Abstract
Background: Low muscle mass is associated with worse cancer treatment outcomes. Although dual-energy X-ray absorptiometry or computerized tomography-based analysis have both been widely studied in this clinical setting, studies in the use of bioelectrical impedance analysis (BIA) remain limited. The aim of this prospective study was to investigate for association between body composition estimated by BIA and hematologic adverse events in early-stage breast cancer patients receiving chemotherapy. Methods: A total of 144 female patients were enrolled. Before the first cycle of chemotherapy, body weight and fat-free mass were measured by a BIA device and then those values were converted into body mass index and fat-free mass index. Association between fat-free mass index and composite adverse events (CAEs), including grade 4 neutropenia, febrile neutropenia, or relative dose intensity <85%, was explored. Results: CAEs occurred in 85 patients (59%), and point biserial correlation showed an inverse correlation between the fat-free mass index and CAE. No included patients were sarcopenic (fat-free mass index <11.4 kg/m2). Receiver operating characteristic curve analysis revealed <14.85 kg/m2 as the cutoff value indicating a low fat-free mass index. Using this cutoff, 85 patients were classified as having a low fat-free mass index, and 62 of those patients (72.9%) had CAE (relative risk: 1.86, P < .001). After adjusting for other factors, a low fat-free mass index was found to be independently associated with a high CAE (adjusted odds ratio: 4.562, 95% CI: 2.162-9.627, P < .001). Conclusion: Low fat-free mass index is an independent predictor of increased risk of hematologic adverse events in early-stage breast cancer patients receiving chemotherapy. Estimation of fat-free mass index by BIA may identify at-risk patients so that interventions can be considered to improve treatment outcomes.
Keywords: low fat-free mass index, bioelectrical impedance analysis, hematologic adverse events, early-stage breast cancer patients, chemotherapy
Introduction
Breast cancer is the most common cancer, and the leading cause of cancer death among women worldwide, including in Thailand.1,2 Patients with early-stage breast cancer (EBC) usually require multimodality treatments, including surgery, adjuvant radiotherapy, and systemic therapy, which has successfully decreased breast cancer mortality. 3 Adjuvant systemic therapy includes endocrine therapy, human epidermal growth factor receptor 2 (HER2) targeted therapy, and chemotherapy. Although adjuvant chemotherapy improves survival, toxicity remains a major concern. Based on data from randomized controlled trials of adjuvant chemotherapy in node-positive breast cancer patients, grade 4 leukopenia was found in 17% of patients, and grade 3 or 4 non-hematologic adverse events occurred in 20% of patients. 4 Moreover, a retrospective cohort study of EBC patients treated with chemotherapy at our center found that 35% of patients developed grade 4 neutropenia, and febrile neutropenia occurred in 9%. 5
According to the Impact of Neutropenia in Chemotherapy—European Study Group (INC-EU) Prospective Observational European Neutropenia Study, older age, lower weight, higher planned dose intensity and number of planned cycles of anthracycline or taxane, vascular comorbidity, lower baseline white blood cell count, and higher baseline bilirubin were identified as independent risk factors for grade 4 neutropenia. 6 Moreover, a previous retrospective study by our group and a systematic review of the effect of body weight on chemotherapy-induced toxicity in EBC patients found that obese patients experienced significantly fewer neutropenic events when compared with the number of neutropenic events experienced by lean patients.5,7 This finding may be due to differences in nutritional status and body composition.
Body composition is a term used to describe the relative amounts of body components including fat mass, muscle mass, bone mass, and body water. Dual-energy X-ray absorptiometry (DXA) is a 3-compartment model that is widely used for body composition analysis. Other validated tools for measuring body composition include computerized tomography (CT)-defined skeletal muscle at the third lumbar vertebra (L3) level, and bioelectrical impedance analysis (BIA). A prior meta-analysis demonstrated low muscle mass, or sarcopenia to be associated with poor survival outcomes in patients with solid tumors. 8 Sarcopenia was also reported to be associated with toxicity outcome and time-to-treatment failure in metastatic breast cancer patients receiving taxane-based chemotherapy. 9 Furthermore, lower muscle mass and higher fat mass were found to be associated with increased risk of toxicity-induced modifications of treatment in EBC patients receiving chemotherapy.10,11
Most previous studies in cancer patients performed body composition measurement using either CT-based analysis or DXA scan. However, BIA is a widely available, easy to use, and less expensive method for predicting fat-free mass (FFM) with no radiation-associated risks to the patient. Moreover, BIA was evaluated and validated against DXA scan in both healthy persons and cancer patients.12,13 Accordingly, the aim of this study was to investigate for association between body composition estimated by BIA and hematologic adverse events in EBC patients receiving chemotherapy.
