, and irradiation preservation of foods (7, 11, 23, 36). Furthermore, several works have indicated that acid tolerance of E. coli O157:H7 is enhanced or sustained longer upon refrigerated storage (10, 12, 22, 31, 33). Finally, it is thought that acid resistance and/or induction of acid tolerance may better enable pathogens to survive gastrointestinal acidity and ultimately cause disease and that it may enhance virulence (1, 15, 26, 35).
Clearly, acid resistance and the development of acid tolerance by food-borne pathogenic bacteria may be significant at several points along the farm-to-table continuum of food production. It is important that we understand how previous environment and processing conditions can affect the acid tolerance status of food-borne E. coli O157:H7 in order to devise strategies for better control of the occurrence, growth, or survival of this organism in foods. Cattle are a reservoir of E. coli O157:H7, and raw or undercooked beef and milk, as well as food products likely contaminated with bovine feces containing this organism, have been incriminated in many food-borne illness episodes (21). Solutions of lactic and acetic acid are commonly used by the beef slaughter industry as antimicrobial spray wash interventions to reduce the microbial load on freshly slaughtered beef carcasses. Because of the potential for acid adaptation in the bovine gastrointestinal tract due to exposure to low pH and organic acids (10, 15, 29), and because bovine feces are a common source of bacterial contamination of carcasses, our objective was to determine if acid adaptation can affect the ability of 2% (vol/vol) acetic acid (2% AA) spray washes to reduce populations of E. coli O157:H7 on prerigor beef carcass surface tissue (BCT). Initial experiments involved the assessment of acid resistance characteristics of a selection of E. coli O157:H7 strains available for these experiments.
MATERIALS AND METHODS
Microorganisms and inoculum preparation.
The E. coli O157:H7 strains used in this study are listed in Table 1. Acid-adapted (A) and unadapted (NA, not adapted) stationary-phase cells of each strain were prepared by cultivation in Trypticase soy broth with 1% glucose (TSB+G; BBL, Becton Dickinson Microbiology Systems, Cockeysville, Md.) and without glucose (TSB−G), respectively, according to the method described by Buchanan and Edelson (6). To prepare inocula for both acid challenge and spray wash experiments, 0.1-ml volumes from frozen (−20°C) 25% glycerol stock cultures were inoculated into 10 ml of TSB+G and TSB−G and incubated for 18 h at 37°C prior to use.
TABLE 1.
E. coli O157:H7 strains evaluated in this study
| Strain | Source | Description of isolate (reference) | Acid resistance categorya |
|---|---|---|---|
| ATCC 43895 | ATCCb | Ground beef isolate | Resistant |
| ATCC 43894 | ATCC | Human feces isolate | Resistant |
| ATCC 35150 | ATCC | Human feces isolate | Resistant |
| ATCC 43889 | ATCC | Human feces isolate | Adaptable |
| ATCC 43890 | ATCC | Human feces isolate | Sensitive |
| ATCC 43888 | ATCC | Human feces isolate | Sensitive |
| B6914-MS1 | Centers for Disease Control and Prevention, N. Strockbine | Human feces isolate | Sensitive |
| MARCS-1 | Roman L. Hruska U.S. Meat Animal Research Center culture collection | Streptomycin (250 μg/ml)-resistant B6914-MS1 isolate (19) | Sensitive |
| 2886-75 | E. coli Reference Center | Human feces isolate | Resistant |
| 86-24 | National Animal Disease Center, E. Nystrom | Human feces isolate (27) | Resistant |
| NADC 5570 | National Animal Disease Center, E. Nystrom | Streptomycin (100 μg/ml)-resistant E. coli O157:H7 86-24 isolate (27) | Resistant |
| NADC 4477 | National Animal Disease Center, T. Casey | Nalidixic acid (50 μg/ml)-resistant E. coli O157:H7 86-24 isolate (27) | Adaptable |
Based on survival and injury patterns of cells grown in TSB+G and TSB−G, following exposure to pH 2.5 for 6 h.
ATCC, American Type Culture Collection.
Determination of acid resistance characteristics of E. coli O157:H7 strains.
