Abstract
Background/purpose
Mechanical stress plays a vital role in osteogenic differentiation of periodontal ligament stem cells (PDLSCs). Cyclic mechanical stress may up-regulate reactive oxygen species (ROS) level. N-acetylcysteine (NAC) possesses powerful antioxidant capacity. However, it is undefined the impact of NAC on osteogenic differentiation stimulated by cyclic mechanical stress in PDLSCs. The aim of our research was to study the effect of NAC on PDLSCs during osteogenic differentiation under cyclic mechanical stress.
Materials and methods
The expression levels of osteogenesis markers were used to examine the osteogenic differentiation of PDLSCs. ROS production were measured by flow cytometry. The levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were analyzed. We also examined the changes of alveolar bone and periodontal ligament (PDL) tissues in orthodontic rats by micro-computed tomography (micro-CT) system and immunohistochemistry (IHC) staining. The nuclear factor erythroid-2-related factor-2 (Nrf2) expression was examined.
Results
NAC could enhance the osteogenic differentiation and up-regulate the GSH level as well as the ratio of GSH/GSSG, while down-regulate ROS generation and Nrf2 expression induced by cyclic mechanical stress in PDLSCs. NAC had beneficial effects on the microstructure of alveolar bone and enhanced the expression levels of osteogenesis markers, such as alkaline phosphatase (ALP) and collagen type 1 (COL1) in PDL in orthodontic rats at the tension side.
Conclusion
NAC could improve the osteogenic differentiation stimulated by cyclic mechanical stress in PDLSCs and in orthodontic rats, suggesting a potential therapeutic approach for alveolar bone remodeling in orthodontics.
Keywords: Mechanical stress, N-acetylcysteine, Orthodontic tooth movement, Periodontal ligament, Reactive oxygen species
Introduction
Orthodontic tooth movement (OTM) under the biomechanical force caused by orthodontic appliances is based on the alveolar bone remodeling,1 including bone formation and deposition by osteoblasts at the tension side and bone resorption by osteoclasts at the pressure side. Osteogenic differentiation in periodontal ligament stem cells (PDLSCs) induced by mechanical stress is the crucial process in alveolar bone remodeling at the tension side in orthodontics. However, unwanted iatrogenic sequelae, including alveolar bone loss, gingival recession and dehiscence in orthodontic patients, remain to be resolved.2,3 It is pivotal to study the relative molecular mechanism underlying osteogenic differentiation of PDLSCs under mechanical stress in orthodontics, which may contribute to the development of orthodontic therapeutic approach to cause minimum tissue damage to the patients and improve the alveolar bone remodeling in orthodontics.
Cyclic mechanical stress is a known inducer of the increased reactive oxygen species (ROS) level.4,5 ROS, as intracellular signaling molecules, can be essential for regulation of cell physiological functions, while excessive accumulation of ROS can disrupt physiological functions and cause numerous pathophysiological processes.6 Numerous studies confirmed that ROS had a critical influence on bone cell function as well as the pathophysiology of bone diseases. The heightened levels of ROS are involved in bone remodeling and disrupt bone homeostasis, causing more bone resorption caused by osteoclast differentiation while restraining osteoblastic differentiation and osteogenesis.7,8 The excessive ROS alters the process of bone remodeling contributing to an imbalance between osteoclast and osteoblast function, which can be implicated in bone diseases including osteoporosis.9 Antioxidant administration has a vital effect on protecting bone health and preventing bone loss as well as restoring the physiological bone remodeling.9
N-acetylcysteine (NAC), a greatly applied antioxidant, possesses powerful antioxidant capacity by scavenging ROS and facilitating the generation of glutathione. NAC could enhance osteoblastic phenotypic expression in osteoblastic culture and accelerate bone defect healing in the rat femoral critical size defects.10 Supplementation of NAC played a protective role in orchiectomy-induced osteoporosis through the increase of osteoblastic bone formation and inhibition of the increased ROS and osteoclastic bone resorption.11 These studies revealed the potential clinical therapy of NAC for improving bone forming and regeneration. The biological and pharmacological characteristics of NAC, including antioxidation, anti-inflammatory activity and antimicrobial activity, render it a potential therapeutic candidate in dental and oral disorders.12 However, it is undefined the therapeutic potential of NAC in orthodontic tooth movement.
The aim of our research was to study the impact of NAC on cyclic mechanical stress-induced osteogenic differentiation in PDLSCs. We further attempted to evaluate the therapeutic potential of NAC in orthodontic rats by using micro-computed tomography (micro-CT) system and immunohistochemistry (IHC) staining. We also investigated the effect of NAC on nuclear factor erythroid-2-related factor-2 (Nrf2) expression, the crucial transcription regulator regulating the expression of numerous antioxidants.
Materials and methods
The culture of PDLSCs
The present study was carried out in accordance with Declaration of Helsinki. And all experimental protocols and procedures were approved and authorized by the Medical Ethical Committee of the School of Stomatology, Shandong University (Protocol number: 20201206). The informed consents were obtained from the subjects and subjects' guardians. The method was consistent with our previous study.13 Human periodontal ligament (PDL) tissues were isolated and collected from premolars, extracted from participants, aged 12–16 years and in good physical health, for orthodontic treatment reasons at the Stomatological Hospital of Shandong University. After the extraction, the premolars were collected and placed immediately in minimum essential medium-α (α-MEM) (Biological Industries Israel Beit-Haemek Ltd., Kibbutz Beit-Haemek, Israel) containing 5% antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin, Sigma–Aldrich, St Louis, MO, USA), and washed with phosphate-buffered saline (PBS). The human PDL tissues at the middle third of the premolar root surface were scraped off, acquired and cut into small fragments (1–2 mm3) by the surgical blade, and then placed and attached to the bottom of 25 cm2 culture flask. The primary cells were cultured in α-MEM with 20% fetal bovine serum (FBS) (BI) and 1% antibiotics at 37 °C in a 5% CO2 incubator. The culture medium was replaced every 3 days. The cells were separated with 0.25% trypsin (Solarbio, Beijing, China) and passaged. PDLSCs at passages 3–6 were used in the following experiments.
The surface markers of PDLSCs
The method was consistent with our previous study.13 The detection and analysis of surface markers, including STRO-1, CD146, CD34 as well as CD45 (ProteinTech Group, Chicago, IL, USA), of the human PDLSCs at passage 6 were performed by BD Accuri™ C6 flow cytometer (BD Biosciences, Milan, Italy).
