Abstract
Background/purpose
Tongue pressure plays an important role in swallowing function. The purpose of this study is to investigate whether decreased tongue pressure is associated with dysphagia and the development of pneumonia in the elderly requiring long-term care.
Materials and methods
Tongue pressure measurement and swallowing videoendoscopic (VE) examination were performed in 60 hospitalized elderly people (33 males and 27 females, with an average age of 84.3 years) to investigate the relationship with the clinical course. Factors related with dysphagia was analyzed by Fisher's exact test and one-way ANOVA, followed by multivariate logistic regression. The relationship between each variable and survival were analyzed by cox regression.
Results
Twenty-one patients had dysphagia by VE examination. Multivariate analysis showed that smaller BMI and reduced tongue pressure were significantly correlated with dysphagia. Smaller number of remaining teeth and dysphagia were significantly related to pneumonia-related death. No patients with tongue pressure of larger than 20 kpa died by pneumonia within one year, while in those with tongue pressure of smaller than 20 kpa, one-year cumulative survival rate by pneumonia was 44.3%.
Conclusion
Decreased tongue pressure was significantly associated with dysphagia and may increase the risk of pneumonia-related death in the elderly requiring long-term care.
Keywords: Tongue pressure, Dysphagia, Pneumonia, Mortality
Introduction
Tongue pressure examination has been widely used to evaluate oral function such as swallowing or pronunciation function. Tongue pressure decreases with age, and decreases as the number of teeth or functional tooth units decreases.1,2 Some investigators have attempted to improve tongue pressure by oral rehabilitation,3, 4, 5, 6 but there is no established method for preventing reduction of tongue pressure or for strengthening tongue pressure. It is believed that lowering tongue pressure reduces swallowing function, but no studies have investigated the association between decreased tongue pressure and dysphagia in elderly people using objective instruments such as swallowing videoendoscopic (VE) or swallowing videofluoroscopic (VF) examination. Furthermore, it is unclear whether reduced tongue pressure actually increases the frequency of aspiration pneumonia and death in elderly people.
Tongue pressure examination is a simple, quick and non-invasive test. In Japan, which has entered a super-aging society, prevention of oral frailty is thought to contribute to oral feeding support and extension of healthy life expectancy. If it becomes clear that decreased tongue pressure leads to dysphagia, aspiration pneumonia, and death, it is expected that it will become more widespread as a screening test for elderly people requiring long-term care, and it is also necessary to consider actively incorporating tongue function training into the rehabilitation plan.
The purpose of this study is to investigate whether decreased tongue pressure is associated with dysphagia using VE examination and whether it affects survival rate in patients in a geriatric hospital, as a preliminary study.
Materials and methods
Patients
The subjects were 60 patients (33 males and 27 females) who received a request for VE examination of from their physicians in charge of internal medicine at Hirono Kogen Hospital from October 2018 to April 2020. The patient had a chronic phase illness rather than an acute phase illness. Patients with neuromuscular diseases, such as Parkinson's disease, spinocerebellar degeneration, amyotrophic lateral sclerosis, myasthenia gravis, muscular dystrophy, or polymyositis, were excluded from the study.
Variable
The following factors were investigated from the medical records; age, gender, medical disease, presence or absence of dementia, BMI, serum albumin level, Eastern Cooperative Oncology Group (ECOG) performance status (PS),7 consciousness status, number of remaining teeth, denture use, tongue pressure, swallowing VE examination, and prognosis. The state of consciousness was classified using the Japan Coma Scale (JCS).8 For the tongue pressure test, a tongue pressure measuring device JMS-TPM (JMS Co. Ltd, Hiroshima, Japan) (Fig. 1) was used. The probe was held by the anterior teeth, the balloon was instructed to be sandwiched between the tongue and the palate and crushed. After practice, the measurement was performed 3 times and the average value was used. Patients wearing dentures measured tongue pressure with dentures on. Dysphagia was judged by VE examination9 as follows, after consultation by dentists (YS and GO). Dysphagia: those who are judged to be unable to eat orally or only to be directly trained with jelly. No dysphagia: those judged to be able to eat orally. The prognosis was determined on August 1, 2020, and classified into “survival” and “death”. The cause of death was divided into pneumonia-related death and others (cancer, cerebrovascular accidents, myocardial infarction, etc.).
