Abstract
Protocatechuate 3,4-dioxygenase (EC 1.13.11.3) catalyzes the ring cleavage step in the catabolism of aromatic compounds through the protocatechuate branch of the β-ketoadipate pathway. A protocatechuate 3,4-dioxygenase was purified from Streptomyces sp. strain 2065 grown in p-hydroxybenzoate, and the N-terminal sequences of the β- and α-subunits were obtained. PCR amplification was used for the cloning of the corresponding genes, and DNA sequencing of the flanking regions showed that the pcaGH genes belonged to a 6.5-kb protocatechuate catabolic gene cluster; at least seven genes in the order pcaIJFHGBL appear to be transcribed unidirectionally. Analysis of the cluster revealed the presence of a pcaL homologue which encodes a fused γ-carboxymuconolactone decarboxylase/β-ketoadipate enol-lactone hydrolase previously identified in the pca gene cluster from Rhodococcus opacus 1CP. The pcaIJ genes encoded proteins with a striking similarity to succinyl-coenzyme A (CoA):3-oxoacid CoA transferases of eukaryotes and contained an indel which is strikingly similar between high-G+C gram-positive bacteria and eukaryotes.
The intradiol or ortho ring cleavage pathway commonly known as the β-ketoadipate pathway, named after the intermediate β-ketoadipate (3-oxoadipate) (57), is widely distributed among taxonomically diverse soil microorganisms, including both eubacteria and fungi. It is considered a “major utility pathway” playing a significant role in the processing and degradation of aromatic compounds from plant material found in soil, such as those originating from the solubilization of lignin (27). The pathway consists of two branches, one starting at catechol and the other at protocatechuic acid, which are cleaved by catechol 1,2-dioxygenase and protocatechuate 3,4-dioxygenase (3,4-PCD), respectively. In bacteria the two branches of the pathway converge at the intermediate β-ketoadipate enol-lactone. The β-ketoadipate pathway is biochemically conserved, and the structural genes encoding enzymes of this pathway (Fig. 1) in widely differing bacterial species are similar. The genes of the β-ketoadipate pathway have been cloned and sequenced from different bacteria, including Acinetobacter sp. strain ADP1 and Pseudomonas putida, two organisms whose G+C contents differ by 20%, but amino acid sequence identities for isofunctional Pca enzymes range from 45 to 68% (27). Despite this conservation, diversity in the β-ketoadipate pathways has evolved in pathway branching, inducing metabolites, genetic organization, operon clustering, and regulation (48).
FIG. 1.
The protocatechuate and catechol branches of the β-ketoadipate pathway. Gene products catalyzing reactions in the pathway are given in parentheses. For the reactions common to both branches, some bacteria possess only a single set of genes, while others have separate pca and cat genes for one or more of the steps.
In addition to its role in the degradation of aromatic compounds derived from lignin and other plant compounds that are recalcitrant or resistant to degradation, the enzymes of the β-ketoadipate pathway are required for the degradation of chlorocatechols by the modified ortho pathway. Although a 3,4-dihydroxychlorobenzoic acid ortho-cleaving enzyme has not been found, two protocatechuate 3,4-dioxygenase isozymes that oxidize 4-sulfocatechol were identified recently from two members of a sulfanilic acid (4-aminobenzenesulfonate)-degrading, mixed culture of Agrobacterium radiobacter strain S2 and Hydrogenophaga palleronii strain S1 (23). No protein or nucleic acid sequences were reported for these enzymes, but the broad substrate specificity suggests that they were related to “classical” protocatechuate 3,4-dioxygenases.
Protocatechuic acid is an intermediate in the metabolism of vanillic acid and p-hydroxybenoic acid in Streptomyces spp. (21, 60, 61). In Streptomyces sp. strain D7, vanillic acid is converted to guaicol by a specific vanillate decarboxylase (VDC) and is not metabolized further (7), whereas in Streptomyces setonii, guaicol is converted to catechol before being mineralized (49). Of the above reactions, gene sequence information has so far been obtained only for the VDC enzyme. In this study, Streptomyces sp. strain 2065 was found to utilize vanillic acid or p-hydroxybenzoic acid as the sole carbon source. Induction of synthesis of protocatechuate 3,4-dioxygenase by either of these aromatic acids was observed. The dioxygenase has been purified and the pcaGH genes have been cloned and sequenced from Streptomyces sp. strain 2065; these genes were found to be part of a larger pcaIJFHGBL gene cluster. Zaborina et al. (66) purified and characterized a 6-chlorohydroxyquinol 1,2-dioxygenase from Streptomyces rochei 303, but no molecular studies have been reported.
MATERIALS AND METHODS
Bacterial strains, plasmids, and cultivation conditions.
Streptomyces sp. strain 2065 was obtained from the British Columbia Research Institute collection of actinomycetes (kindly provided by P. Axelrood) isolated from the bark of coastal British Columbia trees; it was chosen for its ability to grow on vanillic acid or p-hydroxybenzoic acid as the sole carbon source.
Streptomycetes were routinely grown on ISP medium 4 (Difco Laboratories Inc., Detroit, Mich.), soy mannitol agar, or tryptic soy agar plates (Difco) at 30°C. Spores were resuspended and stored in sterile 20% glycerol at −20°C. For DNA isolation the streptomycetes were grown in liquid yeast extract-malt extract medium (YEME) cultures (28). For enzyme assays and protein purification, cells were first grown in tryptic soy broth (TSB) to early- to mid-log phase and then washed in isotonic medium before being transferred to mineral salts medium with yeast extract (MSMYE), pH 7.2, containing (per liter) 0.1 g of (NH4)2SO4, 0.1 g of NaCl, 0.2 g of MgSO4 · 7H2O, 0.01 g of CaCl2, 0.5 g of yeast extract, 1.0 g of K2HPO4, and 0.5 g of KH2PO4 and supplemented with 0.3% p-hydroxybenzoic acid or vanillic acid (Sigma Chemicals, Oakville, Ontario, Canada). Streptomycete liquid cultures were grown in baffled Erlenmeyer flasks or flasks containing steel springs on a rotary shaker at 260 rpm.
Escherichia coli DH5α and E. coli XL-1 Blue (P2) from Stratagene (La Jolla, Calif.) and derivatives were grown at 37°C on Luria-Bertani (LB) medium containing appropriate antibiotics at standard concentrations (52). The λ DASH II bacteriophage vector, pBluescript KS(+), and the TA cloning vector were obtained from Stratagene.
Characterization of actinomycete isolates.
Actinomycetes were screened for activity against various aromatic acids on minimal medium agar containing trace elements, bromothymol blue, and the aromatic acid (1.5 to 3 g/liter), which was phosphate buffered to pH 7.2 (10). Degradation of the aromatic acid was indicated by an increase in pH, resulting in a color change from green (at pH 7.2) to blue (greater than pH 7.2). Aromatic acids tested were benzoic acid, cinnamic acid, 2-chlorobenzoic acid, 3-chlorobenzoic acid, 4-chlorobenzoic acid, p-hydroxybenzoic acid, vanillic acid, isovanillic acid, and veratric acid. Isolates which were positive in this assay were grown in liquid minimal medium in the presence of the aromatic acid of interest as a sole carbon source. Culture supernatants were sampled over time and analyzed by UV/visual spectrophotometry. Removal of the aromatic acid from culture was detected as a decrease in absorbance at the λmax for the particular aromatic acid tested. TerraGen Diversity Inc. (Vancouver, British Columbia, Canada) provided cell wall fatty acid methyl ester (FAME) analysis and 16S ribosomal DNA (rDNA) sequence determinations.
Harvesting of cells, cell disruption, and preparation of cell extracts.
Cells were harvested by centrifugation and washed with 50 mM Tris-HCl, pH 8.5 (buffer A). The cell paste was frozen at −20°C until further use. The following steps were performed at 4°C unless otherwise noted. For enzyme assays using crude cell-free extracts from small-scale cultures, cells from 50-ml cultures were harvested and resuspended in 2 ml of buffer A containing 1 mg of lysozyme/ml, 100 μg of DNase I/ml, 100 μg of RNase A/ml, and 1 mM phenylmethysulfonyl fluoride (PMSF). The cell suspension was incubated at 37°C for 1 h and homogenized on ice with a tissue grinder, and the extract was centrifuged at 25,000 × g for 5 min to remove cellular debris. For protein purification, cells from 6 liters of culture were resuspended in buffer A containing 100 μg of DNase I/ml, 100 μg of RNase A/ml, and 1 mM PMSF. The cells were disrupted with a single passage through a French press operated at 20,000 lb/in2. The cellular debris was removed by centrifugation at 8,000 × g for 30 min.
