Abstract
Background/purpose
To investigate the associations between treated and untreated dental caries and periodontitis in young adults.
Materials and methods
The study enrolled 1289 participants aged 18–45 years in Taiwan. Localized periodontitis was categorized into healthy and stage II/III (n = 936 and n = 353, respectively) based on the 2017 criteria of the World Workshop. Multivariable logistic regression analysis with adjustments for sex, age, tobacco smoking status, betel nut consumption status, metabolic syndrome, and total white blood cell count was used to determine the associations.
Results
Decayed tooth numbers were positively associated with localized stage II/III periodontitis [odds ratio (OR): 1.15 (95% confidence intervals (CI): 1.06–2.25)], while filled tooth numbers were inversely associated with localized stage II/III periodontitis in young adults [OR: 0.96 (95% CI: 0.92–0.99)].
Conclusion
Our study confirms the relationship between dental caries and periodontitis by direct evidence that the more decayed teeth there are, the higher the risk of periodontitis and by indirect evidence that the more treated decayed teeth there are, the lower the risk of periodontitis in young adults.
Keywords: Dental caries, Localized periodontitis, Young adults
Introduction
The oral cavity is most often affected by periodontal disease and dental caries.1 If left untreated, these diseases might cause some negative impacts. Both periodontitis and dental caries result in tooth loss, reduced masticatory function, poor nutritional status, low self-esteem, low quality of life and negative general health impacts. There is also evidence of an association between oral health and all-cause mortality.2,3 In addition, both periodontitis and dental caries are complex diseases with multiple and diverse exposures that impact the risk of disease initiation or progression of existing systemic diseases. Exposures include those that are inherited (e.g., genetic variants), those that are acquired, such as social, educational and economic factors, the local environment (e.g., biofilm load or composition), other diseases (e.g., suboptimally controlled diabetes) and lifestyle factors (e.g., smoking, consumption of sugars, and carbohydrate intake).4 These may arise in different combinations in different individuals, and at an individual patient level, they may also have differentially weighted effects.4
Most previous studies indicated a positive association between periodontal disease and untreated dental caries.5, 6, 7, 8 However, findings concerning the simultaneous occurrence of periodontal disease and dental caries are conflicting. Some studies have revealed a negative association or no association.9, 10, 11, 12 In addition, whether there is a higher risk of localized periodontitis associated with treated dental caries in young adults has rarely been investigated before. Therefore, the aim of this study was to clarify the association between periodontitis and treated and untreated dental caries in young adults.
Materials and methods
Study population
A cohort of 1289 military participants (1129 males and 160 females) was retrospectively collected from the cardiorespiratory fitness and hospitalization events in armed forces (CHIEF) oral study in eastern Taiwan for the analysis.13 These participants had an average age of 30.28 years (18–45 years). They were without diabetes and were not taking any medications. They were subsequently divided into two subsets, periodontal healthy/localized stage I periodontitis and localized stage II/III periodontitis, based on the 2017 the World Workshop of the American Academy of Periodontology and the European Federation of Periodontology.14 All participants received a comprehensive health examination requiring information on service specialty, highest level of education (senior high school degree, college or university degree, and postgraduate degree), betel nut chewing status (never, former and current) and tobacco smoking status (never, former and current).
Clinical and demographic measures
The physical health examination included anthropometric measurements of height, weight, body mass index (weight, kg/height, m2; assessed in a standing position) and waist circumference. The hemodynamic data of systolic and diastolic blood pressure (SBP and DBP, respectively) [in the right upper arm; measured in a sitting position after rest for at least 15 min, using the FT-201 automated blood pressure monitor (Parama-Tech Co Ltd, Fukuoka, Japan)] were determined. The laboratory data, including serum concentrations of total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and fasting plasma glucose (FPG), were measured by an auto analyzer (Olympus AU640 auto analyzer, Olympus, Kobe, Japan), and the hemoglobin level was measured by the XT-2000-I automated hematology analyzer (Sysmex Corporation, Kobe, Japan).
