Abstract
Background:
Osteochondral lesions of the talus (OLTs) are a common condition found in patients with chronic ankle pain after previous ankle sprains. Surgical management is indicated after conservative management has failed.
Hypothesis/Purpose:
This study evaluates the influence of body mass index (BMI) on the early clinical outcomes of arthroscopic debridement and microfracture of OLTs.
Methods:
A total of 252 patients with symptomatic OLTs who failed conservative management underwent arthroscopic debridement and microfracture of OLTs over the affected ankle between 2007 and 2017. Patients from this cohort were divided into 2 groups based on BMI: the normal BMI group (NB Group) (BMI 18.5-25.0) and overweight and obese BMI group (OB Group) (BMI ≥25). Visual analogue scale (VAS), American Orthopaedic Foot & Ankle Society (AOFAS) hindfoot score, and the physical and mental component summaries of the 36-Item Short-Form Health Survey (PCS and MCS, respectively) were prospectively collected from the cohort during their standard postoperative outpatient follow-up.
Results:
The NB Group (n=105) and OB Group (n=147) were well matched demographically. The operative duration was significantly shorter for the NB Group compared to the OB Group. Patients from both groups had significant improvements in VAS, AOFAS, and PCS scores postoperatively at 6 and 24 months after surgery (P < .05). Between both groups, patients had comparable VAS, AOFAS, and PCS scores at preoperation, 6 months postoperation, and 24 months postoperation (P > .05). However, MCS in the OB Group was lower at 24 months postoperatively compared with the NB Group (P < .05). The OB Group reported better satisfaction scores (82.4% vs 72.6%, P < .05), and a greater proportion had their expectations met (88.2% vs 77.9%, P < .05).
Conclusion:
A BMI ≥25 was not associated with worse postoperative pain and functional outcomes, but rather was found to be associated with greater satisfaction and fulfillment. However, patients with BMI ≥25 required longer procedure duration and had poorer MCS scores at 24 months after surgery.
Level of Evidence: Level III, retrospective cohort study.
Keywords: ankle cartilage, general sports trauma, osteochondral lesions, obesity, bone marrow stimulation, ankle arthroscopy
Introduction
Osteochondral lesions of the talus (OLTs) are a common condition found in patients with chronic ankle pain after a traumatic ankle-twisting injury. Incidence of cartilage injury of the talus after an ankle sprain can be as high as 50%. 36
Multiple treatment options are available for the treatment of OLTs. Conservative measures such as analgesics, physical therapy, activity modification, and immobilization of the affected ankle are usually applied as first-line treatments. Surgical management is indicated after conservative management has failed.2,31
Surgical treatment techniques such as arthroscopic bone marrow stimulation, direct fixation of OLTs, autologous tissue transplantation (eg, osteochondral autograft transfer, mosaicplasty, and autologous chondrocyte implantation), allograft osteochondral transplantation, as well as the use of biologic scaffolds and corrective osteotomy have been discussed in literature.2,27,31,35,36,39 However, operative treatment options for OLTs are controversial, with no strong evidence to guide best practice.14,27 Loveday et al 27 in a recent Cochrane review concluded that there was a lack of evidence to determine which surgical intervention was most appropriate treatment for OLTs. 39 Currently, arthroscopic bone marrow stimulation remains the surgical modality of choice for most surgeons, especially for smaller lesions—due to it being widely studied, reproducing good clinical outcomes and minimal morbidity associated with the procedure.14,19,20,34,37
There is considerable literature reporting poorer outcomes in lesions greater than 1.5 cm2 in size9,11 or uncontained defects of OLTs.7,11,15 Other commonly discussed predictors such as age and location of OLTs, however, remain widely debated.1,3,8,43 The effect of BMI on the outcome of arthroscopic treatment of OLT treatment outcomes remain unexplored. The only study to date was a cohort of 45 patients by Becher et al, who found that BMI greater than 25 was a negative prognostic factor. However, it is important to note that this was a heterogenous population with both osteochondral and degenerative chondral defects. 3 Because of the paucity of literature guiding best practice, studying the impact of obesity on arthroscopic debridement and microfracture of OLTs will shape how OLTs are managed.
This study aims to evaluate the influence of being overweight on the early clinical outcomes of arthroscopic debridement and microfracture of OLTs. The authors hypothesize that an elevated BMI negatively affects outcomes of patients with OLTs.