Enzyme assays.
To screen for the presence of intradiol ring cleavage dioxygenase activity in crude cell extracts, the colorimetric Rothera reaction (58) was performed following the method of Ottow and Zolg (42). Development of a deep purple color indicated the presence of the ortho pathway intermediate β-ketoadipate.
Protocatechuate 3,4-dioxygenase activity was measured spectrophotometrically as described by Stanier and Ingraham (56) with slight modifications. The assay mixture contained 50 mM Tris-HCl (pH 8.5), an appropriate amount of enzyme (for example, 20 to 50 μg of crude cell extract), and 160 μM protocatechuic acid in a total volume of 300 μl. The reaction was initiated by the addition of substrate, and a decrease in absorbance at 290 nm at 25°C was recorded on a Varian-Cary 1 Bio spectrophotometer (Varian Canada Inc., Edmonton, Alberta, Canada). One unit of enzyme activity is defined as the amount that oxidizes protocatechuic acid at an initial rate of 1 μmol per min. Reaction rates were calculated using an extinction coefficient of 2.3 mM−1 · cm−1 for the conversion of protocatechuic acid to β-carboxy-cis,cis-muconic acid. Specific activity was expressed in units per milligram of protein. Protocatechuate 4,5-dioxygenase was monitored by an increase in absorbance at 410 nm (63). Catechol 2,3-dioxygenase activity was measured by an increase in absorbance at 375 nm (32). Catechol 1,2-dioxygenase activity was measured by an increase in absorbance at 260 nm (5), and gentisate 1,2-dioxygenase activity was measured by an increase in absorbance at 334 nm (9).
Protein purification.
Protocatechuate 3,4-dioxygenase was purified at room temperature by fast protein liquid chromatography (FPLC) (Pharmacia Biotech Inc., Baie d'Urfé, Quebec, Canada). Protein eluates were detected spectrophotometrically at 280 nm. The crude cell extract was first precipitated with (NH4)2SO4 on ice. Protein which precipitated between 40 and 60% (NH4)2SO4 was resuspended and dialyzed in buffer A at 4°C and batch purified on a 3- by 6-cm (diameter by height) chromatography column packed with Q-Sepharose Fast Flow resin (Pharmacia Biotech Inc.) and equilibrated in the same buffer. Protein eluting between 350 and 450 mM NaCl was concentrated, exchanged into buffer A, and passed through a 0.45-μm-pore-size filter before being purified by FPLC. This preparation was chromatographed on a Mono-Q HR 5/5 (Pharmacia) column equilibrated in buffer A and was eluted with a 250 to 550 mM NaCl gradient in the same buffer. The active fractions were pooled, concentrated, and exchanged into buffer A with 1.7 M (NH4)2SO4. This preparation was chromatographed on a Phenyl Superose HR5/5 (Pharmacia) column equilibrated in buffer A with 1.7 M (NH4)2SO4, and the dioxygenase was eluted with a 700 to 200 mM (NH4)2SO4 gradient. The active fractions were again pooled, exchanged into buffer A with 100 mM NaCl, and chromatographed through a Superose 6 HR 10/30 (Pharmacia) column equilibrated in the same buffer. The final active fractions were pooled, concentrated, and stored at 4°C.
Molecular mass determination.
For determination of native molecular mass, a Superose 6 column (Pharmacia) was equilibrated in buffer A with 100 mM NaCl. High-molecular-weight gel filtration standards were purchased from GIBCO BRL (Burlington, Ontario, Canada).
Protein electrophoresis.
Protocatechuate 3,4-dioxygenase was concentrated and exchanged into appropriate buffers using Centricon and Centriprep concentrators (Amicon, Bedford, Mass.). The concentration of protein in cell extracts and throughout the enzyme purification was measured by the bicinchoninic acid method (54) (Sigma Chemicals Ltd.). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a Bio-Rad (Mississauga, Ontario, Canada) Miniprotein II apparatus with 13% polyacrylamide gels using the modified procedure of Laemmli (2). Samples were boiled with SDS for 5 min and before separation. For native PAGE, 10% polyacrylamide was used and electrophoresis solutions contained no SDS or denaturing agents. Gels were silver stained to visualize proteins. Coomassie blue R-250 staining was used only for blotted protein prior to N-terminal sequencing. Prestained protein molecular weight markers were purchased from GIBCO BRL.
N-terminal protein sequencing.
The standard procedure for SDS-PAGE was used (2) with the following exceptions. All gel solutions, excluding the running buffer, were filtered through a 0.45-μm-pore-size filter. Samples were solubilized with sucrose-containing sample buffer instead of urea and heated to 37°C for 10 to 15 min prior to electrophoresis. The gel was blotted onto Immobilon-PSQ polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, Mass.) using a Milliblot semidry graphite electroblotter (Millipore). A three-buffer protocol was used in which ɛ-amino-n-caproic acid was substituted for glycine according to the manufacturer's instructions. The membrane was stained with Coomassie blue R-250. Protein sequencing was performed using the Edman degradation procedure by the Nucleic Acid and Protein Sequencing (NAPS) Unit at the University of British Columbia (UBC) using the Applied Biosystems (ABI) (Mississauga, Ontario, Canada) model 476A Protein Sequencer.
Phage λ library.
A Streptomyces sp. strain 2065 genomic library was prepared using a lambda DASH II/BamHI Vector Kit from Stratagene. Sau3AI partially digested total genomic DNA was treated with calf intestinal phosphatase (CIP) to suppress the formation of tandem inserts and was then ligated to the vector DNA. Lawns of plaques were obtained by infecting E. coli XL-1 (P2) with a recombinant λ phage library as described by the manufacturer. The library was amplified in the same bacterial host according to the manufacturer's instructions, and the titer was determined to be 6.3 × 105 plaques/μg of DNA.
Manipulation of DNA.
Restriction digests, ligation reactions, DNA analysis on agarose gels, and other procedures mentioned were performed according to standard protocols unless otherwise stated (2, 52). Restriction enzymes and DNA-modifying enzymes were purchased from GIBCO BRL, New England Biolabs Ltd. (Mississauga, Ontario, Canada), and Promega Corp. (Nepean, Ontario, Canada). Total genomic DNA from Streptomyces sp. strain 2065 was purified by cell lysis with pretreatment with lysozyme and proteinase K (28) and cesium chloride-ethidium bromide density gradient centrifugation (38). Bacteriophage DNA was purified by a standard protocol which included a polyethylene glycol (PEG) precipitation step (52). Plasmid DNA from E. coli strains was purified using QIAwell Miniprep columns (Qiagen, Chatsworth, Calif.). E. coli strain DH5α was transformed by electroporation using a model 165-2076 Gene Pulser Transfection Apparatus and Pulse Controller (Bio-Rad) according to the instructions of the manufacturer.
PCR amplification of the protocatechuate 3,4-dioxygenase genes.
PCRs were performed on a model PTC-150 Minicycler (MJ Research, Inc., Watertown, Mass.). Primers used for PCR and/or DNA sequencing were prepared by DNA oligonucleotide synthesis services from TerraGen Diversity Inc. and the NAPS Unit at UBC. Degenerate primers, P34OAfor [CT(C/G) AC(C/G) CAG CAC GAC ATC GAC CT] and P34OBCrev [C(C/G)G G(C/G)C G(C/G)(C/G) (A/T)(C/G)T GTC GAT (C/G)GT (C/G)GT], were designed from N-terminal sequences from the purified streptomycete 3,4-PCD α and β subunits. After the 800-bp PCR product had been cloned and sequenced, nondegenerate primers, βfor (GCC GAG CAC GCG ACG TAC GAG AAG C) and αrev (ACG TGT CGA TGG TCG TCA TGG C), were designed to amplify the pcaH gene. Streptomycete-specific 16S rDNA primers, S16S2 and S16S3 (62), were used for control PCRs. PCR using streptomycete DNA were performed according to Webb and Davies (62). Reaction mixtures were heated to 95°C for 2 min before 1.25 U of Taq polymerase was added, and cycling parameters used for streptomycete DNA were described previously (38).