Initial screening of the E. coli O157:H7 strains to assess their stationary-phase acid resistance and acid adaptation characteristics was done by examining the survival of A and NA cells inoculated into pH-adjusted broth medium (6). Brain heart infusion broth was adjusted to pH 2.5 with concentrated HCl (BHI-2.5; Difco Laboratories, Detroit, Mich.). The BHI-2.5 was dispensed in 10-ml volumes to test tubes and sterilized by autoclaving, and the test tubes were preequilibrated to 37°C prior to experiments.
For the experiments, BHI-2.5 tubes were inoculated with a 0.1-ml volume of an 18-h culture of either A or NA E. coli O157:H7 cells. Each tube was sampled immediately following inoculation and mixing by vortexing and then returned to 37°C to incubate statically for 6 h, when the tube was sampled again. At each sampling time, the BHI-2.5 samples were diluted if necessary in buffered peptone water (BPW) and spiral-plated in duplicate on both tryptic soy agar (TSA) and MacConkey sorbitol agar (SMAC) plates, using a model D spiral plater (Spiral Systems Instruments, Bethesda, Md.). All plates were incubated at 37°C for 24 h prior to enumeration. Using spiral-plating procedures, the minimum detection level was 1.30 log10 CFU/ml. Experiments were duplicated on separate days for A and NA cells of each E. coli O157:H7 strain.
Spray wash experiments.
On each day of a spray wash experiment, fresh bovine feces were collected from three different cows on a corn silage ration. For each individual A and NA E. coli O157:H7 strain, 75-g samples of the three fecal specimens were pooled in a sterile beaker and mixed well with 75 ml of sterile 0.85% NaCl. To reduce interference by indigenous E. coli when we enumerated E. coli O157:H7, fecal slurries were autoclaved at 121°C for 2 min and rapidly cooled on ice, with occasional stirring using a sterile tongue depressor. An additional 35 ml of 0.85% NaCl was added to the cooled feces, and the mixtures were held at room temperature until beef tissue inoculation. Immediately prior to tissue inoculation, 2 ml of an 18-h A or NA E. coli O157:H7 culture was added to the fecal slurry and mixed well. Prepared in this fashion, the fecal slurries contained 6 log10 CFU of E. coli O157:H7 per g and yielded ca. 5 log10 CFU of E. coli O157:H7 per cm2 when inoculated with a paintbrush onto the external surface of a 15- by 20-cm piece of lean BCT (17).
Lean BCT was obtained from the cutaneous trunci of prerigor carcasses immediately after slaughter at a local cow and bull processing facility. The BCT was placed in plastic bags in an insulated container to minimize cooling and transported to the laboratory at the Roman L. Hruska U.S. Meat Animal Research Center for immediate use in experiments. The BCT was aseptically trimmed to 15- by 20-cm pieces. The entire external surface of each piece was inoculated with the appropriate fecal slurry, prepared as described above, with a sterile 5.1-cm-wide paintbrush. The inoculated tissues were allowed to stand for 15 min prior to spray washing; inoculated untreated control tissues were allowed to stand for 15 min prior to sampling for enumeration.
An insertable pod of a commercial carcass washer, modified for use in a biological safety hood, was used to apply the spray washing treatments to inoculated BCT (19). Individual BCT samples were mounted on the surface of a stainless steel plate and, as appropriate to treatment, were spray washed with 25 ± 2°C sterile tap water (W) or 25 ± 2°C 2% AA prepared with W. The spray washes were delivered at 125 lb/in2 for 15 s, with a spray nozzle oscillation rate of 60 cycles/min.
Immediately following the spray wash treatments, BCT was placed on sterile trays. A 5- by 5-cm sample was aseptically excised from each tissue piece and placed in sterile side-filter sample bags (Spiral Biotech, Bethesda, Md.) for bacterial enumeration. The BCT surface pH was measured using a flat-surface combination probe (Corning, Inc., Corning, N.Y.). Each tray was then covered with an inverted sterile tray and stored at 4°C for 48 h. At 48 h, BCT was again sampled for enumeration and pH determination. At least two additional 5- by 5-cm areas were excised at this time, placed in vacuum packaging bags (3.2-mil nylon-copolymer bags with an oxygen transmission rate at 23°C of 52 cm3/m2; Hollymatic, Inc., Countryside, Ill.), and vacuum sealed (model LV10G; Hollymatic, Inc.). The vacuum-packaged BCT was stored at 4°C and removed for sampling at 7 and 14 days.
