Abstract
Conventional farming systems are highly reliant on chemical fertilizers (CFs), which adversely affect soil quality, crop production and the environment. One of the major current challenges of current agriculture is finding ways to increase soil health and crop yield sustainably. Manure application as a substitute for CF is an alternative fertilization strategy for maintaining soil health and biodiversity. However, little is known about the complex response of soil bacterial communities and soil nutrients to manure and CFs application. This study reports the response of soil nutrients, rice yield, and soil microbial community structure to 2 years of continuous manure and CFs application. The study consisted of six treatments: no N fertilizer control (Neg-Con); 100% CF (Pos-Con); 60% cattle manure (CM) + 40% CF (High-CM); 30% CM + 70% CF (Low-CM); 60% poultry manure (PM) + 40% CF (High-PM), and 30% PM + 70% CF (Low-PM). We used high-throughput sequencing of 16S ribosomal RNA gene amplicons to characterize the soil bacterial communities. Results revealed that the addition of manure significantly altered the soil bacterial community composition and structure; and enhanced the relative abundance of phyla Proteobacteria, Chloroflexi, Firmicutes, Acidobacteria, and Planctomycetes. Organic fertilizer treatments, particularly high CM and PM had the highest measured soil bacterial diversity of all treatments. Similarly, integrated application of manure and CFs increased the soil biochemical traits [i.e., pH, total N (TN), soil organic C (SOC), microbial biomass N (MBN), and microbial biomass C (MBC)] and rice grain yield. Average increases in SOC, TN, MBN, and MBC were 43.66, 31.57, 24.34, and 49.45%, respectively, over the years in the High-PM compared with Pos-Con. Redundancy analysis showed that the dominant bacteria phyla were correlated with soil pH, SOC, TN, and microbial biomass, but the relative abundance of Proteobacteria was strongly correlated with environmental factors such as soil pH, SOC, TN, and MBC. We employed a structural equation model to examine the relationship between microbial biomass, soil nutrients and grain yield among treatments. This analysis supported the hypothesis that soil nutrient content and availability directly affect rice grain yield while soil bacteria indirectly affect grain yield through microbial biomass production and nutrient levels. Overall, the findings of this research suggest that the integrated application of CF and manure is a better approach for improving soil health and rice yield.
Keywords: paddy field, organic manure, chemical fertilizer, soil chemical peripeties, bacterial community, grain yield
Highlights
-
-
Manure plus mineral fertilizer improved rice yields and enhanced soil bacterial community composition in a dual cropping system.
-
-
All of the soil bacterial communities in soils were dominated by Acidobacteria, Proteobacteria, and Chloroflexi.
-
-
Manure inputs enhanced soil microbial-derived C and N relative to chemical fertilization.
-
-
Soil pH and soil organic C were the keys to regulating soil microbial community structure.
Introduction
Rice (Oryza sativa L.) is grown worldwide and is a staple food for more than 65% of the Chinese (Chauhan et al., 2017). An appropriate fertilization plan and sufficient soil quality are required for high crop production. To enhance crop production, farmers have increased the application of CFs. Although CFs, particularly nitrogen (N), has been one of the main factors driving rice yield enhancements over the last four decades, the low N-use efficiency caused by N overuse has led to serious environmental problems (e.g., soil acidification, degradation, biodiversity loss, water eutrophication, declining soil productivity, and increasing greenhouse gas emissions) that have attracted global attention (Duan et al., 2019; Zhang et al., 2019). A change in the source of N fertilizer is urgently needed to reduce the negative effects of CF. However, more research is needed to identify methods for increasing soil fertility, achieving sustainable and stable rice production, and reducing the use of synthetic N.
Prior studies have demonstrated that an effective way of improving soil health and crop productivity is to use a combination of manure and CFs (Iqbal et al., 2019; Oladele et al., 2019). Manure application can change a soil’s physical and biochemical properties, improve soil enzymatic activity, and decrease or eliminate harmful impacts of the CF-only overuse on soil health (Gu et al., 2015; Qaswar et al., 2020). Moreover, manure could deliver further benefits over synthetic fertilizers, such as improving soil structure, fertility, and sustaining soil health (Ali et al., 2020a; Luo et al., 2020) without sacrificing, and sometimes increasing, crop yields and grain quality (Cercioglu, 2017; Ali et al., 2020b). The increase in grain yield induced by manure fertilization is mainly due to enhancements in soil nutritional status, environmental variables, and microbial community composition (Jarvan et al., 2014; Zhang et al., 2021). However, these previous investigations were performed on a weight basis rather than the fertilization of organic fertilizer on a specific N percentage integrated with CFs in the paddy field. This has developed a gap in examining the influence of combined organic and inorganic nitrogen fertilizer on soil biochemical properties and microbial population based on particular concentration instead of weight.
Soil microorganisms play an important role in farming systems by controlling biogeochemical processes such as organic matter decomposition, nitrogen mineralization, and recycling (Xu et al., 2013), as well as promoting plant nutrient uptake and aboveground plant growth (Harris, 2009; Chaparro et al., 2012; Wagg et al., 2019). The soil bacterial community is critical for soil fertility and function due to their involvement in decomposing organic residues, releasing enzymes into the soil, recycling nutrients, and water (Ingham, 2009;Stroobants et al., 2012). Fertilizer application can alter belowground bacterial communities by changing soil’s physical and biochemical characteristics (Yu et al., 2019; Li P. et al., 2020). Therefore, belowground microorganisms are influenced by fertilizer application and can impact plant growth by altering soil nutrient turnover and status (van der Heijden et al., 2008; Wang et al., 2021). The regulation and improvement of soil microbial community diversity and activity may thus serve as a potential fertilization approach for improving soil health and crop yield while limiting the negative environmental impact of synthetic N fertilization, thus contributing to the sustainability of the farming system.
The structure and composition of soil bacterial communities require time to stabilize (Hartmann et al., 2015). Understanding how soil bacterial communities react to continuous fertilization is crucial for directing the development of an effective rice field fertilization plan. Prior studies have reported that continued fertilization of organic manure alone or combined with CF can increase soil microbial communities’ diversity and abundance and improve soil quality (Kumar et al., 2017; Cui et al., 2018; Pérez-Valera et al., 2022). Appropriate manure application can regulate the soil microbial community and improve the soil’s micro-ecological environment (Ikoyi et al., 2020; Li T. et al., 2020; Hou et al., 2022). Cui et al. (2018) found that applying organic fertilizer combined with CFs enhanced the abundance of Chloroflexi, Proteobacteria, Actinobacteria, Planctomycetes, and Firmicutes. Some research has concentrated on dryland soil agro-ecosystems (Wei et al., 2017), and only a few studies have published bacterial community characteristics in paddy soils. However, the effect of combined treatment of organic fertilizer and CFs on the bacterial community structure and its relationships with soil environmental variables in paddy fields have not been examined. Thus, characterize the variations in soil microbial biomass and bacterial community structure under different fertilizer treatments before implementing novel fertilization strategies to improve soil ecosystem health and plant productivity.
The double-rice cropping pattern is an important rice cultivation system in southern China, where low rice yields and high CFs application are common (Zhu et al., 2019). This study is based on an ongoing experiment in a rice field, with the following objectives: (i) explore the effect of organic and chemical N fertilization on rice yield, soil properties, and bacterial community structure (ii) examine the influence of integrated fertilization on the correlation among soil bacterial community and soil biochemical traits; and (iii) assess the contributions of organic–inorganic N fertilization, soil properties, and microbes to increase in rice yield. We assumed for the current study that animal manure combined with CFs could enhance soil biochemical traits and bacterial community composition, which has a positive role in higher soil quality and rice grain yield. The main objective of this study is to provide a theoretical background for scientific fertilization practices and reasonable rice cultivation while aiming to reduce chemical fertilizer (CF) use and environmental degradation.
Materials and Methods
Experimental Site
The field study at the rice research station of Guangxi University, Nanning, China, was started in 2019. This location has a sub-tropical monsoon climate, with approximately 1,300 mm of annual rainfall and an average annual temperature of 24.6°C. The soil is defined as Ultisols (United States Department of Agriculture soil classification) and is acidic (pH 5.96). A soil test showed that the total nitrogen (TN) was 1.66 g kg–1 and soil organic carbon (SOC) was 18.74 g kg–1; other soil properties are provided in Table 1.
TABLE 1.