Southern blot hybridizations and cloning strategy.
The Streptomyces sp. strain 2065 Sau3AI λ phage total genomic library was screened by plaque hybridization screening, and phage clones that hybridized to the probe were isolated, purified and analyzed. DNA fragments produced by PCRs were cloned using the TA Cloning System (Invitrogen, San Diego, Calif.). Southern blot hybridizations (55) were performed using the DIG system (Boehringer Mannheim Biochemica, Laval, Quebec, Canada) according to the manufacturer's recommendations. Nonradioactive DNA labeling was performed by PCR incorporation of digoxigenin-11-dUTP (36) into the ca. 800-bp insert. Purified DNA was blotted onto positively charged nylon membranes (Boehringer Mannheim Biochemica) by the alkaline upward capillary transfer method. Plaque lifts were blotted onto Hybond N+ positively charged membranes from Amersham (Oakville, Ontario, Canada), and DNAs were fixed by baking membranes at 80°C for 2 h. Hybridizations were performed in glass tubes in a Hybaid Micro-4 hybridization oven and rotisserie (Interscience, Inc., Markham, Ontario, Canada). DIG-labeled DNA was detected colorimetrically using nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP).
DNA sequencing and analysis.
Plasmids containing cloned genes were sequenced using M13 Universal forward and reverse primers, T3 and T7 primers, and successive synthesized oligonucleotides. For 16S rDNA sequencing, primers 7F, 530F, 1100F (12), and 1491R (modified from Li and DeBoer [35]) were used. Sequencing reactions were performed by Taq cycle sequencing using the DyeDeoxy terminator method (Applied Biosystems Inc.) according to the instructions of the manufacturer. Sequencing reaction products were analyzed on an ABI model 373A DNA Sequencer. Computer-based sequence analysis were performed with the Genetics Computer Group (GCG, Madison, Wis.) package and PC/GENE (Intelligenetics Inc., Mountain View, Calif.); alignments were done using open and unit gap costs set to 10. BLAST 2.0 was used to identify similarity to other sequences.
Nucleotide sequence accession number.
The 6,551-bp sequence containing pcaIJFHGBL on pKQ1 and pKQ2 appears in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession number AF109386.
RESULTS
Isolation and characterization of Streptomyces sp. strain 2065.
A collection of soil actinomycetes was screened for the ability to degrade aromatic acids. Among the 45 isolates screened, 11 were found to have activity against vanillic and/or p-hydroxybenzoic acid. Streptomyces sp. strain 2065 degraded both vanillic acid and p-hydroxybenzoic acid and was selected for further analysis. When this isolate was grown in liquid culture with vanillic acid or p-hydroxybenzoic acid added as the sole carbon source, removal of the aromatic acid from the culture supernatants was observed by spectrophotometric monitoring. The rates of degradation were comparable to those previously observed with Streptomyces sp. strain D7 (7).
When grown on ISP4 medium agar, Streptomyces sp. strain 2065 had light-gray mycelia and lighter-gray spores and produced a blue, diffusible pigment. On soy mannitol agar it formed beige colonies and white spores. The 16S rDNA BLAST sequence analysis of this isolate showed 98% identity (identical in 1,438 out of 1,464 positions) to an unspecified Streptomyces sp. Cell wall FAME analysis performed by gas chromatography confirmed its identity as a member of the genus Streptomyces that was weakly related to Streptomyces halstedii with a fatty acid profile similarity of 44.8%, which indicated that the species group to which this isolate belongs has not been previously characterized in the FAME database.
Investigation of enzyme activity.
To determine whether vanillic acid and p-hydroxybenzoic acid were degraded through an ortho or a meta aromatic catabolic pathway and whether the ring cleavage intermediate was catechol or protocatechuic acid, enzyme assays were performed on crude cell extracts of Streptomyces sp. strain 2065 grown in the presence of these compounds. When extracts from cells grown in minimal medium with vanillic acid or p-hydroxybenzoic acid were incubated with protocatechuic acid or catechol, there was no development of a yellow meta-cleavage product. By subsequent Rothera reaction, a positive reaction was observed only with protocatechuate as the substrate, indicating that the mode of cleavage was ortho via the intermediate protocatechuic acid. These results were confirmed by spectrophometric assays (A290) detecting the disappearance of protocatechuate and the appearance of β-carboxy-cis,cis-muconic acid.
No protocatechuate 4,5-dioxygenase, catechol 2,3-dioxygenase, catechol 1,2-dioxygenase, or gentisate 1,2-dioxygenase activity was detected by spectrophotometric assays of cell extracts from Streptomyces sp. strain 2065 grown on vanillic acid or p-hydroxybenzoic acid. This is consistent with ortho cleavage, indicating that catechol was not the ring cleavage intermediate. Higher levels of total enzyme activity and specific enzyme activity were seen in liquid cultures of Streptomyces sp. strain 2065 grown in MSMYE with p-hydroxybenzoic acid. Maximal levels were detected at 11 h after induction with p-hydroxybenzoic acid, and the dioxygenase was purified from cells grown under these conditions.
Purification of protocatechuate 3,4-dioxygenase.
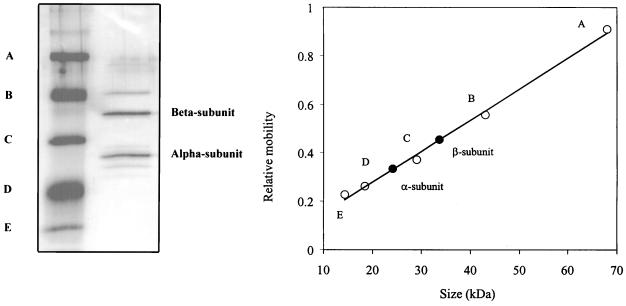
Enzyme was purified from 6 liters of cells induced with p-hydroxybenzoic acid. Throughout the FPLC purification, the enzyme activity was seen to correlate to a single protein peak. The final purified dioxygenase had a specific activity of 106 U/mg. This compares with a final specific activity of 105 U/mg for the A. radiobacter 3,4-PCD (23). Lower specific activities (ranging from 7 to 47 U/mg) have been observed for most purified 3,4-PCDs from other bacteria (4, 6, 14, 15, 18, 23, 29, 33, 59, 64). The final purified streptomycete 3,4-PCD on a native PAGE gel was observed as a single protein band (data not shown), and on a corresponding SDS-PAGE gel, two proteins with approximate masses of 24.2 and 33.7 kDa were observed (Fig. 2). These proteins were present in apparently equimolar amounts, indicating the presence of two subunits.
FIG. 2.
SDS-PAGE and subunit molecular weight determination of protocatechuate 3,4-dioxygenase. SDS-PAGE was performed as described in Materials and Methods. The α and β subunits of the streptomycete dioxygenase are represented as solid circles. A, bovine serum albumin (molecular size, 68 kDa); B, ovalbumin (43 kDa); C, carbonic anhydrase (29 kDa); D, β-lactoglobulin (18.4 kDa); E, lysozyme (14.3 kDa). The molecular sizes of the α and β subunits of the streptomycete dioxygenase are 24.2 and 33.7 kDa, respectively.
The 33.7- and 24.2-kDa proteins isolated on SDS-PAGE were blotted onto PVDF membranes, and N-terminal protein sequence information was obtained. Thirteen and 27 amino acids were obtained from the N-terminal regions of the α and β subunits, respectively; N-terminal methionine residues were absent. The α and β subunits were TTIDTSRPEEVQP and TLTQHDIDLEIAAEHATYEKRVADGAP, respectively.
Properties of protocatechuate 3,4-dioxygenase.
The purified streptomycete 3,4-PCD was chromatographed over a calibrated Superose 6 column, and its retention time was compared with those for high-molecular-weight standards; the native size of the protein was calculated to be approximately 158 kDa (Fig. 3). This falls in the lower end of the molecular size range of other 3,4-PCDs, 150 to 700 kDa (4, 6, 14, 15, 18, 23, 29, 33, 59, 64). Based on an α-subunit size of 21.8 kDa and a β-subunit size of 29.3 kDa, as predicted by the gene products below, and what is known of characterized 3,4-PCDs, the native streptomycete enzyme is proposed to contain three αβ protomers.
FIG. 3.
