Abstract
Objective:
The present work aims to analyse the effectiveness of platelet-rich plasma (PRP) in degenerative knee pathology based on real-world data and to evaluate possible factors influencing the response to treatment.
Methods:
In total, 531 cases were analysed collecting data on gender, age, body mass index, pathology location, severity, number of cycles and route of administration. Clinical outcome was evaluated at 6 and 15 months after treatment, using the Knee injury and Osteoarthritis Outcome Score (KOOS) and obtaining percentages of Minimal Clinically Important Improvement (MCII). Blood and PRP samples were randomly tested as a quality control measure to ensure the correct properties. Comparative statistical tests and multivariate regression were performed for the analysis of the variables.
Results:
The PRP applied had a platelet concentration factor of 1.67, with no leukocytes or erythrocytes. The percentage of patients with MCII at 6 and 15 months after PRP application was 59.32% and 70.62%, respectively. Patients with MCII were younger (p = 0.0246) and with lower body mass index (p = 0.0450). The treatment had a better response in mild/moderate cases than in severe cases (p = 0.0002). Intraosseous PRP application in severe cases improved the effect of intraarticular PRP (p = 0.0358). The application of a second cycle of PRP only improved the response in patients without MCII at 6 months (p = 0.0029), especially in mild/moderate cases (p = 0.0357).
Conclusion:
The applications of PRP in degenerative knee pathologies is an effective treatment, but this effectiveness nonetheless depends on several variables. Real-world data can complement that from clinical trials to provide valuable information.
Keywords: intraarticular, intraosseous, knee joint degeneration, platelet-rich plasma, real-world evidence
Introduction
Knee joint degeneration is a highly disabling disease, especially in the elderly population, with a prevalence of more than 15% worldwide, and more than 40% in patients over 40 years of age. 1 Today’s habits such as sedentarism, obesity and an ageing population will inevitably lead to an increase in prevalence in the coming years, not only in the elderly population but also in young patients. 2 Its complexity is another obstacle that makes it a challenge for health systems, because other structures besides the cartilage are involved, namely, the synovial membrane and the subchondral bone. 3 The lack of a clear therapeutic target and its degenerative nature make it difficult to apply effective treatment to stop or slow its progression. Current treatments, such as oral or intraarticular pharmacology, achieve symptom relief but do not resolve this disease, making total knee arthroplasty the definitive solution for these patients. However, this surgical intervention is contraindicated in patients of advanced age or with multiple comorbidities, not to mention the inherent surgical risks and associated costs.4,5
Treatments based on regenerative medicine, such as platelet-rich plasma (PRP) or cell therapies, aim to expand therapeutic arsenal so as to avoid or delay surgery as far as possible. While cell therapy is still in its infancy and has to overcome several challenges, PRP has been applied for more than 15 years with a consolidated position in the treatment of this disease. 6 It is based on obtaining the plasma fraction from the patient’s blood with a concentration of platelets similar to or higher than in blood levels. PRP contains high levels of biomolecules that participate in different biological processes that favouring cellular repair. 7
An increasing number of randomized clinical trials (RCTs) are being conducted to draw firm conclusions regarding the efficacy and safety of PRP, with promising results. 8 Although RCTs are the cornerstone of evidence-based medicine, their use for the study of PRP has certain limitations. The term PRP encompasses a range of products of different compositions, which makes a proper comparison between the different studies impossible and leads to contradictory results. This also prevents the aggregation of patients from different RCTs for the analysis of large population samples. On the contrary, the information obtained from real-world evidence (RWE) studies may be a useful complement to the data obtained from RCTs. RWE can be defined as the collection of clinical data from patients in routine clinical practice. Although it does not have all the strengths of RCTs, it allows the real-world data collection from a large volume of patients and the assessment of various factors that may influence treatment.9,10 The combination of both types of study provides the medical and scientific community with valuable information for the further study of pathologies and treatments. However, there are hardly any RWE studies on knee degeneration and PRP.11,12
We hypothesize that conducting RWE studies could provide information to help optimize treatment protocols. Thus, the present work aims to analyse the effectiveness of PRP in knee degenerative pathology, based on a large number of patients, and to evaluate possible factors influencing the response to treatment.
Methods
Study design, patients and data collection
The study was designed as a prospective observational study to analyse PRP application in knee degenerative pathology. This study was carried out in accordance with the International Declaration of Helsinki in Fortaleza, Brazil (2013), Good Clinical Practice Regulations and the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement. 13 Ethical approval for this study (Protocol No. EPA2015046) was obtained from the Ethics Committee of the Basque Country (September 2015), as was written informed consent.
The eligible patients were enrolled consecutively between 2015 and 2020 in the same medical centre. They met the following inclusion criteria: patients of both sexes over 18 years old, diagnosed with knee joint degeneration and a complete follow-up for a minimum of 12 months. The exclusion criteria were as follows: associated joint pathologies or systemic autoimmune rheumatic disease, and any knee intervention or intraarticular infiltrations in the past 12 months or during PRP treatment and follow-up.
Age, sex, body mass index (BMI), number of PRP cycles (one or two), route of administration (intraarticular or intraosseous), presence of synovial fluid, location and pathological severity were collected. Imaging studies assessed pathological severity using the Ahlbäck and Outbridge scales for osteoarthritis and chondropathies, respectively. Patients were divided into two categories: mild or moderate grade (Ahlbäck: I–II; Outbridge: 1–2) and severe grade (Ahlbäck: III–IV, Outbridge 3–4). Patients completed the Knee injury and Osteoarthritis Outcome Score (KOOS) to assess their response to treatment. Concurrent medication such as paracetamol was forbidden 48 h before assessment. All data were collected through the use of electronic medical records.