Soil and organic manure physical and chemical properties before the experimentation.
| Properties | Soil | Cattle | Poultry |
| manure | manure | ||
| pH (water) | 5.97 | 7.83 | 7.99 |
| Moisture content (%) | 12.03 | – | – |
| Bulk density (g cm–3) | 1.34 | 0.80 | 0.74 |
| SOC (g kg–1) | 18.74 | 161 | 145 |
| Total N (g kg–1) | 1.66 | 9.57 | 12.98 |
| Total P (g kg–1) | 0.85 | 11.10 | 9.35 |
| Total K (g kg–1) | – | 13.83 | 10.06 |
| Available N (mg kg–1) | 158 | – | – |
| Available P (mg kg–1) | 27.83 | – | – |
| Available K (mg kg–1) | 26.73 | – | – |
N, nitrogen; SOC, soil organic carbon; P, phosphorous; K, potassium.
Experimental Setup
The dual cropping seasons ranged from March to July (early season) and July to November (late season). The experimental study was established in a randomized complete block design with three replications. Organic manure [cattle manure (CM) and poultry manure (PM)] and CF urea were used, and the combinations of treatment were: no N fertilizer control (Neg-Con); 100% CF in the form of urea (Pos-Con); 60% CM + 40% CF (High-CM); 30% CM + 70% CF (Low-CM); 60% PM + 40% CF (High-PM), and 30% PM + 70% CF (Low-PM). We grew the Zhenguiai, a broadly cultivated cultivar in Guangxi Province. This cultivar has a short growth duration (approximately 110–120 days) with a morphological structure and high grain filling rate (Li et al., 2006). Rice seeds were grown, and 25-days old seedlings of even size were transferred into the fields. Except for Neg-Con, which received no N fertilizer amendments, the recommended dose of N, P, and K at a rate of 150:75:150 (kg ha–1) was applied to each plot (Sapkota et al., 2020). Table 2 shows the nutrient content of manure and the amount of each treatment. The potassium (KCl) and N fertilizers were delivered in three stages: 50% prior to transplantation, 30% during tillering, and the remaining 20% during the heading period. All phosphorous fertilizer was supplied as a base dose before transplanting. Furthermore, uniform flooding continued from seedling transplantation to rice physiological maturity. Normal agricultural practices, such as irrigation and insecticide application, were performed for all regimes.
TABLE 2.
Content and amount of NPK provided of each plot and fertilization time.
| Treatment | N (kg/ha) | Urea (kg/ha) | CM and PM (kg/ha) | Basal fertilization (kg/ha) | Tillering (kg/ha) | Panicle initiation (kg/ha) |
| Neg-Con: N = 0% (T1) | 0 | 0 | 0 | P2O2: 397, KCl: 128 | KCl: 128 | Urea: 0 |
| Pos-Con: 100% CF (T2) | 150 | 322 | 0 | Urea: 192, P2O2: 397, KCl: 128 | Urea: 65, KCl: 128 | Urea: 65 |
| High CM: 60% CM + 40% CF (T3) | 150 | 130 | 9,188 | Urea: 0, CM: 9188, P2O2: 397, KCl: 128 | Urea: 65, KCl: 128 | Urea: 65 |
| Low CM: 30% CM + 70% CF (T4) | 150 | 225 | 4,572 | Urea: 94, CM: 4572, P2O2: 397, KCl: 128 | Urea: 65, KCl: 128 | Urea: 65 |
| High PM: 60% PM + 40% CF (T5) | 150 | 128 | 6,623 | Urea: 0, PM: 6623, P2O2: 397, KCl: 128 | Urea: 65, KCl: 128 | Urea: 65 |
| Low PM: 30% PM + 70% CF (T6) | 150 | 225 | 3,290 | Urea: 94, PM: 3290, P2O2: 397, KCl: 128 | Urea: 65, KCl: 128 | Urea: 65 |
CF, chemical fertilizer; CM, cattle manure; PM, poultry manure; N, nitrogen; KCl, potassium chloride.
Soil Sampling and Analysis
Soil Properties
Soil samples were collected using a core sampler at depth (0–20 cm) from each plot post rice harvest in 2019–2020. Samples were obtained from various places in each plot and then combined to produce a mixture, separated into two parts: one for nutrient measurements and the other for molecular examination. Soil organic C (SOC) was measured using the K2Cr2O7-H2SO4 oxidation method as described by Wang et al. (2003). In addition, for total N, 200 mg of the soil samples were treated according to Ohyama et al. (1991). Finally, TN was determined by the micro-Kjeldahl method as recommended by Jackson (1956). For soil available N, P, and K and pH were assessed by the procedures of Lu (2000). The fumigation extraction technique was used to examine microbial biomass carbon (MBC) as described by Brookes et al. (1985) and microbial biomass nitrogen (MBN) by the technique of Vance et al. (1987).
Extraction of DNA and PCR Amplification
DNA was extracted from wet soil samples (0.25 g) following the guidelines of the E.Z.N.A. Soil DNA Kit of the Omega, United States. Extracted DNA (50 ng/reaction) was used as a template in standard 16S ribosomal RNA (rRNA) gene amplification targeting the V3–V4 variable regions of microbial 16S rRNA genes, as described previously (Sundberg et al., 2013). The primers used included 341F (CCTACGGGNGGCWGCAG) and 805R (GACTACHVGGGTATCTAATCC) (Klindworth et al., 2013). Primers contained Illumina sequencing adapters and sample-specific barcodes. PCR reactions were performed using mastermix, with the following cycling conditions: 94°C for 3 min, followed by 5 cycles at 94°C for 30 s, 45°C for 20 s, 65°C for 30 s, and then 20 cycles at 94°C for 20 s, 55°C for 20 s, 72°C for 30 s with a final extension of 72°C for 5 min. Library preparation and sequencing were performed by Illumina MiSeq system (Illumina MiSeq, United States) (Claesson et al., 2010).
Illumina MiSeq Sequencing
After PCR, amplicons of the correct size were extracted from 2% agarose gels and purified by an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, CA, United States). The amplicons were quantified via Qubit 3.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, United States). Amplicons were pooled in equimolar levels and sequenced on an Illumina MiSeq sequencer (Illumina, San Diego, CA, United States) following standard sequencing protocols.
Processing of Sequencing Data
Raw paired-end sequence data were merged using the PEAR software package (Zhang et al., 2014). Merged sequence data were processed through the software package QIIME (Caporaso et al., 2010). UCLUST (Edgar, 2010) was used to identify operational taxonomic units (OTUs) based on 97% similarity thresholds (Robert et al., 2010). Chimeric sequences were identified and removed using the USEARCH tool (Edgar, 2010). Taxonomic classification was carried out on representative sequences from each OTU using the SILVA 119 reference database (Quast et al., 2012) using the RPD classifier (Cole et al., 2009) or UCLUST with a 90% confidence threshold level.
Rice Grain Yield
At maturity, the rice was collected from the entire plot and the grain yields. The dry weight of the grain was calculated using an adjusted moisture content of 14% in rice grains.
Statistical Analysis
The changes in soil biochemical characteristics and rice grain yields as affected by fertilization treatment were examined using the ANOVA method in Statistics 8.1 (Analytical Software Tallahassee, FL, United States). First, the data were subjected to standard tests to ensure they met the assumptions of normality. Furthermore, percentages data were arcsine transformed before analysis to normalize the variables. Tukey’s post hoc test was performed to compare means for the variables where the effects of treatments were significant. A Venn diagram was used to illustrate the number of related and distinctive OTUs in the samples and determine the similarity and coincide in the number of OTUs between the samples. Microbial alpha diversity indices, including Simpson’s and Shannon indices, were calculated using MOTHUR (Schloss et al., 2009). Species richness rarefaction curves were plotted against the series of data using the MicrobiomeAnalyst (Dhariwal et al., 2017). RDA was used to analyze the relationship strength between soil traits and soil bacterial diversity using the software package CANOCO5 (Microcomputer Power, Ithaca, NY, United States).
Results
Impact of Fertilization on Soil Biochemical Attributes
Co-fertilization of fields with manure and chemical nutrients significantly increased the SOC, TN, pH, MBC, and MBN compared to CF application: Pos-Con and Neg-Con (Table 3). The effect was greatest in all observed data when manure input was high; no substantial (P < 0.05) variations among CM and PM were observed. Across the seasons, the regimes revealed the same trend. Compared to Pos-Con, High CM and PM treatments increased SOC 26.3 and 31.2%, TN 25.3 and 25.6%, respectively. In addition, over the years, High-CM enhanced treatment increased soil MBN and MBC by 55 and 61%, respectively, relative to Pos-Con. Nevertheless, High CM was statistically (P < 0.05) comparable to High PM. Likewise, low manured input treatments also increased the measured soil biochemical traits as compared to control.
