Abstract
The Beibu Gulf harbors abundant underexplored marine microbial resources, which are rich in diversified secondary metabolites. The genera Vibrio is a well-known pathogenic bacterium of aquatic animals. In this study, 22 fungal strains were isolated and identified from the Beibu Gulf coral via the serial dilution method and internal transcribed spacer (ITS) sequence analysis, which were further divided into three branches by phylogenetic tree analysis. The crude extracts of them via small-scale fermentation were selected for the screening of inhibitory activity against Vibrio alginalyticus, Vibrio coralliilyticus, Vibrio harveyi, Vibrio parahaemolyticus, Vibrio owensii, and Vibrio shilonii. The results showed that eight fungal extracts displayed anti-Vibrio activity via the filter paper disk assay. Several of them showed strong inhibitory effects. Then, two tetramic acid alkaloids, equisetin (1) and 5′-epiequisetin (2), were identified from Fusarium equiseti BBG10 by bioassay-guided isolation, both of which inhibited the growth of Vibrio spp. with the MIC values of 86–132 μg/ml. The scanning electron microscope results showed that cell membranes of Vibrio became corrugated, distorted or ruptured after treatment with 1 and 2. Taken together, this study provided eight fungal isolates with anti-Vibrio potentials, and two alkaloid-type antibiotics were found with anti-Vibrio effects from the bioactive strain F. equiseti BBG10. Our findings highlight the importance of exploring promising microbes from the Beibu Gulf for the identification of anti-Vibrio for future antibiotic development.
Keywords: the Beibu Gulf, coral-derived fungi, anti-Vibrio, phylogenetic tree, equisetin
Introduction
As an important biocenosis in marine ecosystems, a coral reef ecosystem represents an extraordinarily diverse biota in tropical environments, among which corals often constitute a dominant part of the reef biomass. Scleractinian corals harbor diverse and abundant microbial symbionts with different types of interactions, which function as the primary reef ecosystem engineers, constructing the framework and shaping the resource availability for other coral reef-associated organisms (Tang et al., 2020). Manipulation of the coral-associated microbiome was postulated as a key strategy to improve the resilience of reef-building corals. The genera Vibrio, known as Vibrio coralliilyticus and Vibrio mediterranei, are important coral pathogens capable of inducing serious coral damage, which has seriously impacted reef-building corals throughout the oceans as well as global warming (Esther et al., 2020). Recently, coral and its associated microorganisms have been evidenced as promising producers of structurally diverse compounds with a wide range of potent bioactivities, such as anti-inflammatory, cytotoxic, antimicrobial, antivirus, and antifouling activities (Hou et al., 2015; Sang et al., 2019).
The Beibu Gulf, located in the north of the South China Sea, harbors abundant biodiversity in both marine organisms and microorganisms and is regarded as a potential source of new species, new genes, new drugs, and new biological materials (Xu et al., 2020). However, there are relatively few reports about marine natural products from the Beibu Gulf (Carroll et al., 2019, 2020, 2021, 2022; Wang et al., 2019). In continuation of our research program aiming at the discovery of bioactive metabolites from the Beibu Gulf-derived marine fungi, a series of new bioactive compounds with diversified structures have been obtained recently (Luo et al., 2021; Lu et al., 2022; Zhang et al., 2022). Therefore, the main objective of this study was to investigate the anti-Vibrio potential of fungi isolated from scleractinian corals collected from the Weizhou Islands coral reef in the Beibu Gulf and to obtain and evaluate the potential exploitable anti-Vibrio alkaloids, equisetin and 5′-epiequisetin, from Fusarium equiseti BBG10.
Materials and Methods
Sampling and Isolation of Fungi
The corals Porites lutea were collected from the Weizhou Islands coral reef in Guangxi Zhuang Autonomous Region, China, in July 2019. The samples were stored in sterilized polythene bags, transported to the laboratory, and processed immediately for the isolation and cultivation of fungi. The fungi were isolated by the serial dilution method (1:10, 1:100, and 1:1,000) using potato dextrose agar (PDA) medium supplemented with sea salt (20 g/L) and chloramphenicol (20 mg/L). The inoculated plates were cultured at 25°C for 1–3 weeks and observed the growth of fungi intermittently. Fungal isolates were chosen and transferred into another blank agar plates based on their morphological traits. The isolated strains were deposited at the Institute of Marine Drugs, Guangxi University of Chinese Medicine, Nanning, China.
Identification of Fungi
The fungal strains were cultured in PDA medium at 28°C for 5 days. The genomic DNA of the fungal strains was isolated by using the protocol described previously (Shen et al., 2020). The internal transcribed spacer (ITS) sequences were checked and amplified using ITS1-(5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4-(5′-TCCTCCGCTTATTGATATGC-3′) primers. The fungi were identified mainly by analysis of the ITS sequences (as shown in Supplementary Material) in the NCBI BLAST program. The phylogenetic tree was created based on the ITS sequences by MEGA7.