Multipotent differentiation of PDLSCs
The method was consistent with our previous study.13 PDLSCs were cultivated in the osteogenic, adipogenic or chondrogenic induction medium for three weeks. Then cells were performed by alizarin red staining (Sigma–Aldrich; Merck KGaA, Darmstadt, Germany), oil red O staining (Solarbio) and alcian blue staining (Solarbio), respectively.
The application of cyclic mechanical stress
The method was consistent with our previous study.13 PDLSCs at passages 3–6 were cultivated and then seeded in the six-well silicone Bioflex ® culture plates (Flexcell International Corporation, Hillsborough, NC, USA). After PDLSCs reached approximately 80% confluence, 10% and 0.5 Hz cyclic mechanical stress was performed for 3, 12 and 24 h using Flexercell FX-5000 Strain Unit (Flexcell International Corporation).14,15 Non-loaded PDLSCs (0 h) were cultivated and then seeded in the similar culture plates and in the same incubator without cyclic mechanical stress.
Reagent preparation
The human PDLSCs were cultured with the concentrations of NAC (Beyotime, Nantong, China) (0, 2.5, 5 mM), according to the results of the Cell Counting Kit 8 (CCK8) assay in our previous experiments and previous researches.10,13,16
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
The method was consistent with our previous study.13 The primer sequences are listed in Table 1.
Table 1.
The primer sequences used in this study. COL1, collagen type 1; RUNX2, runt-related transcription factor 2; OPN, osteopontin; Nrf2, nuclear factor erythroid-2-related factor-2; HO-1, heme oxygenase-1; NQO1, NADPH dehydrogenase quinone 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
| Genes | Primer | Sequence (5′-3′) |
|---|---|---|
| COL1 | Forward primer (5′-3′) | 5′-TTTGGATGGTGCCAAGGGAG-3′ |
| Reverse primer (5′-3′) | 5′-CACCATCATTTCCACGAGCA-3′ | |
| RUNX2 | Forward primer (5′-3′) | 5′-CACTGGCGCTGCAACAAGA-3′ |
| Reverse primer (5′-3′) | 5′-CATTCCGGAGCTCAGCAGAATA-3′ | |
| OPN | Forward primer (5′-3′) | 5′-TCTCAGAAGCAGAATCTCCTAG-3′ |
| Reverse primer (5′-3′) | 5′-TCTACATCATCAGAGTCGTTCG-3′ | |
| Nrf2 | Forward primer (5′-3′) | 5′-ATCCATTCCTGAGTTACAGTGTCTT-3′ |
| Reverse primer (5′-3′) | 5′-TGTCAGTTTGGCTTCTGGACT-3′ | |
| HO-1 | Forward primer (5′-3′) | 5′-TCTTGGCTGGCTTCCTTACC-3′ |
| Reverse primer (5′-3′) | 5′-GGATGTGCTTTTCGTTGGGG-3′ | |
| NQO1 | Forward primer (5′-3′) | 5′-CTGGTTTGAGCGAGTGTTCA-3′ |
| Reverse primer (5′-3′) | 5′-AGTACATGGAGCCACTGCCA-3′ | |
| GAPDH | Forward primer (5′-3′) | 5′-AGAAGGCTGGGGCTCATTTG-3′ |
| Reverse primer (5′-3′) | 5′-AGGGGCCATCCACAGTCTTC-3′ |
COL1, collagen type 1; RUNX2, runt-related transcription factor 2; OPN, osteopontin; Nrf2, nuclear factor erythroid-2-related factor-2; HO-1, heme oxygenase-1; NQO1, NADPH dehydrogenase quinone 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blotting
The method of western blotting was consistent with our previous study.13 These antibodies were used in our study: collagen type 1 (COL1) (14695-1-AP, ProteinTech), runt-related transcription factor 2 (RUNX2) (ab23981, Abcam, Cambridge, MA, USA), osteopontin (OPN) (22952-1-AP, ProteinTech), Nrf2 (ab137550, Abcam), heme oxygenase-1 (HO-1) (10701-1-AP, ProteinTech), NADPH dehydrogenase quinone 1 (NQO1) (11451-1-AP, ProteinTech), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sc-25778, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Lamin B1 (12987-1-AP, ProteinTech).
The measurement of alkaline phosphatase (ALP) activity
The measurement of ALP activity of each sample was carried out by using the ALP assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer's instructions. ALP staining was performed using the NBT/BCIP staining kit (Beyotime).
The intracellular ROS
The ROS levels were measured with 2',7'-dichlorofluorescin diacetate (DCFH-DA) (Beyotime). The method was consistent with our previous study.13 The cells were collected and analyzed by BD Accuri™ C6 flow cytometer (BD Biosciences).
Cellular glutathione
Glutathione levels in PDLSCs were detected with GSH and GSSG Assay Kit (Beyotime).
Experimental animals
The animal experiments were performed complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal experiments were approved and authorized by the Medical Ethical Committee of School of Stomatology, Shandong University (Protocol number: 20201205). The male Wistar rats (8-week-old) were kept in our research.
Establishing the tooth movement model
The method was consistent with our previous study.13 The orthodontic force, 25 g,17,18 was performed to move the maxillary molar mesially and the two maxillary incisors were used as the anchorage.
The rats were randomly divided into the control and NAC groups. NAC (Beyotime) was dissolved in 0.9% saline. The rats were injected intraperitoneally with the NAC solution (225 mg/kg/d).19 The control rats were treated with saline injection.
At day 7, 14 or 28, 6 rats in each group were sacrificed under the overdose of pentobarbital anaesthesia, and then fixed with 4% paraformaldehyde by cardiac perfusion. The maxilla was separated, collected as well as fixed in 4% paraformaldehyde.
Micro-CT scanning
The method was consistent with our previous study.13 The CT images of the specimens were exported into Mimics software (Materialise, Leuven, Belgium) to create the three-dimensional (3D) images.
Evaluating the microstructural parameters
We selected the alveolar bone around distal to the cervial third of mesiobuccal root of the maxillary first molar as the region of interest (ROI) for the following analysis.13,18,20,21 We measured the microstructure of alveolar bone, including bone volume/total volume (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular separation (Tb.Sp, μm) as well as microstructure model index (SMI), using the CT Analyser software (Version 1.17.7.2, Bruker, Kontich, Belgium).