Figure 1.
Tongue pressure measuring device.
Statistical analysis
All statistical analyses were performed using SPSS software (version 26.0; Japan IBM Co., Ltd., Tokyo, Japan), and a p-value ˂0.05 was considered significant. A univariate analysis was performed by Fisher's exact test or One way ANOVA, on the relationship between the two, with dysphagia as the dependent variable and other variables as the independent variables. After that, the factors that were significant in the univariate analysis were input and the multivariate analysis was performed by logistic regression analysis. Furthermore, the association between each factor and mortality or pneumonia-related mortality was analyzed by Cox regression analysis, and the association between decreased tongue pressure and mortality was examined by the Kaplan–Meier method and log rank test.
Ethics
The study was approved by the institutional review board of Kansai Medical University Medical Center, and the research plan and guaranteed opt-out opportunity were published on the homepage of the official website of the hospital. As this was a retrospective observational study, it was not registered.
Results
The patients consisted of 33 males and 27 females, with an average age of 84.3 years. Medical diseases included cerebrovascular disease in 31 patients, dementia in 24, respiratory disease in 24, heart disease in 18, hypertension in 17, and diabetes in 15 cases (Table 1). The majority were in poor general condition. Average level of BMI is 18.9, average level of serum albumin is 2.96 g/dl, PS 0–2 is not present, PS 3 is 14 cases, PS 4 is 46 cases, consciousness level (JCS) is 1 digit in 46 cases, 2 digits in 14 cases.
Table 1.
Patient characteristics.
| Factor | Number of patients / mean±SD | |
|---|---|---|
| Age | years | 84.31±7.97 |
| Sex | male | 33 |
| female | 27 | |
| BMI | 18.9±3.85 | |
| Serum albumin | g/dL | 2.96±0.53 |
| Performance Status | PS 3 | 14 |
| PS 4 | 46 | |
| Consciousness level | 1-3 | 46 |
| 10 | 14 | |
| General disease | cerebrovascular disease | 31 |
| dementia | 24 | |
| respiratory disease | 24 | |
| heart disease | 18 | |
| hypertension | 17 | |
| diabetes | 15 | |
| digestive disease | 9 | |
| renal / urinary disease | 9 | |
| malignant neoplasm | 8 | |
| epilepsy | 8 | |
| disuse syndrome | 7 | |
| others | 9 | |
| Number of remaining teeth | 12.5±10.19 | |
| Denture use | (-) | 45 |
| (+) | 15 | |
| Tongue pressure | 11.7±9.99 | |
| VE findings | no dysphagia | 39 |
| dysphagia | 21 |
Abbreviation: BMI: body mass index, VE: swallowing videoendoscopic examination.
Twenty-one patients had dysphagia by VE examination. By univariate analysis, dysphagia was significantly associated with smaller BMI (p = 0.011) and lower tongue pressure (p = 0.019) (Table 2). Lower consciousness level tended to have more dysphagia, but there was no significant difference (p = 0.061). Multivariate analysis revealed that BMI (p = 0.020, odds ratio [OR]: 1.251, 95% confidence interval [CI]: 1.036–1.511) and tongue pressure (p = 0.029, OR: 1.099, 95% CI: 1.010–1.195) were independent risk factors for dysphagia (Table 3).
Table 2.