Oral status measures
Impacted teeth and third molars were excluded from this study. Dental caries were assessed by recording the caries index DMFT (number of decayed, missing and filled teeth) according to the criteria recommended by the WHO.15
The 2017 World Workshop of both the American Academy of Periodontology and the European Federation of Periodontology was used to classify the severity of periodontitis.14 In addition, the extension of periodontitis was localized in the present study (<30% of teeth involved).14 This study design and protocol were reviewed and approved by the human subject ethics board of Mennonite Christian Hospital (No. 16-05-008) in Hualien City, Taiwan, and written informed consent was obtained from all participants. Additionally, this study was performed in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Definition of metabolic syndrome
Metabolic syndrome was defined based on the International Diabetes Federation (IDF) definition.16 The subjects who satisfied at least three of the following five criteria were classified as having metabolic syndrome: (1) high triglycerides (≥150 mg/dL) or on lipid-lowering therapy; (2) low HDL-C (<40 mg/dL in men and <50 mg/dL in women) or on lipid-lowering therapy; (3) SBP ≥130 mmHg and/or DBP ≥85 mmHg or on antihypertensive treatments; (4) fasting glucose ≥100 mg/dL or on antidiabetic therapy; and (5) waist circumference ≥90 cm in men and ≥80 cm in women.
Statistical analysis
The clinical variables are expressed as the mean ± standard deviation (SD) for continuous data and numbers (percentages) for categorical data. Continuous variables were compared by one-way ANOVA, and categorical variables were compared by the chi-square test. Multivariable logistic regression analysis was used to determine the odds ratio (OR) and 95% confidence intervals (CI) of the DMFT index and decayed, missing and filled teeth with localized stage II/III periodontitis. The covariates were stepwise adjusted in two models for localized stage II/III periodontitis. In Model 1, sex, age, betel nut chewing status and tobacco smoking status were adjusted. In Model 2, metabolic syndrome and an inflammation marker, blood total leukocyte cell count, were further adjusted. A value of p < 0.05 was considered significant. SPSS statistical software was used for all statistical analyses (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. International Business Machines Corporation, Armonk, NY, USA).
Results
Table 1 presents the characteristics of the healthy participants and participants with localized stage I periodontitis (N = 936) and localized stage II/III periodontitis (N = 353). Participants with localized stage II/III periodontitis were relatively older and had a greater prevalence of males, betel-nut chewing status, metabolic syndrome. They also had greater values for SBP, DBP, body mass index and waist circumference. They had higher serum concentrations of total cholesterol, LDL-C, triglycerides, uric acid, liver enzymes, total leucocyte counts, hemoglobin, and mean PPD and lower serum HDL-C levels. They also had higher percentages of sites with PPD within 4–5 mm, percentages of sites with PPD ≥6 mm, mean CAL, percentages of sites with CAL within 3–4 mm and percentages of sites with CAL ≥5 mm. In addition, those with localized stage II/III periodontitis had relatively more decayed and missing teeth.
Table 1.
Baseline clinical characteristics (N = 1289).