Screening of Fungi Fermentation and Extracts
The small-scale fermentations of 22 fungal isolates (BBG1–BBG22) were carried out in rice solid medium (50 g rice, 1.2 g artificial sea salt, and 60 mL H2O) employing with 1-L Erlenmeyer flasks at room temperature for 30 days. The fermented cultures were overlaid and extracted three times with EtOAc. All the fungal extracts (10 mg/mL dissolved in methanol) were analyzed by high-performance liquid chromatography (HPLC; Shimadzu Prominence-i LC-2030) using a PDA detector and an ODS column (YMC-pack ODS-A, 4.6 mm × 250 mm, 5 μm). In addition, the organic extracts were combined and evaporated in vacuo as a total crude extract for further anti-Vibrio assays.
Anti-Vibrio Assay
All the fungal extracts, along with two isolated tetramic acid alkaloids, equisetin (1) and 5′-epiequisetin (2), were screened for antibacterial activity against Vibrio alginalyticus, Vibrio coralliilyticus, Vibrio harveyi, Vibrio parahaemolyticus, Vibrio owensii, and Vibrio shilonii, by using a K–B disk agar diffusion method (Zhang et al., 2022). The strain Vibrio parahaemolyticus was kindly provided by Prof. Nan Li (Nanning Normal University, Nanning, China), while other Vibrio strains were kindly provided by Prof. Chang Chen (South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China). Each fungal extract was dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 25 mg/ml, and the two compounds (1–2) were prepared at a concentration of 10 mg/ml. The positive control chloramphenicol was prepared at a concentration of 150 μg/mL in DMSO. The Vibrio strains were cultivated in Luria Bertani (LB) broth medium (10 g/L peptone, 5 g/L yeast extract, and 20 g/L NaCl, pH adjusted to 7.0) and were incubated at 28°C, and the cultures were incubated to logarithmic phase with the optical density at 600 nm (OD600) reaching 0.8. The exponential-phase cells were added to LB agar medium (40°C–45°C) at a final concentration of 5 × 104 cfu/mL. After solidification for 20 min, sterile filter paper impregnated with 3 μL of sample solution was placed on the plates and incubated at 28°C for 12 h. The anti-Vibrio effects were checked and recorded.
The minimal inhibitory concentration (MIC) assay of equisetin (1) and 5′-epiequisetin (2) toward these Vibrio strains was further determined with minor modification as described previously (Zhang et al., 2022). The Vibrio strains were cultivated in LB broth medium at 28°C, and the culture was incubated to exponential phase with the optical density at 600 nm (OD600) reaching 0.8. Briefly, the OD600 of exponential-phase cells of Vibrio was adjusted to 0.01 with LB broth medium. Thereafter, 150 μL of the Vibrio cells suspension was transferred into the wells of a 96-well microplate with different concentrations of 1 or 2. DMSO (1%, v/v) was served as the negative control. The microplate was incubated at 28°C for 16 h and checked after incubation (Wiegand et al., 2008). The MIC values were defined as the lowest concentrations of 1 or 2 that inhibited the growth of Vibrio spp. (OD600 < 0.05).
Scanning electron microscopy (SEM) was further performed to investigate the morphological changes of Vibrio treated with compounds 1 and 2. The strain V. parahaemolyticus was incubated on the LB as described above. Thereafter, compounds 1 and 2 (10 mg/mL) were added to the Vibrio cells suspension at a final concentration of 1.0 × MIC and incubated at 28°C for 12 h. The treated cells were collected for the SEM assay. The treated cells were washed three times by PBS, and fixed in 2.5% glutaraldehyde for 2 h. Then, the treated cells dehydrated successively in an ethanol series of 30%, 50%, 70%, 80%, 90%, and 100% tert-butyl alcohol for 10 min at each stage. The freeze-dried samples were analyzed with an SEM (Zeiss, Sigma 300) operated at 3 kV.
