Abstract
Four subpopulations of a Plutella xylostella (L.) strain from Malaysia (F4 to F8) were selected with Bacillus thuringiensis subsp. kurstaki HD-1, Bacillus thuringiensis subsp. aizawai, Cry1Ab, and Cry1Ac, respectively, while a fifth subpopulation was left as unselected (UNSEL-MEL). Bioassays at F9 found that selection with Cry1Ac, Cry1Ab, B. thuringiensis subsp. kurstaki, and B. thuringiensis subsp. aizawai gave resistance ratios of >95, 10, 7, and 3, respectively, compared with UNSEL-MEL (>10,500, 500, >100, and 26, respectively, compared with a susceptible population, ROTH). Resistance to Cry1Ac, Cry1Ab, B. thuringiensis subsp. kurstaki, and B. thuringiensis subsp. aizawai in UNSEL-MEL declined significantly by F9. The Cry1Ac-selected population showed very little cross-resistance to Cry1Ab, B. thuringiensis subsp. kurstaki, and B. thuringiensis subsp. aizawai (5-, 1-, and 4-fold compared with UNSEL-MEL), whereas the Cry1Ab-, B. thuringiensis subsp. kurstaki-, and B. thuringiensis subsp. aizawai-selected populations showed high cross-resistance to Cry1Ac (60-, 100-, and 70-fold). The Cry1Ac-selected population was reselected (F9 to F13) to give a resistance ratio of >2,400 compared with UNSEL-MEL. Binding studies with 125I-labeled Cry1Ab and Cry1Ac revealed complete lack of binding to brush border membrane vesicles prepared from Cry1Ac-selected larvae (F15). Binding was also reduced, although less drastically, in the revertant population, which indicates that a modification in the common binding site of these two toxins was involved in the resistance mechanism in the original population. Reciprocal genetic crosses between Cry1Ac-reselected and ROTH insects indicated that resistance was autosomal and showed incomplete dominance. At the highest dose of Cry1Ac tested, resistance was recessive while at the lowest dose it was almost completely dominant. The F2 progeny from a backcross of F1 progeny with ROTH was tested with a concentration of Cry1Ac which would kill 100% of ROTH moths. Eight of the 12 families tested had 60 to 90% mortality, which indicated that more than one allele on separate loci was responsible for resistance to Cry1Ac.
Microbial products based on the insecticidal crystal (Cry) proteins of the bacterium Bacillus thuringiensis are regarded as the safest pesticides to nontarget organisms (7, 8). The development of resistance to B. thuringiensis strains is seriously threatening their life expectancy as pest control agents, particularly with the introduction of commercially grown transgenic crops expressing insecticidal proteins which increase the risk of resistance by providing a temporary constant selection pressure (49). Laboratory-based resistance to B. thuringiensis has been reported in a number of species (3, 47). To date, reports of field resistance to B. thuringiensis subspp. kurstaki and aizawai (23, 36, 40, 44, 58) have been limited to the diamondback moth, Plutella xylostella.
Some insect species can be readily selected for resistance to several different B. thuringiensis toxins (28). For example, it has been shown that Plodia interpunctella can be selected for resistance to the toxins Cry1Aa, Cry1Ab, Cry1Ca, Cry1Da, and possibly others contained in B. thuringiensis subsp. aizawai (31). Cross-resistance between B. thuringiensis toxins has also been reported in Heliothis virescens (16, 17) and P. xylostella (47). Cross-resistance among Cry1A toxins is not surprising, owing to their structural and functional similarities (35), and studies have shown that these toxins may bind to the same receptor in most of the insect species tested (2, 9, 55).
Most studies of resistance of P. xylostella to B. thuringiensis have focused on resistance to B. thuringiensis subsp. kurstaki. Resistance to B. thuringiensis subsp. kurstaki in P. xylostella is autosomally inherited and partially to completely recessive (13, 18, 52). In a resistant strain of P. xylostella from Hawaii, a single recessive gene conferred resistance to the toxins Cry1Aa, Cry1Ab, and Cry1Ac and cross-resistance to Cry1F (49).
The successful management of insecticide resistance will depend on a thorough knowledge of its genetic basis and the mechanisms involved. The mode of inheritance helps in resistance detection, monitoring, modeling, and risk assessment (27, 45). Some management strategies are particularly effective when resistance is inherited as a recessive trait. For example, one of the most promising tactics is to provide a spatial refuge from exposure to B. thuringiensis to increase survival of susceptible pests and slow the evolution of resistance (15, 32, 41, 42). If the resistance is recessive, heterozygous offspring produced by mating between resistant and susceptible individuals can be killed by B. thuringiensis toxins, delaying the evolution of resistance (15, 32, 41, 42).
In the present study, a population of P. xylostella from the Melaka region of Malaysia which evolved resistance to B. thuringiensis subsp. kurstaki in the field was examined for cross-resistance in the laboratory using subpopulations selected with Cry1Ab, Cry1Ac, B. thuringiensis subsp. kurstaki, and B. thuringiensis subsp. aizawai. In the second part of the study, alteration of the binding of Cry1A toxins to larval midgut binding sites was tested as a possible mechanism of resistance. Finally, maternal effects, sex linkage, and dominance were evaluated by measuring the response to hybrid progeny from crosses between Cry1Ac resistance-selected and Cry1Ac-susceptible populations.
MATERIALS AND METHODS
B. thuringiensis products.
B. thuringiensis subsp. kurstaki HD-1 (Dipel; 32,000 IU mg−1 [wettable powder]) and B. thuringiensis subsp. aizawai (Xentari; 35,000 diamondback moth U mg−1 [wettable powder; 15,000 IU mg−1]) were supplied by Abbott Laboratories, Chicago, Ill., and stored at room temperature. Toxins Cry1Ab, Cry1Ac, and Cry1C were obtained from recombinant B. thuringiensis strains EG7077, EG11070, and EG1081, respectively (Ecogen Inc.). Bacteria were grown in CCY medium (37) supplemented with 10 μg of tetracycline ml−1 (for Cry1Ab-producing bacteria) or with 3 μg of chloramphenicol ml−1 (for Cry1Ac- and Cry1C-producing bacteria) at 30°C with continuous shaking for 2 to 3 days, until most of the cells had sporulated. Crystals were recovered (together with spores) by centrifugation at 9,700 × g for 10 min at 4°C. The pellet was resuspended in ice-cold 1 M NaCl–5 mM EDTA and centrifuged again. This washing procedure was performed twice. The final pellet was resuspended and the crystals were solubilized in 50 mM carbonate buffer–10 mM dithiothreitol, pH 10, by incubation at room temperature for 1 h with continuous shaking. Solubilized Cry1A proteins were separated from spores by centrifugation at 9,700 × g for 10 min at 4°C. Activation of protoxins was performed by addition of trypsin at a ratio of 1:10 (trypsin-protoxin) and incubation for 2 h at 37°C. Any insoluble material was removed by centrifugation at 15,800 × g for 5 min at room temperature, and the activated toxins were stored at −20°C until used. Each test product was freshly prepared in distilled water with Triton X-100 (50 μg ml−1) added as a surfactant (58).
Insects.