Materials and Methods
Study Design and Participants
This prospective observational cohort study was conducted at the Division of Medical Oncology of the Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand during June 2019 to December 2019. Female patients aged 18 years or older with histologically confirmed stage I-III breast cancer treated with (neo)adjuvant anthracycline-based and/or taxane-based chemotherapy were eligible for inclusion. Patients with cardiac pacemaker, cardioverter-defibrillator, orthopedic prosthesis, presence of ascites or pleural effusion, and patients who received primary prophylaxis with granulocyte colony-stimulating factor were excluded from this study. Patients who were lost to follow up before completing the fourth cycle (which prevented determination of the patient’s neutropenic status) were withdrawn from the final analysis. We conducted and reported the study in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines. 14 Written informed consent were obtained from all patients prior to enrollment in the study. We have de-identified all patient details to protect the privacy of the patients. The study protocol was approved by the Siriraj Institutional Review Board (SIRB) (Protocol no. 286/2562(EC3), COA no. Si346/2019), and was registered in the Thai Clinical Trials Registry (TCTR reg. no. TCTR20190702004).
Procedure
Demographic and clinical data including age, comorbidity, menopausal status, performance status, TNM stage, and tumor characteristics were collected at baseline. Before administration of the first cycle of chemotherapy, all participants were evaluated using a BIA device to measure the components of body composition, including body weight, fat mass, FFM, bone mass, and phase angle (PA). FFM includes muscle mass and vital organ mass, excluding fat mass and bone. PA reflects the relative contribution of fluid (resistance) and cell membranes (reactance) which is positively associated with reactance and negatively associated with resistance. Thus, higher PA suggests large quantities of intact cell membranes. 15 Body mass index (BMI) was calculated by dividing body weight in kilogram by height in meters squared (formula: weight (kg) / [height (m)]2). The formula for calculating fat-free mass index (FFMI) was FFM (kg) / [height (m)]2. In the original protocol, the plan was to measure each participant with 2 different BIA devices (InBody 770; InBody Co., Ltd; and, Tanita RD-800; Tanita Corporation). However, due to problems with the availability of the InBody 770, the protocol was amended to body composition measurement with at least one BIA device. Complete blood count was assessed before each cycle of chemotherapy and at the nadir period after the first cycle of chemotherapy. Neutropenia related adverse events were graded using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. The total dose of each chemotherapy, and the duration from the first cycle to the end of the chemotherapy were recorded and analyzed. Relative dose intensity (RDI) was calculated using the following equation:
Outcome
The primary objective was to investigate the association between a low FFMI and the composite adverse event (CAE) rate. CAE was defined as grade 4 neutropenia, febrile neutropenic event, and a dose reduction or delay due to neutropenia leading to an RDI <85%. The exploratory outcomes were the appropriate FFMI cut point, the sensitivity and specificity of the FFMI, and the effect size as defined by odds ratio, particularly in obese patients with a low FFMI. Agreement in the results between the 2 BIA devices used in this study was included as an exploratory endpoint in the amended protocol.
Sample Size Calculation and Statistical Analysis
A sample size of greater than 122 patients was calculated using the formula published by Green 16 with a 95% confidence interval and medium size of relationship with the following 9 factors included in the equation: age, performance status, comorbidity, stage, chemotherapy regimen, baseline white blood cell count, BMI, FFMI, and PA.
Patient baseline demographic and clinical characteristics were summarized using descriptive statistics, including number and percentage, median and range, and mean and range. Association between FFMI and CAE was investigated using point-biserial correlation estimation. Univariate and multivariate analyses were performed using binomial logistic regression analysis to identify factors independently associated with the CAEs outcome. The results of those analyses are presented as odds ratio (OR) and adjusted odds ratio (aOR)—both with their 95% confidence interval (CI). Regarding exploratory outcomes, prediction analysis of FFMI was performed using receiver operating characteristic (ROC) curve analysis, and the appropriate FFMI cutoff value was estimated using Youden’s index. In the amended protocol, agreement between the 2 BIA devices used in this study was analyzed using linear correlation, Bland-Altman plot, and paired Student’s t-test for mean comparison. Predictive performance parameters, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), were also examined. All statistical analyses were performed using PASW Statistics (formerly SPSS Statistics) version 18.0 (SPSS Inc.), and a P-value less than .05 was considered statistically significant for all tests.
