Abstract
Background/purpose
Oral submucous fibrosis (OSF) has been regarded as a premalignant disorder of oral cancer, and myofibroblasts are the main cells that are responsible for pathological fibrosis. Hence, elucidation of the molecular mechanism underlying myofibroblast activation is important to treat OSF. MicroRNA-21 (miR-21) is a well-known fibrosis non-coding RNA, and its role in the development of OSF remains largely unclear.
Materials and methods
Luciferase reporter assay was used to confirm the direct interaction between miR-21 and its target programmed cell death 4 (PDCD4). The expression level of PDCD4 in OSF was examined by qRT-PCR. Myofibroblast activities were assessed by collagen gel contraction and transwell migration assays.
Results
Our result validated the direct binding of miR-21 to PDCD4. We showed the expression of PDCD4 was downregulated in OSF specimens and negatively correlated with miR-21. Our results suggested that overexpression of PDCD4 in fibrotic buccal mucosal fibroblasts (fBMFs) mitigated the myofibroblast activities, including collagen gel contractility and migration capacity. Moreover, we showed miR-21 contributed to myofibroblast activation of BMFs through repression of PDCD4.
Conclusion
Our results suggest that the miR-21/PDCD4 axis mediates the myofibroblast activation of BMFs, and targeting this axis may exert an anti-fibrosis effect.
Keywords: MicroRNA-21, Myofibroblast, Oral submucous fibrosis, Programmed cell death 4
Introduction
Oral submucous fibrosis (OSF) is a progressive fibrosis and inflammatory disease affecting any part of the oral cavity. Patients with OSF experience burning sensation, pain, and ulceration. Moreover, the gradual deposition of extracellular matrix (ECM) components in the buccal mucosa often leads to the difficulty of mouth opening. Additionally, OSF has been recognized as a premalignant disease, which may transform into oral cancer.1 Various studies have focused on the pathogenesis of OSF in order to prevent it from malignant transformation. It has been shown that the habit of areca nut consumption is a major etiological factor,2 and the activation of transforming growth factor-beta (TGF-β) pathway by areca nut constituents has been known to implicate in the development of OSF.3 Like other fibrosis diseases, the elevation of TGF-β signaling has been proven to induce myofibroblasts transdifferentiation from oral fibroblasts.3 Our previous work has shown that TGF-β activation mediated the areca nut-associated myofibroblast characteristics via upregulation of microRNA-21 (miR-21).4
MiRs are non-coding RNAs that are not translated into proteins, these short RNA transcripts of approximately 22 nucleotides have been found to modulate target genes through recognizing the 3′-untranslated region (UTR) of its target mRNAs.5 MiR-21 has been shown to participate in the development of various types of fibrosis diseases and can be used to discriminate oral tongue cancer from normal subjects.6 Our previous study has found that miR-21 is overexpressed in OSF tissues and the upregulation of miR-21 can be induced by arecoline, a natural alkaloid in areca nut, in buccal mucosal fibroblasts (BMFs).4 Here, we sought to examine the downstream factor of miR-21 that mediates the myofibroblast transdifferentiation in response to areca nut stimulation. It was reported that miR-21 post-transcriptionally inhibited the tumor suppressor programmed cell death 4 (PDCD4) in colorectal cancer.7 Also, downregulation of PDCD4 by miR-21 has been shown to increases tumor aggressiveness in oral cancer.8 As such, we aimed to investigate whether the miR-21/PDCD4 mediated the progression of premalignant OSF as well.
In the current study, we examined the relationship between miR-21 and PDCD4 and the expression level of PDCD4 in OSF tissues. Moreover, the functional role of PDCD4 in myofibroblast activation was investigated. Besides, we assessed if miR-21 modulated the myofibroblast transdifferentiation via repression of PDCD4. Our results will demonstrate how the miR-21/PDCD4 axis contributes to the development of OSF.