Native molecular mass determination of protocatechuate 3,4-dioxygenase by Superose 6 column chromatography. Gel filtration was performed as described in Materials and Methods. A, thyroglobulin (molecular mass, 670 kDa); B, immunoglobulin G (150 kDa); C, ovalbumin (44 kDa); D, myoglobin (17 kDa); E, vitamin B12 (1.35 kDa). The streptomycete dioxygenase is represented as a filled circle (158 kDa).
The enzyme was stable at room temperature for several days and at 4°C for several weeks with only a slight reduction in activity. The streptomycete 3,4-PCD was tested for activation or inactivation after a brief incubation (1 min) with various compounds. Fe2+ was seen to inhibit (28%) and Fe3+ to increase (17%) activity, suggesting that the streptomycete dioxygenase contains Fe3+, consistent with what has been found for other 3,4-PCDs. Enzyme activity was slightly reduced (10%) by ascorbate addition, but no reduction in activity was observed with dithiothreitol (DTT) and no change in activity was observed with H2O2. The slight decrease in activity due to ascorbate, a reductant, is a property consistent with the requirement for Fe3+. DTT inactivated the Burkholderia cepacia dioxygenase slowly over time (4); while for the Rhizobium trifolii enzyme, inactivation by an iron chelator was accelerated by addition of DTT (6). The Azotobacter vinelandii 3,4-PCD was not affected by brief incubation with a low concentration of DTT but was partially inactivated by H2O2 (14).
Tiron (4,5-dihydroxy-m-benzenedisulfonic acid), a Fe3+ chelator, reduced activity by 47% at 10 mM and by 90% at 20 mM. A Fe2+ chelator, 2,2-dipyridyl, reduced activity by 11% at 2 mM, 42% at 5 mM, and 100% at 10 mM. No effect was seen with up to 20 mM EDTA, a nonspecific iron chelator, similar to what was seen for the 3,4-PCDs from A. vinelandii, R. trifolii, and B. cepacia (4, 6, 16). Inhibition of the streptomycete enzyme was observed at high concentrations of Tiron and 2,2-dipyridyl, suggesting that the iron is fairly tightly held. The A. vinelandii enzyme was slightly inhibited by low concentrations of 2,2-dipyridyl and 1,10-phenanthroline but was significantly inhibited by a 1-h preincubation with 1.7 mM Tiron (14). In comparison, the 3,4-PCD from B. cepacia had an unchanged iron content after incubation with Tiron at room temperature for several days, indicating that its iron was very tightly held (4). The fact that 2,2-dipyridyl inhibited the streptomycete enzyme at lower concentrations than Tiron reflects the fact that these compounds are not entirely specific for Fe2+ and Fe3+ and may be due to differences in iron accessibility.
The relative activity of 3,4-PCD was seen to increase more than 4.5-fold as the pH was increased from 6.5 to 9.5 at increments of 0.5, and maximum relative activity was detected at pH 9.5 (Fig. 4). The pH optimum may be higher, since above pH 9.0 protocatechuic acid undergoes nonenzymatic oxidation (56). In this respect the streptomycete 3,4-PCD behaves similarly to the enzymes from B. cepacia (4), Acinetobacter calcoaceticus (29), and R. trifolii (6) and has a higher pH optimum than the enzymes from A. vinelandii (14) or Pseudomonas putida (18).
FIG. 4.
Effect of pH on the activity of protocatechuate 3,4-dioxygenase. The following buffers were used in each pH region by addition of protocatechuate 3,4-dioxygenase to the cuvette in the presence of the usual Tris-HCl (pH 8.5) buffer: pH 6.5 to 7.5, phosphate; pH 8.0 to 9.0, Tris-HCl; pH 9.5, carbonate-bicarbonate.
Cloning and identification of pcaHG genes.
The 800-bp product obtained when the primer combination P34OAfor and P34OBCrev was used in PCRs indicated the order of the genes to be pcaHG. This DNA fragment was cloned into a TA cloning vector and sequenced using M13 universal and reverse sequencing primers. Translation of the sequence from the insert indicated an amino acid sequence that had similarity to PcaHs from other bacteria. This DNA fragment was labeled nonradioactively with Dig-11-dUTP by PCR incorporation and then used as a nucleic acid probe in the DNA hybridization studies described below.
The pcaHG-containing DNA fragments were isolated from a bacteriophage λ genomic library of Streptomyces sp. strain 2065 prepared using Sau3AI partially digested total DNA. In a primary screen of 30,000 plaques, 30 were chosen that hybridized to the probe. Ten isolates were obtained in a tertiary screen, and DNA was isolated from these 10 clones and digested with SalI; three clones (KQ3, -4, and -6) possessed a 4.5-kb SalI fragment that hybridized to the pcaH probe. A 16-kb EcoRI fragment from KQ6 which hybridized to the probe was subcloned into the EcoRI site of pUC18 to give pKQ1. When total genomic DNA was digested with SalI, the pcaH probe hybridized to a DNA fragment of the same size (data not shown); the 4.5-kb SalI fragment was subcloned from KQ6 into the SalI site of pBluescript KS(+) to obtain pKQ2.
Nucleotide sequence of the 6.5-kb fragment.
DNAs isolated from plasmids pKQ1 and pKQ2 were used to obtain the complete sequence of a 6.5-kb DNA fragment on both strands. Analysis of this fragment identified a gene cluster that contains structural genes, including the pcaHG genes, involved in protocatechuate catabolism in Streptomyces sp. strain 2065. The cluster contains seven genes transcribed in the same direction in the order pcaIJFHGBL (these designations are given in reference to their sequence similarity to previously characterized pca genes) (Fig. 5; Table 1).
FIG. 5.
Gene organization of pca gene clusters from different bacteria. The gene cluster structures for Streptomyces sp. strain 2065 and Streptomyces coelicolor are identical; therefore, this gene cluster has been labeled with the genus name Streptomyces alone. Gene designations are given in Table 1. C refers to pcaC, encoding γ-carboxymuconolactone decarboxylase, while pcaD encodes β-ketpadipate enol-lactone hydrolase. pcaU, pcaR, and pcaQ are regulatory genes described in the text. pcaK is a proposed aromatic acid transport gene, and pcaT is proposed to encode a β-ketoadipate transporter. pobA encodes p-hydroxybenxzoate hydroxylase, while pobR is a regulatory gene.
TABLE 1.
Streptomyces sp. 2065 strain pca genes and predicted gene products
| ORF | Gene | Gene product | Length (aa) | Molecular mass (kDa) | Protein sequence matches (% identity, % similarity) |
|---|---|---|---|---|---|
| 1 | pcaI | β-Ketoadipate succinyl-CoA transferase, α-subunit | 251 | 26.2 | S. coelicolor PcaI (85, 89) |
| M. tuberculosis OxctA (69, 76) | |||||
| 2 | pcaJ | β-Ketoadipate succinyl-CoA transferase, β-subunit | 214 | 22.1 | S. coelicolor PcaJ (85, 91) |
| M. tuberculosis OxctB (70, 81) | |||||
| 3 | pcaF | β-Ketoadipyl CoA thiolase | 400 | 41.4 | S. coelicolor PcaF (87, 93) |
| A. calcoaceticus PcaF (50, 68) | |||||
| 4 | pcaH | Protocatechuate 3,4-dioxygenase, β subunit | 257 | 29.3 | S. coelicolor PcaH (91, 96) |
| P. marginata PcaH (53, 66) | |||||
| 5 | pcaG | Protocatechuate 3,4-dioxygenase, α subunit | 201 | 21.8 | S. coelicolor PcaG (80, 86) |
| P. marginata PcaG (37, 50) | |||||
| 6 | pcaB | β-Carboxymuconate cycloisomerase | 444 | 46.6 | S. coelicolor PcaB (80, 83) |
| R. opacus PcaB (44, 53) | |||||
| 7 | pcaL | β-Ketoadipate enol-lactone hydrolase/γ-carboxymuconolactone decarboxylase | 373 | 39.6 | S. coelicolor PcaL (83, 90) |
| R. opacus PcaL (44, 58) |
Distribution of pcaHG genes in other streptomycetes.