TABLE 3.
Changes in soil biochemical traits under the combined manure and mineral N fertilization.
| pH (water) | SOC (g kg–1) | TN (g kg–1) | MBC (mg kg–1) | MBN (mg kg–1) | ||
| Year | Treatment | |||||
| 2019 | Neg-Con | 5.93c | 14.74b | 1.40c | 170d | 23.34d |
| Pos-Con | 5.91c | 15.08b | 1.41c | 220c | 31.44c | |
| High-CM | 6.25a | 19.24a | 1.78a | 340a | 44.67a | |
| Low-CM | 6.12b | 16.81b | 1.67b | 310b | 38.43b | |
| High-PM | 6.23a | 18.92a | 1.77a | 341a | 43.89a | |
| Low-PM | 6.13b | 16.88b | 1.64b | 315b | 39.44b | |
| 2020 | Neg-Con | 5.95d | 14.88c | 1.40d | 175d | 24.52e |
| Pos-Con | 5.94d | 15.52c | 1.44c | 235c | 34.45d | |
| High-CM | 6.31a | 24.74a | 1.99a | 370a | 51.42ab | |
| Low-CM | 6.18c | 22.25b | 1.81b | 355b | 43.36c | |
| High-PM | 6.28ab | 25.05a | 1.98a | 374a | 54.58a | |
| Low-PM | 6.16c | 21.77b | 1.80b | 311b | 41.35c |
SOC, soil organic carbon; TN, total nitrogen; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; Neg-Con, control; Pos-Con, 100% chemical fertilizer (CF); High-CM, 60% cattle manure (CM) + 40% (CF); Low-CM, 30% CF + 70% CF; High-PM, 60% poultry manure (PM) + 40% CF; Low-PM, 30% PM + 70% CF.
The mean comparison was made using Tukey tests for treatment mean in both years and the lettering was done based on the Tukey HSD test at 5% using a simple effect.
Values followed by the same letters within the column are statistically the same at P ≤ 0.05.
In addition, significant increases were measured for SOC, TN content, MBC, and MBN in the subsequent year; the average enhancement in SOC, TN, MBC, and MBN during 2020 was 26.4, 9.50, 8.9, and 22%, respectively, relative to 2019.
Impact of Combined Fertilization on Bacterial Diversity and Abundance Index
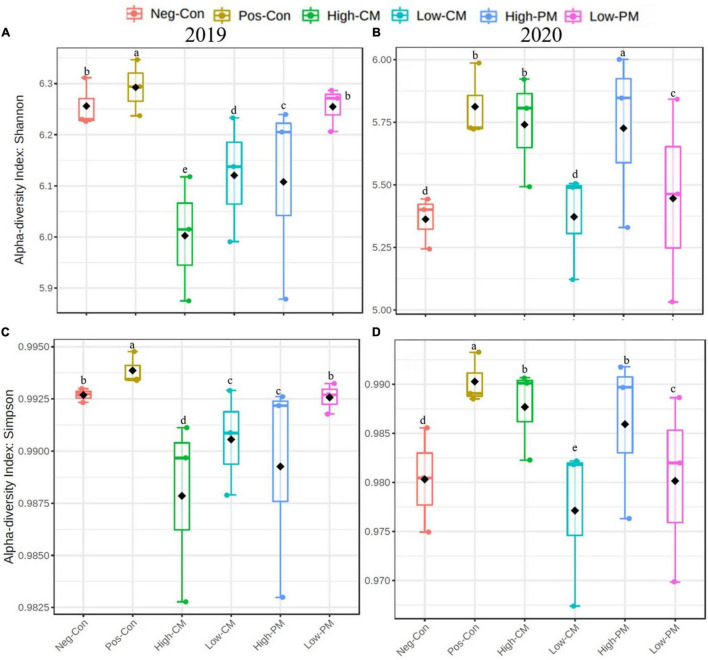
In this study, the co-applied manure and synthetic N significantly influenced the soil bacterial community composition and diversity (Figures 1A–H). The box plots based on the Simpson and Shannon index exhibited significant variations in bacterial α-diversity detected in the regimes. Co-fertilization of manure and CF resulted in the highest bacterial diversity and abundance relative to Pos-Con. In addition, the bacterial diversity and abundance were significantly higher in the High manured plots.
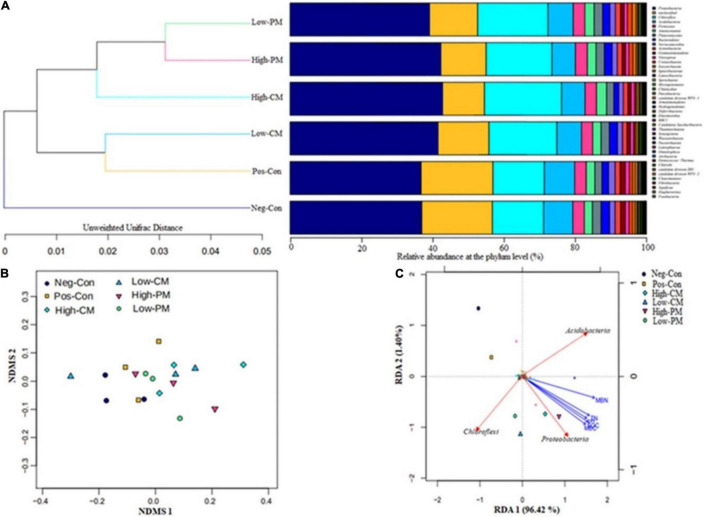
FIGURE 1.
Comparison of the soil bacterial communities based on PCR-amplified 16S rRNA gene analysis. Box plots for α-diversity estimated Shannon (A,B) and Simpson (C,D) indices of the bacterial community under six treatments. The ends of the whiskers represent minimum and maximum, the bottom and top of the box are the first and third quartiles, and the black dot inside the box is the median. Bars show the standard error of the mean, and bars with different letters are significantly different at P < 0.05. Please see Table 3 for the treatment combination.
Impact of Combined Fertilization on Soil Bacterial Community Diversity and Composition
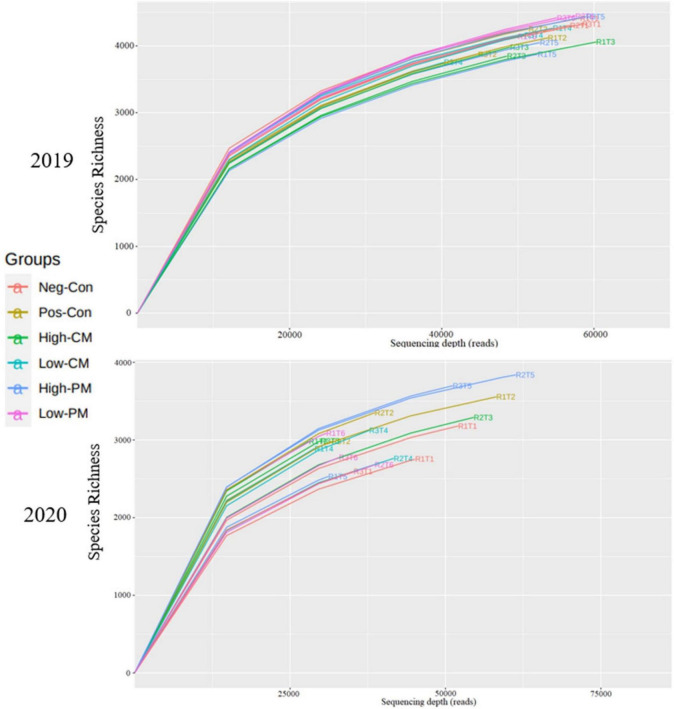
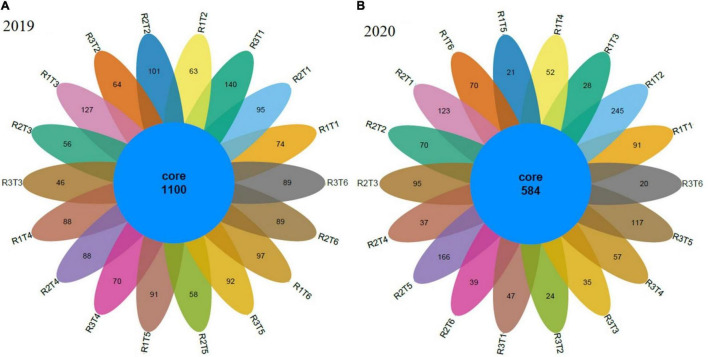
To determine rarefaction curves, richness, and diversity of bacteria, OTUs were identified at 97% of genetic similarity. The rarefaction curves exhibited that the sequencing effort was enough to describe most of the diversity in soil samples (Figure 2). The Venn diagram shows that in 2019, the number of unique OTUs in Neg-Con, Pos-Con, High-CM, Low-CM, High-PM, and Low-PM treatments was 309, 228, 229, 246, 241, and 275, correspondingly, and the number of shared OTUs was 1,100 (Figure 3). In 2020, the number of unique OTUs was 261, 339, 158, 146, 304, and 129, respectively, and the number of mutual OTUs was 584.
