Abstract
Aryl metabolite biosynthesis was studied in the white rot fungus Bjerkandera adusta cultivated in a liquid medium supplemented with l-phenylalanine. Aromatic compounds were analyzed by gas chromatography-mass spectrometry following addition of labelled precursors (14C- and 13C-labelled l-phenylalanine), which did not interfere with fungal metabolism. The major aromatic compounds identified were benzyl alcohol, benzaldehyde (bitter almond aroma), and benzoic acid. Hydroxy- and methoxybenzylic compounds (alcohols, aldehydes, and acids) were also found in fungal cultures. Intracellular enzymatic activities (phenylalanine ammonia lyase, aryl-alcohol oxidase, aryl-alcohol dehydrogenase, aryl-aldehyde dehydrogenase, lignin peroxidase) and extracellular enzymatic activities (aryl-alcohol oxidase, lignin peroxidase), as well as aromatic compounds, were detected in B. adusta cultures. Metabolite formation required de novo protein biosynthesis. Our results show that l-phenylalanine was deaminated to trans-cinnamic acid by a phenylalanine ammonia lyase and trans-cinnamic acid was in turn converted to aromatic acids (phenylpyruvic, phenylacetic, mandelic, and benzoylformic acids); benzaldehyde was a metabolic intermediate. These acids were transformed into benzaldehyde, benzyl alcohol, and benzoic acid. Our findings support the hypothesis that all of these compounds are intermediates in the biosynthetic pathway from l-phenylalanine to aryl metabolites. Additionally, trans-cinnamic acid can also be transformed via β-oxidation to benzoic acid. This was confirmed by the presence of acetophenone as a β-oxidation degradation intermediate. To our knowledge, this is the first time that a β-oxidation sequence leading to benzoic acid synthesis has been found in a white rot fungus. A novel metabolic scheme for biosynthesis of aryl metabolites from l-phenylalanine is proposed.
Consumer preferences for products with a natural origin have led to the exploitation of microbial sources that produce natural aroma compounds (16, 28). Among the potential aroma producers, white rot basidiomycetes are probably the most versatile microorganisms. These fungi are able to produce a wide variety of volatile aryl metabolites of commercial interest, such as vanillin, benzaldehyde (bitter almond aroma), and cinnamaldehyde (1, 7, 12). Therefore, fermentation of natural substrates, such as l-phenylalanine or tyrosine, by white rot fungi can offer alternative routes for biosynthesis of a wide spectrum of aryl metabolites (11, 21). Other biosynthetic precursors, like aromatic acids, stimulate the production of aryl metabolites in Bjerkandera adusta BOS55 (21).
The metabolism of l-phenylalanine has been studied in several white rot fungi (13, 17). Among the extracellular aromatic compounds that these organisms produce, veratryl alcohol has received special attention because it is known to be a substrate and possibly a mediator in lignin biodegradation (6, 15). This compound is the major aryl metabolite formed in Phanerochaete chrysosporium cultures supplemented with l-phenylalanine (13).
To a large extent, the variety and amount of aryl metabolites depend on the fungus and its enzymatic repertoire (10, 15, 18). Enzymes like peroxidases, lignin peroxidase (LiP), and manganese peroxidase (MnP), which are present in a wide variety of white rot fungi, including B. adusta and P. chrysosporium (26), are also involved in biosynthesis of hydroxylated and methoxylated aromatic compounds, such as veratryl alcohol (15). Aryl alcohol oxidase (AAO), an enzyme that oxidizes aryl alcohols (e.g., benzyl, anisyl, and veratryl alcohols) to the corresponding aldehydes (22), has been purified and characterized in B. adusta (22) and fungi belonging to the genus Pleurotus (8). AAO activity is also significantly increased in immobilized cells of B. adusta, and the increase corresponds with high benzaldehyde yields (18). Guillén and Evans (9) have suggested that intracellular dehydrogenases, such as aryl-aldehyde dehydrogenase (AADD), reduce aromatic acids to aldehydes and that aryl-alcohol dehydrogenase (AAD) reduces aromatic aldehydes to alcohols in Pleurotus eryngii. An intracellular AAD was purified from the fungus P. chrysosporium (23). This enzyme reduced veratraldehyde and other aryl aldehydes to veratryl alcohol and corresponding aryl alcohols by using NADPH as a cofactor.