PRP preparation
Depending on whether infiltration was intraarticular or intraosseous, 32 or 80 ml of venous blood was extracted from the patient, respectively. The blood was withdrawn into 9-ml tubes containing 3.8% (w/v) sodium citrate and centrifuged at 580 × g for 8 min at room temperature (BTI Biotechnology Institute, Vitoria-Gasteiz, Spain). The 2-ml plasma fraction located above the red blood fraction, but not including the buffy coat, was collected. This plasma fraction contained a moderate concentration of platelets (1.5–2.5 times compared with peripheral blood) and an absence of erythrocytes and leukocytes. Calcium chloride (10% w/v) was added as an activator. All procedures were performed under sterile conditions. 14
PRP quality control
During routine clinical practice, blood and PRP samples are collected randomly and periodically from patients undergoing treatment. Both types of samples are analysed in the Sysmex XS-1000i haematology analyser (Sysmex, Kobe, Japan) to verify that the PRP is elaborated correctly and complies with the parameters indicated by the manufacturer.
Treatments
The intraarticular administration consisted of 8 ml of PRP infiltrated into the articular space after evacuating the totality of the synovial fluid. One PRP cycle consisted of three intraarticular infiltrations on a weekly basis.
In the first treatment visit, intraosseous administration included three different injections of 2 ml (patella) and 5 ml (femoral head and tibial plateau) into different anatomical locations, conducted in the operating room. Following one PRP intraarticular injection, two PRP intraosseous injections were performed depending on the location of the degeneration, in accordance with the technique described by Sánchez et al. 15 Two more intraarticular PRP infiltrations were performed over the 2 weeks following the first visit to complete the PRP administration cycle. 16
In both cases, patients could opt for a second PRP cycle approximately 6 months after the first, depending on the physician’s recommendation after a follow-up visit which consisted of a clinical and physical evaluation.
Outcome evaluation
Patients filled out KOOS at baseline, 6 months and 15 months (a follow-up window of between 12 and 18 months) after the third injection of the first cycle of PRP. The primary efficacy criterion was a change from baseline in joint pain, measured using the KOOS pain subscale. Success rates were calculated according to a reduction in the pain score of at least 10 points from baseline (Minimal Clinically Important Improvement (MCII)). 17 Secondary variables included changes in KOOS subscales for symptoms, activities of daily living (ADL), function in sport and recreation (Sport/Rec) and knee-related quality of life (QOL).
Statistical analyses
Demographic and medical variables were determined by the mean and standard deviation for parametric data, and median and 95% confidence interval (CI) for non-parametric data. A comparison of the patients’ success rate percentages was carried out using the χ2 test. Comparisons were performed by Student’s t test for independent or paired parametric data, Wilcoxon signed-rank test for paired non-parametric data and Mann–Whitney U test for independent non-parametric data. Multivariate logistic regression was performed to analyse the influence of the different variables considered collectively, calculating coefficients (B), p value, odds ratios (ORs) and 95% CI. Distribution of the samples was assessed by Shapiro–Wilk’s test. Data were considered statistically significant when p < 0.05. Statistical analysis was performed with SPSS 20.0 (SPSS, Chicago, IL, USA).
Results
PRP characterization
A total of 445 blood samples and corresponding PRP sample were analysed at random. The median PRP platelet concentration was 309 × 103 platelets/ml (CI: 297–327), reaching a concentration factor of 1.67 (CI: 1.63–1.73), and with no leukocytes or erythrocytes. In accordance with the latest coding system and minimum reporting requirements for PRP studies, the PRP used in this study was 13-00-11, and the characteristics of the PRP are reported in Table 1. 18
Table 1.
Characteristics of platelet rich-plasma.
| Parameter | Values |
|---|---|
| PRP preparation | |
| Initial blood volume | 32 ml (intraarticular) or 80 ml (intraosseous) |
| Anticoagulant | Sodium citrate 3.8% (w/v) |
| System | Close |
| Centrifugation | Yes |
| Number | 1 |
| Speed | 580 × g for 8 min |
| Final PRP volume | 8 ml (intraarticular) or 20 ml (intraosseous) |
| PRP characteristics | |
| PRP type | 13-00-11 |
| MPV | 9.60 fl (CI: 9.50–9.80) |
| Red blood cells | <0.01 × 106/µl |
| White blood cells | <0.05 × 106/µl |
| Neutrophils | – |
| Lymphocytes | – |
| Monocytes | – |
| Eosinophils | – |
| Basophils | – |
| Activation | CaCl2 (10% w/v) |
| Application characteristics | |
| Formulation type | Liquid |
| Administration route | Intraarticular or intraosseous |
| Dosage | 3 infiltrations on a weekly basis |
| Volume | Intraarticular injection: 8 ml Intraosseous injection: 3–5 ml |
| Dose (range of platelets) | Intraarticular injection:
2.37 × 109–2.62 × 109
Intraosseous injection: 0.89 × 109–1.64 × 109 |
| Tissue | Cartilage, synovium, subchondral bone |
| Pathology | Knee joint degeneration |
CI, confidence interval; PRP, platelet-rich plasma; MPV, mean platelet volume.
Demographics, the overall effectiveness of PRP and influence of patient factors
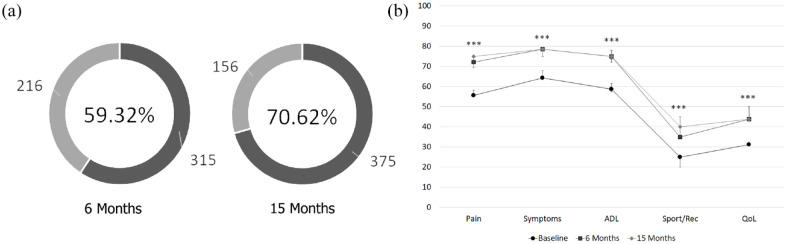
The study analysed a total of 441 patients (531 knees; Figure 1). The median age was 60.47 years (CI: 59.41–61.87), with a mean BMI of 28.65 (CI: 28.09–29.25) and a percentage of females of 47.47%. The percentage of cases who showed a pain reduction of at least 10 points (MCII) from baseline to 6 months was 59.32% (315 out of 531), and 70.62% (375 out of 531) by 15 months. All KOOS scores showed a significant statistical increase at 6 months post-treatment, with improvement maintained at 15 months post-treatment (p < 0.0001; Figure 2).