For E. coli O157:H7 enumeration, the excised 5- by 5-cm BCT samples were pummeled for 2 min with 25 ml of BPW containing 0.1% (vol/vol) Tween 20 using a Stomacher lab blender (model 400; Tekmar, Inc., Cincinnati, Ohio). Following pummeling, the filtered samples were serially diluted in BPW as necessary and spiral plated or spread plated in duplicate onto SMAC plates containing 0.05 mg of cefixime per liter and 2.5 mg of potassium tellurite (CT-SMAC) per liter. CT-SMAC plates were incubated for 24 h at 37°C and enumerated.
Statistical analyses.
Six replications of each spray wash experiment were done, with three replications being done on each of two separate days for each A and NA E. coli O157:H7 strain. Numbers of bacteria from duplicate plates of spray wash experiments were averaged and converted to log10 CFU per square centimeter. Least squares means of bacterial populations were analyzed as a completely randomized factorial design (six organisms [three A and three NA] by three treatments by five sampling periods) using the general linear model procedure of SAS (version 6.12; SAS Institute Inc., Cary, N.C.). Statistical significance is defined as a P of ≤0.01 unless otherwise noted.
RESULTS AND DISCUSSION
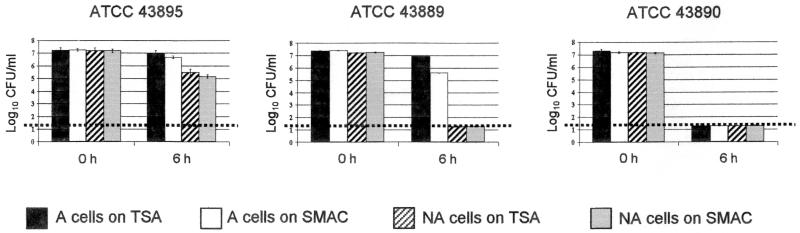
To identify strains for use in spray wash experiments, stationary-phase acid resistance and the ability to adapt to acidic conditions were determined for a selection of E. coli O157:H7 strains utilizing the method described by Buchanan and Edelson (6). After 18 h of growth in TSB−G, the final pHs of the various cultures ranged from 6.7 to 7.2. Alternatively, after 18 h of growth in TSB+G, glucose fermentation by the cultures resulted in final pHs ranging from 4.3 to 4.8, which is within the pH range reported to induce acid tolerance in E. coli (5, 23, 30, 31). Final pHs of the TSB+G and TSB−G cultures were similar to those previously reported for E. coli O157:H7 in the same media (6, 36). As other researchers have observed, there is a range of responses to acidic conditions among different strains of this organism (1, 6, 14, 33). In addition, comparison of levels of recovery of the acid-challenged cells on both nonselective TSA and the selective SMAC was useful for assessing the degrees of both the acid resistance and the acid injury of the strains. Based on these survival and injury patterns following exposure to pH 2.5 for 6 h, and for our particular objectives, the E. coli O157:H7 strains tested were grouped into three broad categories as either acid resistant, acid adaptable, or acid sensitive (Table 1). Initial numbers and survival of A and NA E. coli O157:H7 cells following a 6-h exposure to pH 2.5 are shown in Fig. 1 for strains ATCC 43895, ATCC 43889, and ATCC 43890, which were categorized as acid resistant, adaptable, and sensitive, respectively. These results are representative of those seen with the other E. coli O157:H7 strains in the same acid resistance categories (Table 1). For all acid-resistant strains, adaptation to acidic conditions did improve survival at pH 2.5; however, high numbers of NA cells remained viable after the 6-h exposure. Members of this acid-resistant group may be similar to E. coli strains that have been described as exhibiting pH-independent acid tolerance or being constitutively acid tolerant (6). For strains categorized as acid adaptable, growth in TSB+G and the resultant adaptation to acidic conditions were important to the survival of these strains upon exposure to pH 2.5. For adaptable strains, A cell counts recovered on TSA after the 6-h exposure approximated initial counts, although recovery on SMAC indicated ca. 90% cell injury. Alternatively, NA cell counts of the adaptable strains approached or were below detectable levels on both TSA and SMAC following the exposure to pH 2.5. For strains in the acid-sensitive group, adaptation to acidic conditions did not enhance cell survival to the pH 2.5 acid challenge. Following the 6-h exposure, both A and NA cells of acid-sensitive strains typically were below detectable levels on both TSA and SMAC.