Factors related to dysphagia (univariate analysis).
| Variable | Dysphagia | No dysphagia | p-value | |
|---|---|---|---|---|
| Age | years | 83.9±8.04 | 84.5±8.03 | 0.772 |
| Sex | male | 13 | 20 | 0.587 |
| female | 8 | 19 | ||
| BMI | 17.3±2.90 | 19.89±4.03 | ∗0.011 | |
| Serum albumin | g/dL | 2.91±0.584 | 2.99±0.492 | 0.599 |
| Performance status | PS 3 | 3 | 11 | 0.340 |
| PS 4 | 18 | 28 | ||
| Consciousness level | 1-3 | 13 | 33 | 0.061 |
| 10 | 8 | 6 | ||
| Dementia | (-) | 12 | 24 | 0.787 |
| (+) | 9 | 15 | ||
| Number of remaining teeth | 14.1±10.9 | 11.8±9.87 | 0.419 | |
| Denture use | (-) | 18 | 27 | 0.218 |
| (+) | 3 | 12 | ||
| Tongue pressure | 7.75±6.45 | 14.0±10.9 | ∗0.019 |
∗ significant.
Abbreviation: BMI: body mass index.
Table 3.
Factors related to dysphagia (multivariate logistic regression analysis).
| Variable | Odds ratio | 95% Confidence interval | p-value |
|---|---|---|---|
| BMI | 1.251 | 1.036-1.511 | ∗0.020 |
| Tongue pressure | 1.099 | 1.010-1.195 | ∗0.029 |
∗ significant.
Abbreviation: BMI: body mass index.
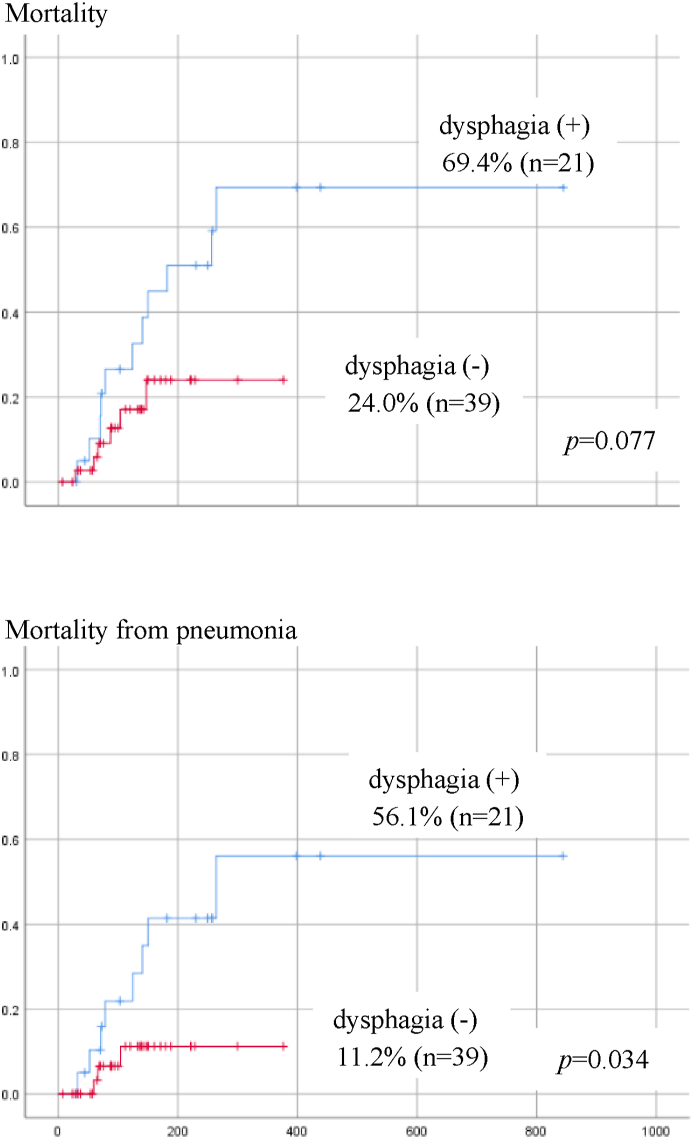
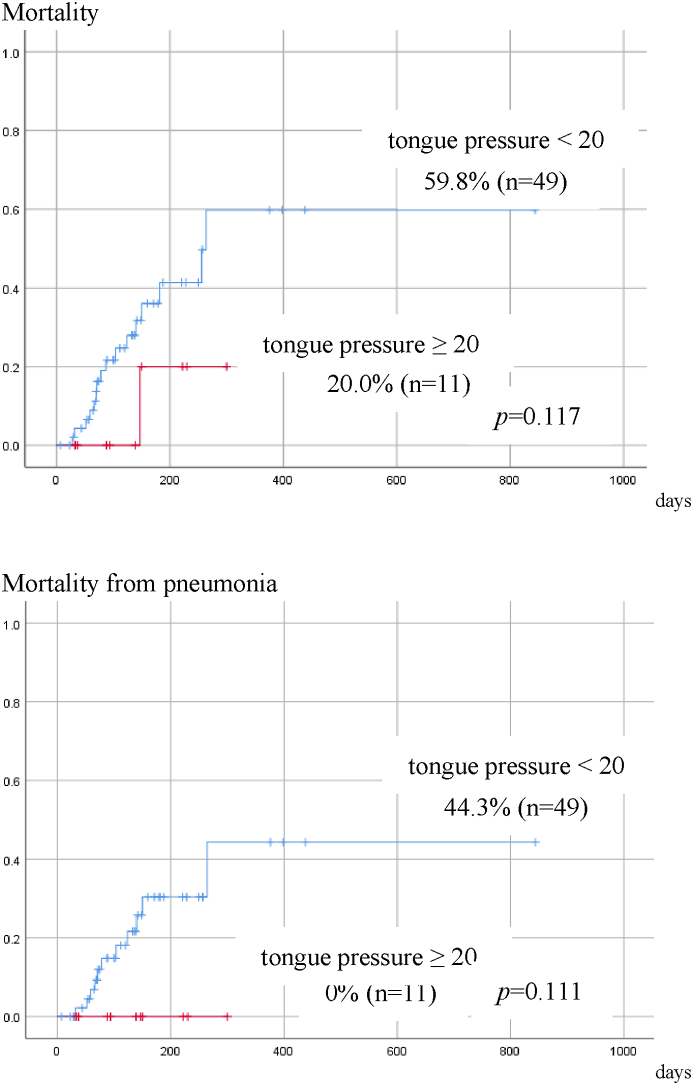
Seventeen patients died during observation, and among them 11 died of pneumonia. The causes of death in the other 6 cases were cancer in 2 cases, cerebral infarction in 2, heart failure in 1 and aortic dissection in 1. The relationship between each factor and mortality was analyzed by univariate Cox regression. There were no significant factors associated with overall mortality. However, the smaller number of remaining teeth (p = 0.045), and dysphagia (p = 0.048) were significantly related to pneumonia-related death (Table 4). Patients with dysphagia tended to have lower overall survival (p = 0.077), and had a significantly higher pneumonia-related mortality rate (p = 0.034) that those without dysphagia (Fig. 2). When the tongue pressure was divided into 2 categories, 20 kpa or more and less than 20 kpa, according to Utanohara,10 the overall mortality rate and pneumonia-related mortality were higher in patients with tongue pressure of less than 20 kpa. No patients whose tongue pressure was 20 kpa or more died by pneumonia within one year, while one-year cumulative survival rate by pneumonia was 44.3% in those with tongue pressure smaller than 20 kpa, although there was no significant difference probably due to the small number of cases (Fig. 3).
Table 4.