Materials and methods
OSF tissues sample collections and primary cell culture
All subjects were followed the tenets of the Declaration of Helsinki and were reviewed by the Institutional Review Board of Human Subjects Research Ethics Committee at Chung Shan Medical University, Taichung, Taiwan, and obtain the informed written consent from each individual. Primary cultures including BMFs and fBMFs were cultivated as previously described and the third and eighth passages were used in OSF study.4
Quantitative real-time PCR (qRT-PCR)
Total RNA is prepared from cells using Trizol reagent according to the manufacturer's protocol (Invitrogen Life Technologies, Carlsbad, CA). qRT–PCR of mRNAs are reverse-transcribed using the Superscript III first-strand synthesis system for RT–PCR (Invitrogen Life Technologies, Carlsbad, CA). qRT-PCR reactions on resulting cDNAs will be performed on an ABI StepOne™ Real-Time PCR Systems (Applied Biosystems).4
Western blot analysis
Cell lysates were harvested and analyzed for the expression of PDCD4 by western blotting analysis. Equal amounts of total cellular protein from different samples were separated by SDS/PAGE and blotted onto nitrocellulose membrane (Amersham, Arlington Heights, IL). The primary antibodies against PDCD4 (Cell Signaling, Beverly, MA) and GAPDH (Millipore, Billerica, MA) were used and the protein bands were subsequently visualized according to chemiluminescence reagents (Amersham Biosciences Co., Piscataway, NJ).
Overexpression of PDCD4
PDCD4 cDNA will be cloned into pLV-EF1a-MCS-IRES-Puro (BioSettia, Cat. No: cDNA-pLV01; San Diego, CA). Lentivirus production will be performed by co-transfection of plasmid DNA mixture with lentivector plus helper plasmids (VSVG and Gag-Pol) into 293T cells (American Type Culture Collection, Manassas, VA) using Lipofectamine 2000 (LF2000, Invitrogen, Carlsbad, CA).
Reporter construction and assay
The 3′UTR sequence of PDCD4 was cloned into the pMIR-REPORT vector (Life Technologies, Grand Island, NY) to generate the wild type reporter (WT). A mutant reporter (MUT) construct was generated from the WT by replacing the targeted sequence. Firefly luciferase activity after normalizing to transfection efficiency represented reporter activity. Transfectin lipid transfection reagent was purchased from BioRad Lab (Hercules, CA).
Collagen contraction assay
Effect of myofibroblast differentiation was measured with collagen contraction assay (Sigma–Aldrich, St. Louis, MO). The photograph images of contraction of the gels were measure the area with ImageJ software (NIH, Bethesda, MD).4
Transwell migration assay
In brief, BMFs or fBMFs were placed in the upper chamber of transwell (Corning, Acton, MA) with serum free medium. Medium containing 10% FBS will be added to the lower chamber and cells will be incubated for 24 h. Cells attached to the other side of the membrane were stained with crystal violet and randomly selected five fields were counted.4
Statistical analysis
Statistical Package of Social Sciences software (version 13.0) (SPSS, Inc., Chicago, IL) was used for statistical analysis. Data from at least triplicate analysis was shown as mean ± SD. Student's t test was used to determine statistical significance of the differences between experimental groups; p values less than 0.05 will be considered statistically significant.
Results
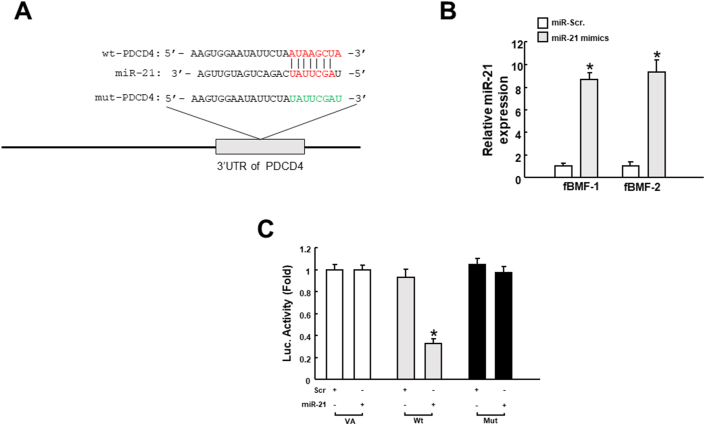
In order to confirm the direct relationship between miR-21 and PDCD4, we employed the luciferase reporter assay to validate the target site. Fig. 1A illustrated the complementarity between the 3′-UTR of PDCD4 and miR-21 to pinpoint the target sequence of miR-21. Reporter plasmids containing either full-length (Wt-PDCD4) or mutated (mut-PDCD4) forms of the miR-21-binding region were constructed and co-transfected with miR-21 mimics into fBMFs. The miR-21 overexpression stimulated by miR-21 mimic in fBMFs was demonstrated by real-time qRT-PCR analysis (Fig. 1B). We showed that the luciferase activity of Wt-PDCD4 vector was reduced when co-transfected with miR-21 mimics, whereas no significant change was observed in the mut-PDCD4 vector in fBMFs (Fig. 1C). These results showed that miR-21 binds through imperfect base pairing to the 3′UTR of PDCD4.