Immunohistochemistry (IHC) examinations
IHC staining was made by using the SPlink Detection kit (catalog no. SP9000, ZSGB-BIO, Beijing, China). The following antibodies were used in this study: ALP (1:400, 11187-1-AP, ProteinTech) and COL1 (1:1000, 14695-1-AP, ProteinTech) at 4 °C overnight. We selected the PDL around distal to the cervial third of mesiobuccal root of the maxillary first molar to evaluate the expression levels of osteogenesis markers ALP and COL1 by IHC staining. The slices were observed by the microscope (Olympus, Tokyo, Japan), and the images were captured and saved by the imaging software (Olympus cellSens Standard 1.17). Integrated optical density (IOD) of the protein expression, including ALP and COL1, in the PDL around distal to the cervial third of mesiobuccal root of the maxillary first molar was analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
Data were expressed as mean ± standard deviation (SD). One-way ANOVA was used to analyze the variances between more than two experimental groups and control group. Student's t test was used to analyze the differences between two groups with the GraphPad Prism software (MacKiev Software, Boston, MA, USA).
Results
The culture and identification of PDLSCs
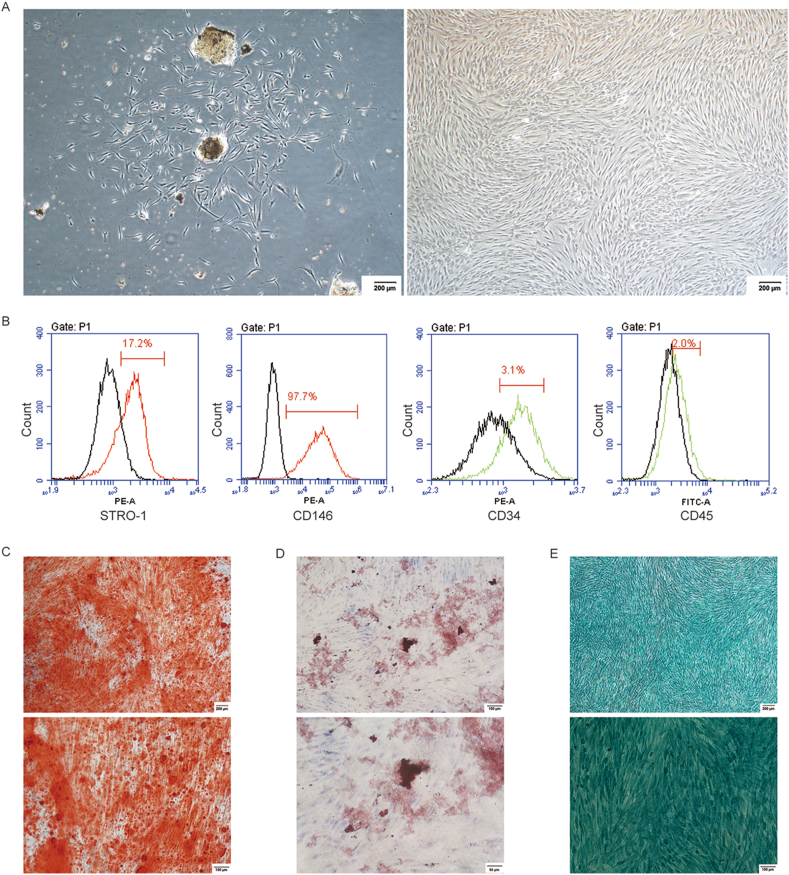
Cultured human PDLSCs were derived from PDL tissues, presenting the fusiform morphology (Fig. 1A). According to flow cytometric analyses, PDLSCs were positive for STRO-1 and CD146, the mesenchymal stem cell (MSC)-specific markers, but were negative for CD34 as well as CD45, the hematopoietic and endothelial cell-specific markers (Fig. 1B). And PDLSCs possessed multipotentiality properties, as evidenced by the formation of alizarin red nodules (Fig. 1C), lipid droplets (Fig. 1D) and alcian blue staining (Fig. 1E).
Figure 1.
The culture and identification of PDLSCs. (A) The human PDLSCs were successfully derived from PDL tissues (left), and cultured at passage five (right), presenting the fusiform morphology (the scale bar represented 200 μm). (B) According to flow cytometric analysis, PDLSCs were positively expressed STRO-1 and CD146, but negatively expressed CD34 as well as CD45. (C) Alizarin red staining (the scale bar represented 100 and 200 μm). (D) Oil red O staining (the scale bar represented 50 and 100 μm). (E) Alcian blue staining (the scale bar represented 100 and 200 μm).
The cyclic mechanical stress-induced osteogenic differentiation in PDLSCs
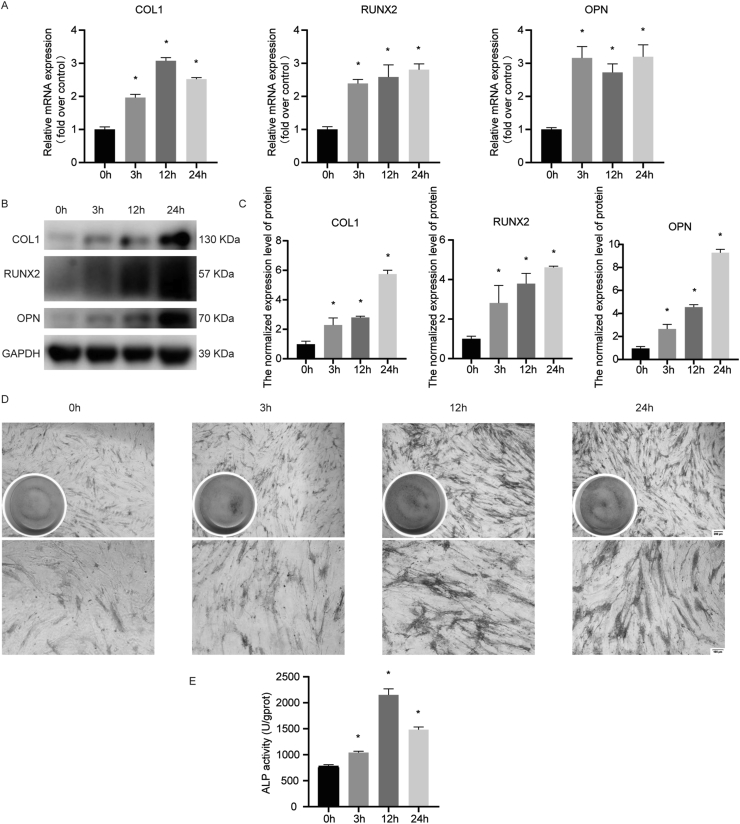
To study the influence of cyclic mechanical stress on osteogenic differentiation in PDLSCs, we examined the expression levels of osteogenic relative markers, including COL1, RUNX2, OPN as well as ALP in response to 10% and 0.5 Hz cyclic mechanical stress for 3 h, 12 h, and 24 h. These results revealed that cyclic mechanical stress increased the osteogenic differentiation tendency in PDLSCs (Fig. 2).
Figure 2.