Methods
A retrospective study of prospectively collected data from a single institution was performed. Between January 2007 and December 2017, a total of 252 patients with symptomatic OLTs who failed at least 6 months of conservative management underwent arthroscopic ankle joint debridement and microfracture of OLTs over the affected ankle. The presence of OLTs in these patients was confirmed using magnetic resonance imaging. Contained lesions less than 1 cm2 in size were included in this study. 9 The size of the lesion was determined intraoperatively and measured in 2 planes (the widest length and its perpendicular axis) using an arthroscopic probe, in a technique previously described by Yasui et al. 42
Patients with previous ankle fractures, previous infection, radiologic features of degenerative joint disease (eg, osteophyte formation, joint space narrowing), lower limb malalignment, and ankle ligament pathology were excluded from this study. Patients who underwent concomitant procedures were also excluded.
According to the World Health Organization classification of BMI, between 18.5 and 24.9 is normal, 25.0 to 29.9 is overweight, and 30.0 and above is considered obese. 41 Patients from this cohort were divided into 2 groups based on their BMI. The normal BMI group (NB Group) (BMI 18.5-24.9) (Control group) and overweight and obese BMI group (OB Group) (BMI ≥ 25) (Study group) were studied.
Visual analog scale (VAS), American Orthopaedic Foot & Ankle Society hindfoot score (AOFAS) as well as the physical and mental component summaries of the 36-Item Short-Form Health Survey (PCS and MCS, respectively) were prospectively collected from patients preoperatively as well as during their postoperative outpatient follow-up at 6 and 24 months by a dedicated senior physiotherapist at our institution. Patients’ overall satisfaction and fulfillment of expectation were captured using a questionnaire adapted from question 53 of the North American Spine Society Questionnaire. 12 Patient satisfaction and expectations were recorded on a Likert scale of 1 to 6 and 1 to 7, respectively—with higher scores indicating a poorer result. Satisfaction score was dichotomized, with a score of 1 to 3 being “satisfied” and 4 to 6 being “not satisfied.” Scores of 1 to 3 were grouped into an “expectations met” category whereas a score of 4 to 7 were grouped into “expectations not met.” 23
Surgical Technique
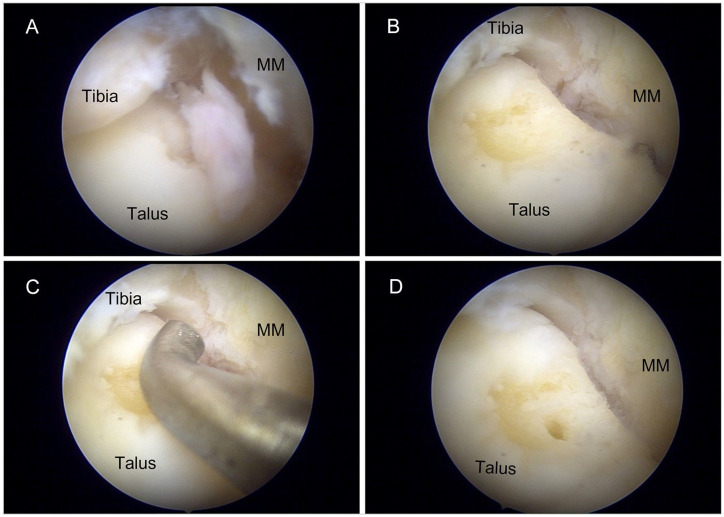
Patients received either general or spinal anaesthesia. Patients were positioned supine with a sandbag placed under the ipsilateral buttock and their heel aligned with the distal edge of the operating bed. Manual distraction of the ankle joint was used to establish routine anteromedial and anterolateral portals. A 4.0-mm arthroscope was used for all procedures. During ankle arthroscopy, the tibiotalar joint was visualized (Figure 1). Loose-body removal was performed. Inflamed synovial tissue as well as flaps of loose cartilage were debrided with a motorized 2.7-mm shaver. Area overlying the OLT was identified by either cartilage softening or cartilage loss (Figure 1A). The surrounding loose cartilage was debrided until smooth healthy edges were observed (Figure 1B). Defect size was then measured using an arthroscopic probe (Figure 1c). Microfracture was performed with aid of arthroscopic awls at 3- to 4-mm intervals, to a depth of 2 to 4 mm until fat globules were noted (Figure 1D and E). 31
Figure 1.
Intraoperative images of arthroscopic debridement and microfracture of OLTs. (A) OLT was identified by the flap of cartilage overlying the chondral defect. (B) Surrounding loose cartilage was debrided until smooth edges were observed. (C) Size of the lesion was measured with the aid of an arthroscopic probe. (D) Microfracture was performed with an arthroscopic awl to a depth of 2 to 4 mm at 3- to 4-mm intervals until fat globules were observed. MM, medial malleolus; OLT, osteochondral lesion of the talus.