Figure 1.
MiR-21 directly interacts with PDCD4. (A) Schematic of miR-21, the putative binding sequence along with the mutant sequence at the 3′-untranslated region (3′UTR) of PDCD4; (B) The miR-21 overexpression effect in fBMFs was demonstrated by real-time qRT-PCR analysis; (C) Luciferase activity decreased when fBMFs were cotransfected with Wt-PDCD4 and miR-21 mimic. ∗p < 0.05.
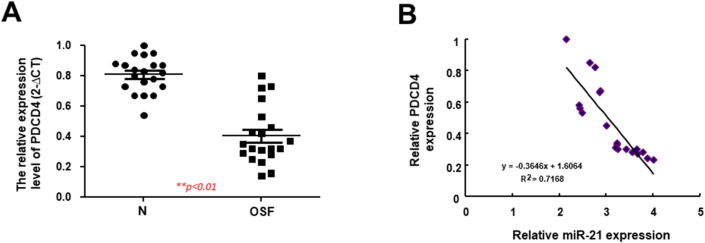
Previously, miR-21 was up-regulated in OSF tissues relative to control normal mucosa.4 Next, we used real-time qRT-PCR to assess the gene expression of PDCD4 in OSF tissues to validate that the overexpressed miR-21 in OSF restrained the expression of PDCD4.4 As expected, the expression level of PDCD4 was downregulated in OSF (n = 20) compared to normal counterparts (Fig. 2A). In addition, the result from the Pearson correlation suggested that there was an inverse correlation between miR-21 and PDCD4 (Fig. 2). The strong negative linear relationship was in conformity with the abovementioned finding that PDCD4 was downregulated in OSF by direct binding of miR-21.
Figure 2.
PDCD4 is downregulated in OSF tissues and inversely related to miR-21. (A) The relative expression of PDCD4 in normal counterparts and OSF specimens (n = 20; ∗∗p < 0.01; un-paired test) (B) The linear relationship between miR-21 and PDCD4 using Pearson correlation coefficient.
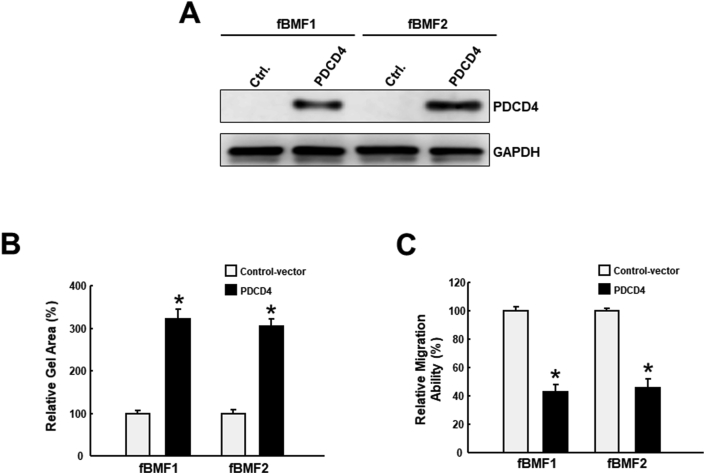
Myofibroblasts are the key players in the development of pathological fibrosis,9 and the persistent activation of buccal mucosa fibroblasts (BMFs) has been shown to be associated with the deposition of collagen in response to the areca nut stimulation.10 Given that miR-21 has been shown to mediate the areca nut-associated myofibroblast activities in BMFs and negatively regulated PDCD4, we then examined if PDCD4 exerted an anti-fibrosis effect in myofibroblast activities.4 Due to their main role in wound healing, the increased collagen gel contraction and cell motility are two common features to examine myofibroblast activities.9 As shown in Fig. 3A, the PDCD4 overexpressed plasmid transfected to fBMF, and confirmed the PDCD4 protein overexpression by western blotting. To investigate if upregulation of PDCD4 suppressed myofibroblast activation, collagen gel contraction, and transwell migration assays were conducted. We observed that the collagen gel contractility was ameliorated in PDCD4-overexpressing fBMFs compared to control (Fig. 3B). Likewise, ectopic expression of PDCD4 down regulated the migration capacity of these two fBMFs (Fig. 3C). Altogether, these results supported that PDCD4 exhibited an inhibitory effect on myofibroblasts.
Figure 3.