Isolation and Structure Characterization of Equisetin (1) and 5′-Epiequisetin (2)
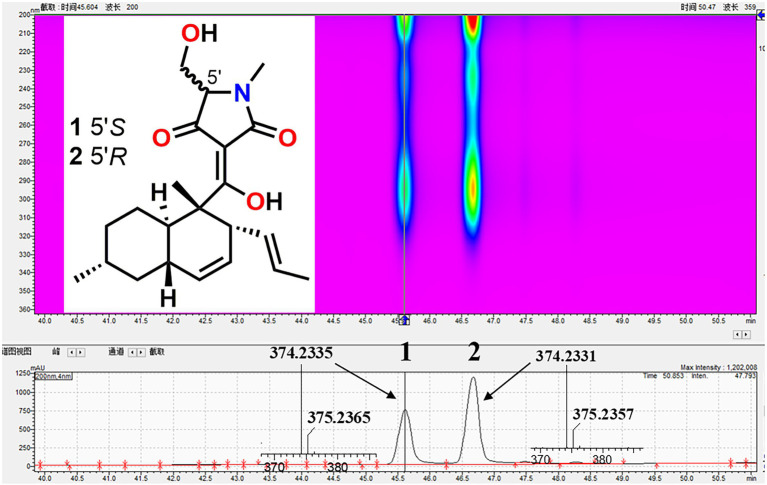
The fungal strain F. equiseti BBG10 was cultured on Müller Hinton broth (MB) agar plates (malt extract 15 g, artificial sea salt 15 g, and agar 20 g) at 25°C for 7 days. Then, it was inoculated in the seed medium (malt extract 15 g and artificial sea salt 15 g in 1.0-L tap distilled H2O, pH 7.4–7.8) at 25°C on a rotary platform shaker at 180 rpm for 48 h. Subsequently, a large-scale fermentation of F. equiseti BBG10 was carried out in modified rice solid medium (150 g rice, 3.0 g artificial sea salt, and 180 mL H2O) employing with 1 L × 20 Erlenmeyer flasks at room temperature for 30 days. The whole fermented cultures were extracted with EtOAc three times to provide a brown extract (50 g). The EtOAc crude extract was fractionated by medium pressure liquid chromatography (MPLC) using a step gradient elution with petroleum ether/CH2Cl2/MeOH (petroleum ether/CH2Cl2, 1:0–0:1; CH2Cl2/methanol, 1:0–1:1, v/v), which afforded 10 fractions (Frs.1 ~ 10) based on thin-layer chromatography (TLC) analysis. Fr.6 was further separated by semipreparative high performance liquid chromatography (HPLC) with MeCN/H2O (80:20, v/v, 5.0 mL/min) to yield compounds 1 (tR = 38 min, 300 mg) and 2 (tR = 43 min, 350 mg). Their structures were confirmed by high resolution-electron spray ionization mass spectrometry (HR-ESIMS) and high-performance liquid chromatography-diode array detection (HPLC-DAD) data analysis as well as comparison with the standard reference substances. HR-ESIMS spectra were collected on a Waters Xevo G2-S TOF mass spectrometer (Waters Corporation, United States).
Results
Identification and Phylogenetic Tree Analysis of Fungal Strains Derived From the Weizhou Islands Coral
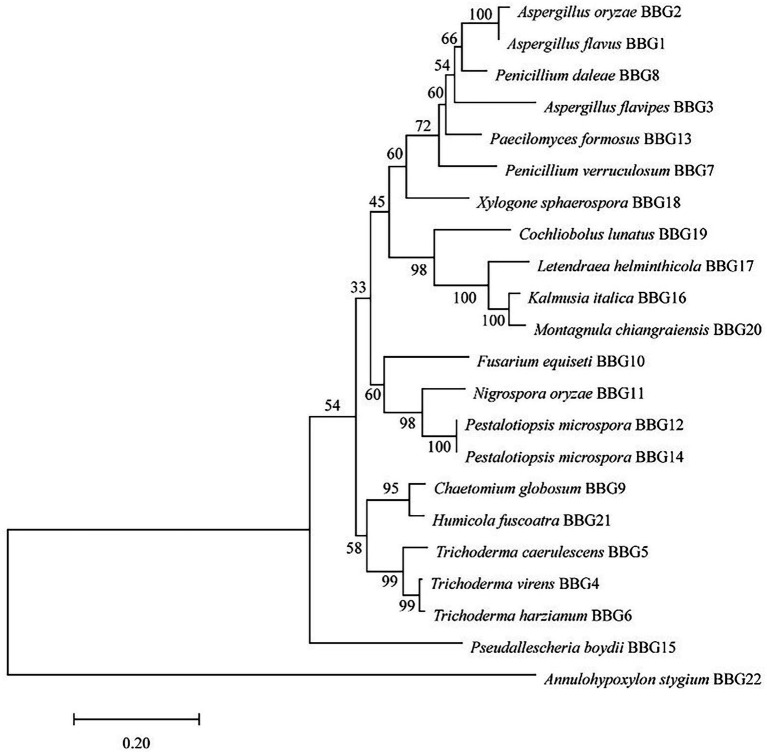
Twenty-two candidate fungal strains were identified on the basis of the molecular protocol by amplification and sequencing of the DNA sequences of the ITS region of the rDNA gene. A classification of 22 strains based on the species name of the closely related species is shown in Figure 1. The predominant genera were Aspergillus and Trichoderma. Based on the ITS sequences, a phylogenetic tree was created using the neighbor-joining method to analyze the genetic relationship between 22 strains. The results showed that these strains could be divided into three major branches, while Annulohypoxylon stygium BBG22 is the most distant from other strains and belongs to a relatively independent branch. Moreover, the morphological property of the potential strain BBG10 was further collected to confirm the identification by using scanning electron microscopy (Supplementary Figure 1).
Figure 1.