To date, we do not know the pathway for biosynthesis of aryl metabolites from l-phenylalanine in B. adusta, a white rot fungus that is an excellent producer of aryl metabolites, such as benzaldehyde, benzyl alcohol, and benzoic acid (18–20). Therefore, our objective was to characterize this biosynthetic pathway in B. adusta by using 14C- and 13C-labelled l-phenylalanine as precursors. The presence and location (intra- or extracellular) of related oxidases and reductases in B. adusta and the possible roles of these enzymes in the biosynthesis of aryl metabolites were also investigated. We used cycloheximide, an inhibitor of protein biosynthesis, to examine induction of these enzymes. Putative intermediates of the l-phenylalanine degradation pathway were added to the fungal culture media in order to determine their effects on aryl metabolite biosynthesis. Based on our findings, we propose two metabolic pathways for aryl metabolite formation in the representative white rot fungus B. adusta. Our results provide evidence that β-oxidation of trans-cinnamic acid to benzoic acid occurs in B. adusta.
MATERIALS AND METHODS
Microorganism.
B. adusta CBS 595.78 was used in this study. This white rot fungus was cultivated at 25°C on potato dextrose agar slants and stored at 4°C.
Chemicals.
All chemicals were purchased from Sigma (Saint Quentin Fallavier, France) or Aldrich (Saint Quentin Fallavier, France).
Media and culture conditions.
An inoculum was prepared in a medium which contained (per liter) 0.2 g of KH2PO4, 0.2 g of MgSO4 · 7H2O, 2 g of l-phenylalanine, 10 g of glucose, 0.01 g of CuSO4 · 5H2O, and 0.5 g of yeast extract. Cultures were grown in a medium which contained (per liter) 0.2 g of KH2PO4, 0.2 g of MgSO4 · 7H2O, 3 g of l-phenylalanine, 0.01 g of CuSO4 · 5H2O, 0.5 g of yeast extract, and 10 g of lecithin. When bioconversion of different aromatic acids was investigated, this medium was supplemented with the following precursors: l-phenylalanine, mandelic acid (α-hydroxyphenylacetic acid) (calcium salt), trans-cinnamic acid, phenylpyruvic acid (α-oxophenylpropionic acid) (sodium salt), and benzoylformic acid (phenylglyoxylic acid). For most of these precursors the initial concentration was 3 g/liter; the only exception was trans-cinnamic acid, whose initial concentration was 100 mg/liter.
Media were adjusted to pH 5.5 with NaOH or HCl before sterilization. Cultures were grown as previously described (18) in 500-ml baffled flasks. Each flask, which contained 125 ml of medium, was inoculated with 2.5 ml of homogenized mycelium (18). The immobilization supports used were polyurethane foam supports (Filtren T45; Recticel, Brussels, Belgium). Twelve foam cubes (2 by 2 by 2 cm) were placed in each flask and sterilized at 120°C for 20 min. Autoclaving the polyurethane foam cubes did not release any inhibitory compounds that could affect fungal growth or biosynthesis of aromatic compounds (19). The cultures were incubated at 25°C and 100 rpm (5-cm-diameter stroke).
When labelled compounds were used, cultures were grown essentially as described above, except that we used 125-ml Erlenmeyer flasks that contained 25 ml of medium and 20 foam cubes (1 by 1 by 1 cm). Cycloheximide (10 mg/liter) was added to the culture medium at the time of inoculation in order to determine the effects of protein biosynthesis on metabolite production and enzymatic activities.
Labelled compounds.
13C is a naturally occurring, low-abundance (1.1%) isotope. In our study, the initial isotope enrichment experiments were performed with 100% ring 13C-labelled l-phenylalanine. Ring 13C-labelled l-phenylalanine (isotopic enrichment, 99%; Leman, Saint Quentin en Yvelines, France) or l-[U-14C]phenylalanine (specific activity, 505.3 μCi/mmol; NEN, Nemours, France) was added at the time of inoculation.
HPLC quantitative analysis of aromatic metabolites.
Aryl metabolites and l-phenylalanine were quantified by performing high-performance liquid chromatography (HPLC) analyses. Samples were filtered through 0.2-μm-pore-size syringe filters (Microgon, Inc., DynaGard, Laguna Hills, Calif.), diluted 10- or 20-fold, and analyzed every day for 10 days. An aromatic compound analysis was performed with a Waters (Saint Quentin en Yvelines, France) column (Symmetry C18 3.5 μm; diameter, 4.6 mm; length, 100 mm). The operating conditions used were as follows: flow rate of 0.6 ml/min, 40°C, and detection at 200 nm with a photodiode array detector (model 996; Waters). A solution containing water, methanol (40%), and acetic acid (0.01%) was used as the eluent (isocratic method). The amounts of radioactivity incorporated into U-14C-labelled aromatic compounds were determined by liquid scintillation counting with a 14C detector (EG&G Berthold, Evry, France), and the HPLC analysis conditions used were the conditions described above.
Aromatic compound extraction and analysis.