Factors related to death (univariate cox regression analysis).
| Variable | Hazard ratio | 95% Confidence interval | p-value | |
|---|---|---|---|---|
| i) All death (17 patients) | ||||
| Age | 1.003 | 0.937-1.073 | 0.939 | |
| Sex | male / female | 0.653 | 0.240-1.772 | 0.403 |
| BMI | 0.949 | 0.832-1.081 | 0.430 | |
| Serum albumin | 0.483 | 0.196-1.191 | 0.114 | |
| Performance status | PS 3 / PS 4 | 4.220 | 0.558-31.893 | 0.163 |
| Consciousness level | JAS 0-3 / JAS 10 | 1.079 | 0.376-3.098 | 0.888 |
| Dementia | (-) / (+) | 0.586 | 0.205-1.678 | 0.319 |
| Number of remaining teeth | 0.974 | 0.926-1.024 | 0.306 | |
| Denture use | (-) / (+) | 0.575 | 0.129-2.569 | 0.469 |
| Tongue pressure | 0.956 | 0.897-1.018 | 0.159 | |
| dysphagia | (-) / (+) | 0.410 | 0.149-1.133 | 0.086 |
| ii) Death related to pneumonia (11 patients) | ||||
| Age | 1.060 | 0.973-1.154 | 0.180 | |
| Sex | male / female | 0.824 | 0.260-2.609 | 0.742 |
| BMI | 0.917 | 0.781-1.077 | 0.292 | |
| Serum albumin | 0.409 | 0.139-1.204 | 0.551 | |
| Performance status | PS 3 / PS 4 | 28.915 | 0.071-11850.083 | 0.273 |
| Consciousness level | JAS 0-3 / JAS 10 | 1.275 | 0.378-4.293 | 0.620 |
| Dementia | (-) / (+) | 0.742 | 0.223-2.474 | 0.627 |
| Number of remaining teeth | 0.934 | 0.874-0.998 | ∗0.045 | |
| Denture use | (-) / (+) | 0.898 | 0.190-4.254 | 0.892 |
| Tongue pressure | 0.953 | 0.884-1.027 | 0.208 | |
| dysphagia | (-) / (+) | 0.258 | 0.068-0.987 | ∗0.048 |
∗ significant.
Abbreviation: BMI: body mass index.
Figure 2.
Cumulative survival rate by dysphasia (Overall survival). Patients with dysphagia have higher overall mortality and pneumonia-related mortality. There is a significant difference in mortality from pneumonia.
Figure 3.
Cumulative survival rate by tongue pressure (Survival due to pneumonia). Patients with tongue pressure of 20 kpa or higher have lower overall mortality and pneumonia-related mortality, although there is no significant difference probably due to a small number of patients.
Discussion
This study showed an association between decreased tongue pressure and dysphagia using VE examination in hospitalized elderly people, and found that decreased tongue pressure may increase the risk of pneumonia-related death.
In the process of swallowing, the tongue forms a bolus, transfers the bolus to the back tongue, is pressed against the palate from the front of the tongue, and plays an important role in pushing the bolus toward the pharynx at once. Tongue pressure examinations, which measure the tongue pressure that makes up the swallowing pressure, are now being used to evaluate oral function.11 It has been reported that the tongue pressure decreases as the age increases and the frailty of the whole body progresses.12 The subjects in the study were elderly people admitted to a geriatric hospital, who had some diseases such as dementia, cerebrovascular accident, and disuse syndrome. Most of them had low albumin, low BMI, and system ic sarcopenia, and VE examinations revealed dysphagia in 21 of 60 patients.