Rehabilitation Protocol
All patients underwent a standardized postoperative rehabilitation protocol. The operated limb is dressed in Jones pressure bandage around the ankle and patients are encouraged to elevate the operated limb to alleviate swelling. Patients are kept nonweightbearing on the operated limb for 2 weeks. From 2 weeks onwards, patients are converted to walking aircast boot with partial weight bearing (less than 50% body weight) on the operated limb. 25 Outpatient physiotherapy was initiated at 4 weeks after surgery and focused on range of motion exercises. Patients were allowed to full weightbear after 6 weeks, if pain free, with physiotherapy focused on proprioceptive and isometric strengthening exercises.
This study was approved by the hospital’s ethics committee (CIRB Ref: 2021/2237) and carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Statistical Analysis
Statistical analysis was performed using SPSS 21.0 (IBM Corporation, Armonk, NY). Independent Student t test was used for parametric variables (eg, age and clinical outcome scores). Chi-squared test used for categorical data (eg, gender, satisfaction, and expectations met). Two-tailed Spearman correlation coefficient was used to assess the relation between BMI and clinical outcome measures. Strength of correlation was interpreted as suggested by Dancey and Reidy. 13 Statistical significance was taken as P <.05.
Results
Total of 252 patients were available for analysis, 105 (42%) in the NB Group and 147 (58%) in the OB Group. The mean BMI for the NB Group was 22.4 ± 1.8 whereas the OB Group was 29.8 ± 3.8. Patients from both groups were well matched demographically (Table 1). In addition, preoperative clinical scores were comparable in both groups (Table 2).
Table 1.
Demographics.
| NB Group (n = 105) (n = 105) |
OB Group (n = 147) (n = 147) |
P Value | |
|---|---|---|---|
| Age, y, mean ± SD | 35.8 ± 13.7 | 41.2 ± 12.6 | n.s. |
| BMI, mean ± SD | 22.4 ± 1.8 | 29.8 ± 3.8 | <.001 |
| Gender, n | |||
| Male | 63 | 91 | n.s. |
| Female | 42 | 56 | |
| Side of surgery, n | |||
| Right | 51 | 80 | n.s. |
| Left | 54 | 67 | |
| Operation duration (min), mean ± SD | 42.0 ± 20.1 | 48.8 ± 20.8 | <.05 |
| Length of stay (d), mean ± SD | 0.6 ± 0.5 | 0.7 ± 0.5 | n.s. |
Abbreviations: BMI, body mass index; NB, normal BMI; OB, obese and overweight BMI; n.s., nonsignificant.
Table 2.
Functional Outcome NB Group vs OB Group.
| NB Group (n = 105) |
OB Group (n = 147) |
P Value | |
|---|---|---|---|
| VAS score, mean ± SD | |||
| Preoperative | 5.6 ± 2.3 | 5.9 ± 2.4 | n.s. |
| 6 mo postop. | 2.3 ± 2.6 | 2.8 ± 2.6 | n.s. |
| P value (6 mo postop. vs preop) | <.001 | <.001 | |
| 24 mo postop. | 2.0 ± 2.9 | 1.7 ± 2.5 | n.s. |
| P value (24 mo postop. vs preop) | <.001 | <.001 | |
| AOFAS score, mean ± SD | |||
| Preoperative | 53.1 ± 16.3 | 50.1 ± 16.7 | n.s. |
| 6 mo postop. | 78.3 ± 19.1 | 75.8 ± 18.1 | n.s. |
| P value (6 mo postop. vs preop) | <.001 | <.001 | |
| 24 mo postop. | 83.2 ± 21.5 | 85.0 ± 17.3 | n.s. |
| P value (24 mo postop. vs preop) | <.001 | <.001 | |
| PCS score, mean ± SD | |||
| Preoperative | 38.9 ± 10.0 | 36.7 ± 9.0 | n.s. |
| 6 mo postop. | 46.2 ± 9.7 | 44.7 ± 10.4 | n.s. |
| P value (6 mo postop. vs preop) | <.001 | <.001 | |
| 24 mo postop. | 47.0 ± 11.2 | 47.9 ± 10.0 | n.s. |
| P value (24 mo postop. vs preop) | <.001 | <.001 | |
| MCS score, mean ± SD | |||
| Preoperative | 55.1 ± 11.7 | 54.8 ± 11.0 | n.s. |
| 6 mo postop. | 54.1 ± 11.3 | 51.2 ± 13.7 | n.s. |
| P value (6 mo postop. vs preop) | n.s. | <.05 | |
| 24 mo postop. | 55.6 ± 11.0 | 51.9 ± 9.9 | <.05 |
| P value (24 mo postop. vs preop) | n.s. | <.05 | |
Abbreviations: AOFAS, American Orthopaedic Foot & Ankle Society hindfoot score; MCS, mental component summary; NB, normal BMI; OB, obese and overweight BMI; PCS, physical component summary; postop., postoperation; preop., preoperation; n.s., nonsignificant; VAS, visual analog scale for pain.