A field population (MEL) of P. xylostella was obtained from the Melaka region of Malaysia in September 1997. An insecticide-susceptible population (ROTH) of P. xylostella was obtained from the Institute of Arable Crops Research, Rothamsted (Harpenden, Hertfordshire, United Kingdom), where it had been maintained in the laboratory for more than 150 generations. Insect larvae were reared and tested on 6- to 8-week-old organically greenhouse-grown Chinese cabbage (Brassica chinensis subsp. pekinensis cv. Tip Top) at 20°C and ca. 65% relative humidity under a 16-h photophase.
Selection with B. thuringiensis products and Cry toxins.
The MEL population was divided into five subpopulations at F4. One subpopulation was left unselected (UNSEL-MEL), while the other four were selected (22) with B. thuringiensis subsp. kurstaki (B. thuringiensis subsp. kurstaki-SEL), B. thuringiensis subsp. aizawai (B. thuringiensis subsp. aizawai-SEL), Cry1Ab (Cry1Ab-SEL), and Cry1Ac (Cry1Ac-SEL), respectively, from F4 to F8. The mean survival of larvae (after 5 days of treatment) during the selection process was 70% for B. thuringiensis subsp. kurstaki, 55% for B. thuringiensis subsp. aizawai, 67% for Cry1Ab, and 81% for Cry1Ac. The Cry1Ac-SEL population was further selected up to F13. The mean survival rate from F4 to F13 was 79%.
Toxicity bioassay.
Bioassays were conducted with third-instar larvae on leaf disks at F3 for Cry1Ac and Cry1Ab and at F4 and F9 for B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai. Test solutions were prepared in distilled water with Triton X-100 (50 μg ml−1) as an additional surfactant (22). Each leaf disk (4.8-cm diameter) was immersed in a test solution for 10 s and allowed to dry at ambient temperature for 1 h (23). Control leaf disks were immersed in distilled water with Triton X-100. The leaf disks were placed in individual petri dishes (5-cm diameter) containing moistened filter paper. Five larvae were placed in each dish, and each treatment was repeated eight times. Mortality was determined after 5 days.
Binding experiments.
Brush border membrane vesicles (BBMV) from ROTH, Cry1Ac-SEL, and UNSEL-MEL whole last-instar larvae were prepared by a slight modification (10) of the differential magnesium precipitation method (56). BBMV were frozen in liquid nitrogen and kept at −80°C until they were used. The protein concentration in the BBMV was measured by the method of Bradford (4). Trypsin-activated Cry1Ab, Cry1Ac, and Cry1C toxins were purified by anion-exchange chromatography in a Mono-Q column using a fast protein liquid chromatography system (Pharmacia). Cry1Ab and Cry1Ac were 125I labeled by the chloramine-T method (54). Cry1C was 125I labeled by the Iodo-bead method (26). Binding assays were performed essentially as described previously (12), in a final volume of 0.1 ml of binding buffer (8 mM Na2HPO4, 2 mM KH2PO4, 150 mM NaCl [pH 7.4], 0.1% bovine serum albumin) containing various concentrations of BBMV and around 20,000 cpm of labeled Cry1A toxins or around 8,000 cpm of Cry1C. Incubations were carried out at room temperature for 60 or 90 min for Cry1A or Cry1C toxins, respectively. Bound toxins were separated from unbound toxins by filtration through fiberglass filters. Cold binding buffer (5 ml/filter) was used to wash the filters, and the radioactivity retained was measured in a model 1282 Compugamma CS gamma counter (LKB). An excess of unlabeled toxin was used to determine the extent of nonspecific binding, which was around 0.5% of the total radioactivity in the assay for Cry1Ac and Cry1Ab and around 1% for Cry1C.
Evolution of maternal effects, sex linkage, and genetic variation.
The response of F1 and F2 progeny to Cry1Ac was evaluated. Mass and single-pair reciprocal crosses between Cry1Ac-SEL and ROTH populations produced the F1 progeny. F2 progeny were produced by single-pair crosses with ROTH. The larvae of both sexes were separated at the fourth instar based on the color of the fifth abdominal segment (25). For mass crosses, 40 females of Cry1Ac-SEL were pooled with 40 males of ROTH and 40 females of ROTH were pooled with 40 males of the Cry1Ac-SEL population. Mass crosses provided enough offspring for multiple-concentration testing and calculation of 50% lethal concentrations (LC50s).
F1 progeny from the single-pair crosses between ROTH and Cry1Ac-SEL were obtained. Single pairs consisted of a ROTH virgin male and a Cry1Ac-SEL virgin female and vice versa. F1 progeny from each family were reared on a separate Chinese cabbage plant. The F1 larvae were tested in a leaf dip bioassay with 0.2 and 1.0 μg of Cry1Ac ml−1. To obtain F2 progeny, single-pair crosses were made between F1 progeny (from mass crosses between Cry1Ac-SEL and ROTH) and ROTH. The F2 progeny from single-pair crosses were tested with 0.04 μg of Cry1Ac ml−1.
Tests of F1 and F2 progeny from single-pair crosses enabled detection of genetic variation within parental strains, which is not possible with mass crosses (49). Detection of genetic variation within parental strains is important because standard methods for estimating dominance assume that the susceptible and resistant parental strains are homozygous (19, 38). If the parental strains are genetically variable at the locus or loci controlling resistance, estimates of dominance may be biased (49).
Estimation of degree of dominance.
The term dominant is applied when a hybrid varies from “identity with the pure resistant (complete dominance) to somewhat more than halfway between pure susceptible and pure resistant” (38). The term recessive is applicable when the hybrid varies from “identity with the pure susceptible (complete recessivity) to somewhat less than half way between pure susceptible and pure resistant” (38). The degree of dominance was calculated as described by Liu and Tabashnik (24).
Statistical analysis.
When necessary, bioassay data were corrected for control mortality (1). Estimates of LC50s and their 95% fiducial limits (FL) were obtained by maximum-likelihood logit regression analysis in a generalized linear modeling using the statistical package GLIM 3.77 (Numerical Algorithms Group, 1985), from which differences between sets were extracted by analysis of deviance (6). Differences in between LC50s of two sets were considered significant (P < 0.01) if their 95% FLs did not overlap. Estimation of heritability, the proportion of phenotypic variance accounted for by additive genetic variation (11), was calculated as described by Tabashnik et al. (52) in order to compare different MEL subpopulations. The average rate of change in response to B. thuringiensis products or to Cry toxins per generation (R) was estimated as R = [log (final LC50) − log (initial LC50)]/n, where n is the number of generations selected. An increase or decrease in resistance is reflected in a positive and negative value of R (46).
In order to find genetic variation between parental strains, we used two separate analyses of variance to test the variation in mortality among families and combined their probabilities as described by Liu and Tabashnik (24).
RESULTS
Toxicity to ROTH and UNSEL-MEL.
Cry1Ac was ca. twofold more toxic to ROTH compared with Cry1Ab; B. thuringiensis subsp. aizawai was ca. fivefold more toxic to ROTH compared with B. thuringiensis subsp. kurstaki (Table 1).
TABLE 1.
Toxicity of B. thuringiensis subspp. kurstaki and aizawai and Cry toxins to the susceptible laboratory P. xylostella population ROTH
| Insecticide | LC50a (95% FL) | Avg slope (SE) | nb |
|---|---|---|---|
| Cry1Ac | 0.0019 (0.0015–0.0025) | 3.91 (0.48) | 156 |
| Cry1Ab | 0.0042 (0.003–0.0057) | 3.52 (0.57) | 234 |
| B. thuringiensis subsp. kurstaki | 0.054 (0.036–0.083) | 2.00 (0.28) | 237 |
| B. thuringiensis subsp. aizawai | 0.011 (0.008–0.017) | 1.85 (0.26) | 240 |
Cry1Ac and Cry1Ab values are in micrograms per milliliter; B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai values are in international units per milligram.