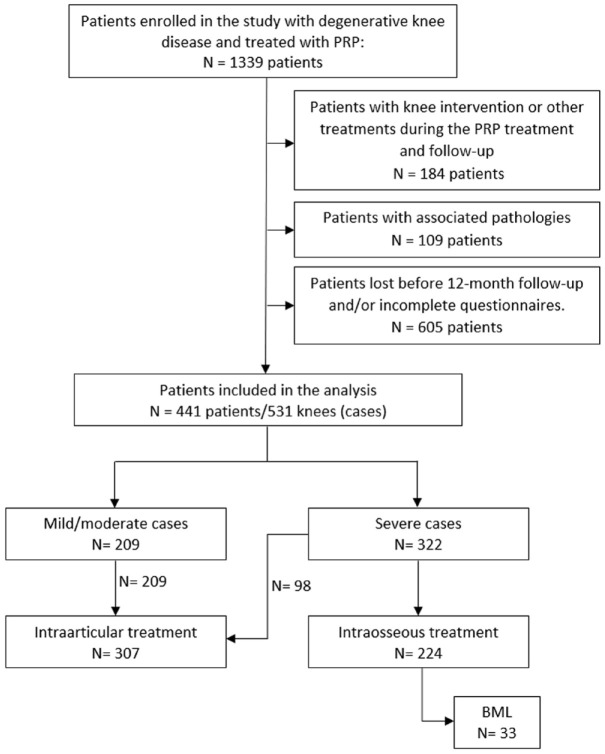
Figure 1.
Study flowchart. Selection of eligible patients and distribution of cases analysed according to severity and treatment.
BML, bone marrow lesion; PRP, platelet-rich plasma.
Figure 2.
Overall effectiveness of platelet-rich plasma. Percentage of MCII patients at 6 and 15 months after treatment (a). KOOS scores before and after treatment (b).
ADL, activities of daily living; CI: confidence interval; KOOS, Knee injury and Osteoarthritis Outcome Score; MCII, Minimal Clinically Important Improvement; QOL, knee-related quality of life; Sport/Rec, function in sport and recreation.
Error bars: CI. *p < 0.05; **p < 0.001; ***p < 0.0001.
Patients with MCII at both 6 and 15 months were significantly younger than those not experiencing clinical improvement, with a median age of 62 years at 6 months (CI: 60–64) compared with 65 years (CI: 63–66) (p = 0.0137). At 15 months, the age of patients with MCII (62; CI: 60–63) was significantly lower than that of patients without MCII (65; CI: 64–67) (p = 0.0246). Concerning BMI, at 15 months, patients with MCII (27.62; CI: 27.00–28.23) presented a BMI significantly lower than that of patients without MCII (28.03; CI: 27.41–29.51) (p = 0.0450).
Influence of pathology factors: severity and location
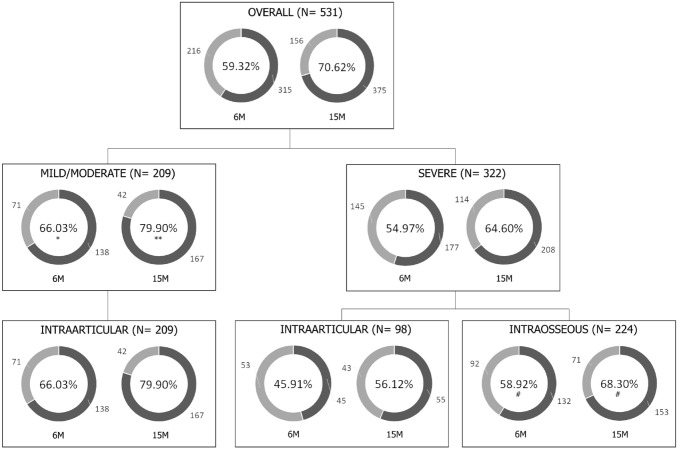
Of the 531 cases, 39.36% (209 out of 531) were mild/moderate, and the response of these was 66.03% at 6 months and 79.90% at 15 months, 11.06 percentage points higher than the severe cases at 6 months (CI: 2.52–19.23; p = 0.0113) and 15.31 percentage points higher at 15 months (CI: 7.52–22.56; p = 0.0002) (Figure 3). The difference in scores for increase in pain (p = 0.0040) and Sport/Rec (p = 0.0457) between these two groups was also significant. There was no difference in age (p = 0.3611) or BMI (p = 0.24.96) between patients in the two severity groups.
Figure 3.
Percentage of patients with MCII according to severity and route of administration.
6M, follow-up at 6 months; 15M, follow-up at 15 months; MCII, Minimal Clinically Important Improvement.
*p < 0.05; **p < 0.001 with respect to severe cases; #p < 0.05 with respect to severe cases treated with intraarticular infiltrations.
Of the cases, 48% presented synovial fluid leakage in the knee (254 out of 531), this being a significant feature in severe osteoarthritis with a difference of 22.05 (CI: 14.41–30.16) percentage points compared with mild/moderate pathologies (p < 0.0001). The presence of synovial fluid did not influence treatment efficacy. At 6 and 15 months post-treatment, the percentage of patients with no more joint effusion was 73.62% and 68.50%, respectively.
The extension of degeneration did not influence the clinical outcomes of PRP (Supplementary Table S1). In unicompartmental pathologies, mild/moderate cases affecting the patellofemoral joint achieved a better result than tibiofemoral cases of the same grade (Supplementary Table S2). Those patients were much younger (39 years; CI: 35–45) and had a lower BMI (25.50; CI: 24.02–25.90) than those with mild/moderate tibiofemoral degeneration (66 years; CI: 64–48 and 28.48; CI: 26.96–30.88). Detailed analysis of all locations showed no difference in treatment response (Supplementary Table S3).