Phylogenetic tree analysis of 22 marine fungal strains.
Anti-Vibrio Activity of the Fungal Extracts
Cultivation of fungi from the Weizhou Islands coral yielded a total of 30 isolates. Reduplicate isolates were excluded under the guidance of observation of morphological differences, including the visible examination of growth characteristics, mycelia, and diffusible pigment. As a result, 22 independent strains (BBG1–BBG22) were selected for the screening of anti-bacterial activity against 6 strains of Vibrio spp. As shown in Table 1 and Supplementary Figure 2, the fungal extracts of 8 isolates (36.3%) displayed potential growth inhibition against Vibrio in the filter paper disk assay (75 μg extracts/per piece). It is worth noting that the fungal extracts of two isolates, Trichoderma virens BBG4 and Trichoderma harzianum BBG6, exhibited strong anti-Vibrio activity, and the sizes of the inhibition zone were larger than that of the positive control, chloramphenicol (150 μg/ml). A previous study reported that T. virens could produce gliotoxin with strong antimicrobial activity (Vargas et al., 2014). Besides, T. harzianum was reported to be a biocontrol bacterium for plant diseases (Altomare et al., 1999). The results also showed that different Vibrio species have divergent susceptibilities to extracts. Additionally, 22 fungal extracts were further analyzed by HPLC for the diversity of secondary metabolites. Therefore, F. equiseti BBG10 with interesting HPLC-DAD profiles (Supplementary Figure 3) of its crude extract was selected as the bioactive target strain to identify the active constituents.
Table 1.
The anti-Vibrio activity of fungal extracts (diameter of inhibition zone, mm).
| Vibrio alginalyticus | Vibrio coralliilyticus | Vibrio harveyi | Vibrio parahaemolyticus | Vibrio shilonii | Vibrio owensii | |
|---|---|---|---|---|---|---|
| Chl | 1.69 ± 0.10 | 2.15 ± 0.22 | 2.00 ± 0.04 | 1.53 ± 0.07 | 2.01 ± 0.20 | 1.66 ± 0.20 |
| Aspergillus oryzae BBG2 | 0.78 ± 0.06 | 0.93 ± 0.08 | 0 | 0.87 ± 0.19 | 0.90 ± 0.10 | 0.89 ± 0.09 |
| Aspergillus flavipes BBG3 | 0 | 1.23 ± 0.21 | 0 | 0.75 ± 0.02 | 0.84 ± 0.07 | 0.72 ± 0.05 |
| T. virens BBG4 | 1.73 ± 0.27 | 2.58 ± 0.03 | 1.96 ± 0.10 | 2.28 ± 0.07 | 2.62 ± 0.04 | 2.26 ± 0.36 |
| T. harzianum BBG6 | 2.10 ± 0.25 | 2.20 ± 0.10 | 1.73 ± 0.15 | 2.36 ± 0.07 | 2.32 ± 0.12 | 1.19 ± 0.01 |
| Chaetomium globosum BBG9 | 0.80 ± 0.04 | 1.64 ± 0.05 | 0.75 ± 0.03 | 0.81 ± 0.06 | 1.26 ± 0.07 | 1.33 ± 0.02 |
| F. equiseti BBG10 | 0.80 ± 0.04 | 1.13 ± 0.08 | 0.71 ± 0.03 | 0.77 ± 0.03 | 0.94 ± 0.03 | 0.78 ± 0.10 |
| Nigrospora oryzae BBG11 | 0.88 ± 0.07 | 1.02 ± 0.18 | 0.82 ± 0.12 | 0.85 ± 0.02 | 1.31 ± 0.06 | 1.28 ± 0.32 |
| A. stygium BBG22 | 0 | 0 | 0.75 ± 0.06 | 0.85 ± 0.17 | 0.83 ± 0.06 | 0.92 ± 0.13 |
Chl, chloramphenicol.
Production of Bioactive Metabolites
To investigate the bioactive constituents of F. equiseti BBG10, a large-scale fermentation was performed in 3 kg of rice solid medium. After harvest, its organic extract was further separated by MPLC and HPLC. Their structures were confirmed by HR-ESIMS and HPLC-DAD data analysis as well as comparison with the standard reference substances, which were identified as equisetin (1) and 5′-epiequisetin (2), respectively (Figure 2).
Figure 2.
The isolation and structures of equisetin (1) and 5′-epiequisetin (2).