Number of larvae used in bioassay, including control.
Cry1Ac and Cry1Ab were similarly toxic to UNSEL-MEL (F3), with resistance ratios of 300- and 121-fold compared with ROTH, respectively (Table 2). B. thuringiensis subsp. kurstaki was less toxic to UNSEL-MEL (F4) compared with B. thuringiensis subsp. aizawai, with resistance ratios of 40- and 13-fold, respectively, compared with ROTH (Table 2).
TABLE 2.
Toxicity of Cry1Ac, Cry1Ab, B. thuringiensis subsp. kurstaki, and B. thuringiensis subsp. aizawai against the UNSEL-MEL population
| Generation | Insecticide | LC50a (95% FL) | Avg slope (SE) | RRb | nc |
|---|---|---|---|---|---|
| F3 | Cry1Ac | 0.57 (0.28–1.42) | 1.33 (0.28) | 300 | 158 |
| F9 | Cry1Ac | 0.21 (0.14–0.32) | 1.68 (0.28) | 110 | 276 |
| F3 | Cry1Ab | 0.51 (0.24–1.30) | 1.32 (0.28) | 121 | 157 |
| F9 | Cry1Ab | 0.22 (0.14–0.35) | 1.70 (0.20) | 52 | 276 |
| F4 | B. thuringiensis subsp. kurstaki | 2.18 (0.93–15.3) | 0.89 (0.32) | 40 | 196 |
| F9 | B. thuringiensis subsp. kurstaki | 0.86 (0.56–1.24) | 2.07 (0.32) | 16 | 237 |
| F4 | B. thuringiensis subsp. aizawai | 0.15 (0.12–0.20) | 4.50 (0.77) | 13 | 160 |
| F9 | B. thuringiensis subsp. aizawai | 0.10 (0.07–0.13) | 3.13 (0.47) | 9 | 271 |
Cry1Ac and Cry1Ab data are in micrograms per milliliter; B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai data are in international units per milligram.
Resistance ratio of LC50s for unselected population compared with ROTH (Table 1).
Number of larvae used in bioassay, including control.
There was no significant (P > 0.01) decrease in LC50s from F4 or F3 to F9 for B. thuringiensis subsp. kurstaki, B. thuringiensis subsp. aizawai, Cry1Ab, and Cry1Ac, respectively (Table 2). There was no significant (P > 0.05) change in the slopes for Cry1Ab, Cry1Ac, and B. thuringiensis subsp. aizawai; however, the slope for B. thuringiensis subsp. kurstaki increased significantly (P < 0.05) from F4 to F9 (Table 2).
Response to selection.
Selection (F4 to F8) increased the resistance ratio for Cry1Ac and Cry1Ab >95- and 10-fold, respectively, compared with UNSEL-MEL (F9) (>10,500- and 500-fold, respectively compared with the ROTH population) (Table 3). There was no significant (P > 0.05) change in the slope for Cry1Ac-SEL (Tables 2 and 3). For Cry1Ab-SEL, there was a significant (P < 0.05) increase in the slope (Tables 2 and 3). Reselection with Cry1Ac from F9 to F13 increased the resistance ratio to >2,400-fold at F15 compared with UNSEL-MEL (>154,000-fold compared with ROTH) (Table 2; see Table 5), but there was no significant (P > 0.05) change in the slope (Table 2; see Table 5).
TABLE 3.
Cross-resistance between Cry1Ac-SEL, Cry1Ab-SEL, B. thuringiensis subsp. kurstaki-SEL, and B. thuringiensis subsp. aizawai-SEL subpopulations of P. xylostella
| Population (generation) | Insecticide | LC50a (95% FL) | Avg slope (SE) | RRb | RRc | nd |
|---|---|---|---|---|---|---|
| Cry1Ac-SEL (F9) | Cry1Ac | >20e | 1.45 (0.49) | >10,500 | >95 | 240 |
| Cry1Ac-SEL (F9) | Cry1Ab | 1.11 (0.85–1.43) | 3.91 (0.37) | 264 | 5 | 298 |
| Cry1Ac-SEL (F9) | B. thuringiensis subsp. kurstaki | 3.17 (2.25–5.96) | 2.31 (0.33) | 59 | 4 | 280 |
| Cry1Ac-SEL (F9) | B. thuringiensis subsp. aizawai | 0.12 (0.09–0.15) | 2.56 (0.55) | 10 | 1 | 238 |
| Cry1Ab-SEL (F9) | Cry1Ac | 13.20 (6.87–38.00) | 1.16 (0.21) | 7,000 | 60 | 282 |
| Cry1Ab-SEL (F9) | Cry1Ab | 2.13 (1.67–2.68) | 3.51 (0.49) | 500 | 10 | 240 |
| Cry1Ab-SEL (F9) | B. thuringiensis subsp. kurstaki | 4.37 (3.17–6.11) | 2.54 (0.32) | 81 | 5 | 242 |
| Cry1Ab-SEL (F9) | B. thuringiensis subsp. aizawai | 0.18 (0.14–0.25) | 2.27 (0.35) | 16 | 2 | 238 |
| B. thuringiensis subsp. kurstaki-SEL (F9) | Cry1Ac | 20.50 (14.60–33.00) | 2.20 (0.39) | 10,700 | 100 | 237 |
| B. thuringiensis subsp. kurstaki-SEL (F9) | Cry1Ab | 3.86 (2.60–7.44) | 2.18 (0.40) | 900 | 18 | 237 |
| B. thuringiensis subsp. kurstaki-SEL (F9) | B. thuringiensis subsp. kurstaki | 6.02 (4.78–7.39) | 3.81 (0.40) | 112 | 7 | 274 |
| B. thuringiensis subsp. kurstaki-SEL (F9) | B. thuringiensis subsp. aizawai | 0.09 (0.07–0.12) | 3.08 (0.40) | 8 | 1 | 280 |
| B. thuringiensis subsp. aizawai-SEL (F9) | Cry1Ac | 13.80 (4.86–129.00) | 0.77 (0.19) | 7,260 | 70 | 208 |
| B. thuringiensis subsp. aizawai-SEL (F9) | Cry1Ab | 1.78 (1.19–3.63) | 2.51 (0.47) | 420 | 8 | 178 |
| B. thuringiensis subsp. aizawai-SEL (F9) | B. thuringiensis subsp. kurstaki | 2.33 (1.57–3.80) | 2.10 (0.35) | 40 | 3 | 173 |
| B. thuringiensis subsp. aizawai-SEL (F9) | B. thuringiensis subsp. aizawai | 0.29 (0.21–0.42) | 2.56 (0.40) | 30 | 3 | 211 |
Cry1Ac and Cry1Ab data are in micrograms per milliliter; B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai data are in international units per microgram.
Resistance ratio of LC50s for selected subpopulation compared with ROTH (Table 1).
Resistance ratio of LC50s for selected subpopulation compared with UNSEL-MEL at F9 (Table 2).
Number of larvae used in bioassay, including control.
Mortality at 20 μg ml−1 = 35%.
TABLE 5.