GC ring 13C-labelled extracts were prepared from centrifuged 10-day-old culture media by the solvent extraction method. Each culture medium was divided into two equal portions. The pH of one portion was adjusted to 7 with NaHCO3, and the pH of the other portion was adjusted to 3 with HCl. The culture media were then extracted three times with 40 ml of dichloromethane (for the neutral samples) or with 40 ml of ethyl acetate (for the acidified samples). The solutions were dried over anhydrous sodium sulfate, and each preparation was concentrated to a volume of 1 ml under an N2 stream. Free acids were converted to methyl esters, which were used for identification by the BF3 methanol technique (3). The concentrates were analyzed by gas chromatography (GC)-mass spectrometry (MS). GC analyses were performed with a DB-FFAP column (length, 30 m; internal diameter, 0.32 mm; film thickness, 0.25 μm; J & W Scientific, Folsom, Calif.) by using a Delsi DI700 chromatograph equipped with a flame ionization detector. The conditions used were as follows: carrier gas, helium; flow rate, 0.75 ml/min; amount injected in split-splitless mode, 2 μl; and a linear temperature gradient that increased from 60 to 240°C at a rate of 5°C/min. GC-MS analyses were performed with a benchtop mass spectrometer (model MSD 5970; Hewlett-Packard, Palo Alto, Calif.) coupled to a model HP 5890 gas chromatograph (Hewlett-Packard) by using helium as the carrier gas. The conditions used were the conditions described above. The ionization energy was 70 eV.
Enzyme assays.
Extracellular and intracellular enzymatic activities were determined at 25°C. To obtain a cellular extract, a pellet was washed with bidistilled water and 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (pH 8), crushed and homogenized in liquid nitrogen, suspended in HEPES buffer containing 100 mM phenylmethylsulfonyl fluoride, a protease inhibitor, and 100 mM EDTA, and centrifuged (10,000 × g, 4°C, 10 min). The resulting extract was used for analysis.
LiP activity was measured by the method of Tien and Kirk (27). MnP activity was quantified by measuring the oxidation of Mn(II) to Mn(III) (25). Laccase activity was quantified by measuring the oxidation of ABTS [2,2′-azinobis(3-ethylbenzthiazoline 6-sulfonate)] (4). AAO activity was quantified by measuring the oxidation of veratryl alcohol to veratraldehyde (22). AAD activity was quantified by measuring the oxidation of NADPH during reduction of veratraldehyde, as previously described (23). AADD activity was measured by using veratric acid as the substrate (9). l-Phenylalanine ammonia lyase (PAL) activity was quantified by measuring the deamination of l-phenylalanine to trans-cinnamic acid as previously described (24). Transaminase activity was measured by using the test for l-phenylalanine:α-ketoglutarate aminotransferase coupled with the colorimetric l-glutamic acid assay (Boehringer, Mannheim, Germany) (29). The protein concentration was determined as described by Bradford (5) by using bovine serum albumin as the standard. When activities were detected, they were expressed in units per liter or units per gram of protein (1 U = 1 μmol/min).
Dry weight measurement.
Dry weight was determined by filtering the mycelium plus foam cubes onto glass fiber filters (type GF/D; diameter, 4.7 cm; Whatman). The mycelium and foam were rinsed twice with bidistilled water and dried at 60°C until the weight was constant. The dry weight of the cubes was determined before the experiment and was subtracted from the weight of the foam plus mycelium. The dry weight was expressed in grams per liter.
RESULTS
Metabolite production and mycelial growth.
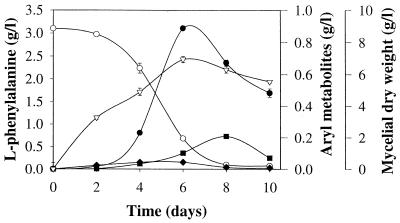
The biomass concentration in B. adusta cultures reached 6.94 g/liter after 6 days of incubation and decreased thereafter (Fig. 1). l-Phenylalanine was totally depleted after 10 days, and the consumption rate was 0.3 g/liter per day. The fungus produced large amounts of benzyl alcohol (887 mg/liter) at a rate of 148 mg/liter per day. The highest benzaldehyde and benzoic acid concentrations observed were 208 and 43 mg/liter, respectively.
FIG. 1.
Kinetics of l-phenylalanine consumption (○) and benzyl alcohol (●), benzaldehyde (■), benzoic acid (⧫), and mycelial dry weight (▿) accumulation in cultures of B. adusta.
Incorporation of U-14C-labelled l-phenylalanine into aryl metabolites.