Anti-Vibrio Activities of Compounds 1 and 2
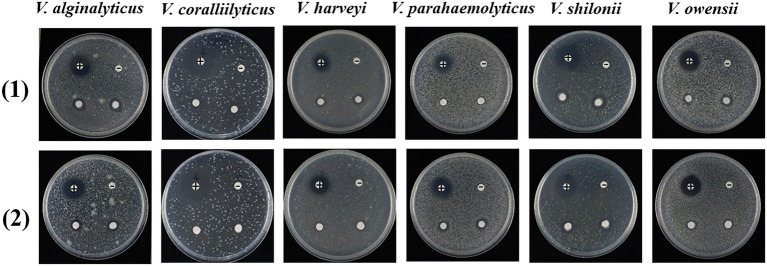
Equisetin has been reported to have various biological actions, including antibacterial (Vesonder et al., 1979), anti-HIV (Hazuda et al., 1999; Clercq, 2000), antiobesity, and selective cytotoxicity to mammalian cells (Yin et al., 2013). However, this is the first report on the anti-Vibrio activity of equisetin (1) and 5′-epiequisetin (2) against V. alginalyticus, V. coralliilyticus, V. harveyi, V. parahaemolyticus, V. owensii, and V. shilonii. The filter paper disk assay showed that equisetin and 5′-epiequisetin exhibited weak bacteriostatic activity against V. alginalyticus, V. parahaemolyticus, V. owensii, and V. shilonii (Figure 3; Supplementary Table 1).
Figure 3.
Inhibitory activities of 1 and 2 against Vibrio. Each plate contains four pieces of paper disk, positive control marks as “+,” negative control marks as “–,” and the other two pieces of paper disk contains compound.
In addition, the MIC assay was further used to test the bacteriostatic ability of equisetin and 5′-epiequisetin. Both equisetin and 5′-epiequisetin showed inhibitory effects on six strains of Vibrio with the MIC values ranging from 86 to 132 μg/ml (Table 2). In order to clearly reflect the effect of compounds on the growth of tested bacteria, one of the Vibrio strain, V. parahaemolyticus, was selected to further investigate the growth curves of 1 and 2 at different concentrations (0.5 × MIC, 1.0 × MIC, and 2.0 × MIC), while the OD600 values were recorded within 16 h. As shown in Supplementary Figure 4, the growth of V. parahaemolyticus in the negative control and 0.5 × MIC (1 and 2) treatment groups entered the logarithmic growth period after 2 h, and the number of bacterial colonies kept growing within 16 h. Notably, the growth of V. parahaemolyticus was almost completely stagnant at the treatments of 1.0 × MIC and 2.0 × MIC, which suggested the bacteria were completely inhibited or even killed after treatment of compounds 1 and 2. The above results indicated that anti-Vibrio effect of compounds 1 and 2 was in a dose-dependent manner.
Table 2.
Anti-Vibrio activity of compounds 1 and 2 (MIC, μg/ml).
| Vibrio alginalyticus | Vibrio coralliilyticus | Vibrio harveyi | Vibrio parahaemolyticus | Vibrio shilonii | Vibrio owensii | |
|---|---|---|---|---|---|---|
| 1 | 119 | 119 | 132 | 119 | 119 | 119 |
| 2 | 86 | 106 | 106 | 106 | 106 | 86 |
| Chl | 1 | 1 | 1.2 | 1.2 | 1 | 1.2 |
Chl, chloramphenicol.
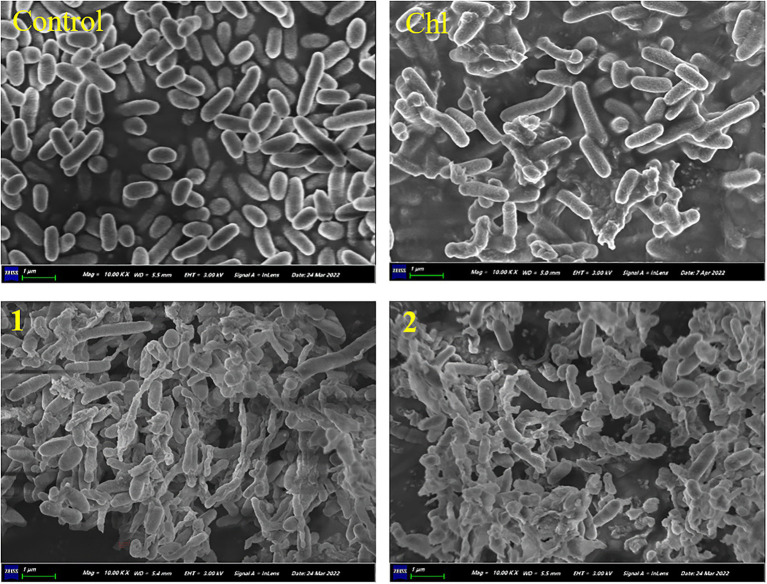
Thereafter, SEM was performed to investigate the morphological changes of V. parahaemolyticus treated with equisetin and 5′-epiequisetin. The results showed that the cell surfaces of the control group were smooth and that the Vibrio cells were plump and round. In contrast, the cell membranes became corrugated, distorted or ruptured after treatment with equisetin or 5′-epiequisetin. Interestingly, the destructive ability of equisetin and 5′-epiequisetin toward Vibrio cells were stronger than that of chloramphenicol. These results indicated that equisetin and 5′-epiequisetin can destroy the structure of Vibrio cell membranes (Figure 4).