The osteogenic differentiation stimulated by cyclic mechanical stress in human PDLSCs. (A) The mRNA levels assay of COL1, RUNX2 and OPN were performed using real-time PCR. (B, C) The protein levels assay of COL1, RUNX2 and OPN were performed. (D) The ALP staining was carried out (the scale bar represented 100 and 200 μm). (E) The ALP activity assay was carried out. ∗P < 0.05 versus 0 h.
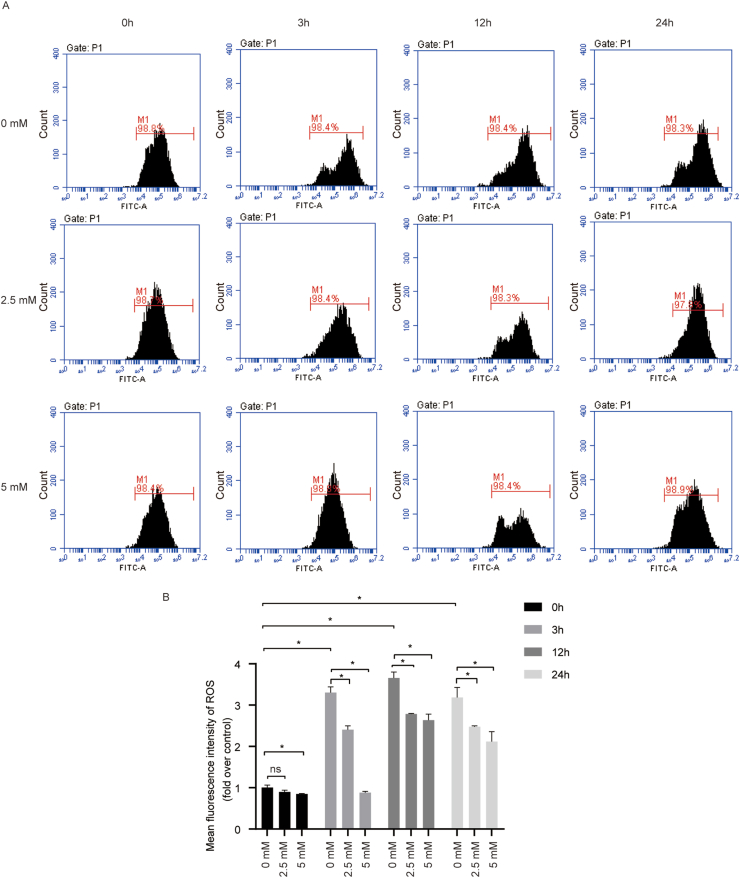
NAC inhibited cyclic mechanical stress-induced ROS generation in PDLSCs
The higher fluorescence intensity of ROS level was observed at 3 h, compared with the control, and the increase was maintained throughout the duration of cyclic stretch. NAC treatment obviously restrained the cyclic mechanical stress-induced ROS generation (Fig. 3). These data demonstrated that cyclic mechanical stress enhanced intracellular ROS production, while NAC treatment inhibited it.
Figure 3.
NAC inhibited cyclic mechanical stress-induced ROS generation in PDLSCs. (A) ROS levels stimulated by cyclic mechanical stress in PDLSCs were measured using DCFH-DA probe by flow cytometry. (B) The quantitative analysis of ROS in PDLSCs based on the flow cytometry results. ∗P < 0.05 versus the control group, respectively; ns, not significant.
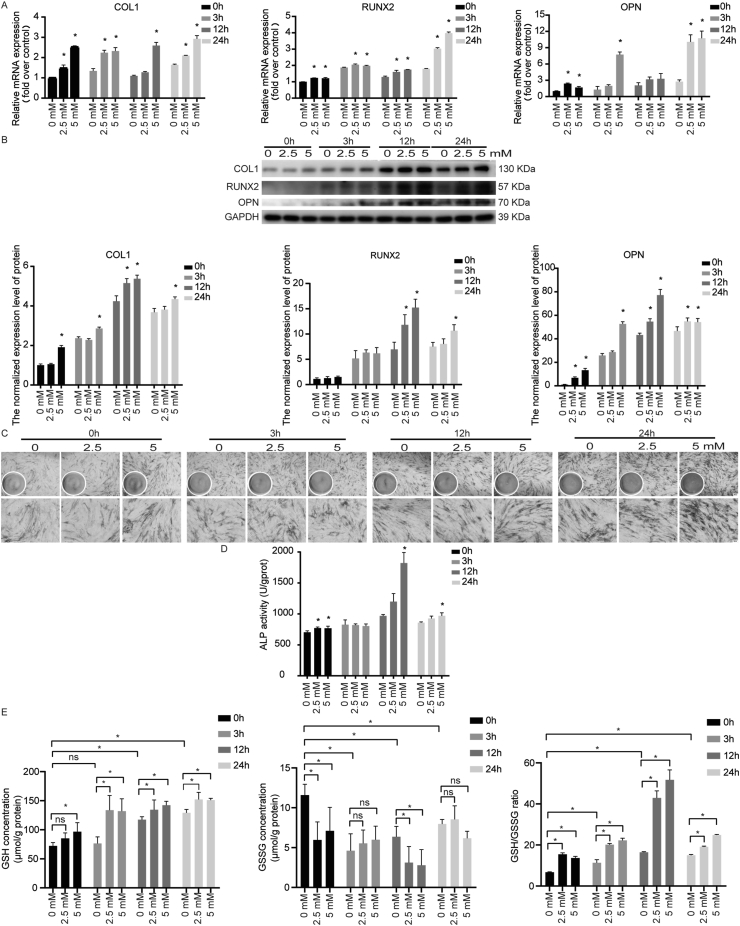
The effect of antioxidant NAC on osteogenic differentiation stimulated by cyclic mechanical stress in PDLSCs
Compared with the controls, the mRNA and protein expression levels of COL1, RUNX2 and OPN were increased with NAC (Fig. 4A and B). And the ALP activity was increased in PDLSCs with NAC treatment under cyclic mechanical stress (Fig. 4C and D). These results revealed that NAC could promote osteogenic differentiation tendency induced by cyclic mechanical stress in PDLSCs.
Figure 4.
NAC promoted osteogenic differentiation and increased the reduced glutathione in PDLSCs stimulated by cyclic mechanical stress. (A) The mRNA levels assay of COL1, RUNX2 and OPN with NAC were performed using real-time PCR. (B) The protein levels assay of COL1, RUNX2 and OPN with NAC were performed. (C, D) The ALP enzyme activity (ALP staining as well as ALP activity tests) increased with NAC in PDLSCs (the scale bar represented 100 and 200 μm). (E) The up-regulated level of reduced glutathione (GSH) and the GSH/oxidized glutathione (GSSG) ratio in PDLSCs with NAC were observed under cyclic mechanical stretch. ∗P < 0.05 versus the control group, respectively; ns, not significant.