FIG. 1.
Survival of A and NA E. coli O157:H7 cells initially (0 h) and after 6 h of exposure in BHI-2.5, as enumerated on TSA and SMAC plates. E. coli O157:H7 strains ATCC 43895, ATCC 43889, and ATCC 43890 were categorized as acid resistant, acid adaptable, and acid sensitive, respectively. The horizontal dotted line at 1.30 log10 CFU/ml denotes the minimum detection level. Error bars indicate standard deviations.
E. coli O157:H7 strains ATCC 43895, ATCC 43889, and ATCC 43890, representing each of the three acid resistance categories, were used to examine the effects of acid adaptation on the ability of acetic acid spray washes to reduce levels of E. coli O157:H7 from beef carcasses. The initial levels of A and NA cells of all three strains on BCT prior to spray washing were the same, at ca. 5 log10 CFU/cm2 (P ≤ 0.01). The pH values of BCT surfaces following treatments and during 4°C storage are shown in Table 2.
TABLE 2.
Average pHs of beef tissue surfaces following the various treatments and during 14 days of storage at 4°C
| Sample day | pH after:
|
||
|---|---|---|---|
| No treatment | W treatment | 2% AA treatment | |
| 0a | 6.84 | 6.75 | 4.61 |
| 2 | 6.35 | 6.13 | 5.35 |
| 7 | 6.03 | 5.87 | 5.38 |
| 14 | 5.98 | 5.86 | 5.36 |
Day 0 pH of beef tissue surfaces measured shortly after wash treatments (<0.5 h).
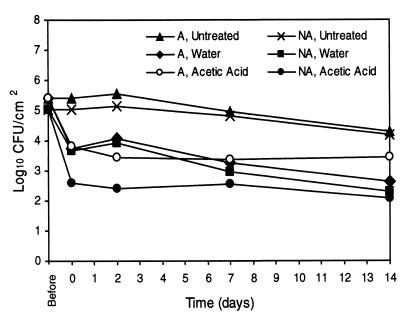
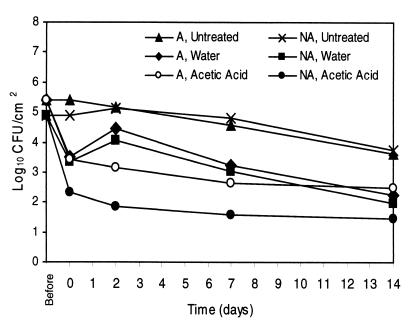
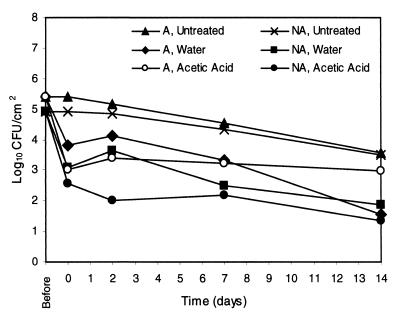
Results of spray washing experiments with A and NA E. coli O157:H7 ATCC 43895 cells are shown in Fig. 2. The acid adaptation of the resistant strain ATCC 43895 was demonstrated to affect the ability of 2% AA spray washes to reduce populations of this strain from BCT. Significantly larger populations of ATCC 43895 A cells than NA cells remained on BCT immediately following 2% AA treatments, and these differences remained throughout the 14 days of refrigerated storage. Instead, populations of A cells immediately following 2% AA washes were the same as those of both A and NA cells remaining on BCT following W washes. Similar results were seen with A and NA cells of the adaptable E. coli O157:H7 strain ATCC 43889 (Fig. 3). Higher levels of A cells than of NA cells remained on the BCT following 2% AA treatments. As seen with strain ATCC 43895, remaining A cell populations of strain ATCC 43889 immediately after 2% AA treatments were similar to those of A and NA cells populations after W treatments. Sizes of populations of A and NA cells on 2% AA-treated tissues were different through the 14 days of storage. Figure 4 shows the effects of 2% AA and W washes on populations of A and NA E. coli O157:H7 ATCC 43890 cells on BCT. Unlike with the resistant and adaptable strains, no differences were seen between levels of A and NA cells of this acid-sensitive strain immediately following 2% AA spray wash treatments. However, during the course of the refrigerated storage, NA cell populations declined, and cell levels of A and NA cells on 2% AA-treated BCT were different at 2, 7, and 14 days.