Figure 4.
Electron microscopic observation of morphological changes in Vibrio parahaemolyticus cells following treatment with 1 and 2. (Chl, chloramphenicol).
Discussion
The marine environment harbors a vast number of underexplored microbial resources. From a natural products perspective, marine microbes are better resources for novel anti-Vibrio lead compounds. Marine natural products represent a rich source of diverse molecules for drug development (Altmann, 2017). According to the Marinlit database, more than 36,000 compounds with diverse structures have been hitherto isolated from marine organisms, while over 1,000 new compounds have been isolated per year in the last decades. Notably, the proportion of novel compounds derived from marine microorganisms is gradually increasing (Carroll et al., 2022). To our knowledge, 15 marine drugs have been approved for marketing, including well-known cephalosporin and rifamycin from marine microorganisms. The Beibu Gulf is located in the tropics and subtropics, which is one of the regions with the most abundant microbial diversity in China. However, the research on microbial resources from the Beibu Gulf is relatively sparse. Therefore, it is promising to isolate and screen microbial resources and their bioactive metabolites from the Beibu Gulf.
Vibrio is a Gram-negative bacterium that is one of the main pathogenic bacteria of fish, shrimp, shellfish, and other marine animals (Letchumanan et al., 2015). Humans can also be infected by eating contaminated seafood, contact with seawater, etc. Vibrio pathogenicity mainly includes V. parahaemolyticus, V. alginolyticus, V. vulnificus, and V. anguillarum (Yu et al., 2012). The application of antibiotics is an effective method of vibrio control. There is an urgent need to find novel antibiotics against Vibrio (Preetha et al., 2015). In this work, we tried to screen new anti-Vibrio natural products from the fungal resources from the Beibu Gulf. We screened 22 fungal crude extracts, and eight fungal crude extracts showed different degrees of anti-Vibrio activity. Among them, the crude extracts of T. virens BBG4 and T. harzianum BBG6 exhibited particularly anti-Vibrio activity, and the size of the inhibition zone was larger than that of chloramphenicol. Two active components, equisetin and 5′-epiequisetin, were further identified from one of the bioactive strain, F. equiseti BBG10.
Equisetin and related derivatives have long been recognized for their wide biological activity against eukaryotic and bacterial cells, including antibacterial (Vesonder et al., 1979), anti-HIV (Hazuda et al., 1999; Clercq, 2000), anti-obesity, and selective cytotoxicity effects on mammalian cells (Yin et al., 2013). Previous reports indicate that equisetin functions in eukaryotic cells by affecting mitochondrial metabolism (Freiberg et al., 2004; Quek et al., 2013). Equisetin could affect malonyl-CoA synthesis as an acetyl-CoA carboxylase inhibitor (Freiberg et al., 2004; Larson et al., 2020). HIV integrase is inhibited by equisetin based upon its metal-binding property (Hazuda et al., 1999; Clercq, 2000).
In this work, equisetin and 5′-epiequisetin were identified from F. equiseti BBG10, and the crude extracts exhibited anti-Vibrio activity. The filter paper disk assay and MIC assay showed that equisetin and 5′-epiequisetin exhibited slight anti-Vibrio activity. Interestingly, the SEM results showed that the cell membranes became corrugated, distorted or ruptured after treatment with equisetin or 5′-epiequisetin. In contrast, the number of cells destroyed by chloramphenicol was less than those of equisetin and 5′-epiequisetin. These results indicated that equisetin and 5′-epiequisetin can more significantly damage the structure of Vibrio cell membranes than chloramphenicol, suggesting that the mechanism by which equisetin inhibits cell growth and kills cells is distinct from that of chloramphenicol. Moreover, T. virens BBG4 and T. harzianum BBG6 are potential strains for finding more potent anti-Vibrio compounds. This will be the focus of our future research.
Conclusion
In summary, 22 fungal strains were isolated and identified from the Beibu Gulf coral via the serial dilution method and ITS sequence analysis, which were further divided into three branches by phylogenetic tree analysis, while eight fungal extracts were screened with potential anti-Vibrio activity via the filter paper disk assay. Further chemical investigation of the extracts of the target strain F. equiseti BBG10 via bioassay-guided isolation led to the characterization of two alkaloid-type antibiotics, equisetin and 5′-epiequisetin, which displayed anti-Vibrio activities against V. alginalyticus, V. coralliilyticus, V. harveyi, V. parahaemolyticus, V. owensii, and V. shilonii. Our research highlights the coral-derived microorganisms may be a large reservoir of bioactive natural products for future agrochemical development, and equisetin and 5′-epiequisetin could be promising lead compounds for the further development of novel anti-Vibrio agents.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XL, BH, and YL conceived and designed the experiments, analyzed the data, wrote the manuscript, and prepared the figures and supplementary materials. XL, SP, and YZ isolated and purified fungi and compounds. XL, XX, and CG identified compounds. BH, SL, and YW performed anti-Vibrio assay and ITS sequencing. BH performed scanning electron microscopy. All authors commented on and approved the manuscript.