Results
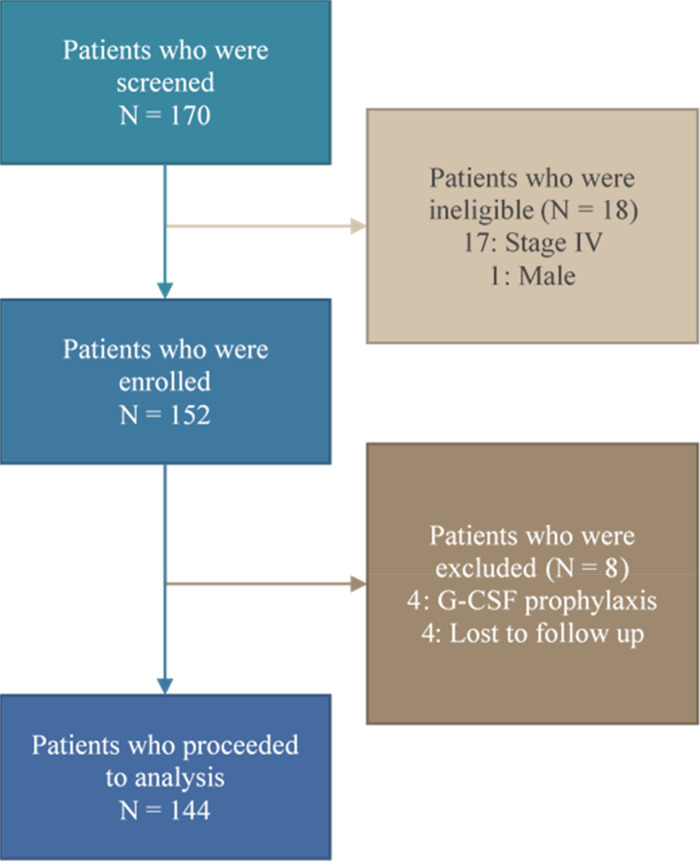
A total of 170 breast cancer patients were screened consecutively during June 2019 to November 2019. Eighteen patients were not enrolled due to advanced stage or male gender. Four patients receiving primary granulocyte colony-stimulating factor prophylaxis were also excluded. The remaining 148 patients were enrolled in this study. Of those, 4 patients were lost to follow up before completing the fourth cycle of chemotherapy, so 144 patients were included in the final analysis (Figure 1).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of the patient enrollment process.
Patient Characteristics
The demographic and clinical characteristics of the 148 enrolled patients are listed in Table 1. The median age was 56 years (range 26-75), 14% of the population was aged 65 years or more. Performance status classified by Eastern Cooperative Oncology Group (ECOG) grading revealed 72% as ECOG0. 57% were postmenopausal. 90% of patients had invasive ductal carcinoma. Hormone receptor positive and HER2 positive breast cancer were detected in 72% and 33% of patients, respectively. Chemotherapy was given as adjuvant treatment setting in 78% of patients. Approximately 10% of patients had a baseline white blood cell equal to or below 5000 cell/mm3.
Table 1.
Baseline Patient Demographic and Clinical Characteristics.
| Characteristics | Number (%) |
|---|---|
| Age (years), median (range) | 56 (26 -75) |
| ≥65 years, n (%) | 20 (13.5%) |
| ECOG, n (%) | |
| 0 | 107 (72.3%) |
| 1 | 41 (27.7%) |
| Menopausal status, n (%) | |
| Pre/perimenopausal | 63 (42.6%) |
| Postmenopausal | 84 (56.8%) |
| Missing | 1 (0.7%) |
| Vascular comorbidity, n (%) a | 56 (37.8%) |
| Disease stage, n (%) | |
| Stage I | 57 (38.5%) |
| Stage II | 36 (24.3%) |
| Stage III | 55 (37.2%) |
| Histology, n (%) | |
| Invasive ductal carcinoma | 133 (89.9%) |
| Invasive lobular carcinoma | 9 (6.1%) |
| Invasive carcinoma, NOS | 6 (4.0%) |
| ER positive, n (%) | 107 (72.3%) |
| HER2 positive, n (%) | 49 (33.1%) |
| Treatment setting, n (%) | |
| Adjuvant | 116 (78.4%) |
| Neoadjuvant | 32 (21.6%) |
| Chemotherapy regimen, n (%) | |
| Anthracycline-containing (AC, AC → T b ) | 136 (91.9%) |
| Non-anthracycline containing (TC b ) | 12 (8.1%) |
| Baseline AST/ALT levels | |
| <1.5 × upper normal limit | 142 (95.9%) |
| 1.5-3 × upper normal limit | 6 (4.1%) |
| Baseline WBC ≤5000 cell/mm3, n (%) | 16 (10.8%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified; ER positive, estrogen receptor positive; HER2 positive, human epidermal growth factor receptor 2 positive; AST, aspartate aminotransferase; ALT, alanine aminotransferase; WBC, white blood cell.