NAC increased glutathione concentration in PDLSCs under cyclic mechanical stress
NAC could facilitate the glutathione synthesis, the most abundant antioxidant thiol in cells. The levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were analyzed. Along with the up-regulated ROS generation induced by cyclic mechanical stress, the enhanced level of GSH as well as the GSH/GSSG ratio were observed (Fig. 4E).
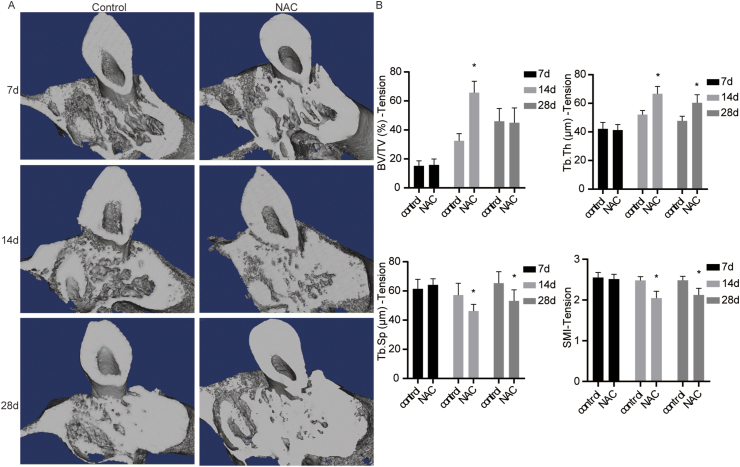
The impact of NAC on the alveolar bone in rats under orthodontic treatment at the tension side
The micro-CT analysis indicated the up-regulation of BV/TV in the NAC group at day 14, compared with the controls. Similarly, Tb.Th was increased at day 14 and day 28 in the NAC group. Additionally, Tb.Sp and SMI were decreased at day 14 and day 28 in the NAC group than that in the control group (Fig. 5).
Figure 5.
The impact of NAC on the alveolar bone at the tension side in rats under orthodontic treatment. (A) The 3D micro-CT images in coronal views of the maxillary first molar. (B) The analysis of BV/TV, Tb.Th, Tb.Sp and SMI by the CT Analyser software (n = 5 specimens/group). ∗P < 0.05 versus the control group, respectively.
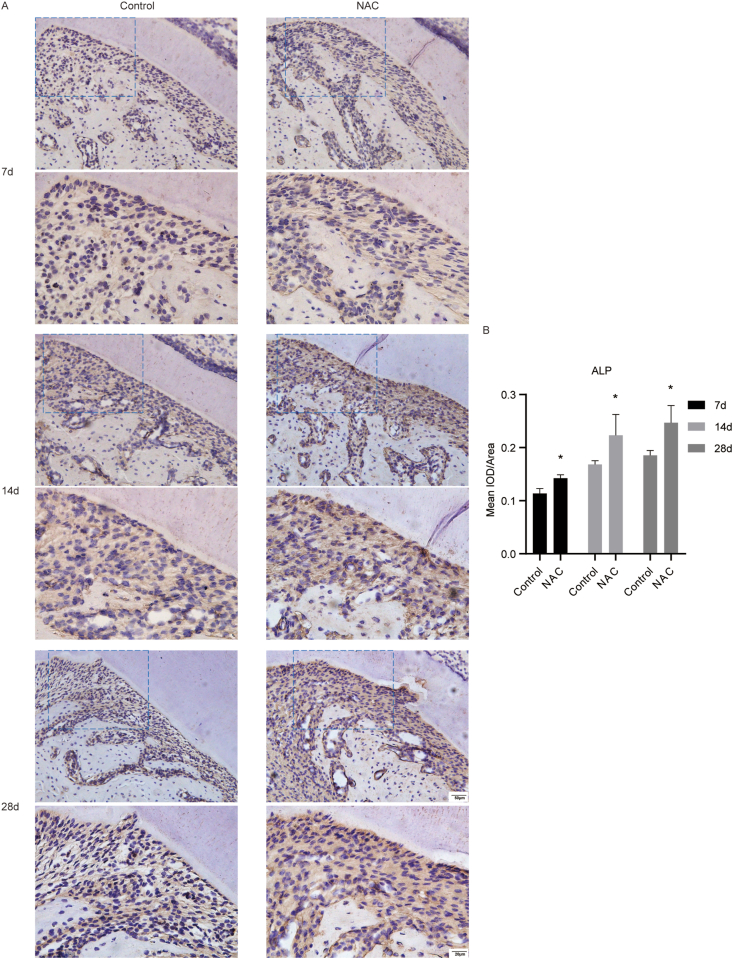
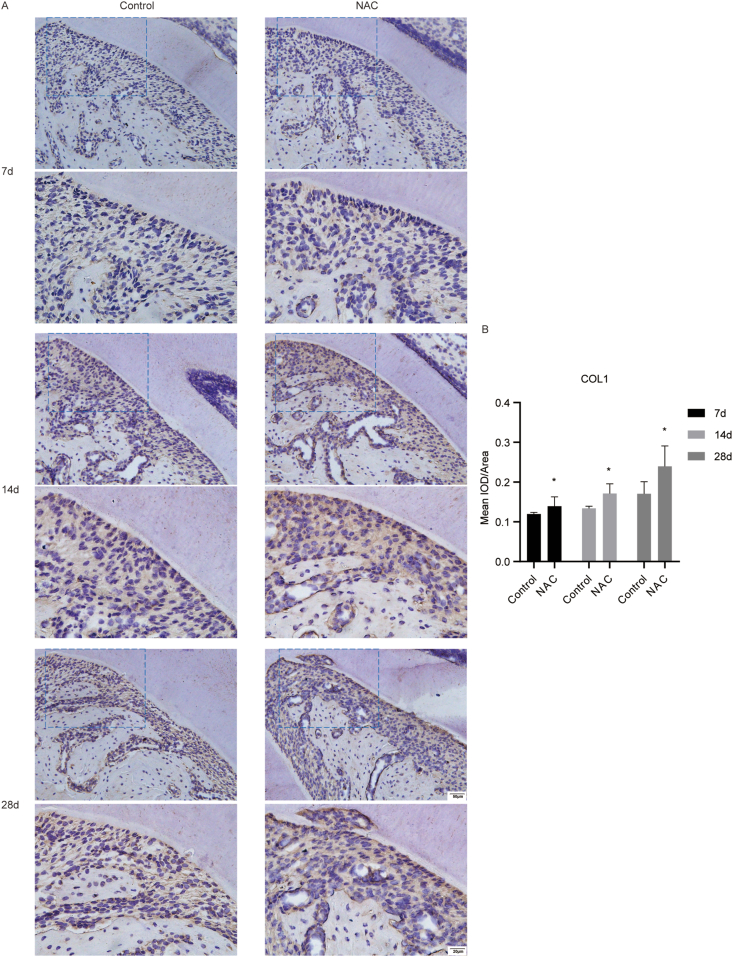
The effect of NAC on the expression levels of osteogenesis markers ALP and COL1 of PDL at the tension side in orthodontic rats
We evaluated the expression levels of osteogenesis markers ALP and COL1 in the PDL tissue around distal to the cervial third of mesiobuccal root of the maxillary first molar. The results of IHC staining showed that the expression levels of ALP and COL1 in the PDL tissue around distal to the cervial third of mesiobuccal root of the maxillary first molar were increased in the NAC group, compared with the controls (Fig. 6 and Fig. 7). The results revealed that NAC could promote the protein expression levels of osteogenesis markers in rats under orthodontic treatment at the tension side.