| Baseline clinical characteristics | Healthy or localized stage I periodontitis (N = 936) | Localized stage II/III periodontitis (N = 353) | p-value |
|---|---|---|---|
| Sex | |||
| Male | 804 [85.9] | 325 [92.1] | 0.003 |
| Female | 132 [14.1] | 28 [7.9] | |
| Age (years) | 29.82 ± 5.95 | 31.51 ± 5.76 | <0.001 |
| Range (min – max) | (18–48) | (19–45) | |
| Service specialty | |||
| Army | 336 [35.9] | 135 [38.2] | 0.43 |
| Navy | 0 [0.0] | 0 [0.0] | |
| Air force | 600 [64.1] | 218 [61.8] | |
| Education level | |||
| Up to senior high school | 304 [32.5] | 97 [27.5] | 0.22 |
| College/University degree | 608 [65.0] | 247 [70.0] | |
| Postgraduate degree | 24 [2.6] | 9 [2.5] | |
| Current betel nut chewer | 42 [4.5] | 34 [9.6] | <0.001 |
| Current tobacco smoker | 163 [17.4] | 75 [21.2] | 0.11 |
| Metabolic syndrome | 157 [16.8] | 96 [27.2] | <0.001 |
| Systolic blood pressure (mmHg) | 121.03 ± 11.96 | 123.29 ± 13.63 | 0.004 |
| Diastolic blood pressure (mmHg) | 73.41 ± 9.71 | 75.09 ± 11.57 | 0.009 |
| Body mass index (kg/m2) | 25.37 ± 3.74 | 26.63 ± 3.75 | <0.001 |
| Waist circumference (cm) | 84.03 ± 10.78 | 88.10 ± 10.75 | <0.001 |
| Blood test | |||
| Total cholesterol (mg/dL) | 179.66 ± 34.73 | 187.59 ± 36.21 | <0.001 |
| HDL-C (mg/dL) | 49.64 ± 10.89 | 47.77 ± 10.55 | 0.006 |
| LDL-C (mg/dL) | 109.11 ± 31.51 | 113.79 ± 30.79 | 0.01 |
| Triglycerides (mg/dL) | 120.39 ± 95.12 | 156.86 ± 125.84 | <0.001 |
| Fasting plasma glucose (mg/dL) | 92.93 ± 15.64 | 93.17 ± 16.06 | 0.80 |
| Serum uric acid (mg/dL) | 6.46 ± 1.44 | 6.86 ± 1.49 | <0.001 |
| White blood cell counts (103/uL) | 6.95 ± 1.69 | 7.26 ± 1.75 | 0.004 |
| PPD (mm) | 2.92 ± 0.05 | 3.03 ± 0.04 | <0.001 |
| % of site with PPD 4–5 mm | 0.50 ± 0.86 | 3.61 ± 1.15 | <0.001 |
| % of site with PPD ≥6 mm | 0.00 ± 0.00 | 0.80 ± 1.00 | <0.001 |
| CAL (mm) | 2.93 ± 0.06 | 3.07 ± 0.06 | <0.001 |
| % of site with CAL 3–4 mm | 0.76 ± 1.01 | 3.92 ± 1.29 | <0.001 |
| % of site with CAL ≥5 mm | 0.00 ± 0.00 | 1.11 ± 1.16 | <0.001 |
| DMFT index | 4.79 ± 4.16 | 4.91 ± 4.27 | 0.66 |
| Decayed teeth | 0.50 ± 1.21 | 0.80 ± 1.70 | 0.001 |
| Missing teeth | 0.75 ± 1.36 | 0.95 ± 1.58 | 0.02 |
| Filled teeth | 3.55 ± 3.49 | 3.16 ± 3.31 | 0.07 |
Continuous variables are expressed as mean ± SD (standard deviation), and categorical variables as N [%].
HDL-C; high-density lipoprotein cholesterol, LDL-C; low-density lipoprotein cholesterol, PPD; probing pocket depth, CAL; clinical attachment loss, DMFT; decayed-missing-filled teeth.
Table 2 demonstrates the estimates of the risk of localized stage II/III periodontitis between the two groups, in which healthy or localized stage I periodontitis was treated as a reference using multivariable logistic regression models. Greater decayed tooth numbers were associated with a higher risk of localized stage II/III periodontitis in Model 1 [OR: 1.16 (95% CI: 1.07–1.26)] and Model 2 [OR: 1.15 (95% CI: 1.05–2.25)]. In contrast, a greater number of filled teeth was inversely associated with a lower risk of localized stage II/III periodontitis in Model 1 [OR: 0.95 (95% CI: 0.92–0.99)] and Model 2 [OR: 0.95 (95% CI: 0.92–0.99)].
Table 2.
Multivariable logistic regression analysis for localized stage II/III periodontitis (healthy or localized stage I periodontitis as reference) with DMFT (decayed-missing-filled teeth) index.