Consisted of diabetes mellitus, hypertension, hypercholesterolemia, ischemic heart disease, or peripheral arterial disease.
AC, doxorubicin/cyclophosphamide; AC → T, doxorubicin/cyclophosphamide followed by paclitaxel; TC, docetaxel/cyclophosphamide.
Events of Interest
Of the 144 patients who received at least 4 cycles of chemotherapy, CAE was observed in 59% of participants. The CAE results, including grade 4 neutropenia, febrile neutropenia, and RDI <85%, are shown in Table 2. Grade 4 neutropenia occurred in 57.6% of total participants. Twelve patients (8.3%) experienced grade 3 febrile neutropenia, but no grade 4 febrile neutropenia was observed. A reduction in RDI to <85% due to neutropenic event occurred in 19 patients (13.2%).
Table 2.
Body Composition and Composite Adverse Event Data.
| Height (cm), mean (range) | 155.4 (140-170) |
| Body weight (kg), mean (range) | 59.5 (35-84.5) |
| Body surface area (m2), mean (range) | 1.59 (1.16-1.98) |
| BMI (kg/m2), mean (range) | 24.64 (17.16-35.03) |
| Tanita RD-800 measurement | |
| Fat-free mass (kg), mean (range) | 35.28 (27.05-44.65) |
| FFMI (kg/m2), mean (range) | 14.59 (12.06-17.25) |
| Inbody 770 measurement a | |
| Fat-free mass (kg), mean (range) | 35.43 (25.2-49.1) |
| FFMI (kg/m2), mean (range) | 14.65 (11.89-18.71) |
| Composite adverse event, n (% total) b | 85 (59.0%) |
| Grade 4 neutropenia | 83 (57.6%) |
| Febrile neutropenia | 12 (8.3%) |
| RDI <85% | 19 (13.2%) |
Abbreviations: BMI, body mass index; FFMI, fat-free mass index; RDI, relative dose intensity.
Based on patients who underwent measurement by both BIA devices (n = 74).
Composite adverse event percentage was calculated based on patients who completed at least 4 cycles of chemotherapy only (n = 144).
Body Composition
Body composition of 148 participants as measured by BIA is shown in Table 2. Only 74 participants were measured with both BIA devices. The average body weight of participants was 59.5 kg (range 35-84.5). The mean BMI was 24.64 kg/m2. The mean FFM by Tanita RD-800 and InBody 770 was 35.28 and 35.43 kg, respectively. The average FFMI was 14.59 kg/m2 by Tanita RD-800 and 14.65 kg/m2 by InBody 770. No patients had an FFMI below 11.4 kg/m2 which is the cutoff for sarcopenia according to international consensus. 17
Agreement Between BIA Devices
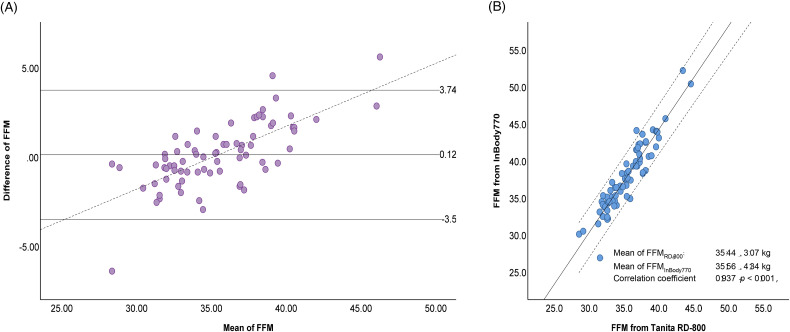
Due to the limited availability of the InBody 770 device, only 74 patients were measured with both devices. FFM agreement analysis between the Tanita RD-800 and the InBody 770 was performed using linear correlation and Bland-Altman plot. The results showed a significant correlation between the FFM results from the 2 BIA devices (R = 0.937, P < .01). The FFM mean comparison did not demonstrate any significant difference, with mean difference ± SD of 0.12 ± 1.81 kg (95% CI −0.30 to 0.54, P = .571). The Bland-Altman plot showed good agreement between FFM estimated by both BIA devices, indicated by no fixed bias and small limits of agreement (−3.50, 3.74 kg). However, a clear proportional bias (r = 0.711, P < .001) was observed, with underestimation and overestimation for low and high values of FFM, respectively (Figure 2).