Decreased tongue pressure is closely associated with dysphagia because the tongue plays an important role in chewing and swallowing. It has been reported that dysphagia occurs due to decreased tongue pressure in Parkinson's patients,13,14 stroke patients,15,16 and ALS patients.17,18 Stierwalt et al. also reported that those with dysphagia secondary to neurogenic etiology (i.e., cerebrovascular accident, traumatic brain injury) showed lower tongue pressure than control people.19 There are also some reports on the association between decreased tongue pressure and dysphagia in the elderly requiring long-term care. Maeda et al. reported that sarcopenia is associated with decreased tongue pressure and dysphagia.20 Shimizu et al. described that decreased tongue pressure was associated with a significant decrease in Mann Assessment of Swallowing Ability (MASA).21 Yoshida et al. reported that measurement of tongue pressure may be useful for the bedside evaluation of swallowing,22 and Namasivayam-MacDonal et al. also stated that saliva swallow pressure measures may be helpful for early identification of dysphagia and nutritional risk.23 However, in these reports, the diagnosis of dysphagia is based on clinical judgment only. As far as we know, this is a first study examining the relationship between tongue pressure and dysphagia in elderly people requiring long-term care.by VE, which is an objective examination modality.
Decreased tongue pressure is not only associated with dysphagia as described above, but can result in the development of aspiration pneumonia and even the prognosis of life. Nakamori et al.24 reported that tongue pressure is a sensitive indicator for predicting pneumonia occurrence in acute stroke patients. Okazaki et al.25 reported that 47 elderly patients with pneumonia showed significantly lower tongue pressure than 35 without pneumonia by univariate analysis, but by multivariate analysis, tongue pressure was not correlated with onset of pneumonia. Furthermore, they stated that patients with lower ADL level, cognitive impairment, impaired modified water-swallowing test, lower maximum static inspiratory pressure, lower serum albumin, smaller BMI, and smaller somatic fat mass were more likely to die within 6 months, but tongue pressure level at baseline did not influence the survival. On the other hand, the current study is the first to show that decreased tongue pressure is associated not only with dysphagia, but also possibly with mortality caused by pneumonia. A relationship between low residual teeth and pneumonia-related mortality was also shown. This may be due to a decrease in tongue pressure as the number of remaining teeth, especially those related to occlusal function, decreases, as reported by Tashiro et al.2 This study suggests that a decrease in residual teeth may result in decreased masticatory function and decreased tongue pressure, resulting in decreased swallowing function and an increased risk of aspiration pneumonia. However, since the number of cases was small and detailed statistical analysis could not be performed, further studies are needed in the future.
Robbins et al.26 reported an increase in tongue pressure and improvement of dysphagia by intervention of training to strengthen tongue pressure by raising the tongue by isometric contraction in patients with dysphagia, and Steele et al.27 also reported improvement in tongue pressure and aspiration by tongue pressure training. On the other hand, there are reports that rehabilitation was not effective in improving swallowing function.4,5 Smaoui et al.28 stated in a systematic review of tongue pressure and training that rehabilitation methods and outcomes were disparate and meta-analysis was not possible, which suggests that rehabilitation methods for dysphagia and decreased tongue pressure have not been established.
As mentioned above, tongue pressure and swallowing function are thought to decrease as systemic sarcopenia progresses, but Chen et al.29 and Shimazaki et al.30 reported that tongue pressure did not necessarily have a significant correlation with systemic sarcopenia, and it was maintained even after systemic sarcopenia. Tashiro et al.2 investigated 745 healthy elderly people living in rural areas and found that decreased tongue pressure was significantly associated with age, decreased number of remaining teeth, decreased number of functioning teeth unit, and decreased grip. They further showed that the decrease in tongue pressure can be prevented by supplementing with a fixed prosthetic device such as a bridge or implant even if the number of remaining teeth is small by propensity score matching analysis. These studies indicate that tongue pressure tends to decrease with aging, low nutrition, and systemic sarcopenia, but the tongue muscle may resist atrophy by continuing to chew a normal diet with healthy teeth or fixed prostheses and may avoid decrease tongue pressure, even if systemic sarcopenia progresses, which suggests that dental management may be important in considering the extension of the healthy life span of the elderly.