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| DMFT index | 0.990 (0.960–1.021) | 0.52 | 0.993 (0.962–1.024) | 0.63 |
| Decayed teeth | 1.159 (1.065–1.262) | 0.001 | 1.149 (1.055–1.252) | 0.001 |
| Missing teeth | 1.039 (0.952–1.133) | 0.39 | 1.037 (0.950–1.132) | 0.41 |
| Filled teeth | 0.952 (0.917–0.989) | 0.01 | 0.958 (0.922–0.995) | 0.02 |
Data are presented as odds ratios and 95% confidence intervals (CI) using multiple logistic regression analysis for.
Model 1: sex, age, betel nut chewing and tobacco smoking adjustments.
Model 2: sex, age, betel nut chewing, tobacco smoking, metabolic syndrome and blood WBC adjustments.
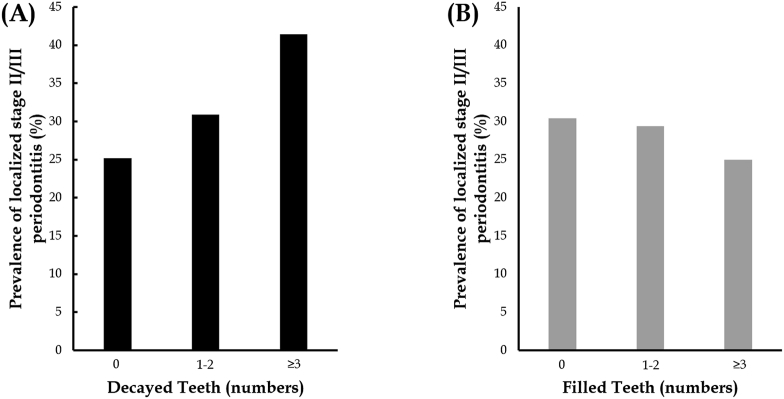
Figure 1 shows the prevalence of localized stage II/III periodontitis and the numbers of decayed teeth (N = 0, 1–2, and ≥3) (A) and filled teeth (N = 0, 1–2, and ≥3) (B). The tendency of having localized stage II/III periodontitis increased in those who had greater numbers of decayed teeth (p = 0.002) or fewer filled teeth (p = 0.06, as the filled teeth, N ≥ 3 compared to no filled teeth N = 0).
Fig. 1.
(A) The prevalence of localized stage II/III periodontitis and the numbers of decayed teeth (N = 0, 1–2, and ≥3) and (B) filled teeth (N = 0, 1–2, and ≥3).
Discussion
The present study first uncovered the relationship between dental caries and periodontitis in young adults. In addition, we found that the risk of localized stage II/III periodontitis was positively associated with the number of decayed teeth but inversely associated with the number of teeth with fillings in young adults.