Tracer studies in which U-14C-labelled l-phenylalanine was used were performed because this technique is very sensitive and nondisruptive. It also allowed us to identify metabolites that were relevant only to aryl metabolite biosynthesis and provided accurate estimates of bioconversion yields because the radioactivity incorporated into the metabolites was measured. The amounts of radioactivity incorporated into benzyl alcohol, benzaldehyde, and benzoic acid after U-14C-labelled l-phenylalanine was added were calculated (Table 1). After 6 days, 39% of the radioactivity was incorporated into benzyl alcohol, 13% was incorporated into benzaldehyde, and 3.2% was incorporated into benzoic acid. We estimated that 18.6% of the l-phenylalanine was mineralized to CO2. After 8 days, the amount of radioactivity incorporated into benzyl alcohol declined to 14%, while the total amount of radioactivity incorporated into benzaldehyde increased to 17.7%. The amount of radioactivity incorporated into benzoic acid decreased to 1.1%, and the estimated amount of radioactivity released as carbon dioxide increased to 62.9%. These results showed that benzoic acid, benzaldehyde, and benzyl alcohol were formed from the upstream precursor l-phenylalanine. However, due to its low sensitivity, HPLC did not allow us to identify trace 14C-labelled aromatic compounds, which could be important metabolic intermediates. Therefore, 13C-labelled intermediates were identified by GC-MS analyses after ring 13C-labelled l-phenylalanine was added.
TABLE 1.
Radioactivity incorporated into the benzyl alcohol, benzaldehyde, and benzoic acid produced by B. adusta after l-[U-14C]phenylalanine was addeda
| Day of harvest | % of radioactivity inb:
|
||||
|---|---|---|---|---|---|
| Residual l-phenylalanine | Benzyl alcohol | Benzaldehyde | Benzoic acid | Estimated 14CO2c | |
| 6 | 26.2 ± 1.3 | 39 ± 2 | 13 ± 0.7 | 3.2 ± 0.2 | 18.6 ± 1 |
| 8 | 4.3 ± 0.2 | 14 ± 1 | 17.7 ± 0.9 | 1.1 ± 0.05 | 62.9 ± 3 |
l-[U-14C]phenylalanine was added at the time of inoculation.
Radioactivity is expressed as the percentage of the total radioactivity added initially (8.29 μCi/ml).
The estimated amount of 14CO2 was calculated by assuming that aryl metabolites and l-phenylalanine could be completely degraded to carbon dioxide.
Identification of ring 13C-labelled aromatic compounds by GC-MS.
The three most representative ring 13C-labelled aromatic compounds identified by GC-MS analyses were benzyl alcohol (58% of the total labelled aromatic compounds), benzaldehyde (24%), and benzoic acid (5%) (Table 2). The relative amounts of several other ring 13C-labelled metabolites (alcohols, aldehydes, and acids) are shown in Table 2. These compounds included para-anisaldehyde (2.5%), acetophenone (0.79%), veratraldehyde (0.70%), 4-hydroxybenzaldehyde (0.55%), 4-hydroxybenzyl alcohol (0.33%), veratryl alcohol (0.27%), and para-anisyl alcohol (0.26%). These results show that the major aromatic compounds biosynthesized from the precursor were transformed to methoxylated and hydroxylated aromatic compounds by the enzymatic complex of the fungus. For instance, the presence of ring 13C-labelled trans-cinnamic acid (Table 3) strongly suggests that l-phenylalanine is converted to trans-cinnamic acid in the first biotransformation step in B. adusta. Furthermore, we also found ring 13C-labelled α- and β-hydroxyphenylpropionic acids (methyl esters) (Table 3), which are oxidation products of trans-cinnamic acid.
TABLE 2.
Relative levels of ring 13C-labelled aryl metabolites identified by GC-MS in a B. adusta culture after ring 13C-labelled phenylalanine was added
| Metabolite | Relative level of 13C-labelled compound (%)a |
|---|---|
| Benzyl alcohol | 58 ± 3 |
| Benzaldehyde | 24 ± 1 |
| Benzoic acid | 5 ± 0.5 |
| para-Anisaldehyde | 2.5 ± 0.12 |
| Acetophenone | 0.79 ± 0.04 |
| Veratraldehyde | 0.70 ± 0.04 |
| para-Anisyl alcohol | 0.26 ± 0.01 |
| Veratryl alcohol | 0.27 ± 0.1 |
| 4-Hydroxybenzaldehyde | 0.55 ± 0.03 |
| 4-Hydroxybenzyl alcohol | 0.33 ± 0.02 |
The levels of all of the metabolites were determined after 10 days. Trace levels of anisic acid, veratric acid, trans-cinnamic acid, 4-hydroxybenzoic acid, phenylpyruvic acid, α-hydroxyphenylpropionic acid, β-hydroxyphenylpropionic acid, phenylacetaldehyde, β-phenylethanol, mandelic acid, benzoylformic acid, phenylacetic acid, and cinnamaldehyde were also found by GC-MS.
TABLE 3.