Responses (mortality) of resistant (Cry1Ac-SEL) and susceptible (ROTH) P. xylostella and their hybrid F1 progeny to Cry1Ac
| Population | LC50a (95% FL) | Avg slope (SE) | RRb | Dc | hd | ne |
|---|---|---|---|---|---|---|
| Cry1Ac-SEL F15 | 293 (198.3–620.70) | 2.30 (0.52) | 154,211 | 241 | ||
| ROTH | 0.0019 (0.0015–0.0025) | 3.91 (0.48) | 1 | 197 | ||
| F1e | 0.037 (0.0195–0.069) | 1.33 (0.18) | 19.47 | 0.49 | 0.75 | 240 |
| F1f | 0.085 (0.047–0.146) | 1.52 (0.20) | 44.74 | 0.36 | 0.68 | 240 |
These values are in micrograms per milliliter.
Resistance ratio (LC50 for selected population divided by LC50 for ROTH).
Degree of dominance = (2X2 − X1 − X3)/(X1 − X3), where X1, X2, and X3 are the log LC50s for the resistant homozygotes, heterozygotes, and susceptible homozygotes, respectively.
Estimate of dominance [h = (D + 1)/2].
F1 progeny of mass crosses between 40 females of ROTH and 40 males of Cry1Ac-SEL.
F1 progeny of mass crosses between 40 females of Cry1Ac-SEL and 40 males of ROTH.
In B. thuringiensis subsp. kurstaki-SEL and B. thuringiensis subsp. aizawai-SEL, the resistance ratios for B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai were 7- and 3-fold greater, respectively, compared with UNSEL-MEL (F9) and 112- and 30-fold greater compared with ROTH (Table 3). The slope increased significantly (P < 0.01) from F4 to F9 for B. thuringiensis subsp. kurstaki-SEL (Tables 2 and 3) but did not change significantly for B. thuringiensis subsp. aizawai-SEL (P > 0.05) (Table 3).
Cross-resistance to insecticides in subpopulations of MEL.
The B. thuringiensis subsp. kurstaki- B. thuringiensis subsp. aizawai- and Cry1Ab-SEL populations had Cry1Ac resistance ratios of 100-, 70-, and 60-fold, respectively, compared with UNSEL-MEL (Table 3). In the B. thuringiensis subsp. kurstaki-, B. thuringiensis subsp. aizawai-, and Cry1Ac-SEL populations, the Cry1Ab resistance ratios were 18-, 8-, and 5-fold, respectively, compared with the unselected population (Table 3). There was little change, if any, in toxicity to B. thuringiensis subsp. aizawai in B. thuringiensis subsp. kurstaki-Cry1Ab-, and Cry1Ac-SEL compared with UNSEL-MEL (Table 3). There was little change in toxicity to B. thuringiensis subsp. kurstaki in the B. thuringiensis subsp. aizawai-, Cry1Ab-, and Cry1Ac-SEL populations (Table 3).
Estimation of h2 for selected MEL subpopulations.
Estimates of realized heritability (h2) based on five generations of selection for three subpopulations (Cry1Ab-SEL, B. thuringiensis subsp. kurstaki-SEL, and B. thuringiensis subsp. aizawai-SEL) were 0.28, and 0.19, and 0.21, respectively (Table 4). The h2 for Cry1Ac-SEL could only be estimated very approximately after five generations of selection because the highest dose of Cry1Ac tested (20 μg ml−1) gave only 35% mortality at F9 (Table 3); after nine generations of selection (at F15), the h2 was 0.34. The number of generations required for a 10-fold increase in the LC50 is the reciprocal of R (Table 4) (50), and for B. thuringiensis subsp. kurstaki, B. thuringiensis subsp. aizawai, and Cry1Ab, it was 11, 18, and 8, respectively.
TABLE 4.
Estimation of h2a of resistance from laboratory selection of the Melaka population of P. xylostella with Cry1Ac, Cry1Ab, B. thuringiensis subsp. kurstaki, and B. thuringiensis subsp. aizawai (F4 to F8) and reselection of Cry1Ac-SEL population with Cry1Ac (F9 to F13)
| Strain | Estimate of mean response/generationb
|
pd | Estimate of mean selection difference/generation
|
h2h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial LC50 | Final LC50 | Rc | ie | Initial slope | Final slope | ςpf | Sg | |||
| Cry1Ac-SEL | 0.573 (−0.241) | >20 (>1.30)j | 0.308 | 25 | 1.271 | 1.33 | 1.45 | 0.719 | 0.913 | 0.34i |
| Cry1Ac-SEL F15 | 0.573 (−0.241) | 293 (2.47) | 0.301 | 21 | 1.372 | 1.33 | 2.30 | 0.551 | 0.756 | 0.40 |
| Cry1Ab-SEL | 0.509 (−0.293) | 2.13 (0.327) | 0.124 | 34 | 1.078 | 1.32 | 3.51 | 0.414 | 0.446 | 0.28 |
| B. thuringiensis subsp. kurstaki-SEL | 2.183 (0.339) | 6.02 (0.78) | 0.088 | 34 | 1.078 | 0.89 | 3.81 | 0.426 | 0.459 | 0.19 |
| B. thuringiensis subsp. aizawai-SEL | 0.356 (−0.449) | 0.68 (−0.17) | 0.056 | 41 | 0.948 | 4.50 | 2.56 | 0.283 | 0.268 | 0.21 |
Reference 52.
Initial and final LC50s were determined at F3 and F9, respectively (for Cry1Ac-SEL F15, initial and final LC50s were determined at F3 and F15, respectively).
Response to selection (R) = log (final LC50) − log (initial LC50)/n.
Percentage of the population with values above the selection threshold (percentage surviving selection).
Intensity of selection (11).
Phenotypic deviation [0.5 (initial slope + final slope)]−1.
Selection differential (difference in mean phenotype between selected parents and entire parental generation, s = iςp).
h2 = R/S.
Approximately calculated, as LC50 is approximated (Table 3).
Log of LC50s.
Specific binding of 125I-labeled toxins to BBMV.
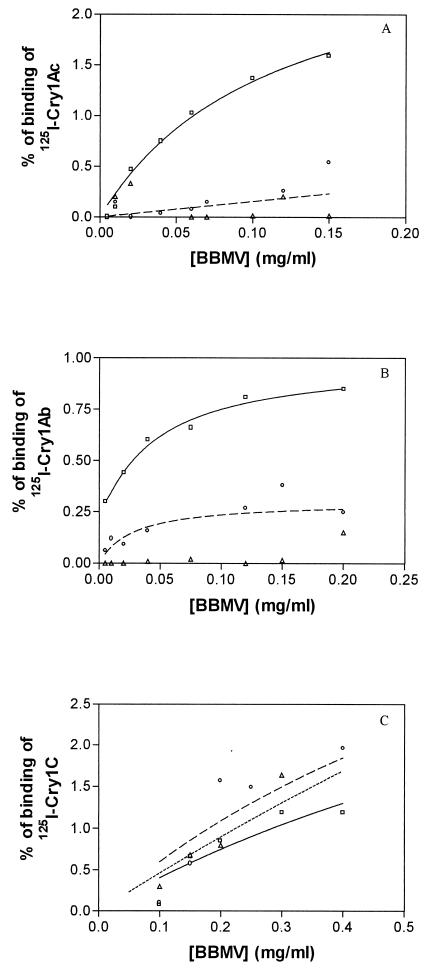
Binding of labeled Cry1Ab, Cry1Ac, and Cry1C to BBMV was evaluated in three subpopulations: ROTH, Cry1Ac-SEL, and UNSEL-MEL (Fig. 1). BBMV from ROTH insects showed saturable binding with the two Cry1A toxins with a maximum specific binding of 0.85% for Cry1Ab and 1.6% for Cry1Ac. In contrast, BBMV from Cry1Ac-SEL insects showed an almost complete absence of binding with either toxin. For UNSEL-MEL (at generation F9), binding of both toxins was considerably reduced, although not completely absent (maximum specific binding was around 0.25% for Cry1Ab and Cry1Ac). Binding of Cry1C was similar in all populations.