Genomic DNAs from 12 streptomycetes were digested with SalI and hybridized with the Streptomyces sp. strain 2065 pcaHG gene probe (Fig. 6). Streptomyces sp. strain 2065 showed one 4.5-kb DNA band which hybridized to the probe; Streptomyces coelicolor showed strong hybridization with DNA bands at 4 and 0.8 kb, a pattern also observed with Streptomyces lividans, indicating their close relationship. Other strains that hybridized strongly include Streptomyces avermertilis, Streptomyces viridosporus, Streptomyces griseolus, and Streptomyces setonii. Streptomyces sp. strain D7 (7) and Streptomyces lavendulae only showed a weakly hybridizing DNA band of 3.5 kb, while Streptomyces baclius, Streptomyces griseus, and Streptomyces hygroscopicus appear to lack the pcaHG genes.
FIG. 6.
Southern blot of streptomycete genomic digests hybridized with an 800-bp pcaH probe. All DNA was digested with SalI. Lane 1, the cloned 4.5-kb SalI fragment that contained pcaH; lane 2, Streptomyces sp. strain 2065; lane 3, S. viridosporus; lane 4, Streptomyces sp. strain D7; lane 5, S. setonii, lane 6, S. lavendulae; lane 7, S. hygroscopicus; lane 8, S. griseus; lane 9, S. griseolus; lane 10, S. coelicolor A(3)2; lane 11, S. baclius; lane 12, S. avermertilis; lane 13, S. lividans TK24. The blot was washed with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 60°C.
DISCUSSION
The actinomycetes are common denizens of soil and represent a large and diverse group of filamentous, gram-positive, high-G+C bacteria (20). Among these are the streptomycetes, which have been employed extensively for their ability to produce potent biologically active small molecules which include the major antibiotics (3) used in the treatment of infectious diseases. The presence of these microbes in soil implies that they play important roles in organic recycling (7). However, the streptomycetes have been little investigated for their catabolic capabilities, in particular for the degradation of naturally lignin-derived aromatic compounds (19) and xenobiotics (53, 65). We have undertaken an analysis of the genetics and biochemistry of the pathways by which streptomycetes degrade aromatic acids, especially those derived from lignin (7). Meta cleavage of aromatic compounds has not been observed in Streptomyces and is considered either not to exist or to be rare in this genus of bacteria (21). The β-ketoadipate pathway is known to be a common route for the degradation of aromatic compounds in soil by microorganisms (27, 48). In particular, phenolic compounds derived from lignin, such as coniferyl alcohol, ferulate, vanillate, and 4-coumarate are seen to be processed by the protocatechuate branch of this pathway (11, 47).
We have described studies on the degradation of protocatechuic acid by Streptomyces sp. strain 2065. Induction of protocatechuate 3,4-dioxygenase was observed upon growth in the presence of p-hydroxybenzoic acid or vanillic acid. This streptomycete dioxygenase is similar in subunit and native enzyme structure to those characterized from other bacteria. Preliminary characterization studies indicate, as with other intradiol-cleaving dioxygenases, that the enzyme contains a tightly held Fe3+ required for catalysis and is active over a wide pH range (pH 6.5 to 9.5).
Open reading frame 4 (ORF4) and ORF5 were identified as pcaH and pcaG, as their deduced gene products matched the N-terminal protein sequences of the purified protein and shared similarity to the β and α subunits of other protocatechuate 3,4-dioxygenases. The order of the genes encoding 3,4-PCD has been conserved. The gene encoding the α subunit, pcaG, has been reported to be located downstream from pcaH, the gene for the β subunit, for Agrobacterium tumefaciens, B. cepacia, Acinetobacter spp., and Pseudomonas spp. (representatives of the α-, β-, and γ-Proteobacteria), as well as for Rhodococcus opacus, a gram-positive nocardioform actinomycete (15, 16, 25, 44, 50, 67). Putative Shine-Dalgarno sequences, GCAGG and GGACG, preceded the ATG start codons of pcaG and pcaH at distances of 8 and 10 nucleotides, respectively. The G+C contents of pcaG and pcaH were 71.4 and 68.7 mol%, respectively, the highest of all the pcaGH genes sequenced so far; the percentage of codons ending in G or C was 93.2%. In streptomycetes TTA codons for leucine are rare, and indeed they are absent in the pcaGH gene sequences from isolate 2065.
The streptomycete PcaG and PcaH subunits were found to be similar to the proteins from Acinetobacter sp. strain ADP1, A. calcoaceticus, B. cepacia DBO1, Pseudomonas sp. strain HR199, Pseudomonas marginata, P. putida, P. putida NCIM9869, and R. opacus 1CP. The β subunits lined up with 22.6% identity and 27.2% similarity, while the α subunits lined up with 13.4% identity and 22.4% similarity. The streptomycete enzyme was most similar to the one from P. marginata (49.6 and 15.3% identity for PcaH and PcaG, respectively) (Table 1; Fig. 7). The higher identity/similarity seen among the PcaH proteins may reflect the fact that it is under stronger evolutionary constraints in order to maintain enzyme function, since the β subunit contains the ligands for the catalytic iron. Regions of highest sequence identity are those encoding iron-binding ligands, substrate binding residues, and conserved amino acid residues that form the binding pocket. Some of these residues, including those that bind the catalytic iron, are also conserved in all intradiol-cleaving dioxygenases. From the P. putida 3,4-PCD crystal structure, of the 22 residues found in the active site (41), 16 are conserved in the enzyme from Streptomyces sp. isolate 2065.
FIG. 7.
Dendrograms showing the relatedness of the α and β subunits of protocatechuate 3,4-dioxygenases. Accession numbers for the published sequences are L05770 (Acinetobacter sp. strain ADP1), M30791 (B. cepacia), U33634 (P. marginata), L14836 (P. putida), AF003947 (R. opacus), and AF109386 (Streptomyces sp. strain 2065).
The DNA sequence upstream from Streptomyces sp. strain 2065 pcaHG contains 3 ORFs. ORF1 is the putative pcaI gene, and ORF2 is the putative pcaJ gene. The start codon of ORF2 (ATG) overlaps with the stop codon of ORF1 (TGA) by 1 bp, indicating these two genes are translationally coupled. The first two ORFs were designated pcaIJ after comparison of the amino acid sequences deduced from the first two ORFs revealed extensive similarity to succinyl-, acetate-, and butyrate-coenzyme A (CoA) transferases from different organisms, including PcaIJ, a β-ketoadipate succinyl-CoA transferase, from A. calcoaceticus (46% and 53% identity) and P. putida (45% and 53% identity). The predicted protein sequence of ORF3 upstream of pcaHG shows similarity to A. calcoaceticus PcaF, a β-ketoadipyl CoA thiolase (50% identity [8]), P. putida PcaF (50% identity [26]), PhaD (40) from P. putida (51% identity), a 3-ketoacyl-CoA thiolase from E. coli (51% identity [1]), and numerous other thiolases (identities of less than 50%). The other characterized PcaFs have lengths of approximately 400 amino acids. The start codon (GTG) of pcaF overlaps with the stop codon (TGA) of pcaJ by 4 bp, indicating translational coupling. The stop codon (TAG) of pcaF is separated from the putative start codon (ATG) of pcaH by 23 bp.
Downstream of the streptomycete pcaHG there are two consecutive ORFs, ORF6 and ORF7, that have predicted gene products showing similarity to PcaB, β-carboxymuconate cycloisomerase, and PcaL, a fused β-ketoadipate enol-lactone-hydrolase and γ-carboxymuconolactone decarboxylase. ORF6 showed the strongest similarity to the PcaB of R. opacus (44% identity [15]), with descending degrees of similarity to Bradyrhizobium japonicum (42% identity [37]), P. putida (38% identity), and A. calcoaceticus (37% identity). This protein also shares significant homology with adenylosuccinate lyases (identities of less than 35%) and fumarases. ORF7 is similar to the putative PcaL of S. coelicolor (79% identity), a second homologous gene in the same organism (44% identity), and the PcaL from R. opacus (44%). These proteins and putative proteins are the only examples where PcaD and PcaC are fused and are assumed to be more closely related than their unfused homologues. The putative start for ORF6 (pcaB) is a GTG that overlaps the stop codon (TGA) of pcaG by 4 bp; the putative stop of ORF6 is a TGA that overlaps with the putative start codon (TTG) of ORF7 (pcaL) by 4 bp. ORF7 appears to start with TTG and terminates with TAA, which are both rarely used codon assignments in streptomycetes. Downstream of ORF7, strong secondary structure prevented further sequence determination.