Figure 6.
The impact of NAC on the expression levels of ALP in PDL at the tension side in rats under orthodontic treatment. (A) Representative IHC images of ALP in the PDL tissue around distal to the cervial third of mesiobuccal root of the maxillary first molar (the scale bar represented 20 and 50 μm). (B) Quantitative analysis of mean integrated optical density (IOD)/Area for ALP in the PDL tissue around distal to the cervial third of mesiobuccal root of the maxillary first molar by Image-Pro Plus 6.0 software. N = 3 specimens/group. ∗P < 0.05 versus the control group, respectively.
Figure 7.
The impact of NAC on the expression levels of COL1 in PDL at the tension side in rats under orthodontic treatment. (A) Representative IHC images of COL1 in the PDL tissue around distal to the cervial third of mesiobuccal root of the maxillary first molar (the scale bar represented 20 and 50 μm). (B) Quantitative analysis of mean integrated optical density (IOD)/Area for COL1 in the PDL tissue around distal to the cervial third of mesiobuccal root of the maxillary first molar by Image-Pro Plus 6.0 software. N = 3 specimens/group. ∗P < 0.05 versus the control group, respectively.
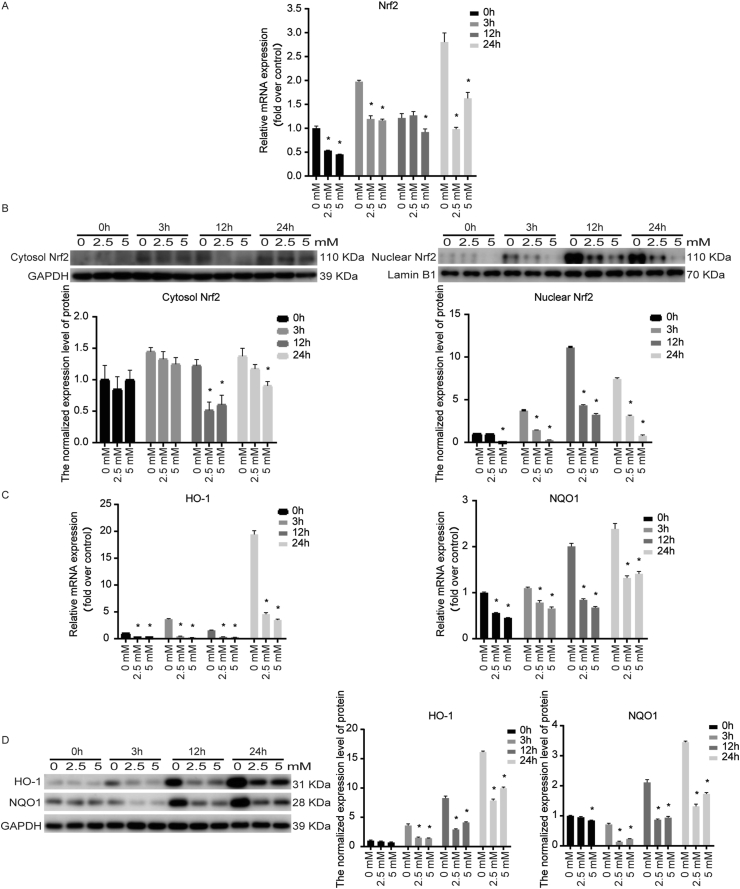
NAC down-regulated the Nrf2 expression level in PDLSCs stimulated by cyclic mechanical stress
Nrf2 participated in modulating the expression of many protective antioxidants, including HO-1 and NQO1. Nrf2 mRNA and the cytosolic and nuclear Nrf2 protein expression were down-regulated with NAC under cyclic mechanical stress (Fig. 8A and B). We also observed that NAC significantly inhibited the mRNA and protein expression level of HO-1 and NQO1 (Fig. 8C and D).
Figure 8.
NAC down-regulated the Nrf2 expression level in PDLSCs stimulated by cyclic mechanical stress. (A) Nrf2 mRNA were down-regulated with NAC under cyclic mechanical stress. (B) The cytosolic and nuclear Nrf2 protein levels were decreased with NAC. (C) NAC significantly inhibited the mRNA expression of HO-1 and NQO1. (D) NAC significantly inhibited the protein expression of HO-1 and NQO1. ∗P < 0.05 versus the control group, respectively.
Discussion
Many studies revealed that NAC might be a potential therapeutic candidate in dental and oral disorders, owing to the characteristics of NAC, including antioxidation, anti-inflammatory activity and antimicrobial activity.12 However, it is undefined the therapeutic potential of NAC in orthodontic tooth movement. To avoid the undesirable side-effects, such as alveolar bone loss, gingival recession and dehiscence, in orthodontics, many orthodontists are extremely interested in studying the molecular mechanism in osteogenesis under mechanical stress. Therefore, it is valuable and pivotal to study the impact of NAC on osteogenic differentiation of PDLSCs under cyclic mechanical stress, which may contribute to the development of effective and promising orthodontic therapeutic approach to improve the alveolar bone remodeling and reduce the undesirable side-effects in orthodontics.
Our study indicated that 10% and 0.5 Hz cyclic mechanical stress enhanced ROS generation, and promoted osteogenic differentiation in PDLSCs. NAC treatment restrained the ROS generation and the expression of Nrf2 in response to cyclic mechanical stress, while reinforced the GSH synthesis and enhanced osteogenic differentiation.