There are several limitations in this study. First, the number of cases was small and sufficient multivariate analysis could not be performed. It is also unclear whether the results obtained using single-center patients can be generalized. However, to the best of our knowledge, this study is a first one that clarified the relationship between decreased tongue pressure and dysphagia using VE examination in elderly people requiring nursing care, and suggested that reduced tongue pressure might associated with not only dysphagia but also pneumonia-related death. We would like to conduct a more detailed study using a large number of cases in the future.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
We greatly appreciate all patients who participated in this trial. We thank Editage (www.editage.jp) for their English language editing services.
References
- 1.Satake A., Kobayashi W., Tamura Y., et al. Effects of oral environment on frailty: particular relevance of tongue pressure. Clin Interv Aging. 2019;14:1643–1648. doi: 10.2147/CIA.S212980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tashiro K., Soutome S., Funahara M., et al. The relationship between dental findings and tongue pressure: a survey of 745 community-dwelling adults and elderly persons in Japan. Gerontology. 2021;67:517–524. doi: 10.1159/000513599. [DOI] [PubMed] [Google Scholar]
- 3.Namiki C., Hara K., Tohara H., et al. Tongue-pressure resistance training improves tongue and suprahyoid muscle functions simultaneously. Clin Interv Aging. 2019;14:601–608. doi: 10.2147/CIA.S194808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakabayashi H., Matsushima M., Momosaki R., et al. The effects of resistance training of swallowing muscles on dysphagia in older people: a cluster, randomized, controlled trial. Nutrition. 2018;48:111–116. doi: 10.1016/j.nut.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Oh J.C. Effects of resistive jaw-opening exercise with elastic resistance bands on suprahyoid muscle activation and tongue strength in the elderly: a pilot study. Folia Phoniatrica Logop. 2020;28:1–8. doi: 10.1159/000509441. [DOI] [PubMed] [Google Scholar]
- 6.Park T., Kim Y. Effects of tongue pressing effortful swallow in older healthy individuals. Arch Gerontol Geriatr. 2016;66:127–133. doi: 10.1016/j.archger.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Oken M.M., Creech R.H., Tormey D.C., et al. Toxicity and response criteria of the eastern cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 8.Okada Y., Kiguchi T., Iiduka R., Ishii W., Iwami T., Koike K. Association between the Japan Coma Scale scores at the scene of injury and in-hospital outcomes in trauma patients: an analysis from the nationwide trauma database in Japan. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staff D.M., Shaker R. Videoendoscopic evaluation of supraesophageal dysphagia. Curr Gastroenterol Rep (Journal abbreviation should be written in italic letters. 2001;3:200–205. doi: 10.1007/s11894-001-0022-7. [DOI] [PubMed] [Google Scholar]
- 10.Utanohara Y., Hayashi R., Yoshikawa M., et al. Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia. 2008;23:286–290. doi: 10.1007/s00455-007-9142-z. [DOI] [PubMed] [Google Scholar]
- 11.Minakuchi S., Tsuga K., Ikebe K., et al. Oral hypofunction in the older population: position paper of the Japanese Society of Gerodontology in 2016. Gerodontology. 2018;35:317–324. doi: 10.1111/ger.12347. [DOI] [PubMed] [Google Scholar]
- 12.Machida N., Tohara H., Hara K., et al. Effects of aging and sarcopenia on tongue pressure and jaw-opening force. Geriatr Gerontol Int. 2017;17:295–301. doi: 10.1111/ggi.12715. [DOI] [PubMed] [Google Scholar]
- 13.Minagi Y., Ono T., Hori K., et al. Relationships between dysphagia and tongue pressure during swallowing in Parkinson's disease patients. J Oral Rehabil. 2018;45:459–466. doi: 10.1111/joor.12626. [DOI] [PubMed] [Google Scholar]