Upregulation of PDCD4 diminishes the myofibroblast activities in fBMFs. (A) Overexpression of PDCD4 in two fibrotic buccal mucosal fibroblasts (fBMFs); Collagen gel contraction (B) and Transwell migration (C) assays were employed to compare the myofibroblast activities between PDCD-overexpressing cells and cells with control-vector. ∗p < 0.05.
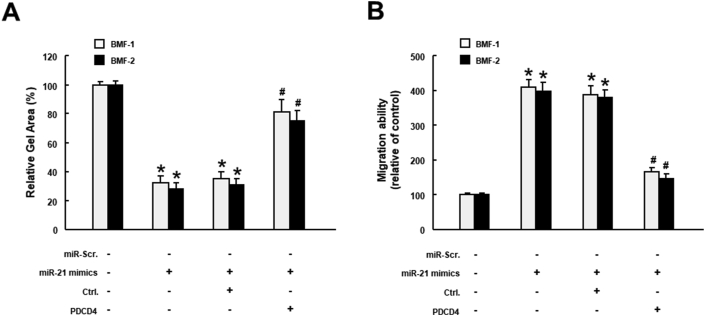
Subsequently, we sought to substantiate that miR-21 contributed to myofibroblast activation through the suppression of PDCD4. As shown in Fig. 4A, the collagen gel contractility was enhanced in normal BMFs transfected with miR-21 mimic, but this phenomenon was reversed when PDCD4 was overexpressed. Similarly, the migration ability was increased in BMFs with miR-21 mimic, whereas overexpression of miR-21 abolished this effect (Fig. 4B). Collectively, we showed that the miR-21/PDCD4 axis regulates the myofibroblast activation.
Figure 4.
Overexpression of PDCD4 rescues the miR-21-induced myofibroblast activation in normal BMFs. Collagen gel contraction (A) and Transwell migration (B) assays were conducted in BMFs transfected with miR-21 mimic in the absence or presence of PDCD4 overexpression. ∗p < 0.05 miR-21 mimic compared to miR-Scr. group. #p < 0.05 miR-21 mimic + PDCD4 compared to the miR-21 mimic group.
Discussion
In the current study, we showed that the expression of PDCD4 was downregulated in premalignant OSF tissues compared to the normal counterparts and this reduction may be due to the direct repression from miR-21. This finding was consistent with a previous study showing that PDCD4 was absent or under-expressed in oral cancer tissues, and they also demonstrated that miR-21 directly targeted the 3′UTR of PDCD4.8 Reis et al. showed that the expression of PDCD4 suppressed the invasive potential of oral cancer cells,8 which was in conformity with our result showing that ectopic expression of PDCD4 downregulated the cell motility of fBMFs. Altogether, these findings indicated that the miR-21/PDCD4 axis not only participates in the progression of oral cancer but also implicates the development of precancerous OSF.
To date, the research regarding the role of PDCD4 in fibrosis disease is still limited. It has been shown that there was a feedback loop of miR-21/PDCD4/activation protein-1 (AP-1) that drove the development of hepatic and renal fibrosis.11,12 PDCD4 has the potential to inhibit AP-1 activity and downregulation of AP-1 reduced the expression of type I collagen.11 Zhang et al. showed that upregulation of miR-21 enhanced the TGF-β signaling pathway during the activation of hepatic stellate cells.11 Another study showed that TGF-β1 induced the expression of miR-21, and suppression of miR-21 diminished the TGF-β1-induced myofibroblast differentiation.13 They also demonstrated that PDCD4 functioned as a negative regulator of various phenotypic genes of myofibroblasts,13 which was consistent with our data showing overexpression of PDCD4 downregulated the myofibroblast features. Moreover, one of the recent studies has shown that knockdown of PDCD4 partly rescued the decreased proliferation of myofibroblasts in response to miR-21 inhibition,14 which was also in line with our finding. Besides, Wang et al. demonstrated that elevation of PDCD4 expression markedly decreased JNK/c-Jun activity,14 a known pathway that may antagonize the TGF-β1/Smad2 signaling.15 PDCD4 has been shown to involve in the miR-21-regulated cardiac epithelial-to-mesenchymal transition (EMT),16 a possible source of myofibroblast.9 Of note, the biological function of PDCD4 may not be solely responsible for preventing fibrosis. It has been reported that PDCD4 protein expression in the myocardium of diabetic rats was overexpressed and high glucose increased its expression in cardiac myoblast H9c2 cells.17 Zhang et al. showed that PDCD4 was involved in the development of diabetic cardiomyopathy and deficiency of PDCD4 attenuated myocardial fibrosis,17 suggesting that PDCD4 may play a controversial role in the progression of different types of fibrosis diseases.