Influence of protocol factors: PRP cycles and administration route
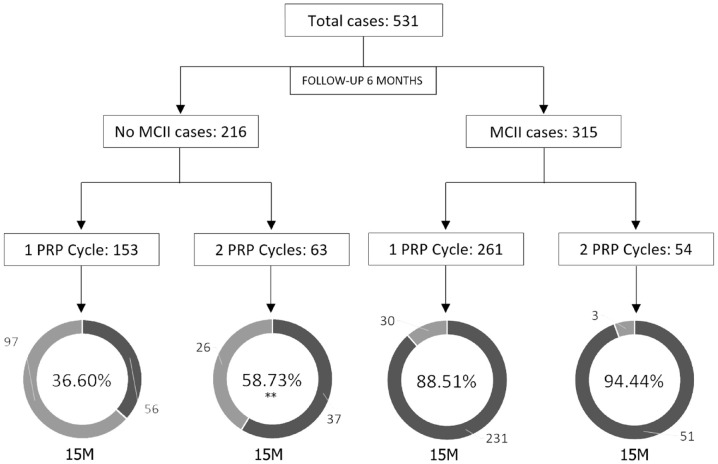
Of all the cases analysed, 117 opted for a second PRP cycle after the first follow-up period, 63 without MCII at 6 months and 54 with a positive response (Figure 4). Of the first 63 patients, 37 achieved MCII after the second PRP cycle (58.73%), 22.13 points higher than the group showing no MCII at 6 months and that did not receive a second cycle of PRP (CI: 7.51–35.54; p = 0.0029). This improvement was only observed in mild/moderate pathologies, with 25.25 percentage points more in the group with the second cycle of PRP (CI: 1.93–44.82.54; p = 0.0357; Supplementary Table S4). In terms of the effect of a second PRP cycle in sustaining the effect of treatment over time, there was no difference between the 54 patients with MCII at 6 months who opted for a second PRP cycle versus those who did not repeat the PRP cycle (p = 0.1951; Supplementary Table S5).
Figure 4.
Percentage of patients with MCII according to number of cycles of PRP.
15M, follow-up at 15 months; MCII, Minimal Clinically Important Improvement; PRP, platelet-rich plasma.
*p < 0.05 with respect to the group receiving one PRP cycle.
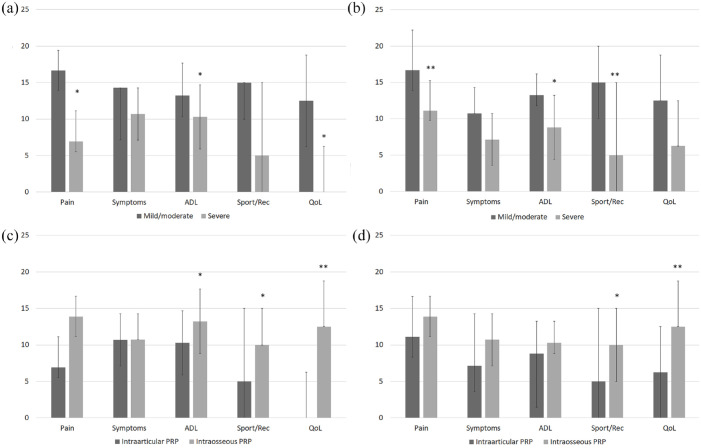
Of the 531 cases, 307 received intraarticular treatment, with 59.61% of patients (183 out of 307) showing MCII at 6 months and 72.31% at 15 months (222 out of 307). This improvement was greater in mild/moderate pathology cases, at 66.03% (138 out of 209) and 79.90% (167 out of 209), respectively. However, in severe pathologies, MCII was 45.91% (45 out of 98) at 6 months and 56.12% (55 out of 98) at 15 months (Figure 3). The increase in KOOS scores was also significantly greater in mild/moderate cases than in severe cases (Figure 5(a) and (b)).
Figure 5.
Differences in response according to severity and route of administration. Differences in the increase in KOOS scores according to severity after intraarticular PRP treatment at 6 (a) and 15 (b) months follow-up. Differences in the increase of KOOS scores according to the type of treatment in severe pathologies at 6 (c) and 15 (d) months follow-up.
ADL, activities of daily living; KOOS, Knee injury and Osteoarthritis Outcome Score; QOL, knee-related quality of life; Sport/Rec, function in sport and recreation.
Error bars: CI. *p < 0.05; **p < 0.001; ***p < 0.0001.
The effectiveness in treating severe pathologies was significantly improved when PRP was administered via intraosseous route. Of the 531 cases, 224 received intraosseous treatment, all with severe pathology. When comparing the clinical outcome in severe pathology, the group receiving intraosseous PRP had an MCII rate of 58.92% at 6 months, 13.01 percentage points higher compared with patients receiving intraarticular treatment (CI: 1.19–24.39; p = 0.0311). The MCII rate at 15 months was 68.30%, 12.18 percentage points higher than the response to intraarticular PRP (CI: 0.82–23.59; p = 0.0358; Figure 3) (Supplementary Table S6). Increases in KOOS scores were also greater in cases treated with intraosseous PRP (Figure 5(c) and (d); Supplementary Table S7).
Bone marrow lesions
In 33 of the 531 cases, bone marrow lesions (BMLs) were detected by magnetic resonance imaging (MRI) and treated with intraosseous PRP. The average age of this group of patients was 49 ± 14.82 years with a BMI of 26.96 ± 4.25 and a female percentage of 42.42%.