- 14.Fukuoka T., Ono T., Hori K., et al. Tongue pressure measurement and videofluoroscopic study of swallowing in patients with Parkinson's Disease. Dysphagia. 2019;34:80–88. doi: 10.1007/s00455-018-9916-5. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.H., Kim H.S., Yun D.H., et al. The relationship between tongue pressure and oral dysphagia in stroke patients. Ann Rehabil Med. 2016;40:620–628. doi: 10.5535/arm.2016.40.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konaka K., Kondo J., Hirota N., et al. Relationship between tongue pressure and dysphagia in stroke patients. Eur Neurol. 2010;64:101–107. doi: 10.1159/000315140. [DOI] [PubMed] [Google Scholar]
- 17.Hiraoka A., Yoshikawa M., Nakamori M., et al. Maximum tongue pressure is associated with swallowing dysfunction in ALS patients. Dysphagia. 2017;32:542–547. doi: 10.1007/s00455-017-9797-z. [DOI] [PubMed] [Google Scholar]
- 18.Easterling C., Antinoja J., Cashin S., Barkhaus P.E. Changes in tongue pressure, pulmonary function, and salivary flow in patients with amyotrophic lateral sclerosis. Dysphagia. 2013;28:217–225. doi: 10.1007/s00455-012-9436-7. [DOI] [PubMed] [Google Scholar]
- 19.Stierwalt J.A., Youmans S.R. Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16:148–156. doi: 10.1044/1058-0360(2007/019). [DOI] [PubMed] [Google Scholar]
- 20.Maeda K., Akagi J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia. 2015;30:80–87. doi: 10.1007/s00455-014-9577-y. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu A., Maeda K., Nagami S., et al. Low tongue strength is associated with oral and cough-related abnormalities in older inpatients. Nutrition. 2021;83:111062. doi: 10.1016/j.nut.2020.111062. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida M., Kikutani T., Tsuga K., Utanohara Y., Hayashi R., Akagawa Y. Decreased tongue pressure reflects symptom of dysphagia. Dysphagia. 2006;21:61–65. doi: 10.1007/s00455-005-9011-6. [DOI] [PubMed] [Google Scholar]
- 23.Namasivayam-MacDonald A.M., Morrison J.M., Steele C.M., Keller H. How swallow pressures and dysphagia affect malnutrition and mealtime outcomes in long-term care. Dysphagia. 2017;32:785–796. doi: 10.1007/s00455-017-9825-z. [DOI] [PubMed] [Google Scholar]
- 24.Nakamori M., Hosomi N., Imamura E., et al. Association between stroke lesions and videofluoroscopic findings in acute stroke patients. J Neurol. 2021;268:1025–1035. doi: 10.1007/s00415-020-10244-4. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki T., Suzukamo Y., Miyatake M., et al. Respiratory muscle weakness as a risk factor for pneumonia in older people. Gerontology. 2021:1–10. doi: 10.1159/000514007. [DOI] [PubMed] [Google Scholar]
- 26.Robbins J., Gangnon R.E., Theis S.M., Kays S.A., Hewitt A.L., Hind J.A. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53:1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 27.Steele C.M., Bailey G.L., Polacco R.E., et al. Outcomes of tongue-pressure strength and accuracy training for dysphagia following acquired brain injury. Int J Speech Lang Pathol. 2013;15:492–502. doi: 10.3109/17549507.2012.752864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smaoui S., Langridge A., Steele C.M. The effect of lingual resistance training interventions on adult swallow function: a systematic review. Dysphagia. 2020;35:745–761. doi: 10.1007/s00455-019-10066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y.C., Chen P.Y., Wang Y.C., Wang T.G., Han D.S. Decreased swallowing function in the sarcopenic elderly without clinical dysphagia: a cross-sectional study. BMC Geriatr. 2020;20:419. doi: 10.1186/s12877-020-01832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimazaki Y., Nonoyama T., Tsushita K., Arai H., Matsushita K., Uchibori N. Oral hypofunction and its association with frailty in community-dwelling older people. Geriatr Gerontol Int. 2020;20:917–926. doi: 10.1111/ggi.14015. [DOI] [PubMed] [Google Scholar]