Relative intensities of majority ions of ring 13C-labelled aryl metabolites produced by B. adusta following addition of ring 13C-labelled l-phenylalanine and relative intensities of majority ions of unlabelled aryl metabolites when unlabelled l-phenylalanine was added
| Aromatic compound | Relative intensity (%) |
|---|---|
| Unlabelled benzaldehyde | m/z 106(75), 105(80), 77(100), 51(80), 29(35) |
| [13C]benzaldehyde | m/z 112 (70), 111 (80), 83(100), 55 (75), 29 (40) |
| Unlabelled benzyl alcohol | m/z 108(50), 79(99), 78(60), 51 (49), 39(30), 29(30) |
| [13C]benzyl alcohol | m/z 114(52), 85(99), 55(49), 42 (25), 29(43) |
| Unlabelled phenylpyruvic methyl ester | m/z 178(45), 118(35), 91(99), 65(37) |
| [13C]phenylpyruvic methyl ester | m/z 184(20), 124(25), 97(99), 69(45) |
| Unlabelled mandelic methyl ester | m/z 166(10), 107(99), 78(60), 79(76) |
| [13C]mandelic methyl ester | m/z 172(15), 113(99), 84(55), 85(99) |
| Unlabelled trans-cinnamic acid | m/z 147(65), 141(6), 126(10), 91(77), 41(100) |
| [trans-13C]cinnamic acid | m/z 153(60), 147(20), 133(20), 97(35), 43(100) |
| Unlabelled α-hydroxyphenylpropionic methyl ester | m/z 180(0), 162(38), 121(10), 103(18), 91(99), 65(17) |
| [α-13C]hydroxyphenylpropionic methyl ester | m/z 186(0), 168(25), 97(99), 109(20), 127(10), 69(17) |
| Unlabelled β-phenylpropionic methyl ester | m/z 180(35), 120(10), 107(99), 79(90) |
| [β-13C]phenylpropionic methyl ester | m/z 186(40), 126(20), 113(99), 85(95) |
| Unlabelled acetophenone | m/z 120(25), 105(90), 77(99), 51(50) |
| [13C]acetophenone | m/z 126(25), 111(95), 83(99), 57(55) |
Bioconversion of different aromatic acids to aryl metabolites.
l-Phenylalanine and several putative precursors, including phenylpyruvic, phenylacetic, mandelic, and benzoylformic acids, were added to B. adusta cultures. For each precursor, the maximum amount of aryl metabolite produced (expressed as a percentage of the amount of precursor initially added) and the day on which maximum production occurred are shown in Table 4. When the precursor was l-phenylalanine, 45% of this compound was converted to benzyl alcohol; when trans-cinnamic acid, phenylpyruvic acid, mandelic acid, and benzoylformic acid were the precursors, the corresponding values were 42, 22, 9, and 11%, respectively. In comparison, 21, 11, 8, 5, and 2% of the precursor were converted to benzaldehyde when the precursors were trans-cinnamic acid, l-phenylalanine, phenylpyruvic, mandelic acid, and benzoylformic acid, respectively. Of the precursors tested, trans-cinnamic acid was by far the precursor that was most efficiently bioconverted to benzoic acid (63%). Other precursors were poorly converted (≤2%) to benzoic acid.
TABLE 4.
Aromatic compounds produced by B. adusta in media supplemented with different putative precursors
| Precursora | Final mycelial dry wt (mg/liter)b | Amt of metabolites produced (%)c
|
||
|---|---|---|---|---|
| Benzaldehyde | Benzyl alcohol | Benzoic acid | ||
| l-Phenylalanine | 5.54 ± 0.25 (10) | 11 ± 0.3 (8) | 45 ± 0.03 (6) | 2 ± 0.2 (6) |
| trans-Cinnamic acid | 5.65 ± 0.03 (10) | 21 ± 0.07 (8) | 42 ± 3 (4) | 63 ± 1 (8) |
| Phenylpyruvic acid | 5.10 ± 0.26 (10) | 8 ± 0.6 (6) | 22 ± 1 (8) | 2 ± 0.1 (4) |
| Mandelic acid | 3.84 ± 0.34 (10) | 5 ± 0.1 (6) | 9 ± 0.5 (10) | 0.8 ± 0.01 (8) |
| Benzoylformic acid | 4.25 ± 0.82 (10) | 2 ± 0.5 (8) | 11 ± 0.9 (10) | 2 ± 0.08 (10) |
Precursors were added at the time of inoculation. The concentrations of precursors were 3 g/liter for l-phenylalanine, phenylpyruvic acid, mandelic acid, and benzoylformic acid and 0.1 g/liter for trans-cinnamic acid.
The values in parentheses indicate the day on which biomass was harvested; this was not necessarily the day on which the maximum biomass concentration was reached.
The amounts of aryl metabolites produced are expressed as percentages of the amount of precursor initially added. The values in parentheses indicate the days on which the maximum amounts of benzaldehyde, benzyl alcohol, and benzoic acid were produced.