The operative duration was significantly shorter for the NB Group compared with the OB Group (42.0 ± 20.1 minutes vs 48.8 ± 20.8 minutes; P < .05). Patients from both groups had significant improvement in VAS, AOFAS, and PCS scores postoperatively at 6 and 24 months after surgery (P < .05) (Table 2). However, MCS in the OB Group showed significant deterioration at 6 and 24 months postoperatively (54.8 ± 11.0 vs 51.2 ± 13.7 and 51.9 ± 9.9 respectively, both P < .05). Neither group reported any complications from surgery.
VAS, AOFAS, and PCS scores between both NB Group and OB Group at 6 months postoperation and 24 months postoperatively were also comparable (P > .05) (Table 2). At 24 months, the OB Group had a significantly poorer MCS compared to the NB Group (51.9 ± 9.9 vs 55.6 ± 11.0, P < .05) (Figure 2).
Figure 2.
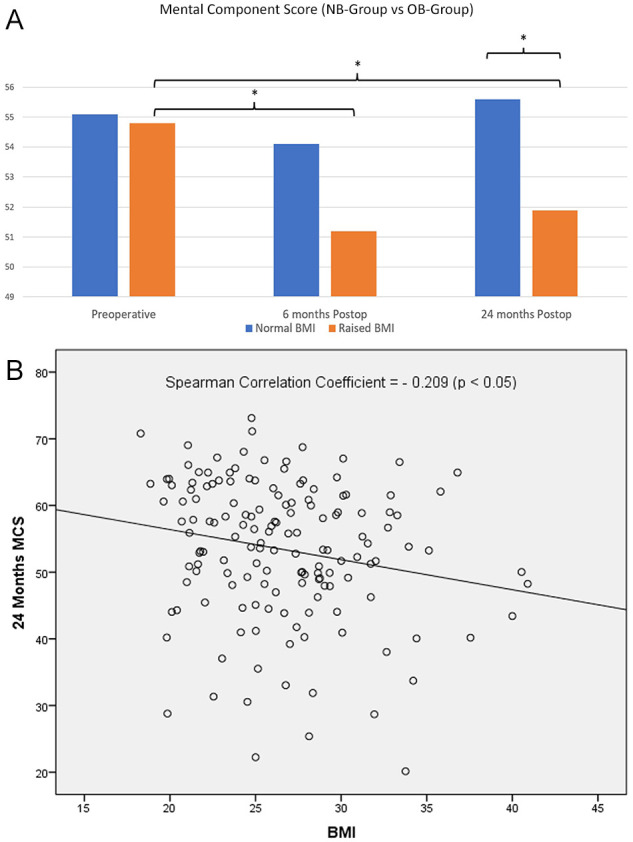
Relationship of BMI and MCS. (A) Interval worsening of MCS scores in the OB Group. At 24 months, MCS was significantly poorer in the OB Group compared to the NB Group. (B) There is a weak but significant negative correlation (–0.209) between BMI and MCS at 24 months postoperatively. BMI, body mass index; MCS, mental component summary; NB, normal BMI; OB, obese and overweight BMI.
* P <.05 denotes statistical significance.
Spearman correlation analysis showed a statistically significant but weak negative correlation between BMI and 24-month MCS scores (correlation coefficient = −0.209, P < .05). No correlation was observed between BMI and VAS, AOFAS, and PCS at 24 months.
The OB Group had a significantly higher proportion of patients reporting satisfaction compared with the NB Group (82.4% vs 72.6%, P < .05). The OB Group also had a greater proportion of patients who report that their expectations were met (88.2% vs 77.9%, P < .05) (Table 3).
Table 3.