Biofilms play an important role in both periodontitis and dental caries. In periodontitis, plaque accumulation at the gingival margin causes inflammation and affects the supporting tissue of teeth, resulting in loss of alveolar bone, cementum and periodontal ligament.13 Unsatisfactory oral hygiene can increase the risk of periodontitis by two-to fivefold.17 In caries, the exposure of dental biofilms to dietary sugars and their fermentation to organic acids results in the phasic demineralization and remineralization of dental hard tissues.18 Theoretically, the etiologies of caries and periodontal diseases are mutually independent.19 Microbial diversity appears to be lower in caries than in healthy individuals, which may reflect the ecological pressure of a more acidic environment. Characteristic features of these organisms include acidogenicity, acid tolerance and extracellular polysaccharide formation from dietary sucrose. Classically recognized organisms, such as mutans streptococci and lactobacilli, Bifidobacterium dentium, Scardovia wiggsiae, Schlegelella or Pseudoramibacter, appear to be present in dentin caries lesions.20, 21, 22 Unlike caries, dysbiosis in periodontal diseases is associated with an increase in microbial diversity, which could be the result of impaired local immune function, increased availability of nutrients or diverse environmental niches at the sampling site of the periodontal pocket.23,24 In contrast, periodontal diseases are commonly associated with gram-negative proteolytic anaerobic species, including Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia and Aggregatibacter actinomycetemcomitans.19 Mechanical and chemical plaque control with fluoridation is essential to prevent the development of periodontitis and dental caries.6 In addition, sociobehavioral inequalities within/between populations have also been shown to be possible causative factors for both of these diseases.25, 26, 27
Similar to our findings, a recent report from a national survey in Finland found a significant positive association between periodontitis and untreated dental caries in adults,5 and data from a recent national survey in Germany indicated that in adults, there was significantly higher attachment loss and probing depths at sites where caries occurred than at sites in which caries did not occur.6 Interestingly, a previous study revealed a negative association between dental caries and advanced periodontitis but not moderate to severe periodontitis.9 In addition, a few studies also reported a null association between dental caries and periodontitis in adults.10, 11, 12 The conflicting results might be due to various definitions for periodontitis and dental caries used in these studies. Frentzen et al. used the community periodontal index of treatment needs (CPITN) and decayed-missing-filled surfaces (DMFS) index.11 Kinane et al. used radiographic assessment of bone loss in quarters of optimum bone height and decayed and filled teeth,12 and Skier and Mandel used the gingival index, plaque index and periodontal destruction index.10 In addition, treatment of dental caries might prevent the occurrence of periodontitis, which was shown in the present study, and this effect was not taken into consideration in prior studies.
The present study has some limitations. First, it was impossible to exclude all residual confounders, which might lead to bias. Second, since this study was a cross-sectional design, no conclusions regarding the causal relationship between periodontitis and dental caries or regarding the incidence of these dental diseases can be made on the basis of the present study. Therefore, further prospective longitudinal studies should be performed for clarification. In contrast, there were several strengths in the present study. First, we used the latest periodontal classification for analysis. Second, our study had a large sample size that could provide sufficient power to make associations between periodontitis and caries. In conclusion, our study confirms the relationship between dental caries and periodontitis by direct evidence that the more decayed teeth there are, the higher the risk of periodontitis and by indirect evidence that the more treated decayed teeth there are, the lower the risk of periodontitis in young adults.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by the Medical Affairs Bureau Ministry of National Defense; Hualien Armed Forces General Hospital, Taiwan, under grants MND-MAB-110-148 and HAFGH-D-110008.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2021.10.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.GBD 2015 disease and injury incidence and prevalence collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;vol. 388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia R.I., Krall E.A., Vokonas P.S. Periodontal disease and mortality from all causes in the VA dental longitudinal study. Ann Periodontol. 1998;3:339–349. doi: 10.1902/annals.1998.3.1.339. [DOI] [PubMed] [Google Scholar]