Figure 2.
Fat-free mass (FFM) estimated by Tanita RD-800 and InBody 770. (A) Bland-Altman plot; mean difference (95% limits of agreement (± 2SD)) 0.12 (−3.5, 3.74) kg. The dotted line represents the regression line between mean FFM Tanita RD-800 and FFM InBody 770 and difference between FFM Tanita RD-800 and FFM InBody 770 (r = 0.711, P < .001). (B) Scatter plots (R2 = 0.874, SEE = 1.09628).
Abbreviations: SD, standard deviation; R2, R squared; SEE, standard error of the estimate.
ROC Curve Analysis
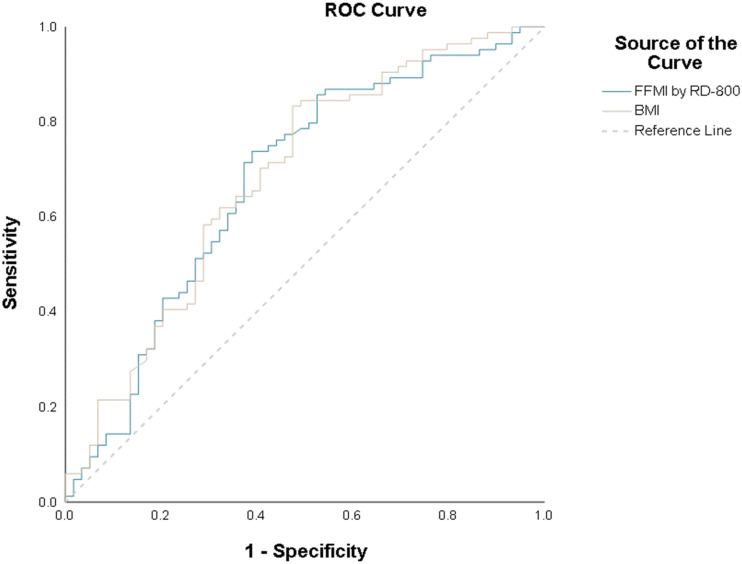
Prediction analysis of FFMI and CAE was performed using ROC curve analysis. The area under the ROC curve (AUC) for FFMI and CAE was 0.675 (Figure 3). Youden’s index had the highest value when FFMI was 14.85 kg/m2. At this value, the sensitivity, specificity, PPV, and NPV were 72.9%, 61%, 72.9%, and 61%, respectively, and the relative risk was 1.86 (power of 97.8% at an alpha error of 0.05). Compared to FFMI, a BMI less than 25 kg/m2 showed lower predictive power with sensitivity, specificity, PPV, and NPV values of 68.2%, 59.3%, 70.7%, and 56.5%, respectively.
Figure 3.
Receiver operating characteristic (ROC) curve analysis of fat-free mass index (FFMI) and body mass index (BMI).
Association Between FFMI and the Primary Outcome
The primary endpoint showed a significant inverse correlation between FFMI and CAE with a correlation coefficient of −0.289. The results of univariate and multivariate analyses to identify factors independently associated with CAE are shown in Table 3. The magnitude of the effect of CAE in the 4 different FFMI quartiles is shown in Table 4. Univariate analysis found low FFMI (<14.85 kg/m2) and low BMI (<25 kg/m2) to be significantly associated with the occurrence of a CAE. Subsequent multivariate analysis revealed low FFMI to be the only independent predictor of CAE (aOR: 4.562, 95% CI: 2.162-9.627; P < .001). Additional analysis using data of the 74 patients with body composition estimated by InBody 700 revealed that low FFMI and lower PA were also significantly associated with CAE (aOR: 4.096 [95% CI: 1.286-13.046; P = .017] and 4.886 [95%CI: 1.541-15.490; P = .007]), respectively.
Table 3.