FIGURE 2.
Rarefaction curves of 16S rRNA sequencing depth and number of species numbers in soil depth (0–20 cm). Neg-Con (R1T1, R2T1, and R3T1), Pos-Con (R1T2, R2T2, and R3T2), High-CM (R1T3, R2T3, and R3T3), Low-CM (R1T4, R2T4, and R3T4), High-PM (R1T5, R2T5, and R3T5), and Low-PM (R1T6, R2T6, and R3T6). See Table 3 for the treatments combination.
FIGURE 3.
Venn diagram showing the bacterial unique and operational units (OTUs) during 2019 (A) and 2020 (B) under organic and inorganic fertilization. Neg-Con (R1T1, R2T1, and R3T1), Pos-Con (R1T2, R2T2, and R3T2), High-CM (R1T3, R2T3, and R3T3), Low-CM (R1T4, R2T4, and R3T4), High-PM (R1T5, R2T5, and R3T5), and Low-PM (R1T6, R2T6, and R3T6). See Table 3 for the treatments combination.
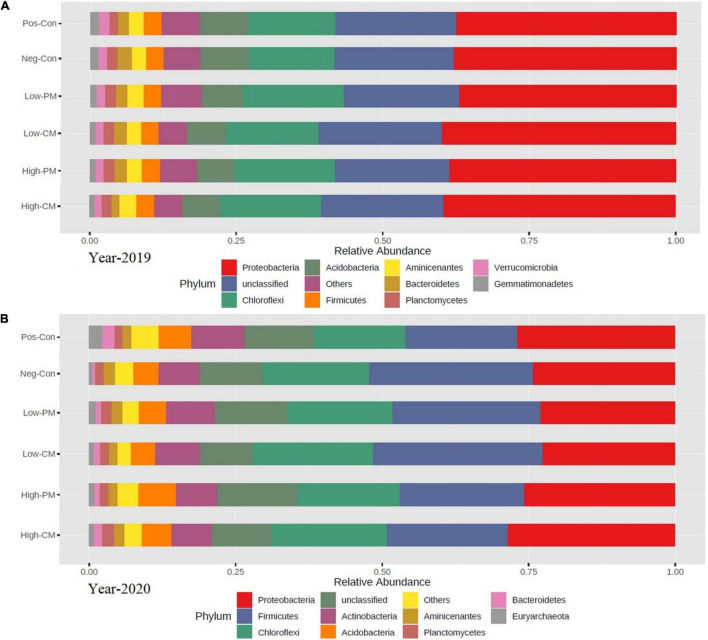
The analysis for bacterial community composition under combined manure and mineral fertilization indicated the presence of a total of 41 phyla, and the average relative abundance of 11 phyla exceeded 1% (Figures 4, 5). Compared to Pos-Con, the integrated application of manure with mineral fertilizer significantly increased the soil bacterial community composition. The top five dominant phyla in all treatments were Chloroflexi, Proteobacteria, Firmicutes, Acidobacteria, and Planctomycetes, which reported more than 70% of the relative abundance of the bacterial communities. Chloroflexi and Proteobacteria were the most abundant among all these dominant phyla in the High manured treatments compared to the Neg-Con and Pos-Con. Likewise, low CM and PM treatments also increased the abundance of Proteobacteria and Chloroflexi compared with control. Furthermore, Actinobacteria, Acidobacteria, and Firmicutes were the second most abundant phyla in the experiment across all treatments.
FIGURE 4.
Based on the 16S rRNA gene the relative abundance of soil bacterial community composition at phylum level during year 2019 (A) and year 2020 (B) in six treatments. Each strip represents the mean of three replicates. See Table 3 for treatments combination.
FIGURE 5.
The similarity between soil bacterial communities of different fertilizers treatments is confirmed by cluster analysis (A), NDMS analysis based on unweighted Unifrac distance at OTU levels (B), and redundancy ordination analysis (RDA) (C) showing the strength of association between the different treatments, environmental factors (represented by yellow arrows), and dominant bacteria at the phylum level (represented by red arrows). For fertilization treatment combination details, see Table 3.
The cluster analysis (Figure 5A) demonstrates that bacterial communities in the six fertilization regimes are divided into main groups. One is made up of Neg-Con, the other is composed of High-CM, High-PM, Low-CM, Low-PM, and Pos-Con, indicating that bacterial communities in different fertilization regimes share a high similarity and the cluster. Moreover, the similarity among bacterial communities of all fertilization regimes is further proved by the NDMS analysis as shown in Figure 5B. In the two-dimensional NDMS plot, a small unweighted UniFrac distance indicates a similar bacterial community. As projected, soil samples in Neg-Con and Pos-Con are closely occurred and clustered and separated from other manured amendments along NMDS axes.
Impact of Combined Fertilization on Rice Yield
The joined manure and synthetic N fertilization considerably affected the rice yield in both years (Figure 6). Combined fertilization increased rice yield considerably across the years compared with control, and over both years, the regimes exhibited a similar trend. The Low PM treatment increased the grain yield by 17 and 32% in 2019 and 2020, correspondingly compared to the control. Nevertheless, there were no substantial differences (P < 0.05) in grain yield between the Low manured regimes. Likewise, High manured treatments also significantly enhanced rice yield relative to the Pos-Con. Significant enhancements in yield were noticed over the years, and the average rice yield was improved by 15% in 2020 relative to 2019.
FIGURE 6.
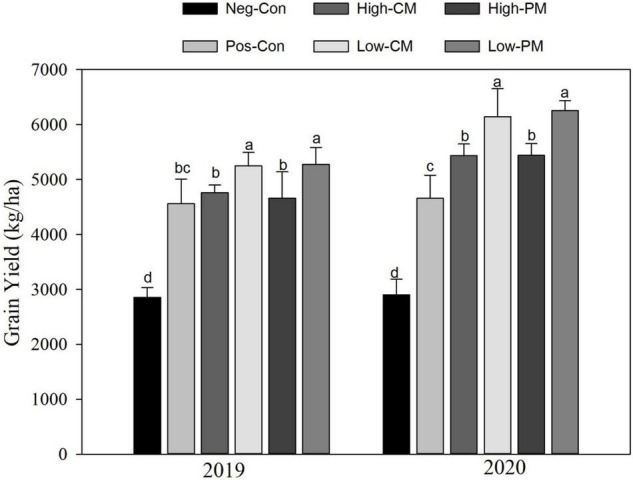
Variation in rice yield as affected by organic and inorganic N application. The mean comparison was made using the Tukey’s post hoc test for treatments at 5%. Different letters on bars are not significantly different at P < 0.05. See Table 3 for treatments combination.
Relationship Between Fertilization Treatments, Soil Traits, and Microorganisms
Redundancy analysis was performed to determine the impact of different fertilization treatments on bacterial community composition and environmental factors and reveal the strength of the relationship between soil traits and soil bacterial community structure (Figure 5C). The RDA shows that the six treatments occurred in distant quadrants show that the treatments had a significant effect on the structure of soil microorganisms and soil properties. The RDA revealed that manure-treated treatments significantly affected soil bacterial community composition compared to non-manure treatments. Proteobacteria, Acidobacteria, and Chloroflexi were strongly associated with soil properties. The RDA outcomes demonstrated that combined organic and inorganic fertilizer application to the rice field has the most substantial influence on soil microbial community composition and environmental properties.