In conclusion, our previous work and the current results demonstrated that the arecoline-induced activation of TGF-β signaling resulted in the elevation of miR-21,4 which suppressed the PDCD4 expression and contributed to the transdifferentiation of BMFs. Our data demonstrated that overexpression of PDCD4 may be a therapeutic strategy to alleviate the progression of OSF.
Declaration of competing interest
All authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by grants from Chung Shan Medical University Hospital (CSH-2021-C-013) and Wan Fang Hospital (108-wf-eva-13) in Taiwan.
Contributor Information
Cheng-Chia Yu, Email: ccyu@csmu.edu.tw.
Ming-Yi Lu, Email: miexyz@gmail.com.
References
- 1.Wang Y.Y., Tail Y.H., Wang W.C., et al. Malignant transformation in 5071 southern Taiwanese patients with potentially malignant oral mucosal disorders. BMC Oral Health. 2014;14:99. doi: 10.1186/1472-6831-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilakaratne W.M., Klinikowski M.F., Saku T., Peters T.J., Warnakulasuriya S. Oral submucous fibrosis: review on aetiology and pathogenesis. Oral Oncol. 2006;42:561–568. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Pant I., Kumar N., Khan I., Rao S.G., Kondaiah P. Role of areca nut induced TGF-β and epithelial-mesenchymal interaction in the pathogenesis of oral submucous fibrosis. PloS One. 2015;10 doi: 10.1371/journal.pone.0129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H.W., Yu C.C., Hsieh P.L., et al. Arecoline enhances miR-21 to promote buccal mucosal fibroblasts activation. J Formos Med Assoc. 2021;120:1108–1113. doi: 10.1016/j.jfma.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Q., Chen Z., Cabay R.J., et al. microRNA-21 and microRNA-375 from oral cytology as biomarkers for oral tongue cancer detection. Oral Oncol. 2016;57:15–20. doi: 10.1016/j.oraloncology.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asangani I.A., Rasheed S.A., Nikolova D.A., et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 8.Reis P.P., Tomenson M., Cervigne N.K., et al. Programmed cell death 4 loss increases tumor cell invasion and is regulated by miR-21 in oral squamous cell carcinoma. Mol Canc. 2010;9:238. doi: 10.1186/1476-4598-9-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.L., Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey W., Scutt A., Meghji S., Canniff J.P. Stimulation of human buccal mucosa fibroblasts in vitro by betel-nut alkaloids. Arch Oral Biol. 1986;31:45–49. doi: 10.1016/0003-9969(86)90112-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z., Zha Y., Hu W., et al. The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J Biol Chem. 2013;288:37082–37093. doi: 10.1074/jbc.M113.517953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Q., Miao J., Luo J., et al. The feedback loop between miR-21, PDCD4 and AP-1 functions as a driving force for renal fibrogenesis. J Cell Sci. 2018:131. doi: 10.1242/jcs.202317. [DOI] [PubMed] [Google Scholar]
- 13.Yao Q., Cao S., Li C., Mengesha A., Kong B., Wei M. Micro-RNA-21 regulates TGF-β-induced myofibroblast differentiation by targeting PDCD4 in tumor-stroma interaction. Int J Canc. 2011;128:1783–1792. doi: 10.1002/ijc.25506. [DOI] [PubMed] [Google Scholar]
- 14.Wang F., Zhao X.Q., Liu J.N., et al. Antagonist of microRNA-21 improves balloon injury-induced rat iliac artery remodeling by regulating proliferation and apoptosis of adventitial fibroblasts and myofibroblasts. J Cell Biochem. 2012;113:2989–3001. doi: 10.1002/jcb.24176. [DOI] [PubMed] [Google Scholar]
- 15.Wu S., Kasisomayajula K., Peng J., Bancalari E. Inhibition of JNK enhances TGF-beta1-activated Smad2 signaling in mouse embryonic lung. Pediatr Res. 2009;65:381–386. doi: 10.1203/PDR.0b013e3181991c67. [DOI] [PubMed] [Google Scholar]
- 16.Brønnum H., Andersen D.C., Schneider M., et al. miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving Programmed Cell Death 4 and Sprouty-1. PloS One. 2013;8 doi: 10.1371/journal.pone.0056280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Zhang M., Yang Z., et al. PDCD4 deficiency ameliorates left ventricular remodeling and insulin resistance in a rat model of type 2 diabetic cardiomyopathy. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2019-001081. [DOI] [PMC free article] [PubMed] [Google Scholar]