The final mycelial dry weights were 5.54, 5.65, 5.10, 3.84, and 4.25 g/liter, respectively, when we used l-phenylalanine, trans-cinnamic acid, phenylpyruvic acid, mandelic acid, and benzoylformic acid, respectively. The various precursors were not autooxidized to aryl metabolites (benzaldehyde, benzyl alcohol, and benzoic acid) in uninoculated controls (Table 4).
Enzymes involved in metabolite biosynthesis.
Several extracellular and intracellular enzymatic activities (Table 5) were monitored with and without cycloheximide, an inhibitor of eucaryotic protein biosynthesis.
TABLE 5.
Effect of cycloheximide on the concentrations of aryl metabolites, extracellular and intracellular enzymatic activities, l-phenylalanine consumption, and mycelial dry weight in B. adustaa
| Concn of inhibitor (mg/liter) | Initial l-phenylalanine concn (mg/liter) | Residual l-phenyl-alanine concn (mg/liter) | Mycelial dry wt (g/liter) | Concn (mg/liter) of:
|
Enzyme activities (U/liter)
|
Enzyme sp act (U/g)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzyl alcohol | Benzaldehyde | Benzoic acid | AAO | LiP | MnP | Laccase | AAO | LiP | PAL | AAD | AADD | Transaminase | MnP | Laccase | ||||
| 0 | 3,100 | 72 ± 12 | 5.54 ± 0.1 | 484 ± 26 | 71 ± 4 | 7 ± 2 | 7.4 ± 2 | 7 ± 2.6 | NDb | ND | 8.75 ± 1 | 37 ± 6 | 25 ± 0.4 | 91 ± 10 | 12 ± 0.8 | ND | ND | ND |
| 10c | 3,100 | 3,000 | 6.3 ± 1.2 | 17 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Activities were determined after 10 days of cultivation.
ND, not detected.
The inhibitor was added at the time of inoculation.
Intracellular and extracellular LiP and AAO activities were detected in a reference culture of B. adusta (Table 5). The AAO activity was 7.4 U/liter in the culture supernatant, and the intracellular activity was 8.7 U/g. In addition, LiP activity was detected extracellularly (7 U/liter), as well as intracellularly (37 U/g). PAL, AAD, and AADD activities were detected only intracellularly (Table 5); the PAL, AAD, and AADD activities were 25, 91, and 12 U/g, respectively, after 10 days of cultivation. Neither extracellular nor intracellular MnP, transaminase, or laccase activity was detected in the reference culture (Table 5).
Cycloheximide completely inhibited enzymatic activities and almost completely inhibited metabolite biosynthesis (Table 5). l-Phenylalanine was not depleted after 10 days of incubation, and 97% of the precursor remained in the culture supernatant (Table 5). In contrast, l-phenylalanine was almost totally consumed when no cycloheximide was added. Cycloheximide did not affect mycelial growth. After 10 days of incubation, the mycelial dry weights were 5.54 g/liter in reference cultures and 6.3 g/liter in cultures supplemented with cycloheximide (Table 5). When cycloheximide was added to B. adusta cultures, little benzyl alcohol (17 mg/liter) was produced, and neither benzaldehyde production nor benzoic acid production occurred.
These results strongly suggest that AAO, LiP, PAL, AAD, and AADD are involved in aryl metabolite biosynthesis in B. adusta. PAL activity, but not transaminase activity, was detected in reference cultures. This shows that PAL initiates l-phenylalanine degradation, which leads to trans-cinnamic acid as the first biotransformation product.
DISCUSSION
This was the first metabolic study of biosynthesis of aryl metabolites in the fungus B. adusta in which tracer experiments were coupled with measurements of intracellular and extracellular enzymatic activities.