Univariate and Multivariate Logistic Regression Analysis to Identify Factors That Independently Predict Composite Adverse Events.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Factors | n (%) | OR (95%CI) | P | Adjusted OR (95%CI) | P |
| Age |
|||||
| <65 years | 76/124 (61.3%) | * | |||
| ≥ 65 years | 9/20 (45.0%) | 0.517 (0.199-1.339) | .174 | 0.776 (0.270-2.229) | .637 |
| Vascular morbidity | |||||
| No | 55/91 (60.4%) | * | |||
| Yes | 30/53 (56.6%) | 0.854 (0.430-1.697) | .652 | ||
| ECOG | |||||
| 0 | 63/104 (60.6%) | * | |||
| 1 | 22/40 (55.0%) | 0.795 (0.381-1.662) | .543 | ||
| Stage | |||||
| I | 55/92 (59.8%) | * | |||
| II–III | 30/52 (57.7%) | 0.917 (0.460-1.829) | .806 | ||
| Chemotherapy | |||||
| Anthracycline | 79/132 (59.8%) | * | |||
| Non-anthracycline | 6/12 (50.0%) | 0.671 (0.205-2.192) | 0.509 | 0.820 (0.220-3.065) | .768 |
| WBC | |||||
| >5000 cell/mm3 | 73/128 (57.0%) | * | |||
| ≤5000 cell/mm3 | 12/16 (75.0%) | 2.260 (0.691-7.389) | .177 | 3.261 (0.921-11.545) | .067 |
| FFMI | |||||
| ≥14.85 kg/m2 | 23/59 (39.0%) | * | |||
| <14.85 kg/m2 | 62/85 (72.9%) | 4.219 (2.076-8.574) | <.001 | 4.562 (2.162-9.627) | <.001 |
| BMI | |||||
| ≥25 kg/m2 | 27/62 (43.5%) | * | |||
| <25 kg/m2 | 58/82 (70.7%) | 3.133 (1.569-6.256) | .001 | ** | |
Abbreviations: OR, odds ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell; FFMI, fat-free mass index; BMI, body mass index.
A P-value <.05 indicates statistical significance.
*Defined as reference.
**BMI was excluded from the multivariate analysis due to suspicion of multicollinearity.
Table 4.
Magnitude of the Effect of CAE in the Different FFMI Quartiles.
| Quartile | OR (95% CI) | P-value |
|---|---|---|
| FFMI <13.85 kg/m2 | 6.566 (2.325-18.539) | <.001 |
| FFMI 13.85-14.57 kg/m2 | 5.372 (1.976-14.601) | .001 |
| FFMI 14.58-15.32 kg/m2 | 3.571 (1.346-9.475) | .011 |
| FFMI >15.32 kg/m2 | Reference | — |
Abbreviations: CAE, composite adverse events; FFMI, fat-free mass index; OR, odds ratio; 95% CI, 95% confidence interval.
A p-value<.05 indicates statistical significance.
After including the 4 patients who were lost to follow-up into association analysis for a best-case and worst-case scenario, the association between FFMI and CAE was similar. Similar to the previous univariate analysis, this univariate analysis also showed significant association between FFMI and CAE. From multivariate analysis, the adjusted ORs in the best-case and worst-case scenarios were 4.505 (95% CI: 2.161-9.389) and 4.219 (95% CI: 2.034-8.752), respectively.
Discussion
This is the first prospective observational cohort study to investigate the association between body composition estimated by BIA and hematologic adverse events in patients with EBC who were treated with adjuvant or neoadjuvant chemotherapy. We found a significant inverse correlation between FFMI and CAE rate, which was defined as grade 4 neutropenia, febrile neutropenia, and a dose reduction or delay due to neutropenia leading to an RDI <85% (aOR: 4.562, 95%CI: 2.162-9.627; P < .001).
The majority of patients (92%) in this study received anthracycline-based regimen, and AC (doxorubicin/cyclophosphamide) followed by paclitaxel was most commonly prescribed (53%), AC was given in 39% and only 8% of patients received non-anthracycline with TC (docetaxel/cyclophosphamide) regimen. The baseline characteristics of our patients (age, menopausal status, comorbidity, and BMI) were similar to those reported from other Asian studies in which EBC patients received anthracycline-based regimen.18,19 The incidence of febrile neutropenia and dose reduction or delay leading to RDI <85% in our study was comparable to that reported from Korean study (8.3% vs 8.5% and 13% vs 11%, respectively). 19 However, the incidence of grade 4 neutropenia in our study was 57.6%, which is higher than the 44.6% reported by Kim HS et al, 19 and the 34% reported from the INC-EU Prospective Observational European Neutropenia Study. 6 The higher incidence of grade 4 neutropenia in our study might be related to ethnicity and body composition. Previous study found a higher percentage of body fat at the same BMI in Chinese population as compared to Caucasian population. 20 In addition, the average FFMI in our study population (14.6 kg/m2) was lower than the average value (16.7 kg/m2) reported in Chinese population. 21
Our analysis demonstrated a significant correlation between low FFMI and the occurrence of a CAE. Multivariate logistic regression analysis revealed an FFMI <14.85 kg/m2 to be an independent risk factor for increased risk of having a hematologic adverse events during chemotherapy in patients with EBC. Our univariate analysis showed BMI to be a significant predictor of CAE; however, did not survive multivariate analysis to achieve independent association. Multicollinearity was suspected since FFM is a part of body weight. Multicollinearity testing was performed, which showed a moderate correlation with R-square value of 0.73 and a variance inflation factor (VIF) of 3.96. The result of multivariate analysis that was performed after excluding FFMI from the regression analysis equation showed BMI to be the only independent factor with an adjusted OR of 2.966 (95%CI: 1.46-6.04, P = .003). Nevertheless, the effect of BMI on CAE was smaller than that exerted by FFMI.