Fulfillment of Expectations and Satisfaction Levels at 24 Months.
| NB Group (n = 105) |
OB Group (n = 147) |
P Value | |
|---|---|---|---|
| Satisfaction | |||
| Satisfied (%) | 72.6 | 82.4 | <.05 |
| Not satisfied (%) | 27.4 | 17.6 | |
| Expectations | |||
| Met (%) | 77.9 | 88.2 | <.05 |
| Not Met (%) | 22.1 | 11.8 | |
Abbreviations: NB, normal BMI; OB, obese and overweight BMI.
Discussion
As a weightbearing joint, the tibiotalar joint experiences considerable forces. Gait analysis studies show that the ankle joint experiences up to 5 times body weight during the stance phase.4,22 The talar dome itself sustains 77% to 90% of load through the ankle joint. 5 Obesity has shown to increase incidence of early complications post arthroscopic surgery.32,38 Nicolay et al 32 studied 141 335 patients from a database evaluating hip, knee, and shoulder arthroscopy complications and found that underweight and class III obesity patients had higher risk for morbidity after arthroscopic procedures.
Contrary to the authors’ hypothesis, a raised BMI did not adversely affect pain and functional outcomes, although it was more technically challenging performing arthroscopy and microfracture in patients with raised BMI as evidenced by the significantly longer operative duration. It did not negatively affect the benefit of arthroscopy and microfracture at 24 months. In fact, a greater proportion of patients with raised BMI reported better satisfaction and fulfillment postoperatively. Of note, quality of life outcomes, MCS, in the raised BMI group was significantly poorer at 6 months to 24 months postoperatively. When compared with the normal BMI group, the raised BMI group had a poorer MCS score at 24 months operatively.
These findings were echoed in similar studies looking at the knee—another weightbearing joint.18,38 Sing et al 38 reported that obesity alone did not increase postoperative complications after knee arthroscopy; instead, other associated comorbidities such as high American Society of Anesthesiologists classification, dependent functional status, and renal comorbidities were independent predictors. Martin et al 30 identified race, prior operation within 30 days, operative time of greater than 1.5 hours, and elderly age as independent risk factors for knee arthroscopy complications. Similarly, we found that BMI did not negatively impact the clinical outcome of arthroscopic debridement and microfracture of OLTs at 24 months.
Bone marrow stimulation through the use of microfracture remains the best treatment option for patients with small, contained OLTs.3,27,36,40,43,44 Tol et al reported high success rates of 85% with the use of bone marrow stimulation compared with just excision and curettage alone. 40 Lee et al reported good to excellent outcomes in 89% of patients with significant improvement in AOFAS, Ankle Activity Score, VAS, as well as PCS scores at a mean follow-up of 33 months. 26 A recent 10-year outcome study by Corr et al 10 found a 93.3% survivorship, with 9 in 10 patients reporting satisfaction 10 years after surgery. Microfracture also allows for early rehabilitation and return to activity. Saxena et al 36 reported early return to activity among high-demand patients treated with microfracture compared with bone grafting. In contrast, Gobbi et al 17 reported a significantly lower numerical pain intensity score at 24 hours postoperatively in patients receiving microfracture and chondroplasty vs patients who received osteochondral autograft transfer.
However, these findings were in contrast to Becher et al, 3 who showed poorer Hannover Scoring System for the Ankle (75.1 ± 17.0 vs 83.8 ± 15.3, P < .05) and VAS pain scores (1.6 ± 2.5 vs 3.0 ± 3.0, P < .04) in the group with BMI greater than 25 at the latest follow-up. This may be attributed to the heterogenous study group observed with both osteochondral and degenerative chondral defects included in the study. Degenerative joint wear may be a confounder in this group, with obese patients experiencing poorer outcomes in the presence of degenerative joint disease.28,33
Despite reports that obese patients tend to have a higher incidence of superficial wound infections, we did not observe any wound complications in both groups, 33 even when operative duration in the OB Group was significantly greater (Table 1). Nevertheless, arthroscopic debridement and microfracture of OLTs did not exceed 1.5 hours in this study—a testament to the technical simplicity and low morbidity associated with this procedure.3,17,19,30,36,40,44
Despite these promising findings, patients with raised BMI experienced poorer quality of life scores as evidenced by deteriorating MCS scores at 6 months and 24 months than before surgery. In addition, patients with raised BMI had a significantly inferior MCS score compared to patients with normal BMI at 24 months. This is likely multifactorial. Obesity negatively influences both the physical and psychosocial aspect of quality of life—especially among the severely obese. 24 Aspects of MCS component of the SF-36 draws significant weight from mental health and social functioning scores. 21 Markowitz et al 29 proposed that obesity-associated behavior such as functional limitations, constant dieting, and mental stress from their body image and poor self-esteem, coupled with social stigma, all play a strong role linking obesity with depression. In addition, patients with raised BMI do experience fatigue, thereby negatively affecting the vitality component of the MCS score. 16 Despite a poorer MCS score, patients experienced improvement in their PCS score. This can be explained by the PCS score drawing greater weight on components such as pain, physical function, and perception of general health. This suggests that patients in the OB Group benefited from surgery functionally and had less pain; however, the negative impacts of obesity may continue to plague their recovery.