The putative streptomycete PcaIJ showed strong similarity to a family of CoA transferases including human succinyl-CoA:3-oxoacid CoA transferase (SCOT) (31) and are 53 and 57% identical to human, pig, and Caenorhabditis elegans SCOTs. In addition, PcaIJs are 69 and 70% identical to the putative Mycobacterium tuberculosis ScoA and ScoB genes. SCOT homologues are highly conserved at the amino acid level (pairwise amino acid identity between any two SCOT sequences is usually greater than 37% over the entire length), and SCOT homologues are present in the major bacterial and eukaryotic taxa. Among bacteria, SCOT homologues have been identified in 7 high-G+C gram-positive species, 3 low-G+C gram-positive species, 13 proteobacteria, 1 bacteriod, and 1 deinococcus. Among eukaryotes, SCOT homologues have been found in human, pig, C. elegans, Dictyostelium discoideum, Trypanosoma brucei, and Candida albicans (no SCOT homologue could be detected in Saccharomyces cerevisiae or archaebacteria). This gene also appears to be absent from bacteria such as Treponema pallidum, Borrelia burgdorferi, Aquifex aeolicus, Mycoplasma spp., Chlamydia spp., and the cyanobacterium Synechocystis sp. strain PCC6803. In bacteria, two separate genes that are transcribed in the order pcaIJ or scoAB encode the two subunits (α and β) of SCOT homologues, whereas in eukaryotic SCOTs the two subunits are fused in a single polypeptide. The high degree of identity between SCOT homologues allowed alignment of these proteins without ambiguity (data not shown).
SCOT homologues of high-G+C gram-positive bacteria have strong amino acid identity to eukaryotic SCOTs (often stronger than their identity to their bacterial homologues), e.g., ScoAB of M. tuberculosis is 54 and 60% identical to human SCOT, PcaIJ of Streptomyces sp. strain 2065 is 52 and 57% identical to human SCOT, and the putative PcaIJ of S. coelicolor is 52 and 54% identical to human SCOT. In particular, the alignment of SCOTs identified a conserved insertion of 18 to 20 amino acids at a unique site in the α subunit of high-G+C gram-positive bacteria and eukaryotes (Fig. 8). As phylogenetic analyses are often unable to resolve deep branching relationships, such specific insertions, or indels, have been considered an alternative and reliable marker in identifying phylogenetic relationships (22). The finding of indels with very similar sequences in both high-G+C gram-positive bacteria and eukaryotes strongly supports the notion that the eukaryotic SCOT genes are related by evolution to those of the high-G+C gram-positive bacteria. The high degree of identity shown by the insertion diminishes the likelihood that they arose independently. Since eukaryotic SCOTs contain other signature sequences (not shown) which are not shared with bacterial SCOT homologues, the possibility of lateral gene transfer from eukaryotes to high-G+C gram-positive bacteria is deemed unlikely. As extensive lateral gene transfers have occurred throughout bacterial genome evolution (30, 34), it is remarkable that such an indel should be confined only to high-G+C gram-positive bacteria. It remains to be seen whether the high-G+C gram-positive organisms made significant contributions to the evolution of the eukaryotic genome, or if the SCOT gene relationships we have identified are anomalous situations.
FIG. 8.
Multiple alignment of SCOT homologues by ClustalW. The cloned pca genes were designated according to their functions in relation to previously characterized pca genes. Selected SCOT homologues representing major taxa are included in the alignments. The species and GenBank accession numbers are as follows: pig, 284562; human, 1519052; C. elegans (Ce), 3874069; Streptomyces sp. strain 2065 (Smp), AF109386 (this work); S. coelicolor (Smc), ALO79355; M. tuberculosis (Mt), 2113937; E. coli (Ec), 1788551; Bacillus subtilis (Bs), 666002; Clostridium acetobutylicum (Ca), 538947; Helicobacter pylori (Hp), 2313815. Highly conserved amino acids are marked by asterisks. Other abbreviations: E, eukaryote; HG+, high G+C gram-positive; LG+, low-G+C gram-positive; G−, gram-negative. Superscripts: a, sequence data for C. albicans (Ca) were obtained from the Stanford DNA Sequencing and Technology Center website at http://www-sequence.stanford.edu/group/candida/; b, preliminary sequence data for T. brucei (Tb) were obtained from The Institute for Genomic Research website at http://www.tigr.org/; c, the sequence data were produced by the Streptomyces coelicolor Sequencing Group at the Sanger Centre and can be obtained from ftp://ftp.sanger.ac.uk/pub/S coelicolor/sequences.
Sequence data produced by the S. coelicolor Sequencing Group at the Sanger Centre have revealed that the chromosome of this streptomycete also contains pca genes (51; http://www.sanger.ac.uk/Projects/S coelicolor). S. coelicolor A3(2) is able to grow on p-hydroxybenzoate, and a 3,4-PCD is induced in the presence of this aromatic compound (data not shown). In fact, sequencing of cosmid clone St4C6 (accession number AL079355.1) identified the same pcaIJFGHBL operon structure seen in Streptomyces sp. isolate 2065 (Fig. 5). The pcaGH genes from these two streptomycetes have an identity of 85%. The deduced protein sequences for PcaG and PcaH for these two streptomycetes have identities of 80 and 91%, respectively. The entire cluster is 84% identical at the DNA sequence level and 86% identical at the amino acid sequence level (Table 1). Ribosomal sequence comparisons indicate that Streptomyces sp. strain 2065 is more closely related to S. griseus (97.54%; identical in 1,428 out of 1,464 positions) than to S. coelicolor (96.28%; identical in 1,411 out of 1,464 positions). S. griseus was not seen to contain the pcaHG genes by DNA hybridization, nor was any 3,4-PCD activity observed with growth in the presence of p-hydroxybenzoic acid (data not shown). This suggests that more distantly related streptomycetes may contain similar pca genes that could have originated from independent horizontal gene transfer events.
All the ORFs within the streptomycete pca gene cluster are transcribed in the same direction and/or separated by small intergenic regions that lacked any discernible secondary structure. This indicates that the genes are most likely transcribed together. The strong secondary structure downstream of pcaL implies a transcriptional stop. A regulatory gene upstream of pcaIJ would likely be present in light of the presence of a divergently transcribed hypothetical transcriptional regulator (gene SC4C6.14; accession number AL079355) upstream of the cluster in S. coelicolor (Sanger Centre website [http://www.sanger.ac.uk/Projects/S coelicolor). Regulatory genes have been found either within or close to pca gene clusters in other bacteria. In fact, preliminary sequence data strongly suggest the presence of a regulatory gene.
In P. putida the pcaGH genes have not been found to cluster with other pca genes (16). In Acinetobacter sp. strain ADP1 the genes are contiguous in a pcaIJFBDKCHG cluster (25), while in A. tumefaciens they are in a pcaDCHGB cluster (45). Analysis of the sequence data indicates that the streptomycete pca gene cluster seems to closely resemble that of R. opacus, in which the 3,4-PCD genes are in a pcaHGBLRF cluster (15), which is similar to the pcaIJFHGBL gene order, the pcaF gene being translocated in the streptomycete cluster. The gene order pcaIJF is conserved within the Acinetobacter and the streptomycete pca gene clusters. It is interesting to find a pcaL homologue in the streptomycete pca gene cluster because the β-ketoadipate enol-lactone-hydrolase and γ-carboxymuconolactone-decarboxylase enzymes are encoded by separate genes, pcaD and pcaC, in Acinetobacter, P. putida, A. tumefaciens, and B. japonicum (25, 27, 37, 45). This is the second report of a pcaL gene; the first resulted from identification of the dual enzyme activity in the protein from R. opacus after which the gene was cloned (15). Eulberg et al. (15) hypothesized that since the evolution of proteins tends to go from simple to more complex, the separate γ-carboxymuconolactone-decarboxylase/β-ketoadipate enol-lactone-hydrolase enzyme arrangement of proteobacteria may be the more ancient, and that the presence of a fused enzyme was a gram-positive trait.
Streptomyces spp. are abundant in soil, and considering the ubiquity of the β-ketoadipate pathway, it is not surprising that this pathway is present in this genus of bacteria. β-Ketoadipate genes are almost always located chromosomally (27), and in the chromosome of S. coelicolor the pca genes are located outside the genetically unstable regions, indicating the underlying importance of the β-ketoadipate pathway in the metabolic activities of this soil microbe. Although catechol 1,2-dioxygenase activity has been reported in other streptomycetes, only the protocatechuate branch was observed in Streptomyces sp. strain 2065. This has also been reported for some members of the rhizobial/agrobacterial phylogenetic groups and an Azotobacter sp. (6, 24, 46). In addition, expression of Streptomyces sp. strain 2065 3,4-PCD in E. coli was not successful (data not shown). Heterologous expression of 3,4-PCD has been achieved in E. coli only for the enzymes from A. calcoaceticus (13) and Pseudomonas sp. strain HR199 (50).