In some cases, like sarcopenic obesity, BMI and FFMI are discordant. Prior studies reported that these patients had a higher chance of developing toxicity from chemotherapy, as well as having poorer survival.22,23 In our study, 62 patients (43%) were obese as defined by BMI ≥25 kg/m2, and 12 of those 62 patients (19.3%) were found to have an FFMI lower than 14.85 kg/m2. Ten of those 12 cases (83.3%) with obesity with low FFMI developed CAE. The odds ratio with continuity correction between low FFMI and high FFMI in obese patients was 8.04 (95%CI: 1.8-35.87). As such, FFMI may be a useful tool for identifying obese patients who are at risk for hematologic adverse events from chemotherapy.
Our study result is consistent with the results of other studies that reported that poor body composition metrics, such as low skeletal muscle gauge measured by CT-based analysis, were significantly associated with increased treatment-related toxicities in EBC patients. 10 Another observational study that used DXA scan to assess body composition in EBC patients receiving chemotherapy found higher absolute fat mass and higher relative fat mass to be significantly associated with increased toxicity that led to the need for treatment modifications. 11 The results of this study and previous study highlight the need for further investigation to identify appropriate interventions for improving body composition, such as exercise and dietary interventions, to reduce the incidence of CAE and improve treatment outcomes.
This is the first prospective study to demonstrate a correlation between body composition estimated by BIA and hematologic adverse events in EBC patients receiving chemotherapy. BIA is more widely available, easier to use, and less expensive compared to CT or DXA scan. FFMI estimated by BIA may be used to identify patients who might be at high risk for developing toxicity from chemotherapy.
Limitations
This study has some mentionable limitations. First, the BIA device that we used for all participants in this study (Tanita RD-800) is a consumer-level product. However, we evaluated and demonstrated agreement between the Tanita RD-800 and the InBody 770, the latter of which is a professional-level device and was validated against DXA. 24 Second, our study enrolled only EBC patients, so the FFMI cutoff value of 14.85 kg/m2 may not be generalizable to women with more advanced stages of breast cancer. Third and last, we did not include male breast cancer patients because males physiologically have a higher proportion of muscle components compared to females. The FFMI cut point in male patients may be higher, so further investigation in different population should be conducted.
Conclusion
Low FFMI is an independent predictor of increased risk of hematologic adverse events in EBC patients receiving chemotherapy. Estimation of FFMI by BIA may identify at-risk patients so that interventions can be considered to improve treatment outcomes.
Acknowledgements
The authors gratefully acknowledge the women who generously agreed to participate in this study, and the nurses at the outpatient chemotherapy infusion unit of Siriraj Hospital for facilitating patient recruitment and data collection.
Abbreviations
- aOR
adjusted odds ratio
- AUC
area under the curve
- BIA
bioelectrical impedance analysis
- BMI
body mass index
- CAE
composite adverse event
- CI
confidence interval
- CT
computed tomography
- CTCAE
common terminology criteria for adverse events
- DXA
dual energy X-ray absorptiometry
- EBC
early-stage breast cancer
- FFM
fat-free mass
- FFMI
fat-free mass index
- HER2
human epidermal growth factor receptor 2
- INC-EU
Impact of Neutropenia in Chemotherapy—European Study Group
- NPV
negative predictive value
- OR
odds ratio
- PA
phase angle
- PPV
positive predictive value
- RDI
relative dose intensity
- ROC curve
receiver operating characteristic curve
- VIF
variance inflation factor
Footnotes
Ethics Statement: The study protocol has been approved by Siriraj Institutional Review Board (Protocol number 286/2562 [EC3, approval number: Si346/2019]). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Availability of Data: All data generated or analyzed during this study are included in this article. The original anonymous data are available upon request from the corresponding author.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research project was supported by a grant from Siriraj Research Fund, Grant number (IO) R016231041, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand. The funder has no role in the design, conduct, and analysis of the study.