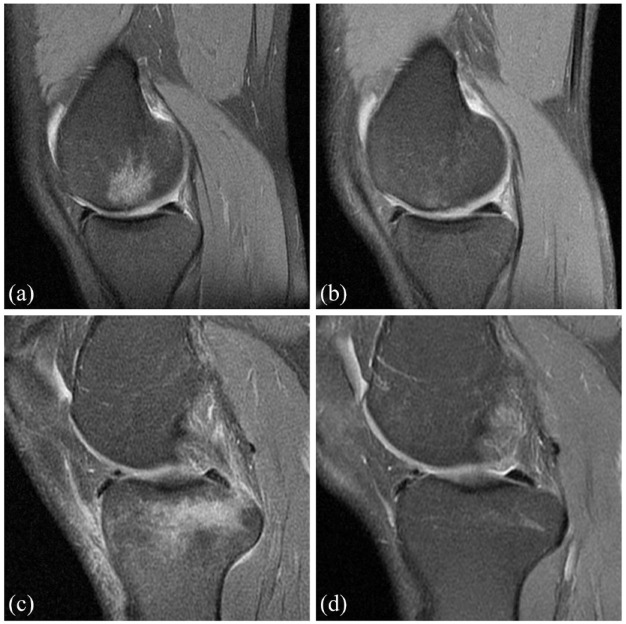
The percentage of patients with MCII at 6 and 15 months post-treatment was 69.70% (23 out of 33) and 78.79% (26 out of 33), respectively. All KOOS scores improved significantly at both 6 and 15 months post-treatment (p < 0.001). During follow-up MRI studies, the decrease (41.94%) and removal (41.94%) of the BML image were observed (Figure 6).
Figure 6.
MRI images of BML. Before (a) and 15 months after (b) intraosseous PRP treatment of a BML in the medial femoral condyle. Before (c) and 15 months after (d) intraosseous PRP treatment of a BML in the tibial plateau.
BML, bone marrow lesion; MRI, magnetic resonance imaging.
Multivariate logistic regression
The multivariate logistic regression model indicated that medium/moderate severity (p = 0.001) and intraosseous application (p = 0.024) significantly favoured positive response to treatment at 6 months (Table 2). The 12-month model (Table 3) showed the strong influence of severity, with a better response in patients with mild/moderate pathologies (p < 0.001).
Table 2.
Multivariate regression analysis for response at 6 months.
| Variable | B | p value | 95% CI | OR |
|---|---|---|---|---|
| Age | –0.008 | 0.291 | 0.977–1.007 | 0.992 |
| BMI | –0.017 | 0.408 | 0.943–1.024 | 0.983 |
| Severity (mild–moderate/severe) | 0.870 | 0.001* | 1.397–4.076 | 2.387 |
| Administration route (IA/IO) | –0.604 | 0.024* | 0.324–0.923 | 0.546 |
B, coefficient; BMI, body mass index; CI, confidence interval; IA, intraarticular; IO, intraosseous; OR, odds ratio.
p < 0.05.
Table 3.
Multivariate regression analysis for response at 15 months.
| Variable | B | p value | 95% CI | OR |
|---|---|---|---|---|
| Age | −0.008 | 0.378 | 0.975−1.009 | 0.992 |
| BMI | −0.039 | 0.086 | 0.921–1.005 | 0.962 |
| Severity (mild–moderate/severe) | 1.145 | <0.001* | 1.757–5.615 | 3.141 |
| Administration route (IA/IO) | −0.473 | 0.088 | 0.362–1.073 | 0.623 |
| PRP cycles | 0.123 | 0.657 | 0.658–1.942 | 1.130 |
B, coefficient; BMI, body mass index; CI, confidence interval; IA, intraarticular; IO, intraosseous; OR, odds ratio; PRP, platelet-rich plasma.
p < 0.05.
Discussion
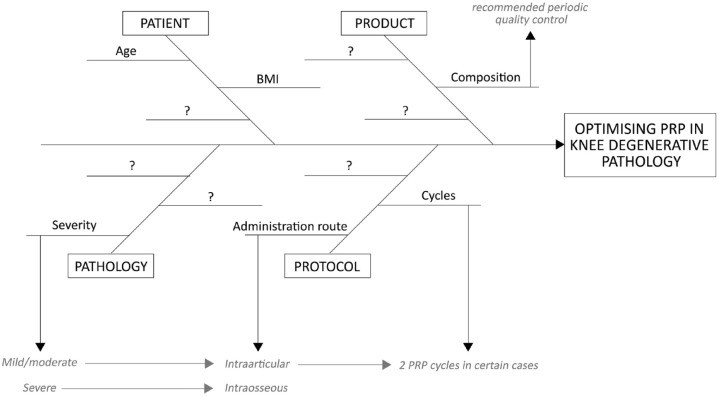
The present study examines 531 cases of degenerative knee pathology treated with PRP and followed up for at least 1 year. The overall effectiveness of PRP was more than 70%, based on the percentage of patients with MCII. This response is influenced by patient, pathology, product and protocol (4P) factors. Thus, these ‘four Ps’ should be taken into consideration in order to achieve an optimal clinical outcome (Figure 7).
Figure 7.
The ‘four Ps’ influencing the effectiveness of PRP. The clinical response to PRP treatment is influenced by many factors related to the patient, the pathology, the product and the protocol, many of which are still unknown.
PRP, platelet-rich plasma.
RWE studies are a valuable tool to analyse the effectiveness of treatment in a real scenario and the factors that may influence it. Although RCTs are essential to evidence-based medicine, they present several limitations that hinder this type of analysis. Concerning PRP, RCTs evaluate a limited number of subjects, and although they are subsequently examined in meta-analyses, the data obtained can be misleading due to the different PRP products and treatment protocols. In contrast, despite their inherent limitations, RWE studies reflect the response to treatment in the real world and allow us to reach a sufficient number of patients to analyse the different variables that could influence the response. The information obtained in both types of studies is useful in helping to find the best possible treatment.9–11
The present study is the prospective observational study to have analysed the largest number of patients to date. The results obtained align with the latest RCTs and meta-analyses published, indicating that PRP is an effective treatment for degenerative knee pathologies. Our work considers clinical outcome the results of the scores and their clinical significance according to the MCII, because a statistically significant difference in the scores does not mean a clinical one.8,10–21 This is becoming increasingly important in the analysis 22 and allows a better interpretation. 23 It should be noted that the type of PRP applied to all the patients in the study was obtained using the same system and with the same cellular composition in accordance with the quality control carried out, so it is a variable that did not interfere in the results analysed.