FIG. 2.
Initial reductions of cell numbers and growth or survival of A and NA E. coli O157:H7 ATCC 43895 (acid-resistant strain) cells on lean BCT stored at 4°C following spray washing treatment with W or 2% AA or after no treatment (n = 6). The standard error of the least squares means was equal to 0.14.
FIG. 3.
Initial reductions of cell numbers and growth or survival of A and NA E. coli O157:H7 ATCC 43889 (acid-adaptable strain) cells on lean BCT stored at 4°C following spray washing treatment with W or 2% AA or following no treatment (n = 6). The standard error of the least squares means was equal to 0.14.
FIG. 4.
Initial reductions of cell numbers and growth or survival of A and NA E. coli O157:H7 ATCC 43890 (acid-sensitive strain) cells on lean BCT stored at 4°C following spray washing treatment with W or 2% AA or following no treatment (n = 6). The standard error of the least squares means was equal to 0.14.
For both A and NA cells of all strains examined, there was a trend that indicated a gradual decline in E. coli O157:H7 populations on W-treated and untreated BCT over the 14 days of refrigerated storage (Fig. 2 to 4). This same trend was not as apparent for populations on 2% AA-treated BCT, which, in comparison, remained constant during the 4°C storage. There are at least two possible reasons for this observation. One possibility is that there were increasing populations of a competing microflora present on W-treated and untreated BCT, compared to those on 2% AA-treated BCT, resulting in a depression of E. coli O157:H7 populations on these samples during storage. General bacterial microflora populations were not monitored in this study; however, previous studies have demonstrated that while both organic acid and water spray washes reduce populations of mesophilic aerobic bacteria, organic acids can contribute the added residual effect of slowing or suppressing the growth of this group of bacteria on beef tissue during storage, compared to their more rapid growth on untreated or water-washed beef (18, 20). A second reason for this observation may be the cross-protective relationship between E. coli O157:H7 acid adaptation and cold temperature. Conner and Kotrola (13) reported that the presence of organic acids, including acetic, citric, and lactic acids, in broth medium held at 4°C enhanced the survival of E. coli O157:H7, compared to its survival in unacidified control medium held at the same temperature. Likewise, other works have demonstrated that once induced, the acid tolerance of this pathogen is enhanced or maintained longer when the organism is held at colder temperatures (10, 12, 22, 31, 33). To determine if A cells of E. coli O157:H7 sustained acid tolerance on BCT during refrigerated storage, an additional experiment was incorporated during the course of spray wash experiments with the acid-adaptable E. coli O157:H7 strain ATCC 43889. When BCTs were sampled for enumeration at 2, 7, and 14 days, 200 μl of the filtered samples, following pummeling, were placed into 3 ml of BHI adjusted to pHs 2.5, 3.5, and 4.0 with HCl (n = 3). The BHI media were incubated for 1 h at 37°C, surviving cells were enumerated on CT-SMAC, and these cell numbers were compared to the initial counts. Because of the low numbers of cells available for this assay and the small volume of inoculum that could be applied without changing the pH of the BHI, the surviving cell populations could only be estimated (data not shown). However, these limited observations suggested that at 2, 7, and 14 days, A cells of ATCC 43889 did not maintain the degree of acid resistance that they had when they were initially inoculated onto the meat, following growth in TSB+G. Counts of surviving cells after 1 h of exposure to pHs 3.5 and 4.0 suggested that there were possible differences in survival between A and NA cells. Further studies are planned to confirm these observations.