FIG. 1.
Specific binding of Cry1Ac (A), Cry1Ab (B), and Cry1C (C) as a function of P. xylostella BBMV concentration. Nonspecific-binding values were subtracted from each datum point. Lines: solid, ROTH; broken, UNSEL-MEL (F9); dotted, Cry1Ac-SEL (F15).
Evaluation of maternal effect and sex linkage.
Following reciprocal mass crosses, the LC50 and slope obtained for Cry1Ac with F1 progeny from Cry1Ac-SEL females were not significantly different (P > 0.05) from those of F1 progeny of ROTH females (Table 5).
Degree of dominance.
Bioassays of F1 progeny from mass and single-pair crosses between Cry1Ac-SEL and ROTH showed that resistance to Cry1Ac depended upon the concentration (Tables 6 and 7). The LC50s of F1 progenies were significantly greater (P < 0.001) than that of ROTH (Table 5). There was no significant difference (P > 0.05) in slope and LC50 between F1 progenies (Table 5). The LC50s of F1 progenies from mass crosses yielded D values of 0.49 and 0.36, respectively, which are equivalent to h values of 0.75 and 0.68 (Table 5), and indicated that resistance showed incomplete dominance at the LC50.
TABLE 6.
Dominance of resistance to Cry1Ac in the Melaka population of P. xylostella as a function of the concentration of Cry1Ac
| Concn (μg ml−1) | Population | Mortalitya (%) | Fitnessb | hc |
|---|---|---|---|---|
| 0.002 | Cry1Ac-SEL | 0 | 1 | |
| ROTH | 23 | 0.77 | ||
| F1d | 13 | 0.87 | 0.43 | |
| F1e | 3 | 0.97 | 0.87 | |
| 0.02 | Cry1Ac-SEL | 0 | 1 | |
| ROTH | 100 | 0 | ||
| F1d | 43 | 0.57 | 0.57 | |
| F1e | 38 | 0.62 | 0.62 | |
| 0.2 | Cry1Ac-SEL | 0 | 1 | |
| ROTH | 100 | 0 | ||
| F1d | 80 | 0.20 | 0.20 | |
| F1e | 60 | 0.40 | 0.40 | |
| 1.0 | Cry1Ac-SEL | 0 | 1 | |
| ROTH | 100 | 0 | ||
| F1d | 83 | 0.17 | 0.17 | |
| F1e | 85 | 0.17 | 0.17 | |
| 1.5 | Cry1Ac-SEL | 0 | 1 | |
| ROTH | 100 | 0 | ||
| F1d | 87 | 0.13 | 0.13 | |
| F1e | 85 | 0.15 | 0.13 |
n = 40 (39 for ROTH at 0.002 μg ml−1).
Fitness is the survival rate of the larvae divided by the survival rate of Cry1Ac-SEL larvae (survival rate is estimated as 100 − percent mortality).
Dominance (h) can vary from 0 (completely recessive resistance) to 1 (completely dominant resistance); h = (w12 − w22)/(w11 − w22), where w11, w12, and w22 are the fitness values for resistant homozygotes, heterozygotes, and susceptible homozygotes, respectively.
F1 hybrid progeny of mass crosses between 40 females of ROTH and 40 males of Cry1Ac-SEL.
F1 hybrid progeny of mass crosses between 40 females of Cry1Ac-SEL and 40 males of ROTH.
TABLE 7.
Dominance resistance to Cry1Ac in the Melaka population of P. xylostella as a function of the concentration of Cry1Ac for single-pair hybrid F1 families
| Population or families | Characteristics of larvae at Cry1Ac concna of:
|
|||||
|---|---|---|---|---|---|---|
| 0.2 μg ml−1
|
1.0 μg ml−1
|
|||||
| Mortality (%) | Fitness | hb | Mortality (%) | Fitness | h | |
| Cry1Ac-SEL | 0 | 1 | 0 | 1 | ||
| ROTH | 100 | 0 | 100 | 0 | ||
| Single-pair F1 families | ||||||
| Cry1Ac-SEL female × ROTH male | 35 | 0.65 | 0.65 | 70 | 0.30 | 0.30 |
| Cry1Ac-SEL female × ROTH male | 43 | 0.57 | 0.57 | 65 | 0.35 | 0.35 |
| Cry1Ac-SEL female × ROTH male | 40 | 0.60 | 0.60 | 70 | 0.30 | 0.30 |
| ROTH female × Cry1Ac-SEL male | 73 | 0.27 | 0.27 | 95 | 0.05 | 0.05 |
| ROTH female × Cry1Ac-SEL male | 93 | 0.07 | 0.07 | 100 | 0 | 0 |
Fitness is the survival rate of the larvae divided by the survival rate of Cry1Ac-SEL larvae. Estimates of dominance (h) can vary from 0 (completely recessive resistance) to 1 (completely dominant resistance).
See Table 6, footnote c.
Estimates of dominance with five concentration of Cry1Ac (Table 6) showed that resistance tended to be dominant as the concentration decreased. At high concentrations, resistance of the F1 progeny was partially recessive (Table 6), whereas it was partially dominant at the lowest concentration, except for ROTH female × Cry1Ac-SEL male F1 progeny, which showed partially recessive resistance at the lowest concentration (Table 6). The families of F1 progeny from single-pair crosses had a mean h value of 0.43 at 0.2 μg ml−1 and 0.2 at 1.0 μg ml−1 (Table 7).
Evaluation of genetic variation within strains by single-pair crosses.
Among five families of F1 progeny from single-pair crosses between Cry1Ac-SEL and ROTH, there were significant differences in mortality among families (df, = 4, 35; F = 3.05; P = 0.02) (Table 7). Among the 12 families of F2 progeny from single-pair crosses between ROTH and F1 progeny, mortality ranged from 80 to 100%, and this variation was not significant (df = 11, 36; F = 1.642; P = 0.13). Combining the probabilities from these separate tests showed significant variation in mortality within the sets of single-pair families (df = 4; χ2 = 11.90; P < 0.01).
DISCUSSION
Estimates of h2 suggest that resistance to Cry1Ac could be selected more rapidly than resistance to Cry1Ab and especially resistance to B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai. The level of resistance in the Cry1Ac-SEL population after nine generations of selection was greater than in any other published studies for Cry1Ac, including that of H. virescens (17). The B. thuringiensis subsp. kurstaki-SEL subpopulation showed moderate (>100-fold) resistance to B. thuringiensis subsp. kurstaki which is comparable to that of other Malaysian strains of P. xylostella (23, 40). The B. thuringiensis subsp. aizawai-SEL population showed a level of resistance (26-fold) similar to that of a Cameron Highland population collected in 1993 (23, 58).