The only study with similar characteristics that can be compared with the present work was that carried out by Korpershoek et al., 24 which analysed 158 cases treated with Autologous Conditioned Plasma (ACP). The authors obtained similar results to ours in terms of KOOS scores, with significant differences at 6 and 12 months from baseline. However, the MCII values are lower, which may be due to several factors such as patient characteristics. While the work of Korpershoek et al. focuses only on knee osteoarthritis, the present work encompasses all joint degeneration. We must underline the high efficacy (more than 90%) of the treatment in cases of chondromalacia patellae, which correspond to mild/moderate cases of patellofemoral pathology. Although the data obtained do not suggest the influence of location on the efficacy of the treatment, the typology of these patients, 25 with early degeneration, young age and low BMI, makes them highly suitable candidates for PRP treatment, as these are key factors in the response to treatment according to the data obtained. In the case of age, the enhanced response could be due to improved health and the molecular composition of the PRP. 26 Very few studies have evaluated the effect of PRP in chondromalacia patellae achieving good results both in imaging and clinical studies.27–29 This treatment should be considered an option not only for the improvement of the patient but also to attempt to prevent more serious conditions such as patellofemoral osteoarthritis.
A second key factor is the type of product and the application protocol. Korpershoek et al. used ACP that is similar to that used in our study. This PRP does not present leukocytes and erythrocytes, which could make it the most suitable for this type of pathology.30,31 However, the platelet concentration reached by Korpersoek et al. doubled ours and was applied in a volume of only 3–5 ml, compared with 8 ml in our protocol. It makes the number of platelets similar in each intraarticular infiltration (around 2.5×109 ) in a different volume. Furthermore, a recent RCT that failed to demonstrate the superiority of PRP versus placebo used a type of PRP with a large variability in concentration factor (1.6×–5×) and a volume of 5 ml. 32 Thus, not only the type of product but also the volume needs to be kept in mind. The intraarticular distribution of the PRP must be adequate and reach all the tissue, which is achieved with volumes of approximately 9 ml. 33 Guillibert et al. 34 observed that a high-volume (8 ml) administration of PRP achieved a clinical improvement of more than 80%, proposing this administration as a better alternative to repeated low-volume infiltration protocols. Furthermore, injecting smaller volumes would also lead to the delivery of less therapeutic content present in the plasma such as exosomes or other biomolecules.35,36
Regarding the treatment protocol, previous clinical studies suggest that repeated weekly PRP injections are more effective than a single dose. 37 In addition, kinetic studies showed a release of biomolecules from fibrin during 1 week. 38 However, the application of repeated PRP cycles over time is still little studied. According to the present work, the effect of a second cycle after the first 6 months could be a useful recommendation in certain cases. In an RCT conducted by Vaquerizo et al., 39 using the same PRP and the same protocol, the authors observed an improvement in symptoms and functionality in patients with two cycles, but not in pain, as in the present study. However, this occurred in cases that had not yet shown a positive clinical response at 6 months. In severe cases, it did not increase efficacy, being effective only in patients with mild/moderate pathology. Therefore, although the application of the second cycle of PRP would be advisable to enhance or accelerate the response in patients with mild/moderate pathology, severe pathologies present characteristics and a degree of complexity for which intraarticular application may be insufficient. 16 Patients with severe pathologies treated with intraosseous PRP showed a significant improvement compared with those who received only intraarticular PRP. In these cases, key tissues such as the subchondral bone are more greatly affected. The data obtained in the multivariate analysis confirmed the importance of the severity of the pathology, as well as the higher impact of the route of administration compared with the repetition of cycles. Direct application of PRP extends the range of action and acts on detrimental processes such as the growth of fibroneurovascular tissue, mesenchymal stem cell alterations or biomolecular imbalance. 40 Recent clinical studies suggest the effectiveness and safety of this technique and are consistent with the data obtained in this study, although further study of this route of administration is needed. 41 In fact, this administration could be used for other products such as cell concentrates, although in these cases numerous variables such as age, dosage, composition, protocol or adjuvant substances must be taken into consideration because, otherwise, optimal clinical results may not be achieved. 42 Intraosseous PRP in patients with BML was also shown to be highly effective in line with a recent study that showed a significant decrease in pain 1 year after treatment. 43 These subchondral bone lesions are associated with cartilage loss, and they can also be the origin of joint degeneration.
The effective results obtained in this work together with the limited adverse effects, characterized by episodes of pain in the infiltration area during the following hours, suggest that the reasonable application of PRP is a valid treatment in the management of these pathologies. Along with RCT and RWE studies, cost-effectiveness should also be taken into account for a full assessment of this treatment. Recent studies indicate that PRP could be cost-effective in the long term, although further research is needed.44,45
The limitations of this study are inherent to RWE studies, which lack the strengths of RTCs, namely, randomization, control and more specific follow-up times. The loss of patients during follow-up is considerable, hampering longer follow-ups which could provide important information as in previous studies, 46 and making it necessary for large-scale recruitment. Furthermore, despite a large number of patients, ‘N’ was insufficient to draw solid conclusions for some subgroups. Finally, this type of study assesses clinical outcomes which may be due to an improvement in symptomatology rather than a modification of the disease. However, findings such as the disappearance of BML or reduction in joint effusion after treatment could suggest an effect on the origin of this pathology. Indeed, the presence of synovial fluid was reduced in more than 60% of patients after treatment, which could be a sign of a positive impact on the progression of the disease. 47 In this regard, a recent work conducted by Boffa et al. 48 reviewed in vivo studies demonstrating disease modification by PRP administration. Evaluation of these modifications in clinical research using imaging or surgical studies 46 would help to clarify the action of PRP and its mechanisms.
Conclusion
The application of PRP in degenerative knee pathologies is an effective treatment, but this effectiveness nonetheless depends on several variables. Far from considering PRP to be a magic bullet, the physician must consider certain variables related to the patient, the pathology, the product and the protocol to optimize this treatment. Complementing the information from RCTs with that obtained from RWE studies can be a valuable tool for advancing our understanding of PRP.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-3-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-4-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-5-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-6-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-7-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors wish to thank M.B. Sánchez, A. Iriondo, M. Montoya, M.J. Arnaiz and C. Pérez de Arrilucea for their involvement in the processing of the PRP samples.