Log reductions in viable cell counts were compared in order to ascertain if differences in degrees of acid resistance between the three E. coli O157:H7 strains affected immediate reductions on BCT by 2% AA spray washes. For NA cells of all three strains, there were no differences in log reductions due to 2% AA spray washes. However, when cells were adapted to acidic conditions, there was a significant difference in log reductions by 2% AA treatments between the acid-resistant strain ATCC 43895 and acid-sensitive strain ATCC 43890 (log reductions of 1.61 and 2.38, respectively; P ≤ 0.01). These results indicate that differences in acid resistance between strains can affect the efficacy of organic acid spray washes to reduce the number of these organisms from BCT. Cutter and Siragusa (14) previously reported E. coli O157:H7 strain differences in resistance to 1, 3, or 5% acetic, lactic, or citric acid washes of lean BCT, when bacteria were enumerated after a 24-h incubation of the tissue at 4°C following washing. Among the strains examined in that study, E. coli ATCC 43895 was observed to exhibit the greatest resistance to the organic acid spray treatments, as was noted in the present study. The present study and other reports have noted the high level of acid resistance of this E. coli O157:H7 isolate (1, 30). In fact, differences in levels of acid resistance or abilities to adapt to acidic conditions of different isolates may account for conflicting reports of the efficacies of organic acid spray washing treatments to reduce populations of E. coli O157:H7 on beef (4, 16, 18). This possibility further emphasizes the importance of determining the resistance characteristics of candidate bacterial strains as relevant to the process under examination, prior to their use in studies to validate food preservation processes.
A recent report concerning the effects of feed ration composition on the development of acid resistance by E. coli in cattle has sparked discussion about the relevance of this possible event to the survival of E. coli O157:H7 in the human gastric environment (15, 28, 29). The pertinence of the development of acid tolerance in the bovine gut to public health has been questioned because of the length of time that typically occurs between the time the pathogen is shed in feces and the time the pathogen may be consumed in food or water. During this interval, the bacteria will experience changes in environment and therefore subsequent changes in adaptive state. In the present work, we have demonstrated that acid adaptation of E. coli O157:H7 can negatively affect the ability of organic acid spray washing to reduce the numbers of this organism from prerigor BCT. Pathogens contaminating freshly slaughtered beef carcasses typically are recent residents of the bovine gastrointestinal tract, arriving in feces or ingesta from intestinal organs that are accidentally damaged during removal from the carcass or from feces or environmental soil from the hide or hooves (2, 24). Organic acid spray washes are used in the early steps of beef carcass processing. They may be applied to carcasses after hide removal and before or after evisceration but prior to chilling of the carcasses. Therefore, the acid tolerance status of E. coli O157:H7 as shed from cattle is significant to the microbial safety of meat products. Work is planned to determine the relative acid tolerance of enterohemorrhagic E. coli as naturally shed from cattle, and current work is focused on determining the variation in levels of acid resistance and the ability to adapt to low pH and acidic conditions among recent livestock isolates of this pathogen.
ACKNOWLEDGMENTS
We thank Rebecca Hartford and Jane Long for technical assistance, Ken Ostdiek and Patty Beska for sample collection, and James Wray for consultation on statistical analyses.
REFERENCES
- 1.Arnold K W, Kaspar C W. Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1995;61:2037–2039. doi: 10.1128/aem.61.5.2037-2039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banwart G J. Basic food microbiology. Westport, Conn: AVI Publishing Co., Inc.; 1981. Indicator organisms; pp. 241–253. [Google Scholar]
- 3.Besser R E, Lett S M, Weber J T, Doyle M P, Barrett T J, Wells J W, Griffin P M. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA. 1993;269:2217–2220. [PubMed] [Google Scholar]