Apart from the selection of resistance, the instability of resistance in the absence of exposure to insecticides is of interest in pest management (5). In the present study, resistance to Cry1Ac, Cry1Ab, B. thuringiensis subsp. kurstaki, and B. thuringiensis subsp. aizawai in UNSEL-MEL appeared to be unstable. The rate of decline of resistance to B. thuringiensis subsp. aizawai was much slower compared with that of resistance to Cry1Ac, Cry1Ab, and B. thuringiensis subsp. kurstaki and was consistent with the decline in B. thuringiensis subsp. aizawai resistance observed in a Thailand population of P. xylostella (22). The more rapid rate of decline in resistance to Cry1Ac, Cry1Ab, and B. thuringiensis subsp. kurstaki in MEL was similar to the Hawaiian NO-P, NO-Q, and NO-R populations of P. xylostella (48). However, Tang et al. (53) found that resistance in a Florida population of P. xylostella stabilized after three generations.
Results obtained from the binding assays showed that at least one of the mechanisms of resistance to Cry1Ab and Cry1Ac in the populations of this study is caused by a reduction of the binding of these toxins to midgut membrane binding sites. Similar results have been reported for other resistant populations of P. xylostella (2, 12, 50, 54, 58). The reduced although significant binding detected in insects from the UNSEL-MEL subpopulation is most likely related to the presence of a considerable number of susceptible insects at the time of the analysis due to reversal of resistance. At F9, the resistance ratios (related to the ROTH population) for UNSEL-MEL were 52 for Cry1Ab and 110 for Cry1Ac (Table 2), whereas these ratios in the Cry1Ac-SEL subpopulation were 264 and >10,500, respectively (Table 3). A similar result was reported for a resistant population from Hawaii in which the reversal of resistance was associated with restoration of binding (48).
Two biochemical phenotypes relating to toxin binding reduction have been described among resistant populations of the diamondback moth (2, 50). In one of them, the Cry1A and Cry1F common binding site suffers a major change which impairs the binding of Cry1Aa, Cry1Ab, Cry1Ac, and probably Cry1F and was found in resistant populations from Hawaii (NO-QA) and Pennsylvania (PEN). The second biochemical phenotype involves a minor change in the common binding site which only affects binding of Cry1Ab and was found previously in a resistant population from Malaysia (SERD3) (56) and in a population from the Philippines (PHI). The type of binding site alteration found in the present study seems to belong to the first category, since binding of both the Cry1Ab and Cry1Ac toxins is reduced.
The B. thuringiensis subsp. kurstaki-, B. thuringiensis subsp. aizawai- and Cry1Ab-SEL subpopulations showed very high levels of cross-resistance to Cry1Ac, while the Cry1Ac-SEL subpopulation showed little reciprocal cross-resistance to Cry1Ab, B. thuringiensis subsp. aizawai, or B. thuringiensis subsp. kurstaki. Cross-resistance between toxins of the Cry1A family might have been expected, as these bind to the same receptor site (2) and share more than 80% homology (14, 35). However, the low levels of resistance and cross-resistance to B. thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai observed, compared with those obtained with the single toxins, is not unexpected. It is known that it is easier to select for resistance to a single component than to a mixture of them, especially if they have different mechanisms of toxicity (such as Cry1A, Cry2A, and spores).
The level of cross-resistance in the B. thuringiensis subsp. kurstaki-SEL population to Cry1Ac (>10,000-fold) and Cry1Ab (900-fold) was greater than the level of resistance to B. thuringiensis subsp. kurstaki. This suggests that the low level of resistance in the B. thuringiensis subsp. kurstaki-SEL subpopulation was due to the presence of Cry2A toxins and spores in B. thuringiensis subsp. kurstaki, as Tabashnik et al. (51) reported that Cry2A toxins showed little or no cross-resistance to B. thuringiensis subsp. kurstaki in a Hawaiian population of P. xylostella. The B. thuringiensis subsp. kurstaki-SEL population showed less cross-resistance (eightfold) to B. thuringiensis subsp. aizawai, which is comparable to studies on Hawaiian and Serdang populations of P. xylostella (46, 58) and P. interpunctella (30). The cross-resistance between B. thuringiensis subsp. kurstaki-SEL and B. thuringiensis subsp. aizawai is most probably due to shared Cry toxins present in these subspecies (46).
The low level of resistance to B. thuringiensis subsp. aizawai in the B. thuringiensis subsp. aizawai-SEL MEL subpopulation probably reflects the relatively limited usage of B. thuringiensis subsp. aizawai in this region of Malaysia. This was also suggested by studies on a P. xylostella population collected from another lowland area (Serdang) of Malaysia in 1993 (23).
The low level of cross-resistance between B. thuringiensis subsp. aizawai and Cry1Ab, compared with the high level of cross-resistance between B. thuringiensis subsp. aizawai and Cry1Ac, was unexpected, since only the former is present in B. thuringiensis subsp. aizawai (21). The most probable explanation is that two mechanisms of resistance are present, a reduction of binding to the common site for Cry1Ab and Cry1Ac and a more exclusive mechanism for Cry1Ac. The latter would be responsible for the >19-fold difference in resistance ratio between Cry1Ac and Cry1Ab in the Cry1Ac-SEL population (Table 3).
The results of bioassays following reciprocal mass crosses between Cry1Ac-SEL and ROTH showed that there was no significant difference in the LC50 or slope between the F1 progeny, indicating that the resistance to Cry1Ac in the MEL population is inherited autosomally. Similar results were reported for other studies on P. interpunctella (28), P. xylostella (18, 25, 52), and H. virescens (17). However, Martinez-Ramirez et al. (27) reported that resistance to Cry1Ab in a population of P. xylostella probably had a parental influence, although sex linkage was also discarded.
Unlike resistance to B. thuringiensis subsp. kurstaki, Cry1Ab, Cry1Ac, and Cry1F, which is recessive in some other populations of P. xylostella (18, 33, 50, 52), resistance to Cry1Ac in MEL showed incomplete dominance. In this respect, it was similar to resistance to Cry1Ac in the CP73-3 population of H. virescens (16), Cry1Aa in a Philippine population of P. xylostella (50) and Cry1Ca in P. xylostella (24). In the present study, the extent of dominance of resistance to Cry1Ac depended upon the concentration of the toxin used. Resistance was completely recessive at the highest dose while almost completely dominant at the lowest dose, except for F1 (ROTH female × Cry1Ac-SEL male), in which resistance was only partially recessive at the lowest dose. The above-described results are in broad agreement with the work of Liu and Tabashnik (24) with a Hawaiian population of P. xylostella. Resistance to Cry1Ac in the CP73-3 strain of H. virescens was reported to be relatively recessive at the lowest dose tested, while at higher concentrations, resistance was inherited as an additive trait (16).
The estimation of dominance was based on the assumption that the resistant population was completely homozygous when F1 progeny were produced. The heterozygotes in a selected population would tend to lower the survival rate of F1 progeny and thus underestimate the degree of dominance, while heterozygotes in a susceptible population would have the opposite effect (24). The significant variation within the sets of single-pair families suggests that the Cry1Ac-SEL population was not homozygous for resistance at the time of the crosses.