Cyclic mechanical stress of 10% and 0.5 Hz was performed in our research, as it was confirmed to be physiological and beneficial for osteogenic differentiation, according to previous researches.13,14,22 And 10% and 0.5 Hz cyclic mechanical stress was adopted in the vitro model of orthodontic tooth movement.23 Our data revealed that cyclic mechanical stress induced the ROS generation and promoted osteogenic differentiation in PDLSCs.
The persistent and excessive production of ROS could alter bone remodeling process leading to an imbalance between osteoclast and osteoblast activities, which might be related to the pathogenesis of osteoporosis.24 NAC, a powerful exogenous antioxidant, is greatly applied for treatment of various diseases, such as chronic bronchitis, ulcerative colitis, and Alzheimer.25 Preincubation of bone marrow-derived mesenchymal stem cells (BMSCs) with NAC was reported to reinforce the mineralization of newly formed bone tissue after MSC transplantation.26 Previous studies showed that NAC-pretreated osteoblast-like cells yielded nearly complete bone defect healing with significantly matured mineralized structure after autologous local transplantation in the rat femur critical-size defect.27 Consistent with the previous researches, our data showed that NAC treatment promoted the expression levels of osteogenesis markers, including ALP, COL1, RUNX2 as well as OPN in PDLSCs under cyclic mechanical stress. And the microarchitectural parameters, including BV/TV, Tb.Th, Tb.Sp as well as SMI, and the expressions of osteogenesis markers ALP and COL1 in PDL at the tension side in orthodontic rats in NAC groups were superior to those in the control groups. NAC might be a promising strategy for promoting osteogenic differentiation at the tension side in orthodontics.
As the continuous and sustained overproduction of ROS can oxidize macromolecules, including DNA, proteins and lipids.28 Glutathione, an intracellular major non-enzymatic antioxidant with low-molecular-mass, could serve as a reducing agent and neutralize ROS by binding to electrophiles.29 Exogenous glutathione is unabsorbable outside the cell.27 NAC, an amino acid derivative with l-cysteine, has a sulfhydryl group acting as a ROS scavenger and exhibiting the antioxidant capability.30 In addition, as the glutathione precursor, NAC facilitates the generation of glutathione to buffer against ROS.31 In this research, we evaluated the impact of NAC on osteogenic differentiation tendency in PDLSCs in response to cyclic mechanical stress, and found that NAC enhanced the GSH level and the GSH/GSSG ratio in PDLSCs. NAC might promote osteogenic activity through increasing glutathione synthesis.32 Glutathione has a vital role in osteoclast and osteoblast differentiation as well as the pathophysiology of various bone diseases.33 The GSH/GSSG ratio was reported to have a pivotal role in osteogenic differentiation. NAC treatment enhanced ALP activity during osteogenic differentiation, which was related to the increased GSH/GSSG ratio in NAC-treated cells.34 Our results presented that NAC inhibited the elevation of ROS production and enhanced the GSH level and the GSH/GSSG ratio in PDLSCs, which might be related to the promotion of osteogenic differentiation induced by cyclic mechanical stress in PDLSCs. The detailed mechanisms remain to be further studied.
The limitation of this study was that the ROS generation in PDL at the tension side in rats under orthodontic treatment was not evaluated. The previous studies showed that the malondialdehyde (MDA) level, the oxidative stress biomarker, was enhanced in rats during orthodontic tooth movement.35 And combined with the results of the ROS generation in vitro in our research, we speculated that the ROS generation in PDL at the tension side in rats under orthodontic treatment might be increased, while NAC, the greatly applied antioxidant, could restrain it. The ROS generation in PDL and the detailed mechanisms of NAC in the alveolar bone remodeling in orthodontic treatment require further in-depth evaluation and verification owing to the complex environment in vivo.
Taken together, NAC could enhance the osteogenic differentiation induced by cyclic mechanical stress in PDLSCs and favor the changes of alveolar bone and the expression levels of osteogenesis markers at the tension side in orthodontic rats, suggesting a potential and promising therapeutic approach for the alveolar bone remodeling in orthodontics.
Declaration of competing interest
The authors declare that no financial or other potential conflicts of interest exists with regard to this research.
Acknowledgments
This research was supported by the grants from Graduate education outstanding achievement training program of Shandong University (No. ZY20190004) and Clinical Research Center of Shandong University (No. 2020SDUCRCA005).
References
- 1.Asiry M.A. Biological aspects of orthodontic tooth movement: a review of literature. Saudi J Biol Sci. 2018;25:1027–1032. doi: 10.1016/j.sjbs.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn H., Moon S.C., Baek S. Morphometric evaluation of changes in the alveolar bone and roots of the maxillary anterior teeth before and after en masse retraction using cone-beam computed tomography. Angle Orthod. 2013;83:212–221. doi: 10.2319/041812-325.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Q.Y., Zhang S.J., Liu H., et al. Three-dimensional evaluation of upper anterior alveolar bone dehiscence after incisor retraction and intrusion in adult patients with bimaxillary protrusion malocclusion. J Zhejiang Univ–Sci B. 2011;12:990–997. doi: 10.1631/jzus.B1100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangwar R., Meena A.S., Shukla P.K., et al. Calcium-mediated oxidative stress: a common mechanism in tight junction disruption by different types of cellular stress. Biochem J. 2017;474:731–749. doi: 10.1042/BCJ20160679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song J., Wang Y.Q., Yuan X., et al. Stretching magnitude-dependent inactivation of AKT by ROS led to enhanced p53 mitochondrial translocation and myoblast apoptosis. Mol Biol Cell. 2019;30:1182–1197. doi: 10.1091/mbc.E18-12-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zemskov E.A., Lu Q., Ornatowski W., et al. Biomechanical forces and oxidative stress: implications for pulmonary vascular disease. Antioxidants Redox Signal. 2019;31:819–842. doi: 10.1089/ars.2018.7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agidigbi T.S., Kim C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int J Mol Sci. 2019;20:3576. doi: 10.3390/ijms20143576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin C.H., Li N.T., Cheng H.S., Yen M.L. Oxidative stress induces imbalance of adipogenic/osteoblastic lineage commitment in mesenchymal stem cells through decreasing SIRT1 functions. J Cell Mol Med. 2018;22:786–796. doi: 10.1111/jcmm.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheweita S.A., Al Samghan A.S., Khoshhal O.K. Osteoporosis in children: possible risk factors and role of antioxidants. J Musculoskelet Surg Res. 2019;3:319–325. [Google Scholar]