The strength of this study is that this is a large study looking at the treatment of OLTs with arthroscopic debridement and microfracture. It also addresses the impact of a common modifiable risk factor—BMI. This study has demonstrated that overweight patients did not necessarily have poorer clinical outcome scores. The evidence is contrary—patients with a raised BMI benefited from the surgery and had greater satisfaction and fulfillment after arthroscopic debridement and microfracture of the OLTs. Nevertheless, a raised BMI continues to negatively impact patient’s quality of life as evidenced by the poorer MCS scores noted in this study. Patients with a raised BMI should not be omitted from beneficial surgical management of OLTs. Instead, patients should be encouraged to normalize their BMI in view of the negative impact on their quality of life and the physical limitations associated with a raised BMI.
This study has a few limitations. First, the design of this study is retrospective in nature. Second, patients were recruited from a single institution and therefore may be subject to selection and observation biases. In addition, most studies evaluating the impact of obesity on surgical outcomes often find significance with extremes in BMI (ie, obesity class II and III).6,32,38 However, this study cohort lacked patients with obesity class II and III, with most of the OB Group having mainly overweight and obesity class I patients. Future prospective, multicenter randomized controlled trials will aid in mitigating these biases and allow the study of a more heterogenous cohort. Although no clinical difference was noted between the 2 groups, outcome scores showed poorer MCS score in the OB Group at 24 months. Longer-term studies are therefore crucial in ascertaining the effect of BMI on arthroscopic debridement and microfractures of OLTs.
Conclusion
Arthroscopic debridement and microfracture is a reliable procedure, providing significant improvement in pain relief, functional improvement, and satisfaction in patients. A BMI ≥25 was not associated with worse pain and functional outcomes. Overweight and obese patients therefore should not be excluded from beneficial surgical management of OLTs.
Footnotes
Ethical Approval: This study was approved by the hospital’s ethics committee (CIRB Ref: 2021/2237) and carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. ICMJE forms for all authors are available online.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Don Thong Siang Koh, MBBS, BSc, MRCS,  https://orcid.org/0000-0002-4777-675X
https://orcid.org/0000-0002-4777-675X
Kae Sian Tay, MBBS, MMed, FRCS,  https://orcid.org/0000-0001-6576-7876
https://orcid.org/0000-0001-6576-7876
References
- 1. Angthong C, Chumchuen S, Khadsongkram A. A systematic review of intermediate-term outcomes and failure rates for total ankle replacements: an Asian perspective. Foot Ankle Surg. 2013;19(3):148-154. doi: 10.1016/j.fas.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 2. Badekas T, Takvorian M, Souras N. Treatment principles for osteochondral lesions in foot and ankle. Int Orthop. 2013;37(9):1697-1706. doi: 10.1007/s00264-013-2076-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):656-663. doi: 10.1007/s00167-009-1036-1 [DOI] [PubMed] [Google Scholar]
- 4. Brockett CL, Chapman GJ. Biomechanics of the ankle. Orthop Trauma. 2016;30(3):232-238. doi: 10.1016/j.mporth.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calhoun JH, Li F, Ledbetter BR, Viegas SF. A comprehensive study of pressure distribution in the ankle joint with inversion and eversion. Foot Ankle Int. 1994;15(3):125-133. doi: 10.1177/107110079401500307 [DOI] [PubMed] [Google Scholar]
- 6. Chen JY, Lo NN, Chong HC, et al. The influence of body mass index on functional outcome and quality of life after total knee arthroplasty. Bone Joint J. 2016;98-B(6):780-785. doi: 10.1302/0301-620X.98B6.35709 [DOI] [PubMed] [Google Scholar]