- 3.Kim J.K., Baker L.A., Davarian S., Crimmins E. Oral health problems and mortality. J Dent Sci. 2013;8:115–120. doi: 10.1016/j.jds.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapple I.L., Bouchard P., Cagetti M.G., et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl 18):S39–S51. doi: 10.1111/jcpe.12685. [DOI] [PubMed] [Google Scholar]
- 5.Mattila P.T., Niskanen M.C., Vehkalahti M.M., Nordblad A., Knuuttila M.L. Prevalence and simultaneous occurrence of periodontitis and dental caries. J Clin Periodontol. 2010;37:962–967. doi: 10.1111/j.1600-051X.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 6.Jepsen S., Blanco J., Buchalla W., et al. Prevention and control of dental caries and periodontal diseases at individual and population level: consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl 18):S85–S93. doi: 10.1111/jcpe.12687. [DOI] [PubMed] [Google Scholar]
- 7.Albandar J.M., Buischi Y.A., Axelsson P. Caries lesions and dental restorations as predisposing factors in the progression of periodontal diseases in adolescents. A 3-year longitudinal study. J Periodontol. 1995;66:249–254. doi: 10.1902/jop.1995.66.4.249. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg P., Jamison H.C. A study of periodontal health and oral hygiene in Norwegian army recruits. J Periodontol. 1964;35:302–307. [Google Scholar]
- 9.Sewon L.A., Parvinen T.H., Sinisalo T.V., Larmas M.A., Alanen P.J. Dental status of adults with and without periodontitis. J Periodontol. 1988;59:595–598. doi: 10.1902/jop.1988.59.9.595. [DOI] [PubMed] [Google Scholar]
- 10.Skier J., Mandel I.D. Comparative periodontal status of caries resistant versus susceptible adults. J Periodontol. 1980;51:614–616. doi: 10.1902/jop.1980.51.10.614. [DOI] [PubMed] [Google Scholar]
- 11.Frentzen M., Schuler N., Nolden R. Correlation between caries prevalence (DMFS) and periodontal condition (CPITN) in more than 2000 patients. Int Dent J. 1990;40:313–318. [PubMed] [Google Scholar]
- 12.Kinane D.F., Jenkins W.M., Adonogianaki E., Murray G.D. Cross-sectional assessment of caries and periodontitis risk within the same subject. Community Dent Oral Epidemiol. 1991;19:78–81. doi: 10.1111/j.1600-0528.1991.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 13.Tsai K.Z., Huang R.Y., Cheng W.C., et al. Comparisons of various anthropometric indexes with localized Stage II/III periodontitis in young adults: the CHIEF oral health study. J Periodontol. 2021;92:958–967. doi: 10.1002/JPER.20-0275. [DOI] [PubMed] [Google Scholar]
- 14.Tonetti M.S., Greenwell H., Kornman K.S. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . 4th ed. 1997. Oral health survey: basic methods. [Google Scholar]
- 16.Alberti K.G., Zimmet P., Shaw J., Group IDFETFC The metabolic syndrome-a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 17.Lertpimonchai A., Rattanasiri S., Arj-Ong Vallibhakara S., Attia J., Thakkinstian A. The association between oral hygiene and periodontitis: a systematic review and meta-analysis. Int Dent J. 2017;67:332–343. doi: 10.1111/idj.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitts N.B., Zero D.T., Marsh P.D., et al. Dental caries. Nat Rev Dis Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 19.Sanz M., Beighton D., Curtis M.A., et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. 2017;44(Suppl 18):S5–S11. doi: 10.1111/jcpe.12682. [DOI] [PubMed] [Google Scholar]
- 20.Mantzourani M., Fenlon M., Beighton D. Association between Bifidobacteriaceae and the clinical severity of root caries lesions. Oral Microbiol Immunol. 2009;24:32–37. doi: 10.1111/j.1399-302X.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- 21.Tanner A.C., Mathney J.M., Kent R.L., et al. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 2011;49:1464–1474. doi: 10.1128/JCM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon-Soro A., Mira A. Solving the etiology of dental caries. Trends Microbiol. 2015;23:76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Dewhirst F.E., Chen T., Izard J., et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camelo-Castillo A.J., Mira A., Pico A., et al. Subgingival microbiota in health compared to periodontitis and the influence of smoking. Front Microbiol. 2015;6:119. doi: 10.3389/fmicb.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwendicke F., Dorfer C.E., Schlattmann P., Foster Page L., Thomson W.M., Paris S. Socioeconomic inequality and caries: a systematic review and meta-analysis. J Dent Res. 2015;94:10–18. doi: 10.1177/0022034514557546. [DOI] [PubMed] [Google Scholar]
- 26.Klinge B., Norlund A. A socio-economic perspective on periodontal diseases: a systematic review. J Clin Periodontol. 2005;32(Suppl 6):314–325. doi: 10.1111/j.1600-051X.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- 27.Boillot A., El Halabi B., Batty G.D., Range H., Czernichow S., Bouchard P. Education as a predictor of chronic periodontitis: a systematic review with meta-analysis population-based studies. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.