Discussion
This study investigated the effect of continued manure substitution on rice field soil biochemical traits and bacterial community structure and composition. The microbial population is the most important indicator of soil biology, as it is responsible for improving soil fertility, health, and crop yield (Luo et al., 2020; Liu et al., 2021).
Soil Properties
In this experiment, animal manure combined with CFs significantly increased soil qualitative traits (SOC, TN, MBC, MBN, and pH) compared with CF-only fertilization (Table 3). We detected that the biodegradation of animal manure gradually released plant nutrients to the soil and exhibited that increasing the rate of manure improves soil qualitative traits. Application of CFs reduced soil pH level, while joint manure amendments increased soil pH level significantly. Alike results were stated by Bhattacharyya et al. (2015), who concluded that continuous use of synthetic fertilizer causes soil acidification. Moreover, the decline in pH level is because the overuse of synthetic N forms H+ through nitrification in soil (Yang et al., 2018). Another possible reason for the lower pH resulting from the mineral N fertilization was the acidic nature of mineral N fertilizers, which could donate to the soil’s lower pH (Adekiya et al., 2020). Organic fertilizer application alters soil acidification because it frequently consists of enough basic cations and carbonate ions to neutralize soil acidity, explaining the increase in soil pH under manure amendment plots (Duruigbo et al., 2007).
The application of animal manure and CF meaningfully enhanced the SOC and TN, P and K status in the paddy soil (Table 3), which is in line with Wei et al. (2017). The main macronutrients (N, P, and K) are important for plant growth and production. Soil N, P, and K scarcity have a negative impact on soil fertility and crop yield (Kaur and Reddy, 2014; Verma et al., 2017). The substantial improvement of soil organic C (SOC) could be associated to the substantial effects of manure fertilization because the soil C variation rate is affected by direct carbon inputs from organic fertilizer and indirect carbon inputs from increased plant biomass return (Bitew and Alemayehu, 2017; Wang et al., 2021), such as crop and root residues. This is primarily due to the addition of manure (i.e., CM or poultry) having a significant effect on the soil TN, TP, and TK. This increment in nutrient content might also be related to incorporating manure remains, which, after decomposition, directly added nutrients to the soil (Zhang et al., 2019; Hou et al., 2022). Moreover, besides improving the physical and biochemical traits of soil, animal manure fertilization, steady releases of plant nutrients and prevent nutrient losses from the synthetic fertilizers by binding to plant nutrients and releasing them (Abedi et al., 2010; Ullah et al., 2020). Thus, the integrated application of manure and CFs improves CF use efficiency and, hence, decreases CF’s rate (Tilahun-Tadesse et al., 2013; Adekiya et al., 2019).
In the present research, organic fertilizer in conjunction with synthetic fertilizer, increased microbial biomass C and N (Table 3). Rises in MBC and MBN may have happened because manure enhanced the biogeochemical properties of the soil in the present study, leading to improved absorption of inorganic N by the plant (Ahmad et al., 2008). Another reason is that organic amendments may have boosted soil nutrient richness and crop biomass accumulation, resulting in enhanced crop residues (Akhtar et al., 2019). Such residues are useful for spreading soil bacteria and may promote the alteration of N and C (Lima et al., 2009).
In this experiment, the residual effects of manure have increased soil biochemical traits. Soil organic C, examined as a vital factor in evaluating soil health and quality, enhanced 26.4% in the residual effect of manure fertilization. A rise in SOC due to organic manure fertilization has a significant impact on soil productivity because SOC is the ultimate source of soil nutrients and soil microorganisms activity in the soil. Moreover, SOC also has a key role in enhancing soil quality, infiltration rate, water holding ability, porosity and aeration (Sarwar et al., 2008; Ali et al., 2020a). Moreover, manure contains macro-and micronutrients, such as N, K, P, Ca, and Mg (Abedi et al., 2010; Hafidi et al., 2012). In this study, a significant increase was noted in TN and MBN in the following year; the average enhancement in TN and MBN in 2020 was 9.5 and 22.2% compared with 2019. The observed residual effect of organic fertilizers or enhancement in nutrient contents of manured plots in our study might be due to the reason that soil nutrients contained in organic fertilizers are revered in soil for a longer period and released steadily, ensuring a long term residual influence, and to solubilization of plant nutrients from soil minerals due to the influence of organic manure organic acids (Sharma et al., 2013).
Bacterial Community Diversity and Composition
Microbes are a key index of soil quality (van Bruggen et al., 2015). The richness and biodiversity of the microbial population are crucial to soil integrity, performance, and soil sustainability; however, they are frequently reduced by conventional agricultural techniques (Zhao et al., 2014). In the current study, the fertilization treatments extensively affected the bacterial community structure and composition, and the soil bacterial communities were influenced by the application of organic amendments (Figures 1, 4). Manure and synthetic fertilizer co-application significantly increased the number of sequences and the species richness (i.e., ACE, Shannon, and Chao 1 index values) of the bacteria related to sole inorganic fertilizer (Table 4 and Figure 1). According to Dong et al. (2014), manure applications not only include a wider range of substrates for bacterial activity than synthetic fertilizers, and they also directly introduce microorganisms found in manure into the soil. Furthermore, Bacteria are broadly considered as critical mediators of the quick pathways of nutrient cycling in soil, and their growth rate is nearly ten times higher than that of fungi (Rousk and Bååth, 2007; Schmidt et al., 2014); thereby, bacterial communities can grow rapidly and diversify under manure addition.
TABLE 4.
Pearson correlation coefficients between soil properties, soil bacterial community, and rice grain yield.
| SOC | TN | AN | MBC | MBN | OTU | |
| TN | 0.99** | |||||
| AN | 0.93** | 0.92** | ||||
| MBC | 0.99** | 0.97** | 0.92** | |||
| MBN | 0.99** | 0.98** | 0.92** | 0.98** | ||
| OUT | 0.56* | 0.27 | 0.12 | 0.62* | 0.57* | |
| GY | 0.79** | 0.71* | 0.71* | 0.84** | 0.79** | 0.21 |
SOC, soil organic carbon; TN, total nitrogen; AN, available nitrogen; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; OUTs, operational taxonomic units; GY, grain yield. *P < 0.05, **P < 0.01.
In the current study, the bacterial community structure in synthetic fertilizers treatment and non-synthetic fertilizer treatment was similar; however, it changed following manure amendments treatment (Figure 4). This is probably because of short-term input of CFs did not considerably affect the soil pH. According to Ganzert et al. (2014), pH is the greatest important factor shaping the soil bacterial community. Furthermore, Geisseler and Scow (2014) stated that the response of CFs on soil microorganisms is carbon and pH-dependent. Organic amendments could enhance soil interspace and resource supply for bacterial growth (Li P. et al., 2020), thereby prompting microbe societies. Moreover, cluster analysis showed a substantial difference in bacterial community composition between manure treated and non-manure treatment (Figure 5A). This is primarily due to livestock manure containing more readily available microbial organic resources that can provide additional metabolic nutrients to soil microbes (Bei et al., 2018).
The integrated use of animal manure and CF increased the relative abundance of Proteobacteria, Chloroflexi, Firmicutes, Acidobacteria, and Planctomycetes, which described for more than 70% of the relative abundance of the soil bacterial communities (Figure 4). The relative abundance of the community’s dominant bacteria, Proteobacteria and a type of nutrient-rich bacteria are associated with soil properties (Merila et al., 2010; Luo et al., 2020). In our research, all soil chemical traits improved with manure fertilization (Table 3), promoting the growth of bacteria. Thus, the relative abundance of Acidobacteria, Chloroflexi, Proteobacteria, Firmicutes, and Planctomycetes, the dominant bacteria in the community, enhanced under the integration of organic and synthetic fertilizer treatments.
Grain Yield
Application of organic and inorganic N significantly improved rice yield related to CF-only application in the current study. Manure application improves the physical and biogeochemical properties of the soil, resulting in increased plant growth and crop yield (Abedi et al., 2010; Hafidi et al., 2012). In this study, higher soil nutrients were observed under manured plots (Table 3), which, in turn, improved rice growth and biomass accumulation by providing adequate nutrients during the growth period. Moreover, The residual effect of 1 year (two consecutive seasons) of manure and CFs application also provided yield benefits. Significant increases in rice grain yield were observed between years, and the average rice yield was enhanced by 14% in the year 2020 relative to the year 2019. Person correlation analysis exhibited that grain yield was strongly correlated with SOC, TN, TP, and AK of soil (Table 4). This correlation shows that deficiency of anyone the three main nutrients (NPK) can decrease grain yield in flooded soil. The structural equation model result showed that differences in soil nutrients explained the greatest proportion of the variation in rice crop yield (Figure 7). These findings collectively show the importance of balanced and enough fertilization of organic and chemical N fertilizers for soil quality and sustainable grain yield of rice. According to Akhtar et al. (2019), changes in crop yield are closely related to soil biogeochemical characteristics and microbial biomass yield.