- 7. Choi WJ, Choi GW, Kim JS, Lee JW. Prognostic significance of the containment and location of osteochondral lesions of the talus: independent adverse outcomes associated with uncontained lesions of the talar shoulder. Am J Sports Med. 2013;41(1):126-133. doi: 10.1177/0363546512453302 [DOI] [PubMed] [Google Scholar]
- 8. Choi WJ, Kim BS, Lee JW. Osteochondral lesion of the talus: could age be an indication for arthroscopic treatment? Am J Sports Med. 2012;40(2):419-424. doi: 10.1177/0363546511423739 [DOI] [PubMed] [Google Scholar]
- 9. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974-1980. doi: 10.1177/0363546509335765 [DOI] [PubMed] [Google Scholar]
- 10. Corr D, Raikin J, O’Neil J, Raikin S. Long-term outcomes of microfracture for treatment of osteochondral lesions of the talus. Foot Ankle Int. 2021;42(7):833-840. doi: 10.1177/1071100721995427 [DOI] [PubMed] [Google Scholar]
- 11. Cuttica DJ, Smith WB, Hyer CF, Philbin TM, Berlet GC. Osteochondral lesions of the talus: predictors of clinical outcome. Foot Ankle Int. 2011;32(11):1045-1051. doi: 10.3113/FAI.2011.1045 [DOI] [PubMed] [Google Scholar]
- 12. Daltroy LH, Cats-Baril WL, Katz JN, Fossel AH, Liang MH. The North American Spine Society Lumbar Spine Outcome Assessment Instrument: reliability and validity tests. Spine. 1996;21(6):741-749. doi: 10.1097/00007632-199603150-00017 [DOI] [PubMed] [Google Scholar]
- 13. Dancey CP, Reidy J. Statistics Without Maths for Psychology. 8th ed. Pearson Education; 2004. [Google Scholar]
- 14. Dekker TJ, Dekker PK, Tainter DM, Easley ME, Adams SB. Treatment of osteochondral lesions of the talus: a critical analysis review. JBJS Rev. 2017;5(3). doi: 10.2106/JBJS.RVW.16.00065 [DOI] [PubMed] [Google Scholar]
- 15. Deol PPS, Cuttica DJ, Smith WB, Berlet GC. Osteochondral lesions of the talus: size, age, and predictors of outcomes. Foot Ankle Clin. 2013;18(1):13-34. doi: 10.1016/j.fcl.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 16. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953-959. doi: 10.7326/0003-4819-121-12-199412150-00009 [DOI] [PubMed] [Google Scholar]
- 17. Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22(10):1085-1092. doi: 10.1016/j.arthro.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 18. Goh GSH, Liow MHL, Mitra AK. Outcome following total knee arthroplasty in obese versus non-obese Asian patients. J Orthop Surg (Hong Kong). 2015;23(3):294-297. doi: 10.1177/230949901502300306 [DOI] [PubMed] [Google Scholar]
- 19. Guney A, Yurdakul E, Karaman I, Bilal O, Kafadar IH, Oner M. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1293-1298. doi: 10.1007/s00167-015-3834-y [DOI] [PubMed] [Google Scholar]
- 20. Hunt SA, Sherman O. Arthroscopic treatment of osteochondral lesions of the talus with correlation of outcome scoring systems. Arthroscopy. 2003;19(4):360-367. doi: 10.1053/jars.2003.50047 [DOI] [PubMed] [Google Scholar]
- 21. Karlsen TI, Tveitå EK, Natvig GK, Tonstad S, Hjelmesæth J. Validity of the SF-36 in patients with morbid obesity. Obes Facts. 2011;4(5):346-351. doi: 10.1159/000333406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim Y, Lee KM, Koo S. Joint moments and contact forces in the foot during walking. J Biomech. 2018;74:79-85. doi: 10.1016/j.jbiomech.2018.04.022 [DOI] [PubMed] [Google Scholar]
- 23. Koh DTS, Woo YL, Yew AKS, Yeo SJ. Kinematic aligned femoral rotation leads to greater patella tilt but similar clinical outcomes when compared to traditional femoral component rotation in total knee arthroplasty. A propensity score matched study. Knee Surg Sports Traumatol Arthrosc. 2021;29(4):1059-1066. doi: 10.1007/s00167-020-06081-7 [DOI] [PubMed] [Google Scholar]
- 24. Kushner RF, Foster GD. Obesity and quality of life. Nutrition. 2000;16(10):947-952. doi: 10.1016/s0899-9007(00)00404-4 [DOI] [PubMed] [Google Scholar]
- 25. Lee DH, Lee KB, Jung ST, Seon JK, Kim MS, Sung IH. Comparison of early versus delayed weightbearing outcomes after microfracture for small to midsized osteochondral lesions of the talus. Am J Sports Med. 2012;40(9):2023-2028. doi: 10.1177/0363546512455316 [DOI] [PubMed] [Google Scholar]