- 4.Brackett R E, Hao Y-Y, Doyle M P. Ineffectiveness of hot acid sprays to decontaminate Escherichia coli O157:H7 on beef. J Food Prot. 1994;57:198–203. doi: 10.4315/0362-028X-57.3.198. [DOI] [PubMed] [Google Scholar]
- 5.Brudzinski L, Harrison M A. Influence of incubation conditions on survival and acid tolerance response of Escherichia coli O157:H7 and non-O157:H7 isolates exposed to acetic acid. J Food Prot. 1998;61:542–546. doi: 10.4315/0362-028x-61.5.542. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan R L, Edelson S G. Culturing enterohemorrhagic Escherichia coli in the presence and absence of glucose as a simple means of evaluating the acid tolerance of stationary-phase cells. Appl Environ Microbiol. 1996;62:4009–4013. doi: 10.1128/aem.62.11.4009-4013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan R L, Edelson S G, Boyd G. Effects of pH and acid resistance on the radiation resistance of enterohemorrhagic Escherichia coli. J Food Prot. 1999;62:219–228. doi: 10.4315/0362-028x-62.3.219. [DOI] [PubMed] [Google Scholar]
- 8.Calicioglu M, Faith N G, Buege D R, Luchansky J B. Viability of Escherichia coli O157:H7 in fermented semidry low-temperature-cooked beef summer sausage. J Food Prot. 1997;60:1158–1162. doi: 10.4315/0362-028X-60.10.1158. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami—Washington and California, 1994. Morbid Mortal Weekly Rep. 1995;44:157–160. [PubMed] [Google Scholar]
- 10.Cheng C M, Kaspar C W. Growth and processing conditions affecting acid tolerance in Escherichia coli O157:H7. Food Microbiol. 1998;15:157–166. [Google Scholar]
- 11.Cheville A M, Arnold K W, Buchrieser C, Cheng C-M, Kaspar C W. RpoS regulation of acid, heat, and salt tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1996;62:1822–1824. doi: 10.1128/aem.62.5.1822-1824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clavero M S, Beuchat L R. Survival of Escherichia coli O157:H7 in broth and processed salami as influenced by pH, water activity, and temperature and suitability of media for its recovery. Appl Environ Microbiol. 1996;62:2735–2740. doi: 10.1128/aem.62.8.2735-2740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conner D E, Kotrola J S. Growth and survival of Escherichia coli O157:H7 under acidic conditions. Appl Environ Microbiol. 1995;61:382–385. doi: 10.1128/aem.61.1.382-385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutter C N, Siragusa G R. Efficacy of organic acids against Escherichia coli O157:H7 attached to beef carcass tissue using a pilot scale model carcass washer. J Food Prot. 1994;57:97–103. doi: 10.4315/0362-028X-57.2.97. [DOI] [PubMed] [Google Scholar]
- 15.Diez-Gonzalez F, Callaway T R, Kizoulis M G, Russell J B. Grain feeding and the dissemination of acid-resistant Escherichia coli from cattle. Science. 1998;281:1666–1668. doi: 10.1126/science.281.5383.1666. [DOI] [PubMed] [Google Scholar]
- 16.Dorsa W J. New and established carcass decontamination procedures commonly used in the beef processing industry. J Food Prot. 1997;60:1146–1151. doi: 10.4315/0362-028X-60.9.1146. [DOI] [PubMed] [Google Scholar]
- 17.Dorsa W J, Cutter C N, Siragusa G R. Effectiveness of a steam-vacuum sanitizer for reducing Escherichia coli O157:H7 inoculated to beef carcass surface tissue. Lett Appl Microbiol. 1996;23:61–63. doi: 10.1111/j.1472-765x.1996.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 18.Dorsa W J, Cutter C N, Siragusa G R. Effects of acetic acid, lactic acid and trisodium phosphate on the microflora of refrigerated beef carcass surface tissue inoculated with Escherichia coli O157:H7, Listeria innocua, and Clostridium sporogenes. J Food Prot. 1997;60:619–624. doi: 10.4315/0362-028X-60.6.619. [DOI] [PubMed] [Google Scholar]
- 19.Dorsa W J, Cutter C N, Siragusa G R. Effects of steam-vacuuming and hot water spray wash on the microflora of refrigerated beef carcass surface tissue inoculated with Escherichia coli O157:H7, Listeria innocua, and Clostridium sporogenes. J Food Prot. 1997;60:114–119. doi: 10.4315/0362-028X-60.2.114. [DOI] [PubMed] [Google Scholar]
- 20.Dorsa W J, Cutter C N, Siragusa G R. Long-term effect of alkaline, organic acid, or hot water washes on the microbial profile of refrigerated beef contaminated with bacterial pathogens after washing. J Food Prot. 1998;61:300–306. doi: 10.4315/0362-028x-61.3.300. [DOI] [PubMed] [Google Scholar]