FIGURE 7.
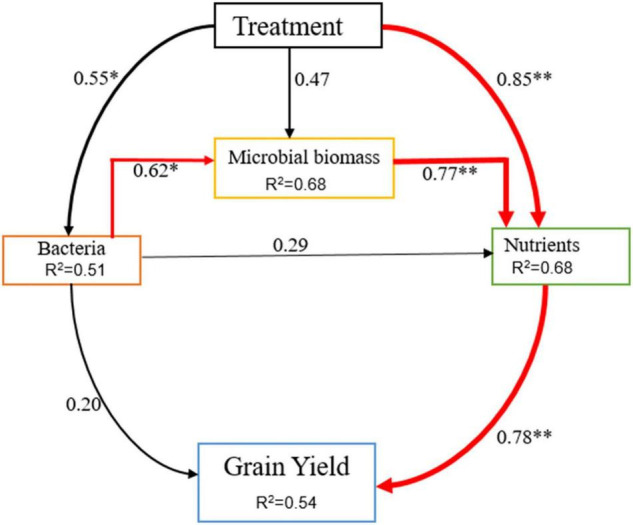
The value above the structural equation model line shows the path coefficient. The red lines represent the positive path coefficient and the black lines represent the non-significant path coefficient. The width of the arrow indicates the significance of the standard path coefficient (**P < 0.01 and *P < 0.05).
Relationships Between Soil Bacterial Communities and Biochemical Traits
Manure fertilization can cause physicochemical variations in soil, resulting in changes in the bacterial community composition (Li et al., 2021). This study discovered that organic amendment treatments dramatically altered soil qualitative traits (Table 3). Moreover, Wu et al. (2020) reported that the structure and composition of the bacterial community were positively associated with soil C and N content. Figure 5C shows the relationship between the soil traits and the bacterial communities for the different fertilizer regimes. In this study, RDA showed that the organic amendments had significant effects on bacterial community and soil qualitative traits. The dominant bacteria at the phyla, notably Proteobacteria, Chloroflexi, and Acidobacteria, had a positive correlation with soil characteristics, but Proteobacteria had a strong correlation with SOC, TN, and pH. Bacterial growth is highly related to the type of fertilizer used, and controlling the type and ratio of animal fertilizer is an effective strategy for increasing bacterial growth. According to what has been discussed thus far, applying organic fertilizer combined with reduced inorganic fertilizers could provide a faster growth environment for soil microbes, improving the bacterial population structure and soil fertility.
Conclusion
Poor soil quality and fertility due to excessive use of CFs is a major threat to agricultural sustainability and food security. Organic fertilizer application as a substitute for CF is an adequate fertilization approach for preserving soil health and biodiversity. In the current study, CF combined with cattle or PM improved soil fertility, bacterial community diversity and composition, and rice grain yield compared to the sole use of CF. A comparison of the structure of the bacterial community and its relationship to soil properties in manured regimes, we noted that manure application enhanced soil nutrients and decreased soil acidification, which contributed directly to higher rice yield. Furthermore, the substitution of animal manure regulated the relationship between soil bacteria and soil biochemical traits, influencing the soil microbial population. Our findings demonstrated that soil biochemical indicators play an important role in forming the structure and stability of soil bacterial communities. Our results indicated that combining manure and CFs applications based on nutrient levels and proper fertilizers composition is the most effective approach for improving bacterial community structure and soil fertility, ensuring yield sustainability.
Data Availability Statement
The datasets presented in this study can be found in the NCBI under accession number PRJNA822677.
Author Contributions
AI and LJ: conceptualization and writing—original draft. AI, IA, and PY: methodology and formal analysis. AI, IA, AK, ZH, and LH: investigation. LJ and SW: resources and supervision. AI, AK, ZH, LH, and PY: data curation. PY, AK, and LJ: writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We want to thank our collaborators at the Agriculture farm of Guangxi University for their assistance in conducting this experiment.
Abbreviations
- OF
organic fertilizer
- PM
poultry manure
- CF
chemical fertilizer
- CM
cattle manure
- TN
total nitrogen
- SOC
soil organic carbon
- AN
available nitrogen
- MBC
microbial biomass carbon
- OTUs
operational taxonomic units.
Funding
National Key Research and Development Project of China, Award Number: 2016YFD030050902.
References
- Abedi T., Alemzadeh A., KazemeIni S. A. (2010). Effect of organic and inorganic fertilizers on grain yield and protein banding pattern of wheat. Aust. J. Crop Sci. 4 384–389. [Google Scholar]
- Adekiya A. O., Agbede T. M., Aboyeji C. M., Dunsin O., Simeon V. T. (2019). Effects of biochar and poultry manure on soil characteristics and the yield of radish. Sci. Hortic. 243 457–463. 10.1016/j.scienta.2018.08.048 [DOI] [Google Scholar]
- Adekiya A. O., Agbede T. M., Ejue W. S., Aboyeji C. M., Dunsin O., Aremu C. O., et al. (2020). Biochar, poultry manure and NPK fertilizer: sole and combine application effects on soil properties and ginger (Zingiber officinale Roscoe) performance in a tropical Alfisol. Open Agric. 5 30–39. 10.1515/opag-2020-0004 [DOI] [Google Scholar]
- Ahmad R., Arshad M., Khalid A., Zahir Z. A. (2008). Effectiveness of organic-/bio-fertilizer supplemented with chemical fertilizers for improving soil water retention, aggregate stability, growth and nutrient uptake of maize (Zea mays L.). J. Sustain. Agric. 31 57–77. 10.1300/J064v31n04_05 [DOI] [Google Scholar]
- Akhtar K., Wang W., Khan A., Ren G., Zaheer S., Sial T. (2019). Straw mulching with fertilizer nitrogen: an approach for improving crop yield, soil nutrients and enzyme activities. Soil Use Manag. 35 526–535. 10.1111/sum.12478 [DOI] [Google Scholar]
- Ali I., He L., Ullah S., Quan Z., Wei S., Iqbal A., et al. (2020a). Biochar addition coupled with nitrogen fertilization impacts on soil quality, crop productivity, and nitrogen uptake under double-cropping system. Food Energy Sec. 9:e208. 10.1002/fes3.208 [DOI] [Google Scholar]
- Ali I., Ullah S., He L., Zhao Q., Iqbal A., Wei S., et al. (2020b). Combined application of biochar and nitrogen fertilizer improves rice yield, microbial activity and N-metabolism in a pot experiment. PeerJ 8:e10311. 10.7717/peerj.10311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei S., Zhang Y., Li T., Christie P., Li X., Zhang J. (2018). Response of the soil microbial community to different fertilizer inputs in a wheat-maize rotation on a calcareous soil. Agri. Ecosyst. Environ. 260 58–69. 10.1016/j.agee.2018.03.014 [DOI] [Google Scholar]
- Bhattacharyya R., Ghosh B. N., Mishra P. K., Mandal B., Rao C. S., Sarkar D., et al. (2015). Soil degradation in India: challenges and potential solutions. Sustainability 7 3528–3570. 10.3390/su7043528 [DOI] [Google Scholar]
- Bitew Y., Alemayehu M. (2017). Impact of crop production inputs on soil health: a review. Asian J. Plant Sci. 16 109–131. 10.3923/ajps.2017.109.131 [DOI] [Google Scholar]
- Brookes P. C., Landman A., Pruden G., Jenkinson D. (1985). Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 17 837–842. 10.1016/0038-0717(85)90144-0 [DOI] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercioglu M. (2017). The role of organic soil amendments on soil physical properties and yield of maize (Zea mays L.). Commun. Soil Sci. Plant Anal. 48 683–691. 10.1080/00103624.2017.1298787 [DOI] [Google Scholar]
- Chaparro J. M., Sheflin A. M., Manter D. K., Vivanco J. M. (2012). Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 48 489–499. 10.1007/s00374-012-0691-4 [DOI] [Google Scholar]
- Chauhan B. S., Jabran K., Mahajan G. (eds) (2017). Rice Production Worldwide, Vol. 247. Cham: Springer International Publishing. 10.1007/978-3-319-47516-5 [DOI] [Google Scholar]