- 26. Lee KB, Bai LB, Chung JY, Seon JK. Arthroscopic microfracture for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):247-253. doi: 10.1007/s00167-009-0914-x [DOI] [PubMed] [Google Scholar]
- 27. Loveday D, Clifton R, Robinson A. Interventions for treating osteochondral defects of the talus in adults. Cochrane Database Syst Rev. 2010;(8):CD008104. doi: 10.1002/14651858.CD008104.pub2 [DOI] [PubMed] [Google Scholar]
- 28. Magliano M. Obesity and arthritis. Menopause Int. 2008;14(4):149-154. doi: 10.1258/mi.2008.008018 [DOI] [PubMed] [Google Scholar]
- 29. Markowitz S, Friedman MA, Arent SM. Understanding the relation between obesity and depression: causal mechanisms and implications for treatment. Clin Psychol Sci Pract. 2008;15(1):1-20. doi: 10.1111/j.1468-2850.2008.00106.x [DOI] [Google Scholar]
- 30. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98.1-10. doi: 10.2106/JBJS.L.01440 [DOI] [PubMed] [Google Scholar]
- 31. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. doi: 10.2106/JBJS.L.00773 [DOI] [PubMed] [Google Scholar]
- 32. Nicolay RW, Selley RS, Terry MA, Tjong VK. Body Mass index as a risk factor for 30-day postoperative complications in knee, hip, and shoulder arthroscopy. Arthroscopy. 2019;35(3):874-882.e3. doi: 10.1016/j.arthro.2018.10.108 [DOI] [PubMed] [Google Scholar]
- 33. Perry KI, MacDonald SJ. The obese patient: a problem of larger consequence. Bone Joint J. 2016;98-B(1 suppl A):3-5. doi: 10.1302/0301-620X.98B1.36415 [DOI] [PubMed] [Google Scholar]
- 34. Polat G, Erşen A, Erdil ME, Kızılkurt T, Kılıçoğlu Ö, Aşık M. Long-term results of microfracture in the treatment of talus osteochondral lesions. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1299-1303. doi: 10.1007/s00167-016-3990-8 [DOI] [PubMed] [Google Scholar]
- 35. Powers RT, Dowd TC, Giza E. Surgical treatment for osteochondral lesions of the talus. Arthroscopy. 2021;37(12):3393-3396. doi: 10.1016/j.arthro.2021.10.002 [DOI] [PubMed] [Google Scholar]
- 36. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35(10):1680-1687. doi: 10.1177/0363546507303561 [DOI] [PubMed] [Google Scholar]
- 37. Schuman L, Struijs P a. A, van Dijk CN. Arthroscopic treatment for osteochondral defects of the talus. Results at follow-up at 2 to 11 years. J Bone Joint Surg Br. 2002;84(3):364-368. doi: 10.1302/0301-620x.84b3.11723 [DOI] [PubMed] [Google Scholar]
- 38. Sing DC, Luan TF, Feeley BT, Zhang AL. Is obesity a risk factor for adverse events after knee arthroscopy? Arthroscopy. 2016;32(7):1346-1353.e1. doi: 10.1016/j.arthro.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 39. Smyth NA, Murawski CD, Adams SB, et al. Osteochondral allograft: proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle Int. 2018;39(1 suppl):35S-40S. doi: 10.1177/1071100718781097 [DOI] [PubMed] [Google Scholar]
- 40. Tol JL, Struijs PA, Bossuyt PM, Verhagen RA, van Dijk CN. Treatment strategies in osteochondral defects of the talar dome: a systematic review. Foot Ankle Int. 2000;21(2):119-126. doi: 10.1177/107110070002100205 [DOI] [PubMed] [Google Scholar]
- 41. WHO. Obesity and overweight. Published April 1, 2020. Accessed May 22, 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 42. Yasui Y, Hannon CP, Fraser EJ, et al. Lesion size measured on MRI does not accurately reflect arthroscopic measurement in talar osteochondral lesions. Orthop J Sports Med. 2019;7(2):2325967118825261. doi: 10.1177/2325967118825261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoshimura I, Kanazawa K, Hagio T, Minokawa S, Asano K, Naito M. The relationship between the lesion-to-ankle articular length ratio and clinical outcomes after bone marrow stimulation for small osteochondral lesions of the talus. J Orthop Sci. 2015;20(3):507-512. doi: 10.1007/s00776-015-0699-3 [DOI] [PubMed] [Google Scholar]
- 44. Zengerink M, Struijs PAA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238-246. doi: 10.1007/s00167-009-0942-6 [DOI] [PMC free article] [PubMed] [Google Scholar]