Funding
This work was financially supported by the Specific Research Project of Guangxi for Research Bases and Talents (AD19110013), the Natural Science Foundation of Guangxi (2021GXNSF DA075010 and 2020GXNSFGA297002), the Guangxi Young and Middle-aged University Teachers’ Scientific Research Ability Enhancement Project (2021KY0315), the Special Fund for Bagui Scholars of Guangxi (YL), the National Natural Science Foundation of China (U20A20101), and the Scientific Research Foundation of Guangxi University of Chinese Medicine (2018006, 2018BS042, 2020QN025, 2019BS021, and 2018ZD005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb. 2022.930981/full#supplementary-material
References
- Altmann K.-H. (2017). Drugs from the Oceans: marine natural products as leads for drug discovery. Chimia 71, 646–652. doi: 10.2533/chimia.2017.646 [DOI] [PubMed] [Google Scholar]
- Altomare C., Norvell W., Björkman T., Harman G. (1999). Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 65, 2926–2933. doi: 10.1128/AEM.65.7.2926-2933.1999, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Copp B. R., Davis R. A., Keyzers R. A., Prinsep M. R. (2019). Marine natural products. Nat. Prod. Rep. 36, 122–173. doi: 10.1039/c8np00092a [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Copp B. R., Davis R. A., Keyzers R. A., Prinsep M. R. (2020). Marine natural products. Nat. Prod. Rep. 37, 175–223. doi: 10.1039/c9np00069k [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Copp B. R., Davis R. A., Keyzers R. A., Prinsep M. R. (2021). Marine natural products. Nat. Prod. Rep. 38, 362–413. doi: 10.1039/D0NP00089B [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Copp B. R., Davis R. A., Keyzers R. A., Prinsep M. R. (2022). Marine natural products. Nat. Prod. Rep. doi: 10.1039/D1NP00076D [DOI] [PubMed] [Google Scholar]
- Clercq E. (2000). Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 20, 323–349. doi: , PMID: [DOI] [PubMed] [Google Scholar]
- Esther R.-P., Martin-Cuadrado A. B., Caraballo-Rodríguez A. M., Rohwer F., Dorrestein P. C., Antón J. (2020). Virulence as a side effect of interspecies interaction in Vibrio coral pathogens. mBio 11, e00201–e002020. doi: 10.1128/mBio.00201-20, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg C., Brunner N., Schiffer G., Lampe T., Pohlmann J., Brands M., et al. (2004). Identification and characterization of the first class of potent bacterial acetyl-CoA carboxylase inhibitors with antibacterial activity. J. Biol. Chem. 279, 26066–26073. doi: 10.1074/jbc.M402989200, PMID: [DOI] [PubMed] [Google Scholar]
- Hazuda D., Blau C., Felock P., Hastings J., Pramanik B., Wolfe A., et al. (1999). Isolation and characterization of novel human immunodeficiency virus Integrase inhibitors from fungal metabolites. Antivir. Chem. Chemother. 10, 63–70. doi: 10.1177/095632029901000202, PMID: [DOI] [PubMed] [Google Scholar]
- Hou X.-M., Xu R.-F., Gu Y.-C., Wang C.-Y., Shao C.-L. (2015). Biological and chemical diversity of coral-derived microorganisms. Curr. Med. Chem. 22, 3707–3762. doi: 10.2174/0929867322666151006093755, PMID: [DOI] [PubMed] [Google Scholar]
- Larson E., Lim A., Pond C., Craft M., Cavuzic M., Waldrop G., et al. (2020). Pyrrolocin C and equisetin inhibit bacterial acetyl-CoA carboxylase. PLoS One 15:e0233485. doi: 10.1371/journal.pone.0233485, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchumanan V., Pusparajah P., Teng Hern T., Yin W.-F., Lee L. H., Chan K.-G. (2015). Occurrence and antibiotic resistance of Vibrio parahaemolyticus from shellfish in Selangor, Malaysia. Front. Microbiol. 6:1417. doi: 10.3389/fmicb.2015.01417, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. M., Tan Y. H., Zhang Y. T., Li Z. H., Chen J. Y., Gao C. H., et al. (2022). Osteoclastogenesis inhibitory phenolic derivatives produced by the Beibu Gulf coral-associated fungus Acremonium sclerotigenum GXIMD 02501. Fitoterapia 159:105201. doi: 10.1016/j.fitote.2022.105201 [DOI] [PubMed] [Google Scholar]