- Claesson M. J., Wang Q., O’Sullivan O., Greene-Diniz R., Cole J. R., Ross R. P., et al. (2010). Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res. 38:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. R., Wang Q., Cardenas E., Fish J., Chai B., Farris R. J., et al. (2009). The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37(Suppl. 1) D141–D145. 10.1093/nar/gkn879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X. W., Zhang Y. Z., Gao J. S., Peng F. Y., Gao P. (2018). Long-term combined application of manure and chemical fertilizer sustained higher nutrient status and rhizospheric bacterial diversity in reddish paddy soil of central South China. Sci. Rep. 8:16554. 10.1038/s41598-018-34685-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhariwal A., Chong J., Habib S., King I. L., Agellon L. B., Xia J. (2017). Microbiome analyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 45 W180–W188. 10.1093/nar/gkx295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W. Y., Zhang X. Y., Dai X. Q. (2014). Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl. Soil Ecol. 84 140–147. 10.1016/j.apsoil.2014.06.007 [DOI] [Google Scholar]
- Duan P., Fan C., Zhang Q., Xiong Z. (2019). Overdose fertilization induced ammoniaoxidizing archaea producing nitrous oxide in intensive vegetable fields. Sci. Total Environ. 650 1787–1794. 10.1016/j.scitotenv.2018.09.341 [DOI] [PubMed] [Google Scholar]
- Duruigbo C. I., Obiefuna J. C., Onweremadu E. U. (2007). Effect of poultry manure rates on the soil acidity in an Ultisol. Int. J. Soil Sci. 2 154–158. 10.3923/ijss.2007.154.158 [DOI] [Google Scholar]
- Edgar R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Ganzert L., Bajerski F., Wagner D. (2014). Bacterial community composition and diversity of five different permafrost-affected soils of Northeast Greenland. FEMS Microbiol. Ecol. 89 426–441. 10.1111/1574-6941.12352 [DOI] [PubMed] [Google Scholar]
- Geisseler D., Scow K. M. (2014). Long-term effects of mineral fertilizers on soil microorganisms–a review. Soil Biol. Biochem. 75 54–63. 10.1016/j.soilbio.2014.03.023 [DOI] [Google Scholar]
- Gu B., Ju X., Chang J., Ge Y., Vitousek P. M. (2015). Integrated reactive nitrogen budgets and future trends in China. Proc. Natl. Acad. Sci. U.S.A. 112:8792. 10.1073/pnas.1510211112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidi M., Amir S., Meddich A., Jouraiphy A., Winterton P., El Gharous M., et al. (2012). Impact of applying composted biosolids on wheat growth and yield parameters on a calcimagnesic soil in a semi-arid region. Afr. J. Biotech. 11 9805–9815. 10.5897/AJB10.1010 [DOI] [Google Scholar]
- Harris J. (2009). Soil microbial communities and restoration ecology: facilitators or followers? Science 325 573–574. 10.1126/science.1172975 [DOI] [PubMed] [Google Scholar]
- Hartmann M., Frey B., Mayer J., Mäder P., Widmer F. (2015). Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 9 1177–1194. 10.1038/ismej.2014.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q., Lin S., Ni Y., Yao L., Huang S., Zuo T., et al. (2022). Assembly of functional microbial communities in paddy soil with long-term application of pig manure under rice-rape cropping system. J. Environ. Manag. 305:114374. 10.1016/j.jenvman.2021.114374 [DOI] [PubMed] [Google Scholar]
- Ikoyi I., Egeter B., Chaves C., Ahmed M., Fowler A., Schmalenberger A. (2020). Responses of soil microbiota and nematodes to application of organic and inorganic fertilizers in grassland columns. Biol. Fertil. Soils 56 647–662. 10.1007/s00374-020-01440-5 [DOI] [Google Scholar]
- Ingham E. R. (2009). “Chapter 4 - Soil biology primer: soil fungus,” in Soil and Water Conservation (Ankeny, IA: Soil & Water Conservation Society; ), 22–23. [Google Scholar]
- Iqbal A., He L., Khan A., Wei S., Akhtar K., Ali I., et al. (2019). Organic manure coupled with inorganic fertilizer: an approach for the sustainable production of rice by improving soil properties and nitrogen use efficiency. Agronomy 9:651. 10.3390/agronomy9100651 [DOI] [Google Scholar]
- Jackson M. L. (1956). ). Soil Chemical Analysis—Advanced Course. Madison, WI: University of Wisconsin, 991. [Google Scholar]
- Jarvan M., Edesi L., Adamson A., Võsa T. (2014). Soil microbial communities and dehydrogenase activity depending on farming systems. Plant Soil Environ. 60 459–463. 10.17221/410/2014-PSE [DOI] [Google Scholar]
- Kaur G., Reddy M. S. (2014). Influence of P-solubilizing bacteria on crop yield and soil fertility at multilocational sites. Eur. J. Soil Biol. 61 35–40. [Google Scholar]
- Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., et al. (2013). Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41:e1. 10.1093/nar/gks808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U., Shahid D. M., Tripathi R., Mohanty S., Kumar A., Bhattacharyya P., et al. (2017). Variation of functional diversity of soil microbial community in sub-humid tropical rice-rice cropping system under long-term organic and inorganic fertilization. Ecol. Indic. 73 536–543. [Google Scholar]
- Li P., Kong D., Zhang H., Xu L., Li C., Wu M., et al. (2021). Different regulation of soil structure and resource chemistry under animal-and plant-derived organic fertilizers changed soil bacterial communities. Appl. Soil Ecol. 165:104020. 10.1016/j.apsoil.2021.104020 [DOI] [Google Scholar]
- Li P., Liu M., Ma X., Wu M., Jiang C., Liu K., et al. (2020). Responses of microbial communities to a gradient of pig manure amendment in red paddy soils. Sci. Total Environ. 705:135884. 10.1016/j.scitotenv.2019.135884 [DOI] [PubMed] [Google Scholar]
- Li T., Zhang Y., Bei S., Li X., Reinsch S., Zhang H., et al. (2020). Contrasting impacts of manure and inorganic fertilizer applications for nine years on soil organic carbon and its labile fractions in bulk soil and soil aggregates. Catena 194:104739. 10.1016/j.catena.2020.104739 [DOI] [Google Scholar]
- Li R., Li M., Ashraf U., Liu S., Zhang J. (2006). Yield analysis of early indica rice Zhenguiai 1 in South China. China Rice 1:17. [Google Scholar]
- Lima D. L., Santos S. M., Scherer H. W., Schneider R. J., Duarte A. C., Santos E. B. H., et al. (2009). Effects of organic and inorganic amendments on soil organic matter properties. Geoderma 150 38–45. 10.1016/j.geoderma.2009.01.009 [DOI] [Google Scholar]
- Liu J., Shu A., Song W., Shi W., Li M., Zhang W., et al. (2021). Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 404:115287. 10.1016/j.geoderma.2021.115287 [DOI] [Google Scholar]
- Lu R. L. (2000). Soil Agricultural Chemical Analysis Method. Beijing: China Agricultural Science and Technology Press, 1–315. [Google Scholar]
- Luo Y., Iqbal A., He L., Zhao Q., Wei S., Ali I., et al. (2020). Long-term no-tillage and straw retention management enhances soil bacterial community diversity and soil properties in Southern China. Agronomy 10:1233. 10.3390/agronomy10091233 [DOI] [Google Scholar]
- Merila P., Malmivaara-Lämsä M., Spetz P., Stark S., Vierikko K., Derome J., et al. (2010). Soil organic matter quality as a link between microbial community structure and vegetation composition along a successional gradient in a boreal forest. Appl. Soil Ecol. 46 259–267. 10.1016/j.apsoil.2010.08.003 [DOI] [Google Scholar]
- Ohyama T., Ito M., Kobayashi K., Araki S., Yasuyoshi S., Sasaki O., et al. (1991). Analytical procedures of N, P, K contents in plant and manure materials using H2SO4-H2O2 Kjeldahl digestion method. Bull. Fac. Agric. Niigata Univ. 43 110–120. [Google Scholar]
- Oladele S. O., Adeyemo A. J., Awodun M. A. (2019). Influence of rice husk biochar and inorganic fertilizer on soil nutrients availability and rain-fed rice yield in two contrasting soils. Geoderma 336 1–11. 10.1016/j.geoderma.2018.08.025 [DOI] [Google Scholar]