If complete or partial dominance exists, at least two different gene interactions can occur (33). When the backcrossed ROTH progeny were exposed to a discriminating dose of Cry1Ac (0.04 μg ml−1) which was eightfold greater than the LC95 (0.005 μg ml−1) and would kill 100% of susceptible insects, 8 families of the 12 tested had 60 to 90% mortality and 4 had 100% mortality. This suggests that resistance to Cry1Ac in this population of P. xylostella is controlled by more than one allele. In addition, epistasis can occur as a consequence of increased or decreased enzyme activities (39), which could increase the ability to tolerate the toxic agent by changing the receptor-ligand kinetics by altering gut acidity or physiology. Epistatic interactions may evolve under close inbreeding, which is the simplest explanation of the selection in the laboratory, where each allele contributes to the resistance and the introduction of a susceptible allele dilutes the effect.
The lack of reciprocal cross-resistance to Cry1Ab, B. thuringiensis subsp. kurstaki, and B. thuringiensis subsp. aizawai in the Cry1Ac-SEL subpopulation is compatible with the idea that more than one resistant mechanism is involved. The best-known mechanism of resistance to Cry1A toxins in P. xylostella is reduced binding in midgut membranes (2, 13). However, Heckel (20) has pointed out that because proteolysis of the toxic fragment involves a gain of function (unlike reduced binding), it is less likely to be inherited recessively. Incomplete dominance might, therefore, involve a proteolytic mechanism of resistance, whereas the cross-resistance observed in the present work may be due to changes in midgut binding sites.
The degree of dominance and cross-resistance profoundly affects the strategies for managing insecticide resistance (49). Refuges or high doses only work when resistance is recessive (24, 41). A high-dose strategy can slow resistance but only when the alleles exist in the population as heterozygotes (53). When the resistance is dominant, then this high-dose strategy accelerates the development of resistance (43).
ACKNOWLEDGMENTS
We thank Dzolkhifli Omar for supply of the field population of P. xylostella from Malaysia and Ecogen Inc. for providing bacterial strains used to prepare Cry toxins.
A.H.S. was supported by the Hundred Scholarship Scheme of the Government of Pakistan. This work was conducted under MAFF licenses PHL 17/2495(11/1997) and PHL 17A/2689(6/1998).
REFERENCES
- 1.Abbot W. A method of computing the effectiveness of insecticide. J Econ Entomol. 1925;18:265–267. [Google Scholar]
- 2.Ballester V, Granero F, Tabashnik B E, Malvar T, Ferré J. Integrative model for binding of Bacillus thuringiensis toxins in susceptible and resistant larvae of the diamondback moth (Plutella xylostella) Appl Environ Microbiol. 1999;65:1413–1419. doi: 10.1128/aem.65.4.1413-1419.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer L S. Resistance: a threat to the insecticidal crystal proteins of Bacillus thuringiensis. Fla Entomol. 1995;78:414–443. [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Brown T M, De Vries D H, Brown A W A. Induction of resistance to insect growth regulators. J Econ Entomol. 1978;71:223–229. [Google Scholar]
- 6.Crawley M J. GLIM for ecologists. London, England: Blackwell; 1993. [Google Scholar]
- 7.Croft B A. Arthropods biological control agents and pesticides. New York, N.Y: John Wiley & Sons, Inc.; 1990. [Google Scholar]
- 8.Entwistle P F, Cory J S, Bailey M J, Higgs S. Bacillus thuringiensis an environmental biopesticide. New York, N.Y: John Wiley & Sons, Inc.; 1993. [Google Scholar]
- 9.Escriche B, Ferré J, Silva F J. Occurrence of a common binding site in Mamestra brassicae, Phthorimaea operculella and Spodoptera exigue for insecticidal crystal proteins Cry1A from Bacillus thuringiensis. Insect Biochem Mol Biol. 1997;27:651–656. doi: 10.1016/s0965-1748(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 10.Escriche B, Silva F J, Ferré J. Testing suitability of brush border membrane vesicles prepared from whole larvae from small insects for binding studies with Bacillus thuringiensis CryIA(b) crystal protein. J Invertebr Pathol. 1995;65:318–320. [Google Scholar]
- 11.Falconer D S. Introduction to quantitative genetics. 3rd ed. New York, N.Y: Longman; 1989. [Google Scholar]
- 12.Ferré J, Real M D, Van Rie J, Jansens S, Peferoen M. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella (L) is due to change in the midgut receptor. Proc Natl Acad Sci USA. 1991;88:5119–5123. doi: 10.1073/pnas.88.12.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferré J, Escriche B, Bel Y, Van Rie J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis insecticidal proteins. FEMS Microbiol Lett. 1995;132:1–7. [Google Scholar]
- 14.Ge A Z, Rivers D, Milne R, Dean D H. Formation of domains of Bacillus thuringiensis insecticidal crystal proteins. J Biol Chem. 1991;266:17954–17958. [PubMed] [Google Scholar]
- 15.Gould F. Evolutionary biology and genetically engineered crops. BioScience. 1988;38:26–33. [Google Scholar]
- 16.Gould F, Martinez-Ramirez A, Anderson A, Ferré J, Silva F J, Moar W J. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc Natl Acad Sci USA. 1992;80:7986–7990. doi: 10.1073/pnas.89.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould F, Anderson A, Reynolds A, Bumgarner L, Moar W. Selection and genetics analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J Econ Entomol. 1995;88:1545–1559. [Google Scholar]
- 18.Hama H. Insecticide resistance characteristics of diamondback moth. In: Talekar N S, Griggs T D, editors. Proceedings of the Second International Workshop, Tainan Taiwan. Taipei, Taiwan: Asian Vegetable Research and Development Center; 1992. pp. 455–463. [Google Scholar]
- 19.Hartl D L. A primer of population genetics. 2nd ed. Sunderland, Mass: Sinauer; 1988. [Google Scholar]
- 20.Heckel D G. The complex genetic basis of resistance to Bacillus thuringiensis toxin insects. Biocontrol Sci Technol. 1994;4:405–418. [Google Scholar]
- 21.Höft H, Whiteley H R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989;53:242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai K, Mori Y. Levels, inheritance and stability of resistance to Bacillus thuringiensis formulation in a field population of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) from Thailand. Appl Entomol Zool. 1999;34:23–29. [Google Scholar]
- 23.Iqbal M, Verkerk R H J, Furlong M J, Ong P C, Rehman S A, Wright D J. Evidence for resistance to Bacillus thuringiensis (Bt) kurstaki HD-1, Bt subspp. aizawai and abamectin in field population of Plutella xylostella from Malaysia. Pestic Sci. 1996;48:89–97. [Google Scholar]
- 24.Liu Y-B, Tabashnik B E. Inheritance of resistance to Bacillus thuringiensis toxin Cry1C in the diamondback moth. Appl Environ Microbiol. 1997;63:2218–2223. doi: 10.1128/aem.63.6.2218-2223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y-B, Tabashnik B E. Visual determination of sex of diamondback moth larvae. Can J Entomol. 1997;129:585–586. [Google Scholar]