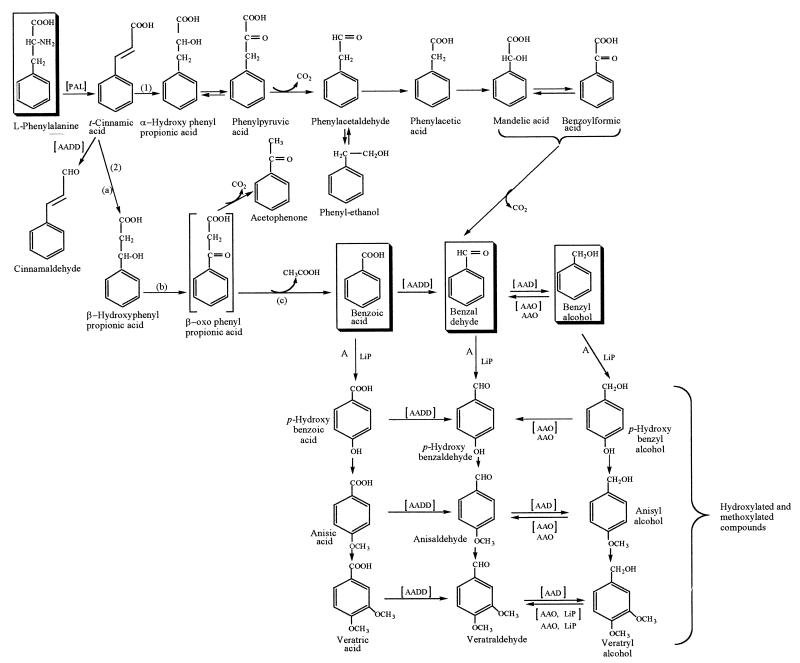
Our results revealed the metabolic pathway that leads from l-phenylalanine to the major aryl metabolites produced by B. adusta. The presence of 13C-labelled trans-cinnamic acid together with PAL activity shows that trans-cinnamic acid is a key pathway intermediate. In addition, trans-cinnamic acid is an efficient precursor of benzoic acid, benzyl alcohol, and benzaldehyde. trans-Cinnamic acid can be subsequently hydroxylated to β-hydroxyphenylpropionic acid (Fig. 2, pathway 2), which in turn can be converted via a β-oxidation step to benzoic acid. This was confirmed by the presence of acetophenone as a degradation product of β-hydroxyphenylpropionic acid. Furthermore, trans-cinnamic acid is the precursor that is most efficiently converted to benzoic acid among the putative precursors which we tested. This confirms that there is a β-oxidation process in B. adusta and that benzoic acid is the major product of this process. β-Oxidation has been found previously in several other fungi (13), although apparently not in B. adusta. To our knowledge, this is the first time that β-oxidation of this nature has been found in a white rot fungus.
FIG. 2.
Proposed pathways for degradation of l-phenylalanine by B. adusta. Pathway 1, nonoxidative l-phenylalanine degradation pathway. Pathway 2, β-oxidation pathway. a, b, and c, β-oxidation sequence. The intermediates in brackets are hypothetical intermediates. The enzymes in brackets are intracellular enzymes.
trans-Cinnamic acid can also be oxidized to α-hydroxyphenylpropionic acid, which can be subsequently oxidized to phenylpyruvic acid (Fig. 2, pathway 1). The latter compound is decarboxylated to phenylacetaldehyde, which is oxidized to phenylacetic acid. Then hydroxylation in the α-position followed by oxidation to the corresponding α-oxo acid and a final decarboxylation leads to mandelic acid, benzoylformic acid, and benzaldehyde. Benzaldehyde is reduced to benzyl alcohol by an intracellular AAD.
The presence of benzaldehyde and benzyl alcohol suggested that there is a mechanism that involves joint activity of AAO and AAD, which converts alcohol to aldehyde and vice versa. The reduction of benzoic acid, which results from β-oxidation of trans-cinnamic acid, to benzaldehyde in B. adusta is carried out by an intracellular AADD, an enzyme found previously in P. eryngii (9).
In addition to benzoic acid, benzaldehyde, and benzyl alcohol, the corresponding para-hydroxyl, para-anisyl, and veratryl compounds were also identified. The hydroxyl- and methoxybenzylic compounds are the result of the activities of LiP, AAO, AAD, and AADD with benzoic acid, benzaldehyde, and benzyl alcohol. Consistent with this, the PAL, AAO, AAD, and AADD activities in B. adusta cultures were totally inhibited after cycloheximide was added, as was aryl metabolite production. The metabolite para-anisaldehyde has been found previously in several other fungi (2, 10), but it was particularly abundant in our study. To date, no complete metabolic sequence for biosynthesis of this metabolite has been described. We also found veratryl alcohol and its corresponding aldehyde and acid in B. adusta cultures. This secondary metabolite is produced by many white rot fungi (13, 14), and its biosynthetic pathway has been studied in P. chrysosporium (13). Jensen et al. (13) suggested that veratryl alcohol biosynthesis proceeds as follows: phenylalanine→cinnamate→benzoate and/or benzaldehyde→veratryl alcohol. These authors did not describe other metabolic intermediates or enzymatic activities that may be associated with veratryl alcohol biosynthesis.
This was the first complete metabolic study in which tracer experiments were performed along with analyses of enzymatic activities involved in l-phenylalanine degradation and aryl metabolite biosynthesis. Two metabolic pathways for benzylic compound formation in B. adusta are described here. In pathway 1 benzaldehyde and benzyl alcohol are the major benzyl metabolites. Pathway 2 involves a β-oxidation which is described for the first time here and leads to benzoic acid formation. Our results also show that trans-cinnamic acid is a key intermediate and that PAL initiates the pathway that leads from l-phenylalanine to benzoic acid, benzaldehyde, and benzyl alcohol. These benzylic compounds are subsequently hydroxylated and/or methoxylated by intracellular enzymatic activities (AAO, LiP, AAD, AADD) and extracellular enzymatic activities (LiP, AAO) which lead to methoxylated and hydroxylated benzyl alcohols, aldehydes, or acids in B. adusta cultures.