ACKNOWLEDGMENTS
S. G. Iwagami and K. Yang were supported by a Forest Renewal British Columbia Grant. This research was also funded in part by NSERC.
We gratefully acknowledge Chris Radomski at TerraGen Diversity, Inc., for assistance with DNA sequencing. FAME analysis of Streptomyces sp. strain 2065 performed at TerraGen Diversity, Inc., is also appreciated. Yossef Av-Gay generously donated his time to assist with computer-based DNA sequence analysis. We are also thankful to Kevin Chow and Meg Pope, who provided helpful advice throughout this work.
REFERENCES
- 1.Aiba H. A 570-kb DNA sequence of the E. coli K-12 genome corresponding to the 28.0–40.1 min region on the linkage map. DNA Res. 1996;3:363–377. doi: 10.1093/dnares/3.6.363. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates; 1991. [Google Scholar]
- 3.Baltz R H. Genetic manipulation of antibiotic-producing Streptomyces. Trends Microbiol. 1998;6:76–82. doi: 10.1016/S0966-842X(97)01161-X. [DOI] [PubMed] [Google Scholar]
- 4.Bull C, Ballou D P. Purification and properties of protocatechuate 3,4-dioxygenase from Pseudomonas putida. J Biol Chem. 1981;256:12673–12680. [PubMed] [Google Scholar]
- 5.Cain R B. Utilization of anthranilic and nitrobenzoic acids by Nocardia opaca and Flavobacterium. J Gen Microbiol. 1966;42:219–235. doi: 10.1099/00221287-42-2-219. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y P, Glenn A R, Dilworth M J. Uptake and oxidation of aromatic substrates by Rhizobium leguminosarum MNF 3841 and Rhizobium trifolii TA1. FEMS Microbiol Lett. 1984;21:201–205. [Google Scholar]
- 7.Chow K T, Pope M, Davies J. Characterization of a vanillic acid non-oxidative decarboxylation gene cluster from Streptomyces sp. D7. Microbiology. 1999;145:2393–2403. doi: 10.1099/00221287-145-9-2393. [DOI] [PubMed] [Google Scholar]
- 8.Collier L S, Nichols N N, Neidle E L. BenK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain AD1. J Bacteriol. 1997;179:5943–5946. doi: 10.1128/jb.179.18.5943-5946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford R L, Hutton S W, Chapman P J. Purification and properties of gentisate 1,2-dioxygenase from Moraxella osloensis. J Bacteriol. 1975;121:794–799. doi: 10.1128/jb.121.3.794-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford R L, Olsen P P. Microbial catabolism of vanillate: decarboxylation to guaiacol. Appl Environ Microbiol. 1978;36:539–543. doi: 10.1128/aem.36.4.539-543.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delneri D, Degrassi G, Rizzo R, Bruschi C V. Degradation of trans-ferulic and p-coumaric acid by Acinetobacter calcoaceticus DSM 586. Biochim Biophys Acta. 1995;1244:363–367. doi: 10.1016/0304-4165(95)00021-3. [DOI] [PubMed] [Google Scholar]
- 12.Dorsch M, Stackebrandt E. Some modifications in the procedure of direct sequencing of PCR amplified 16S rDNA. J Microbiol Methods. 1992;16:271–279. [Google Scholar]
- 13.Doten R C, Ngai K L, Ornston L N. Cloning and genetic organization of the pca gene cluster from Acinetobacter calcoaceticus. J Bacteriol. 1987;169:3168–3174. doi: 10.1128/jb.169.7.3168-3174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durham D R, Stirling L A, Ornston L N, Perry J J. Intergenic evolutionary homology revealed by the study of protocatechuate 3,4-dioxygenase from Azotobacter vinelandii. Biochemistry. 1980;19:149–155. doi: 10.1021/bi00542a023. [DOI] [PubMed] [Google Scholar]
- 15.Eulberg D, Lakner S, Golovleva L A, Schlömann M. Characterization of a protocatechuate catabolic gene cluster from Rhodococcus opacus 1CP: evidence for a merged enzyme with 4-carboxymuconolactone-decarboxylating and 3-oxoadipate enol-lactone-hydrolyzing activity. J Bacteriol. 1998;180:1072–1081. doi: 10.1128/jb.180.5.1072-1081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazee R W, Livingston D M, LaPorte D C, Lipscomb J D. Cloning, sequencing, and expression of the Pseudomonas putida protocatechuate 3,4-dioxygenase genes. J Bacteriol. 1993;175:6194–6202. doi: 10.1128/jb.175.19.6194-6202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frost J W, Drafts K M. Biocatalytic synthesis of aromatics from d-glucose: renewable microbial sources of aromatic compounds. Annu Rev Microbiol. 1995;49:557–579. doi: 10.1146/annurev.mi.49.100195.003013. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa H, Hayaishi O. Protocatechuate 3,4-dioxygenase. J Biol Chem. 1968;243:2673–2681. [PubMed] [Google Scholar]
- 19.Godden B, Ball A S, Helvenstein P, McCarthy A J, Penninckx M J. Towards elucidation of the lignin degradation pathway in actinomycetes. J Gen Microbiol. 1992;138:2441–2448. [Google Scholar]
- 20.Goodfellow M, Cross T. Classification. In: Goodfellow M, Modarski M, Williams S T, editors. The biology of the Actinomycetes. London, England: Academic Press; 1983. pp. 7–164. [Google Scholar]
- 21.Grund E, Knorr C, Eichenlaub H. Catabolism of benzoate and monohydroxylated benzoates by Amycolatopsis and Streptomyces spp. Appl Environ Microbiol. 1990;56:1459–1464. doi: 10.1128/aem.56.5.1459-1464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta R S. What are archaebacteria: life's third domain or monoderm prokaryotes related to gram-positive bacteria? A new proposal for the classification of prokaryotic organisms. Mol Microbiol. 1998;29:695–707. doi: 10.1046/j.1365-2958.1998.00978.x. [DOI] [PubMed] [Google Scholar]
- 23.Hammer A, Stolz A, Knackmuss H. Purification and characterization of a novel type of protocatechuate 3,4-dioxygenase with the ability to oxidize 4-sulfocatechol. Arch Microbiol. 1996;166:92–100. doi: 10.1007/s002030050361. [DOI] [PubMed] [Google Scholar]
- 24.Hardisson C, Sala-Trepat J M, Stanier R Y. Pathways for the oxidation of aromatic compounds by Azotobacter. J Gen Microbiol. 1969;59:1–11. doi: 10.1099/00221287-59-1-1. [DOI] [PubMed] [Google Scholar]
- 25.Hartnett C, Neidle E L, Ngai K, Ornston L N. DNA sequences of the genes encoding Acinetobater calcoaceticus protocatechuate 3,4-dioxygenase: evidence indicating shuffling of DNA sequences within the genes during their evolutionary divergence. J Bacteriol. 1990;172:956–966. doi: 10.1128/jb.172.2.956-966.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harwood C S, Nichols N N, Kim M K, Ditty J L, Parales R E. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol. 1994;176:6479–6488. doi: 10.1128/jb.176.21.6479-6488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harwood C S, Parales R E. The β-ketoadipate pathway and the biology of self identity. Annu Rev Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 28.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, England: The John Innes Foundation; 1985. [Google Scholar]
- 29.Hou C T, Lillard M O, Schwartz R D. Protocatechuate 3,4-dioxygenase from Acinetobacter calcoaceticus. Biochemistry. 1976;15:582–588. doi: 10.1021/bi00648a020. [DOI] [PubMed] [Google Scholar]
- 30.Jain R, Rivera M C, Lake J A. Horizontal gene transfer among genomes: the complexity hypothesis. Proc Natl Acad Sci USA. 1999;96:3801–3806. doi: 10.1073/pnas.96.7.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassovska-Bratinova S, Fukao T, Song X Q, Duncan A M V, Chen H S, Robert M F, Perezcerda C, Ugarte M, Chartrand C, Vobecky S, Kondo N, Mitchell G A. Succinyl CoA-3-oxoacid CoA transferase (SCOT): human cDNA cloning, human chromosomal mapping to 5P13, and mutation detection in a SCOT-deficient patient. Am J Hum Genet. 1996;59:519–528. [PMC free article] [PubMed] [Google Scholar]