- Pérez-Valera E., de Melo Rangel W., Elhottová D. (2022). Cattle manure application triggers short-term dominance of Acinetobacter in soil microbial communities. Appl. Soil Ecol. 176:104466. 10.1016/j.apsoil.2022.104466 [DOI] [Google Scholar]
- Qaswar M., Jing H., Ahmed W., Li D., Liu S., Lu Z., et al. (2020). Yield sustainability, soil organic carbon sequestration and nutrients balance under long-term combined application of manure and inorganic fertilizers in acidic paddy soil. Soil Till. Res. 198 1–6. 10.1016/j.still.2019.104569 [DOI] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 D590–D596. 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C. P., Casella G., Casella G. (2010). Introducing Monte Carlo Methods With R, Vol. 18. New York, NY: Springer. [Google Scholar]
- Rousk J., Bååth E. (2007). Fungal biomass production and turnover in soil estimated using the acetate-in-ergosterol technique. Soil Biol. Biochem. 39 2173–2177. 10.1016/j.soilbio.2007.03.023 [DOI] [Google Scholar]
- Sapkota T. B., Singh L. K., Yadav A. K., Khatri-Chhetri A., Jat H. S., Sharma P. C., et al. (2020). Identifying optimum rates of fertilizer nitrogen application to maximize economic return and minimize nitrous oxide emission from rice–wheat systems in the Indo-Gangetic Plains of India. Arch. Agron. Soil Sci. 66 2039–2054. 10.1080/03650340.2019.1708332 [DOI] [Google Scholar]
- Sarwar G., Schmeisky H., Hussain N., Muhammad S., Ibrahim M., Safdar E. (2008). Improvement of soil physical and chemical improvement with compost application in rice-wheat cropping system. Pak. J. Bot. 40 275–282. [Google Scholar]
- Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hollister E. B., Hartmann M., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75 7537–7541. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S., Nemergut D., Darcy J., Lynch R. (2014). Do bacterial and fungal communities assemble differently during primary succession? Mol. Ecol. 23 254–258. 10.1111/mec.12589 [DOI] [PubMed] [Google Scholar]
- Sharma G. D., Thakur R., Som R., Kauraw D. L., Kulhare P. S. (2013). Impact of integrated nutrient management on yield, nutrient uptake, protein content of wheat (Triticum astivam) and soil fertility in Typic Haplustert. Bioscan 8 1159–1160. [Google Scholar]
- Stroobants A., Degrune F., Olivier C., Roisin C., Bodson B., Portetelle D., et al. (2012). Impact of Depth and Soil Compaction on Bacterial Diversity in Soil. Copenhagen: University of Liège (ORBI). [Google Scholar]
- Sundberg C., Al-Soud W. A., Larsson M., Alm E., Yekta S. S., Svensson B. H., et al. (2013). 454 pyrosequencing analyses of bacterial and archaeal richness in 21 full-scale biogas digesters. FEMS Microbiol. Ecol. 85 612–626. 10.1111/1574-6941.12148 [DOI] [PubMed] [Google Scholar]
- Tilahun-Tadesse F., Nigussie-Dechassa R., Wondimu B., Setegn G. (2013). Effect of farmyard manure and inorganic fertilizers on the growth, yield and moisture stress tolerance of rain-fed lowland rice. Am. J. Res. Com. 1 275–301. [Google Scholar]
- Ullah S., Liang H., Ali I., Zhao Q., Iqbal A., Wei S., et al. (2020). Biochar coupled with contrasting nitrogen sources mediated changes in carbon and nitrogen pools, microbial and enzymatic activity in paddy soil. J. Saudi Chem. Soc. 24 835–849. 10.1016/j.jscs.2020.08.008 [DOI] [Google Scholar]
- van Bruggen A. H. C., Sharma K., Kaku E., Karfopoulos S., Zelenev V. V., Blok W. J. (2015). Soil health indicators and Fusarium wilt suppression in organically and conventionally managed greenhouse soils. Appl. Soil Ecol. 86 192–201. 10.1016/j.apsoil.2014.10.014 [DOI] [Google Scholar]
- van der Heijden M. G., Bardgett R. D., van Straalen N. M. (2008). The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 11 296–310. 10.1111/j.1461-0248.2007.01139.x [DOI] [PubMed] [Google Scholar]
- Vance E. D., Brookes P. C., Jenkinson D. S. (1987). Microbial biomass measurements in forest soils: the use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol. Biochem. 19 697–702. 10.1016/0038-0717(87)90051-4 [DOI] [Google Scholar]
- Verma P., Yadav A. N., Kumar V., Singh D. P., Saxena A. K. (2017). “Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and its impact for crop improvement,” in Plant-Microbe Interactions in Agro-Ecological Perspectives, eds Singh D., Singh H., Prabha R. (Singapore: Springer; ), 543–580. [Google Scholar]
- Wagg C., Schlaeppi K., Banerjee S., Kuramae E. E., van der Heijden M. G. A. (2019). Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 10:4841. 10.1038/s41467-019-12798-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Liu K. L., Zhao X. Q., Zhang H. Q., Li D., Li J. J., et al. (2021). Balanced fertilization over four decades has sustained soil microbial communities and improved soil fertility and rice productivity in red paddy soil. Sci. Total Environ. 793:148664. 10.1016/j.scitotenv.2021.148664 [DOI] [PubMed] [Google Scholar]
- Wang S., Tian H., Liu J., Pan S. (2003). Pattern and change of soil organic carbon storage in China: 1960s–1980s. Tellus B 55 416–427. 10.1034/j.1600-0889.2003.00039.x 11841302 [DOI] [Google Scholar]
- Wei M., Hu G., Wang H., Bai E., Lou Y., Zhang A., et al. (2017). 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 82 27–34. 10.1016/j.ejsobi.2017.08.002 [DOI] [Google Scholar]
- Wu Z. X., Li H. H., Liu Q. L., Changyan Y., Faxin Y. (2020). Application of bio-organic fertilizer, not biochar, in degraded red soil improves soil nutrients and plant growth. Rhizosphere 16:100264. 10.1016/j.rhisph.2020.100264 [DOI] [Google Scholar]
- Xu X., Thornton P. E., Post W. M. (2013). A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 22 737–749. 10.1111/geb.12029 [DOI] [Google Scholar]
- Yang X. D., Ni K., Shi Y. Z., Yi X. Y., Zhang Q. F., Fang L., et al. (2018). Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Environ 252 74–82. 10.1016/j.agee.2017.10.004 [DOI] [Google Scholar]
- Yu Y., Wu M., Petropoulos E., Zhang J., Nie J., Liao Y., et al. (2019). Responses of paddy soil bacterial community assembly to different long-term fertilizations in southeast China. Sci. Total Environ. 656 625–633. 10.1016/j.scitotenv.2018.11.359 [DOI] [PubMed] [Google Scholar]
- Zhang J., Kobert K., Flouri T., Stamatakis A. (2014). PEAR: a fast and accurate illumina paired-end read merger. Bioinformatics 30 614–620. 10.1093/bioinformatics/btt593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. J., Jia J. Q., Hua L. U., Feng M. C., Yang W. D. (2021). Functional diversity of soil microbial communities in response to supplementing 50% of the mineral N fertilizer with organic fertilizer in an oat field. J. Integr. Agric. 20 2255–2264. 10.1016/S2095-3119(20)63331-7 [DOI] [Google Scholar]
- Zhang Y. L., Sun C. X., Chen Z. H., Zhang G. N., Cheng L. J., Wu Z. J. (2019). Stoichiometric analyses of soil nutrients and enzymes in a Cambisol soil treated with inorganic fertilizers or manures for 26 years. Geoderma 353 382–390. 10.1016/j.geoderma.2019.06.026 [DOI] [Google Scholar]
- Zhao J., Zhang R., Xue C., Xun W., Sun L., Xu Y., et al. (2014). Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb. Ecol. 67 443–453. 10.1007/s00248-013-0322-0 [DOI] [PubMed] [Google Scholar]
- Zhu J., Peng H., Ji X., Li C., Li S. (2019). Effects of reduced inorganic fertilization and rice straw recovery on soil enzyme activities and bacterial community in double-rice paddy soils. Eur. J. Soil Biol. 94:103116. 10.1016/j.ejsobi.2019.103116 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in the NCBI under accession number PRJNA822677.