- 21.Doyle M P, Zhao T, Meng J, Zhao S. Escherichia coli O157:H7. In: Doyle M P, Beuchat L R, Montville T J, editors. Food microbiology: fundamentals and frontiers. Washington, D.C.: ASM Press; 1997. pp. 171–191. [Google Scholar]
- 22.Faith N G, Parniere N, Larson T, Lorang T D, Kaspar C W, Luchansky J B. Viability of Escherichia coli O157:H7 in salami following conditioning of batter, fermentation and drying of sticks, and storage of slices. J Food Prot. 1998;61:377–382. doi: 10.4315/0362-028x-61.4.377. [DOI] [PubMed] [Google Scholar]
- 23.Garren D M, Harrison M A, Russell S M. Acid tolerance and acid shock response of Escherichia coli O157:H7 and non-O157:H7 isolates provide cross protection to sodium lactate and sodium chloride. J Food Prot. 1998;61:158–161. doi: 10.4315/0362-028x-61.2.158. [DOI] [PubMed] [Google Scholar]
- 24.Gill C O, McGinnis J C, Badoni M. Assessment of the hygienic characteristics of a beef carcass dressing process. J Food Prot. 1996;59:136–140. doi: 10.4315/0362-028X-59.2.136. [DOI] [PubMed] [Google Scholar]
- 25.Glass K A, Loeffelholz M, Ford J P, Doyle M P. Fate of Escherichia coli O157:H7 as affected by pH or sodium chloride and in fermented dry sausage. Appl Environ Microbiol. 1992;58:2513–2518. doi: 10.1128/aem.58.8.2513-2516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorden J, Small P L C. Acid resistance in enteric bacteria. Infect Immun. 1993;61:364–367. doi: 10.1128/iai.61.1.364-367.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin P M, Ostroff S M, Tauxe R V, Greene K D, Wells J G, Lewis J H, Blake P A. Illnesses associated with Escherichia coli O157:H7 infections. Ann Intern Med. 1988;109:705–712. doi: 10.7326/0003-4819-109-9-705. [DOI] [PubMed] [Google Scholar]
- 28.Hancock D D, Besser T E, Gill C, Bohach C H. Cattle, hay, and E. coli. Science. 1999;284:51–53. doi: 10.1126/science.284.5411.49g. [DOI] [PubMed] [Google Scholar]
- 29.Hovde C J, Austin P R, Cloud K A, Williams C J, Hunt C W. Effect of cattle diet on Escherichia coli O157:H7 acid resistance. Appl Environ Microbiol. 1999;65:3233–3235. doi: 10.1128/aem.65.7.3233-3235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leyer G J, Wang L-L, Johnson E A. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl Environ Microbiol. 1995;61:3752–3755. doi: 10.1128/aem.61.10.3752-3755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Smith M P, Chapin K C, Baik H S, Bennett G N, Foster J W. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1996;62:3094–3100. doi: 10.1128/aem.62.9.3094-3100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massa S, Altieri C, Quaranta V, De Pace R. Survival of Escherichia coli O157:H7 in yoghurt during preparation and storage at 4°C. Lett Appl Microbiol. 1997;24:347–350. doi: 10.1046/j.1472-765x.1997.00067.x. [DOI] [PubMed] [Google Scholar]
- 33.Miller L G, Kaspar C W. Escherichia coli O157:H7 acid tolerance and survival in apple cider. J Food Prot. 1994;57:460–464. doi: 10.4315/0362-028X-57.6.460. [DOI] [PubMed] [Google Scholar]
- 34.Morgan D, Newman C P, Hutchinson D N, Walker A M, Rowe B, Majid F. Verotoxin producing Escherichia coli O157 infections associated with consumption of yogurt. Epidemiol Infect. 1993;111:181–187. doi: 10.1017/s0950268800056880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Driscoll B, Gahan C G M, Hill C. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl Environ Microbiol. 1996;62:1693–1698. doi: 10.1128/aem.62.5.1693-1698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu J-H, Beuchat L R. Influence of acid tolerance responses on survival, growth, and thermal cross-protection of Escherichia coli O157:H7 in acidified media and fruit juices. Int J Food Microbiol. 1998;45:185–193. doi: 10.1016/s0168-1605(98)00165-2. [DOI] [PubMed] [Google Scholar]
- 37.Semanchek J J, Golden D A. Survival of Escherichia coli O157:H7 during fermentation of apple cider. J Food Prot. 1996;59:1256–1259. doi: 10.4315/0362-028X-59.12.1256. [DOI] [PubMed] [Google Scholar]
- 38.Tsai Y-W, Ingham S C. Survival of Escherichia coli O157:H7 and Salmonella spp. in acidic condiments. J Food Prot. 1997;60:751–755. doi: 10.4315/0362-028X-60.7.751. [DOI] [PubMed] [Google Scholar]
- 39.Zhao T, Doyle M P. Fate of enterohemorrhagic Escherichia coli O157:H7 in commercial mayonnaise. J Food Prot. 1994;57:780–783. doi: 10.4315/0362-028X-57.9.780. [DOI] [PubMed] [Google Scholar]