Footnotes
Ethics approval and consent to participate: Ethical approval for this study (Protocol No. EPA2015046) was obtained from the Ethics Committee of the Basque Country.
Consent for publication: Written consent was obtained
Author contribution(s): Mikel Sánchez: Conceptualization; Investigation; Methodology; Writing – review & editing.
Cristina Jorquera: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Leonor López de Dicastillo: Investigation.
Nicolás Fiz: Investigation; Writing – review & editing.
Jorge Knörr: Investigation.
Maider Beitia: Formal analysis; Writing – original draft.
Beatriz Aizpurua: Investigation.
Juan Azofra: Investigation.
Diego Delgado: Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
ORCID iDs: Mikel Sánchez  https://orcid.org/0000-0001-9992-6927
https://orcid.org/0000-0001-9992-6927
Diego Delgado  https://orcid.org/0000-0002-0494-0804
https://orcid.org/0000-0002-0494-0804
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The data underlying this article are available in the article and in its online supplementary material.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mikel Sánchez, Arthroscopic Surgery Unit, Hospital Vithas Vitoria, Vitoria-Gasteiz, Spain; Advanced Biological Therapy Unit, Hospital Vithas Vitoria, Vitoria-Gasteiz, Spain.
Cristina Jorquera, Advanced Biological Therapy Unit, Hospital Vithas Vitoria, Vitoria-Gasteiz, Spain.
Leonor López de Dicastillo, Arthroscopic Surgery Unit, Hospital Vithas Vitoria, Vitoria-Gasteiz, Spain.
Nicolás Fiz, Arthroscopic Surgery Unit, Hospital Vithas Vitoria, Vitoria-Gasteiz, Spain.
Jorge Knörr, Arthroscopic Surgery Unit, Hospital Vithas Vitoria, Vitoria-Gasteiz, Spain.
Maider Beitia, Advanced Biological Therapy Unit, Hospital Vithas Vitoria, Vitoria-Gasteiz, Spain.
Beatriz Aizpurua, Arthroscopic Surgery Unit, Hospital Vithas Vitoria, Vitoria-Gasteiz, Spain.
Juan Azofra, Arthroscopic Surgery Unit, Hospital Vithas Vitoria, Vitoria-Gasteiz, Spain.
Diego Delgado, Advanced Biological Therapy Unit, Hospital Vithas Vitoria, Beato Tomás de Zumarraga 10, 01008 Vitoria-Gasteiz, Spain.
References
- 1. Cui A, Li H, Wang D, et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. eClinicalMedicine 2020; 29–30: 100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Driban JB, Harkey MS, Liu SH, et al. Osteoarthritis and aging: young adults with osteoarthritis. Curr Epidemiol Rep 2020; 7: 9–15. https://link.springer.com/article/10.1007/s40471-020-00224-7 (accessed 16 August 2021). [Google Scholar]
- 3. Sánchez M, Anitua E, Delgado D, et al. A new strategy to tackle severe knee osteoarthritis: combination of intra-articular and intraosseous injections of platelet rich plasma. Expert Opin Biol Ther 2016; 16: 627–643. [DOI] [PubMed] [Google Scholar]
- 4. Ackerman IN, Bohensky MA, Zomer E, et al. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet Disord 2019; 20: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 2015; 67: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andia I, Atilano L, Maffulli N. Moving toward targeting the right phenotype with the right platelet-rich plasma (PRP) formulation for knee osteoarthritis. Ther Adv Musculoskelet Dis 2021; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sánchez M, Beitia M, Pompei O, et al. Isolation, activation, and mechanism of action of platelet-rich plasma and its applications for joint repair. IntechOpen, 2019, https://www.intechopen.com/chapters/70503 [Google Scholar]
- 8. Hong M, Cheng C, Sun X, et al. Efficacy and safety of intra-articular platelet-rich plasma in osteoarthritis knee: a systematic review and meta-analysis. Biomed Res Int 2021; 2021: 2191926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim H-S, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci 2018; 33: e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maissenhaelter BE, Woolmore AL, Schlag PM. Real-world evidence research based on big data: motivation-challenges-success factors. Onkologe (Berl) 2018; 24: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Misra DP, Agarwal V. Real-world evidence in rheumatic diseases: relevance and lessons learnt. Rheumatol Int 2019; 39: 403–416. [DOI] [PubMed] [Google Scholar]
- 12. Betancourt JP, Murrell WD. Leukocyte-poor platelet-rich plasma to treat degenerative meniscal tear: a case report. J Clin Orthop Trauma 2016; 7: 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elm E, von Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sánchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy 2012; 28: 1070–1078. [DOI] [PubMed] [Google Scholar]
- 15. Sánchez M, Fiz N, Guadilla J, et al. Intraosseous infiltration of platelet-rich plasma for severe knee osteoarthritis. Arthrosc Tech 2014; 3: e713–e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sánchez M, Delgado D, Pompei O, et al. Treating severe knee osteoarthritis with combination of intra-osseous and intra-articular infiltrations of platelet-rich plasma: an observational study. Cartilage 2019; 10: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003; 1: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kon E, Di Matteo B, Delgado D, et al. Platelet-rich plasma for the treatment of knee osteoarthritis: an expert opinion and proposal for a novel classification and coding system. Expert Opin Biol Ther 2020; 20: 1447–1460. [DOI] [PubMed] [Google Scholar]
- 19. McLarnon M, Heron N. Intra-articular platelet-rich plasma injections versus intra-articular corticosteroid injections for symptomatic management of knee osteoarthritis: systematic review and meta-analysis. BMC Musculoskelet Disord 2021; 22: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nie LY, Zhao K, Ruan J, et al. Effectiveness of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled clinical trials. Orthop J Sports Med 2021; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med 2021; 49: 249–260. [DOI] [PubMed] [Google Scholar]
- 22. Filardo G, Previtali D, Napoli F, et al. PRP injections for the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Cartilage 2021; 13: 364S–375S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page P. Beyond statistical significance: clinical interpretation of rehabilitation research literature. Int J Sports Phys Ther 2014; 9: 726–736. [PMC free article] [PubMed] [Google Scholar]