ORCID iD: Charuwan Akewanlop https://orcid.org/0000-0003-1022-3745
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65(2):87–108. DOI: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Virani S, Bilheem S, Chansaard W, et al. National and subnational population-based incidence of cancer in Thailand: assessing cancers with the highest burdens. Cancers (Basels) 2017;9(8):108. DOI: 10.3390/cancers9080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365(9472):1687–1717. DOI: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the cancer and leukemia group B experience. J Clin Oncol 2007; 25(24):3699–3704. DOI: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 5.Engkakul T, Thongtang N, Nimmannit A, Chuthapisith S, Akewanlop C. Impact of obesity on outcomes of operable breast cancer: a retrospective cohort study. Asian Pac J Cancer Prev 2020;21(4):953–960. DOI: 10.31557/APJCP.2020.21.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwenkglenks M, Pettengell R, Jackisch C, et al. Risk factors for chemotherapy-induced neutropenia occurrence in breast cancer patients: data from the INC-EU prospective observational European neutropenia study. Support Care Cancer 2011;19(4):483–490. DOI: 10.1007/s00520-010-0840-y. [DOI] [PubMed] [Google Scholar]
- 7.Carroll J, Protani M, Walpole E, Martin JH. Effect of obesity on toxicity in women treated with adjuvant chemotherapy for early-stage breast cancer: a systematic review. Breast Cancer Res Treat 2012;136(2):323–330. DOI: 10.1007/s10549-012-2213-3. [DOI] [PubMed] [Google Scholar]
- 8.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer 2016;57:58–67. DOI: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Shachar SS, Deal AM, Weinberg M, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res 2017; 23(3):658–665. DOI: 10.1158/1078-0432.CCR-16-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shachar SS, Deal AM, Weinberg M, et al. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res 2017;23(14):3537–3543. DOI: 10.1158/1078-0432.CCR-16-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Berg MMGA, Kok DE, Posthuma L, et al. Body composition is associated with risk of toxicity-induced modifications of treatment in women with stage I-IIIB breast cancer receiving chemotherapy. Breast Cancer Res Treat 2019;173(2):475–481. DOI: 10.1007/s10549-018-5014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietrobelli A, Rubiano F, St-Onge MP, Heymsfield SB. New bioimpedance analysis system: improved phenotyping with whole-body analysis. Eur J Clin Nutr 2004; 58(11):1479–1484. DOI: 10.1038/sj.ejcn.1601993. [DOI] [PubMed] [Google Scholar]
- 13.Ræder H, Kværner AS, Henriksen C, et al. Validity of bioelectrical impedance analysis in estimation of fat-free mass in colorectal cancer patients. Clin Nutr 2018;37(1):292–300. DOI: 10.1016/j.clnu.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147(8):573–577. DOI: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 15.Barbosa-Silva MC, Barros AJ. Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Curr Opin Clin Nutr Metab Care 2005;8(3):311–317. DOI: 10.1097/01.mco.0000165011.69943.39. [DOI] [PubMed] [Google Scholar]
- 16.Green SB. How many subjects does it take to do a regression analysis. Multivariate Behav Res 1991;26(3):499–510. DOI: 10.1207/s15327906mbr2603_7. [DOI] [PubMed] [Google Scholar]
- 17.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12(5):489–495. DOI: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 18.Chan A, Chen C, Chiang J, Tan SH, Ng R. Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer 2012;20(7):1525–1532. DOI: 10.1007/s00520-011-1241-6. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Lee SY, Kim JW, et al. Incidence and predictors of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy in Korea. Oncology 2016;91(5):274–282. DOI: 10.1159/000449226. [DOI] [PubMed] [Google Scholar]
- 20.Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord 2000;24(8):1011–1017. DOI: 10.1038/sj.ijo.0801353. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Shu H, Zheng Y, et al. Comparison of fat-free mass index and fat mass index in Chinese adults. Eur J Clin Nutr 2012;66(9):1004–1007. DOI: 10.1038/ejcn.2012.86. [DOI] [PubMed] [Google Scholar]
- 22.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9(7):629–635. DOI: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 23.Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc 2016;75(2):188–198. DOI: 10.1017/S0029665115004279. [DOI] [PubMed] [Google Scholar]
- 24.McLester CN, Nickerson BS, Kliszczewicz BM, McLester JR. Reliability and agreement of various InBody body composition analyzers as compared to dual-energy X-Ray absorptiometry in healthy men and women. J Clin Densitom 2020;23(3):443–450. DOI: 10.1016/j.jocd.2018.10.008. [DOI] [PubMed] [Google Scholar]