- 32.Kojima Y, Itada N, Hayaishi O. Metapyrocatechase: a new catechol-cleaving enzyme. J Biol Chem. 1961;236:2223–2228. [PubMed] [Google Scholar]
- 33.Kurane R, Ara K, Nakamura I, Suzuki T, Fukuoka S. Protocatechuate 3,4-dioxygenase from Nocardia erythropolis. Agric Biol Chem. 1984;48:2105–2111. [Google Scholar]
- 34.Lawrence J G, Ochman H. Molecular archaeology of the Escherichia coli genome. Proc Natl Acad Sci USA. 1998;95:9413–9417. doi: 10.1073/pnas.95.16.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, DeBoer S H. Selection of polymerase chain reaction primers from an RNA intergenic spacer region for specific detection of Clavibacter michiganensis subsp. sepedonicus. Phytopathology. 1995;85:837–842. [Google Scholar]
- 36.Lion T, Haas O A. Nonradioactive labeling of probe with digoxygenin by polymerase chain reaction. Anal Biochem. 1990;188:335–337. doi: 10.1016/0003-2697(90)90616-h. [DOI] [PubMed] [Google Scholar]
- 37.Lorite M J, Sanjuan J, Velasco L, Olivares J, Bedmar E J. Characterization of Bradyrhizobium japonicum pcaBDC genes involved in 4-hydroxybenzoate degradation. Biochim Biophys Acta. 1998;1397:257–261. doi: 10.1016/s0167-4781(98)00048-7. [DOI] [PubMed] [Google Scholar]
- 38.Muth G, Brolle D F, Wohlleben W. Genetics of Streptomyces. In Manual of industrial microbiology and biotechnology. Washington, D.C.: ASM Press; 1999. [Google Scholar]
- 39.Neidle E L, Harnett C, Bonitz S, Ornston L N. DNA sequence of the Acinetobacter calcoaceticus catechol 1,2-dioxygenase I structural gene catA: evidence for evolutionary divergence of intradiol dioxygenases by acquisition of DNA repetitions. J Bacteriol. 1988;170:4874–4880. doi: 10.1128/jb.170.10.4874-4880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olivera E R, Minambres B, Garcia B, Muniz C, Moreno M A, Ferrandez A, Diaz E, Garcia J L, Luengo J M. Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon. Proc Natl Acad Sci USA. 1998;95:6419–6424. doi: 10.1073/pnas.95.11.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orville A M, Lipscomb J D, Ohlendorf D H. Crystal structure of substrate and substrate analog complexes of protocatechuate 3,4-dioxygenase: endogenous Fe3+ ligand displacement in response to substrate binding. Biochemistry. 1997;36:10052–10066. doi: 10.1021/bi970469f. [DOI] [PubMed] [Google Scholar]
- 42.Ottow J C G, Zolg W. Improved procedure and colorimetric test for the detection of ortho- and meta-cleavage of protocatechuate by Pseudomonas isolates. Can J Microbiol. 1974;20:1059–1061. doi: 10.1139/m74-163. [DOI] [PubMed] [Google Scholar]
- 43.Panke S, Sanchez-Romero J M, de Lorenzo V. Engineering of quasi-natural Pseudomonas strains for toluene metabolism through an ortho-cleavage degradation pathway. Appl Environ Microbiol. 1998;64:748–751. doi: 10.1128/aem.64.2.748-751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parke D. Acquisition, reorganization, and merger of genes: novel management of the beta-ketoadipate pathway in Agrobacterium tumefaciens. FEMS Microbiol Lett. 1997;146:3–12. [Google Scholar]
- 45.Parke D. Supraoperonic clustering of pca genes for catabolism of the phenolic compound protocatechuate in Agrobacterium tumefaciens. J Bacteriol. 1995;177:3808–3817. doi: 10.1128/jb.177.13.3808-3817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parke D, Ornston L N. Nutritional diversity of Rhizobiaceae revealed by auxanography. J Gen Microbiol. 1984;130:1743–1750. [Google Scholar]
- 47.Parke D, Rynne F, Glenn A. Regulation of phenolic metabolism in Rhizobium leguminosarum biovar trifolii. J Bacteriol. 1991;173:5546–5550. doi: 10.1128/jb.173.17.5546-5550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parke D, D'Argenio D A, Ornston L N. Bacteria are not what they eat: that is why they are so diverse. J Bacteriol. 2000;182:257–263. doi: 10.1128/jb.182.2.257-263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pometto A L, III, Sutherland J B, Crawford D L. Streptomyces setonii: catabolism of vanillic acid via guaiacol and catechol. Can J Microbiol. 1981;27:636–638. doi: 10.1139/m81-097. [DOI] [PubMed] [Google Scholar]
- 50.Priefert H, Rabenhorst J, Steinüchel A. Molecular characterization of genes of Pseudomonas sp. strain HR199 involved in bioconversion of vanillin to protocatechuate. J Bacteriol. 1997;179:2595–2607. doi: 10.1128/jb.179.8.2595-2607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8Mb Streptomyces coelicolor A3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 53.Sheldon D R, Khader S, Karns J S, Pogell B M. Metabolism of twelve herbicides by Streptomyces. Biodegradation. 1996;7:129–136. doi: 10.1007/BF00114625. [DOI] [PubMed] [Google Scholar]
- 54.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olsen B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 55.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 56.Stanier R Y, Ingraham J L. Protocatechuic acid oxidase. J Biol Chem. 1954;210:799–808. [PubMed] [Google Scholar]
- 57.Stanier R Y, Ornston L N. The β-ketoadipate pathway. Adv Microb Physiol. 1973;9:89–149. [PubMed] [Google Scholar]
- 58.Stanier R Y, Palleroni N J, Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 59.Sterjiades R, Pelmont J. Occurrence of two different forms of protocatechuate 3,4-dioxygenase in a Moraxella sp. Appl Environ Microbiol. 1989;55:340–347. doi: 10.1128/aem.55.2.340-347.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sutherland J B, Crawford D L. Metabolism of cinnamic, p-coumaric, and ferulic acids by Streptomyces setonii. Can J Microbiol. 1983;29:1253–1257. doi: 10.1139/m83-195. [DOI] [PubMed] [Google Scholar]
- 61.Sutherland J B, Crawford D L, Pometto A L., III Catabolism of substituted benzoic acids by Streptomyces species. Appl Environ Microbiol. 1981;41:442–448. doi: 10.1128/aem.41.2.442-448.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Webb V, Davies J. Antibiotic preparations contain DNA: a source of drug resistance genes? Antimicrob Agents Chemother. 1993;37:2379–2384. doi: 10.1128/aac.37.11.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wheelis M L, Palleroni N J, Stanier R Y. The metabolism of aromatic acids by Pseudomonas testeroni and P. acidivorans. Arch Mikrobiol. 1967;59:302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]
- 64.Whittaker J W, Lipscomb J D, Kent T A, Münck E. Brevibacterium fuscum protocatechuate 3,4-dioxygenase: purification, crystallization, and characterization. J Biol Chem. 1984;259:4466–4475. [PubMed] [Google Scholar]
- 65.Zaborina O, Baskunov B, Baryshnikova L, Golovleva L. Degradation of pentachlorophenol in soil by Streptomyces rochei 303. J Environ Sci Health. 1997;32:55–70. doi: 10.1080/03601239709373076. [DOI] [PubMed] [Google Scholar]
- 66.Zaborina O, Latus M, Eberspächer J, Golovleva L A, Lingens F. Purification and characterization of a 6-chlorohydroxyquinol 1,2-dioxyenase from Streptomyces rochei 303: comparison with an analogous enzyme from Azotobacter sp. strain GP1. J Bacteriol. 1995;177:229–234. doi: 10.1128/jb.177.1.229-234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zylstra G J, Olsen R H, Ballou D P. Genetic organization and sequence of the Pseudomonas cepacia genes for the alpha and beta subunits of protocatechuate 3,4-dioxygenase. J Bacteriol. 1989;171:5915–5921. doi: 10.1128/jb.171.11.5915-5921.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]