- 26.Luo K, Adang M J. Removal of adsorbed toxin fragments that modify Bacillus thuringiensis Cry1C delta-endotoxin iodination and binding by sodium dodecyl sulfate treatment and renaturation. Appl Environ Microbiol. 1994;60:2905–2910. doi: 10.1128/aem.60.8.2905-2910.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Ramirez A C, Escriche B, Real M D, Silva F J, Ferré J. Inheritance of resistance to Bacillus thuringiensis toxin in a field population of diamondback moth Plutella xylostella. Pestic Sci. 1995;43:115–120. [Google Scholar]
- 28.McGaughey W H. Implication of cross-resistance among Bacillus thuringiensis toxin in resistance management. Biocontrol Sci Technol. 1994;4:427–435. [Google Scholar]
- 29.McGaughey W H, Beeman R W. Resistance to Bacillus thuringiensis in colonies of Indian meal moth and almond moth (Lepidoptera: Pyralidae) J Econ Entomol. 1988;81:28–33. [Google Scholar]
- 30.McGaughey W H, Johnson D E. Toxicity of different serotypes and toxins of Bacillus thuringiensis to resistant and susceptible Indian meal moth (Lepidoptera: Pyralidae) J Econ Entomol. 1987;80:1122–1126. [Google Scholar]
- 31.McGaughey W H, Johnson D E. Indian meal moth (Lepidoptera: Pyralidae) resistance to different strains and mixture of Bacillus thuringiensis. J Econ Entomol. 1992;85:1594–1600. [Google Scholar]
- 32.McGaughey W H, Whalon M E. Management insect resistance to Bacillus thuringiensis toxins. Science. 1992;258:1451–1455. doi: 10.1126/science.258.5087.1451. [DOI] [PubMed] [Google Scholar]
- 33.Metz T D, Roush R T, Tang J D, Shelton A M, Earle E D. Transgenic broccoli expressing a Bacillus thuringiensis insecticidal crystal protein: implications for pest resistance management strategies. Mol Breed. 1995;1:309–317. [Google Scholar]
- 34.Rahardja U, Whalon M E. Inheritance of resistance to Bacillus thuringiensis subsp. tenebrionis CryIII A δ-endotoxin in Colorado potato beetle (Coleoptera: Chrysomelidae) J Econ Entomol. 1995;88:21–26. doi: 10.1093/jee/88.1.21. [DOI] [PubMed] [Google Scholar]
- 35.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shelton A M, Robertson J L, Tang J D, Perez C, Eigenbrode S D, Prcisler H K, Wilsey W T, Cooley R J. Resistance of diamondback moth (Lepidoptera: Plutellidae) to Bacillus thuringiensis subspecies in the field. J Econ Entomol. 1993;86:697–705. [Google Scholar]
- 37.Stewart G S A B, Johnstone K, Hagelberg E, Ellar D J. Commitment of bacterial spores to germinate. Biochem J. 1981;198:101–106. doi: 10.1042/bj1980101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone B F. A formula for determining degree of dominance in cases of monofactorial inheritance of resistance to chemicals. Bull W H O. 1968;38:325–326. [PMC free article] [PubMed] [Google Scholar]
- 39.Strickberger M W. Genetics. 3rd ed. New York, N.Y: Macmillan; 1985. [Google Scholar]
- 40.Syed A R. Insecticides resistance in the diamondback moth in Malaysia. In: Talekar N S, Griggs T D, editors. Proceedings of the Second International Workshop. Taipei, Taiwan: Asian Vegetable Research and Development Center; 1992. pp. 437–442. [Google Scholar]
- 41.Tabashnik B E. Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol. 1994;39:47–79. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
- 42.Tabashnik B E. Delaying insect adaptation to transgenic plants: seed mixtures and refugia reconsidered. Proc R Soc Lond Ser B. 1994;255:7–12. [Google Scholar]
- 43.Tabashnik B E, Croft B A. Managing pesticide resistance in crop arthropod complexes: interactions between biological and operational factors. Environ Entomol. 1982;11:1137–1144. [Google Scholar]
- 44.Tabashnik B E, Cushing N L, Finson N, Johnson M W. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae) J Econ Entomol. 1990;83:1671–1676. [Google Scholar]
- 45.Tabashnik B E, Finson N, Johnson M W. Managing resistance to Bacillus thuringiensis: lessons from the diamondback moth (Lepidoptera: Plutellidae) J Econ Entomol. 1991;84:49–55. [Google Scholar]
- 46.Tabashnik B E, Finson N, Johnson M W, Moar W J. Resistance to toxins from Bacillus thuringiensis subsp. kurstaki causes minimal cross-resistance to B. thuringiensis subsp. aizawai diamondback moth (Lepidoptera: Plutellidae) Appl Environ Microbiol. 1993;59:1332–1335. doi: 10.1128/aem.59.5.1332-1335.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabashnik B E, Finson N, Johnson M W, Heckel D G. Cross-resistance to Bacillus thuringiensis toxin Cry1F in the diamondback moth (Plutella xylostella) Appl Environ Microbiol. 1994;60:4627–4629. doi: 10.1128/aem.60.12.4627-4629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabashnik B E, Finson N, Groeters F R, Moar W J, Johnson M W, Luo K, Adang M J. Reversal of resistance to Bacillus thuringiensis in Plutella xylostella. Proc Natl Acad Sci USA. 1994;91:4120–4124. doi: 10.1073/pnas.91.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabashnik B E, Liu Y-B, Finson N, Masson L, Heckel D G. One gene in diamondback moth confers resistance to four Bacillus thuringiensis toxins. Proc Natl Acad Sci USA. 1997;94:1640–1644. doi: 10.1073/pnas.94.5.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabashnik B E, Liu Y-B, Malvar T, Heckel D G, Masson L, Ballester V, Granero F, Ménsua J L, Ferré F. Global variation in the genetic and biochemical basis of diamondback moth resistance to Bacillus thuringiensis. Proc Natl Acad Sci USA. 1997;94:12780–12785. doi: 10.1073/pnas.94.24.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tabashnik B E, Malvar T, Liu Y-B, Finson N, Borthakur D, Shin B-S, Park S-H, Masson L, de Maagd R A, Bosch D. Cross-resistance of the diamondback moth indicates altered interactions with domain II of Bacillus thuringiensis toxins. Appl Environ Microbiol. 1996;62:2839–2844. doi: 10.1128/aem.62.8.2839-2844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabashnik B E, Schwartz J M, Finson N, Johnson M W. Inheritance of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae) J Econ Entomol. 1992;85:1046–1055. [Google Scholar]
- 53.Tang J D, Gilboa S, Roush R T, Shelton A M. Inheritance, stability, and lack-of-fitness costs of field selected resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae) from Florida. J Econ Entomol. 1997;90:732–741. [Google Scholar]
- 54.Tang J D, Shelton A M, Van Rie J, De Roeck S, Moar W J, Roush R T, Peferoen M. Toxicity of Bacillus thuringiensis spore and crystal protein to resistant diamondback moth (Plutella xylostella) Appl Environ Microbiol. 1996;62:564–569. doi: 10.1128/aem.62.2.564-569.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins. Importance of specific receptors on the brush border membrane of the midgut of target insects. Eur J Biochem. 1989;186:239–247. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- 56.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis delta-endotoxins. Appl Environ Microbiol. 1990;56:1378–1385. doi: 10.1128/aem.56.5.1378-1385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfersberger M, Luthy P, Maurer A, Parenti P, Sacchi F V, Giordana B, Hanozet G M. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae) Comp Biochem Physiol. 1987;86A:301–308. [Google Scholar]
- 58.Wright D J, Iqbal M, Granero F, Ferré J. A change in a single midgut receptor in the diamondback moth (Plutella xylostella) is only in part responsible for the field resistance to Bacillus thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai. Appl Environ Microbiol. 1997;63:1814–1819. doi: 10.1128/aem.63.5.1814-1819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]