- 24. Korpershoek JV, Vonk LA, De Windt TS, et al. Intra-articular injection with Autologous Conditioned Plasma does not lead to a clinically relevant improvement of knee osteoarthritis: a prospective case series of 140 patients with 1-year follow-up. Acta Orthop 2020; 91: 743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krieger EAG, Karam FC, Soder RB, et al. Prevalence of patellar chondropathy on 3.0 T magnetic resonance imaging. Radiol Bras 2020; 53: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delgado D, Bilbao AM, Beitia M, et al. Effects of platelet-rich plasma on cellular populations of the central nervous system: the influence of donor age. Int J Mol Sci 2021; 22: 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pak J, Lee JH, Lee SH. A novel biological approach to treat chondromalacia patellae. PLoS ONE 2013; 8: e64569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng C, Zhu Q, Liu X, et al. Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. J Tissue Eng Regen Med 2016; 10: 428–436. [DOI] [PubMed] [Google Scholar]
- 29. Cobianchi Bellisari F, De Marino L, Arrigoni F, et al. T2-mapping MRI evaluation of patellofemoral cartilage in patients submitted to intra-articular platelet-rich plasma (PRP) injections. Radiol Med 2021; 126: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riboh JC, Saltzman BM, Yanke AB, et al. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med 2016; 44: 792–800. [DOI] [PubMed] [Google Scholar]
- 31. Kim JH, Park YB, Ha CW, et al. Adverse reactions and clinical outcomes for leukocyte-poor versus leukocyte-rich platelet-rich plasma in knee osteoarthritis: a systematic review and meta-analysis. Orthop J Sports Med 2021; 9: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bennell KL, Paterson KL, Metcalf BR, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the restore randomized clinical trial. JAMA 2021; 326: 2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rastogi AK, Davis KW, Ross A, et al. Fundamentals of joint injection. Am J Roentgenol 2016; 207: 484–494. [DOI] [PubMed] [Google Scholar]
- 34. Guillibert C, Charpin C, Raffray M, et al. Single injection of high volume of autologous pure PRP provides a significant improvement in knee osteoarthritis: a prospective routine care study. Int J Mol Sci 2019; 20: E1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torreggiani E, Perut F, Roncuzzi L, et al. Exosomes: novel effectors of human platelet lysate activity. Eur Cell Mater 2014; 28: 137–151. [DOI] [PubMed] [Google Scholar]
- 36. Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011; 477: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Görmeli G, Görmeli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc 2017; 25: 958–965. [DOI] [PubMed] [Google Scholar]
- 38. Anitua E, Zalduendo MM, Alkhraisat MH, et al. Release kinetics of platelet-derived and plasma-derived growth factors from autologous plasma rich in growth factors. Ann Anat 2013; 195: 461–466. [DOI] [PubMed] [Google Scholar]
- 39. Vaquerizo V, Padilla S, Aguirre JJ, et al. Two cycles of plasma rich in growth factors (PRGF-Endoret) intra-articular injections improve stiffness and activities of daily living but not pain compared to one cycle on patients with symptomatic knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2018; 26: 2615–2621. [DOI] [PubMed] [Google Scholar]
- 40. Delgado D, Garate A, Vincent H, et al. Current concepts in intraosseous platelet-rich plasma injections for knee osteoarthritis. J Clin Orthop Trauma 2019; 10: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Betzler BK, Chee Y-YJ Bin Abd Razak HR. Intraosseous injections are safe and effective in knee osteoarthritis: a systematic review. Arthrosc Sports Med Rehabil 2021; 3: e1557–e1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Centeno C, Cartier C, Stemper I, et al. The treatment of bone marrow lesions associated with advanced knee osteoarthritis: comparing intraosseous and intraarticular injections with bone marrow concentrate and platelet products. Pain Physician 2021; 24: E279–E288. [PubMed] [Google Scholar]
- 43. Lychagin A, Lipina M, Garkavi A, et al. Intraosseous injections of platelet rich plasma for knee bone marrow lesions treatment: one year follow-up. Int Orthop 2021; 45: 355–363. [DOI] [PubMed] [Google Scholar]
- 44. Samuelson EM, Ebel JA, Reynolds SB, et al. The cost-effectiveness of platelet-rich plasma compared with hyaluronic acid injections for the treatment of knee osteoarthritis. Arthroscopy 2020; 36: 3072–3078. [DOI] [PubMed] [Google Scholar]
- 45. Rajan PV, Ng MK, Klika A, et al. The cost-effectiveness of platelet-rich plasma injections for knee osteoarthritis: a Markov decision analysis. J Bone Joint Surg Am 2020; 102: e104. [DOI] [PubMed] [Google Scholar]
- 46. Sánchez M, Jorquera C, Sánchez P, et al. Platelet-rich plasma injections delay the need for knee arthroplasty: a retrospective study and survival analysis. Int Orthop 2021; 45: 401–410. [DOI] [PubMed] [Google Scholar]
- 47. Wang Y, Teichtahl AJ, Pelletier J-P, et al. Knee effusion volume assessed by magnetic resonance imaging and progression of knee osteoarthritis: data from the osteoarthritis initiative. Rheumatology 2019; 58: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boffa A, Salerno M, Merli G, et al. Platelet-rich plasma injections induce disease-modifying effects in the treatment of osteoarthritis in animal models. Knee Surg Sports Traumatol Arthrosc 2021; 29: 4100–4121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-3-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-4-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-5-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-6-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-7-tab-10.1177_1759720X221100304 for Real-world evidence to assess the effectiveness of platelet-rich plasma in the treatment of knee degenerative pathology: a prospective observational study by Mikel Sánchez, Cristina Jorquera, Leonor López de Dicastillo, Nicolás Fiz, Jorge KnKnörrrr, Maider Beitia, Beatriz Aizpurua, Juan Azofra and Diego Delgado in Therapeutic Advances in Musculoskeletal Disease