- 10.Yamada M., Tsukimura N., Ikeda T., et al. N-acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials. 2013;34:6147–6156. doi: 10.1016/j.biomaterials.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 11.Chen L.L., Wang G.T., Wang Q.J., Liu Q., Sun Q. N-acetylcysteine prevents orchiectomy-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Am J Transl Res. 2019;11:4337–4347. [PMC free article] [PubMed] [Google Scholar]
- 12.Pei Y.P., Liu H., Yang Y., et al. Biological activities and potential oral applications of N-acetylcysteine: progress and prospects. Oxid Med Cell Longev. 2018:2835787. doi: 10.1155/2018/2835787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi X., Zhao Y., Liu H., Li Z.X., Chen S., Liu D.X. Nrf2 activation is involved in osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. Exp Cell Res. 2021;403:112598. doi: 10.1016/j.yexcr.2021.112598. [DOI] [PubMed] [Google Scholar]
- 14.Ren D.P., Wei F.L., Hu L.H., Yang S.Y., Wang C.L., Yuan X. Phosphorylation of Runx2, induced by cyclic mechanical tension via ERK1/2 pathway, contributes to osteodifferentiation of human periodontal ligament fibroblasts. J Cell Physiol. 2015;230:2426–2436. doi: 10.1002/jcp.24972. [DOI] [PubMed] [Google Scholar]
- 15.Yang S.Y., Wei F.L., Hu L.H., Wang C.L. PERK-eIF2α-ATF4 pathway mediated by endoplasmic reticulum stress response is involved in osteodifferentiation of human periodontal ligament cells under cyclic mechanical force. Cell Signal. 2016;28:880–886. doi: 10.1016/j.cellsig.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Yan G.Q., Guo Y., Guo J.W., Wang Q., Wang C.Y., Wang X. N-acetylcysteine attenuates lipopolysaccharide-induced osteolysis by restoring bone remodeling balance via reduction of reactive oxygen species formation during osteoclastogenesis. Inflammation. 2020;43:1279–1292. doi: 10.1007/s10753-020-01207-y. [DOI] [PubMed] [Google Scholar]
- 17.Motoji H., Masahiro T., Hidaka K., Masato M. Vitamin C and eggshell membrane facilitate orthodontic tooth movement and induce histological changes in the periodontal tissue. J Oral Biosci. 2020;62:80–87. doi: 10.1016/j.job.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 18.An J.T., Li Y., Liu Z.S., Wang R., Zhang B. A micro-CT study of microstructure change of alveolar bone during orthodontic tooth movement under different force magnitudes in rats. Exp Ther Med. 2017;13:1793–1798. doi: 10.3892/etm.2017.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurulain S.M., Ojha S., Tekes K., Shafiullah M., Kalasz H., Adem A. Efficacy of N-acetylcysteine, glutathione, and ascorbic acid in acute toxicity of paraoxon to wistar rats: survival study. Oxid Med Cell Longev. 2015:329306. doi: 10.1155/2015/329306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki S.S., Garcez A.S., Suzuki H., Ervolino E., Moon W., Ribeiro M.S. Low-level laser therapy stimulates bone metabolism and inhibits root resorption during tooth movement in a rodent model. J Biophot. 2016;9:1222–1235. doi: 10.1002/jbio.201600016. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S.S., Garcez A.S., Reese P.O., Suzuki H., Ribeiro M.S., Moon W. Effects of corticopuncture (CP) and low-level laser therapy (LLLT) on the rate of tooth movement and root resorption in rats using micro-CT evaluation. Laser Med Sci. 2017;33:811–821. doi: 10.1007/s10103-017-2421-5. [DOI] [PubMed] [Google Scholar]
- 22.Tantilertanant Y., Niyompanich J., Everts V., Supaphol P., Pavasant P., Sanchavanakit N. Cyclic tensile force stimulates BMP9 synthesis and in vitro mineralization by human periodontal ligament cells. J Cell Physiol. 2019;234:4528–4539. doi: 10.1002/jcp.27257. [DOI] [PubMed] [Google Scholar]
- 23.Xu H.Y., Nie E.M., Deng G., et al. Periostin is essential for periodontal ligament remodeling during orthodontic treatment. Mol Med Rep. 2017;15:1800–1806. doi: 10.3892/mmr.2017.6200. [DOI] [PubMed] [Google Scholar]
- 24.Manolagas S.C. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokhtari V., Afsharian P., Shahhoseini M., Kalantar S.M., Moini A. A review on various uses of N-acetyl cysteine. Cell J. 2017;19:11–17. doi: 10.22074/cellj.2016.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe J., Yamada M., Niibe K., et al. Preconditioning of bone marrow-derived mesenchymal stem cells with N-acetyl-L-cysteine enhances bone regeneration via reinforced resistance to oxidative stress. Biomaterials. 2018;185:25–38. doi: 10.1016/j.biomaterials.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 27.Yamada M., Watanabe J., Ueno T., Ogawa T., Egusa H. Cytoprotective preconditioning of osteoblast-like cells with N-acetyl-L-cysteine for bone regeneration in cell therapy. Int J Mol Sci. 2019;20:5199. doi: 10.3390/ijms20205199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 29.Siauciunaite R., Foulkes N.S., Calabrò V., Vallone D. Evolution shapes the gene expression response to oxidative stress. Int J Mol Sci. 2019;20:3040. doi: 10.3390/ijms20123040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gould R.L., Pazdro R. Impact of supplementary amino acids, micronutrients, and overall diet on glutathione homeostasis. Nutrients. 2019;11:1056. doi: 10.3390/nu11051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zafarullah M., Li W.Q., Sylvester J., Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jun J.H., Lee S.H., Kwak H.B., et al. N-acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. J Cell Biochem. 2008;103:1246–1255. doi: 10.1002/jcb.21508. [DOI] [PubMed] [Google Scholar]
- 33.Domazetovic V., Marcucci G., Iantomasi T., Brandi M.L., Vincenzini M.T. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. 2017;14:209–216. doi: 10.11138/ccmbm/2017.14.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romagnoli C., Marcucci G., Favilli F., et al. Role of GSH/GSSG redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like SaOS-2 cells. FEBS J. 2013;280:867–879. doi: 10.1111/febs.12075. [DOI] [PubMed] [Google Scholar]
- 35.Vicente A., Bravo-Gonzalez L.A., Navarro J.A., Buendia A.J., Camacho-Alonso F. Effects of diabetes on oxidative stress, periodontal ligament fiber orientation, and matrix metalloproteinase 8 and 9 expressions during orthodontic tooth movement. Clin Oral Invest. 2021;25:1383–1394. doi: 10.1007/s00784-020-03446-7. [DOI] [PubMed] [Google Scholar]