- Luo X. W., Cai G. D., Guo Y. F., Gao C. H., Huang W. F., Zhang Z. H., et al. (2021). Exploring marine-derived ascochlorins as novel human dihydroorotate dehydrogenase inhibitors for treatment of triple-negative breast cancer. J. Med. Chem. 64, 13918–13932. doi: 10.1021/acs.jmedchem.1c01402, PMID: [DOI] [PubMed] [Google Scholar]
- Preetha R., Vijayan K. K., Jayapraksh N. S., Alavandi S. V., Santiago T. C., Bright Singh I. S. (2015). Optimization of culture conditions for mass production of the probiotics Pseudomonas MCCB 102 and 103 antagonistic to pathogenic Vibrios in aquaculture. Probiotics Antimicrob. Proteins 7, 137–145. doi: 10.1007/s12602-015-9185-7, PMID: [DOI] [PubMed] [Google Scholar]
- Quek N., Matthews J., Bloor S., Jones D., Bircham P., Heathcott R., et al. (2013). The novel equisetin-like compound, TA-289, causes aberrant mitochondrial morphology which is independent of the production of reactive oxygen species in Saccharomyces cerevisiae. Mol. BioSyst. 9, 2125–2133. doi: 10.1039/c3mb70056a, PMID: [DOI] [PubMed] [Google Scholar]
- Sang V. T., Dat T. T. H., Vinh L. B., Cuong L. C. V., Oanh P. T. T., Ha H., et al. (2019). Coral and coral-associated microorganisms: A prolific source of potential bioactive natural products. Mar. Drugs 17:468. doi: 10.3390/md17080468, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N. X., Liang Z. Y., Liu Q., Tu C. D., Dong K. M., Wang C. Y., et al. (2020). Antifungal secondary metabolites isolated from mangrove Rhizosphere soil-derived Penicillium Fungi. J. Ocean Univ. China 19, 717–721. doi: 10.1007/s11802-020-4360-1 [DOI] [Google Scholar]
- Tang K. H., Zhan W. N., Zhou Y. Q., Xu T., Chen X. Q., Wang W. Q., et al. (2020). Antagonism between coral pathogen Vibrio coralliilyticus and other bacteria in the gastric cavity of scleractinian coral Galaxea fascicularis. Sci. China: Earth Sci. 63, 157–166. doi: 10.1007/s11430-019-9388-3 [DOI] [Google Scholar]
- Vargas W., Mukherjee P., Laughlin D., Wiest A., Moran-Diez M., Kenerley C. (2014). Role of gliotoxin in the symbiotic and pathogenic interactions of Trichoderma virens. Microbiology 160, 2319–2330. doi: 10.1099/mic.0.079210-0, PMID: [DOI] [PubMed] [Google Scholar]
- Vesonder R., Tjarks L., Rohwedder W., Burmeister H., Laugal J. (1979). Equisetin, an antibiotic from Fusarium equiseti NRRL 5537, identified as a derivative of N-methyl-2,4-pyrrolidone. J. Antibiot. 32, 759–761. doi: 10.7164/antibiotics.32.759, PMID: [DOI] [PubMed] [Google Scholar]
- Wang C., Su Y., Gao Y. K., Tan X. C., Lei F. H., Li H., et al. (2019). The isolation, screening and the active metabolites identification of bioactive fungal strains with antimicrobial activity from samples collected in the Guangxi Beibu gulf region. J. Int. Pharm. Res. 46, 270–276. doi: 10.13220/j.cnki.jipr.2019.04.005 [DOI] [Google Scholar]
- Wiegand I., Hilpert K., Hancock R. (2008). Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substance. Nat. Protoc. 3, 163–175. doi: 10.1038/nprot.2007.521, PMID: [DOI] [PubMed] [Google Scholar]
- Xu X. Y., Yang H., Ning X. Q., Yi X. X., Liu Y. H., Gao C. H. (2020). Research Progress of marine microbial diversity and chemical diversity in Beibu gulf. Guangxi Sci. 27, 433–450, 461. doi: 10.13656/j.cnki.gxkx.20201217.001 [DOI] [Google Scholar]
- Yin J., Kong L. L., Wang C., Shi Y. B., Cai S. J., Gao S. H. (2013). Biomimetic synthesis of Equisetin and (+)-Fusarisetin A. Chem. Eur. J. 19, 13040–13046. doi: 10.1002/chem.201302163, PMID: [DOI] [PubMed] [Google Scholar]
- Yu L.-P., Hu Y.-H., Sun J., Sun L. (2012). C312M: An attenuated Vibrio anguillarum strain that induces immunoprotection as an oral and immersion vaccine. Dis. Aquat. Org. 102, 33–42. doi: 10.3354/dao02544, PMID: [DOI] [PubMed] [Google Scholar]
- Zhang Y. T., Li Z. C., Huang B. Y., Liu K., Peng S., Liu X. M., et al. (2022). Anti-Osteoclastogenic and antibacterial effects of chlorinated polyketides from the Beibu gulf coral-derived fungus Aspergillus unguis GXIMD 02505. Mar. Drugs 20:178. doi: 10.3390/md20030178, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.