ACKNOWLEDGMENTS
C.L. is grateful to INRA (Institut National de la Recherche Agronomique), DRI (Direction des Relations Internationales), for a Ph.D. scholarship.
We thank J. Ouazzani (Institut de Chimie des Substances Naturelles, CNRS) for help in performing 14C-HPLC analyses and G. Feron (Institut National de la Recherche Agronomique, Laboratoire de Recherches Sur les Arômes) and Henry Eric Spinnler (Institut National Agronomique Paris-Grignon, Laboratoire de Génie et Microbiologie des Procédés Alimentaires) for valuable discussions.
REFERENCES
- 1.Berger R G, Neuhäuser K, Drawert F. Characterization of the odour principles of some basidiomycetes: Bjerkandera adusta, Poria aurea, Tyromyces sambuceus. Flavour Fragrance J. 1986;1:181–185. [Google Scholar]
- 2.Berger R G, Neuhaüser K, Drawert F. Biotechnological production of flavour compounds. High productivity fermentation of volatile flavour using a strain of Ischnoderma benzoinum. Biotechnol Bioeng. 1987;30:987–990. doi: 10.1002/bit.260300811. [DOI] [PubMed] [Google Scholar]
- 3.Blau K, Halket J. Handbook of derivatives for chromatography. 2nd ed. New York, N.Y: John Wiley & Sons; 1993. [Google Scholar]
- 4.Bourbonnais R, Paice M. Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) Appl Microbiol Biotechnol. 1992;36:823–827. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Buswell J A, Odier E. Lignin biodegradation. Crit Rev Biotechnol. 1987;6:1–60. [Google Scholar]
- 7.Gallois A, Gross B, Langlois D, Spinnler H-E, Brunerie P. Influence of culture conditions on the production of flavour compounds by 29 ligninolytic basidiomycetes. Mycol Res. 1990;4:494–504. [Google Scholar]
- 8.Guillén F, Martinez A T, Martinez M J, Evans C S. Hydrogen-peroxide-producing system of Pleurotus eryngii involving the extracellular enzyme aryl-alcohol oxidase. Appl Microbiol Biotechnol. 1994;41:465–470. [Google Scholar]
- 9.Guillén F, Evans C S. Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl Environ Microbiol. 1994;60:2811–2817. doi: 10.1128/aem.60.8.2811-2817.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez A, Caramelo L, Prieto A, Martinez M J, Martinez A T. Anisaldehyde production and aryl-alcohol oxidase and dehydrogenase activities in ligninolytic fungi of the genus Pleurotus. Appl Environ Microbiol. 1994;60:1783–1788. doi: 10.1128/aem.60.6.1783-1788.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagedorn S, Kaphammer B. Microbial biocatalysis in the generation of flavor and fragrance chemicals. Annu Rev Microbiol. 1994;48:773–800. doi: 10.1146/annurev.mi.48.100194.004013. [DOI] [PubMed] [Google Scholar]
- 12.Janssens L, De Pooter H L, Schamp N M, Vandamme E J. Production of flavours by microorganisms. Process Biochem. 1992;27:195–215. [Google Scholar]
- 13.Jensen K A, Evans K M C, Kirk T K, Hammel K E. Biosynthetic pathway for veratryl alcohol in the ligninolytic fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1994;60:709–714. doi: 10.1128/aem.60.2.709-714.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai S, Umezawa T, Higuchi T. De novo synthesis of veratryl alcohol by Coriolus versicolor. Wood Res. 1986;73:18–21. [Google Scholar]
- 15.Kirk T K, Farrell R. Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 16.Krings U, Berger R G. Biotechnological production of flavours and fragrances. Appl Microbiol Biotechnol. 1998;49:1–8. doi: 10.1007/s002530051129. [DOI] [PubMed] [Google Scholar]
- 17.Krings U, Hinz M, Berger R G. Degradation of [2H]phenylalanine by the basidiomycete Ischnoderma benzoinum. J Biotechnol. 1996;51:123–129. [Google Scholar]
- 18.Lapadatescu C, Feron G, Vergoignan C, Djian A, Durand A, Bonnarme P. Influence of cell immobilization on the production of benzaldehyde and benzyl alcohol by the white-rot fungi Bjerkandera adusta, Ischnoderma benzoinum and Dichomitus squalens. Appl Microbiol Biotechnol. 1997;47:708–714. [Google Scholar]
- 19.Lapadatescu C, Giniès C, Djian A, Spinnler H-E, Le Quéré J-L, Bonnarme P. Regulation of the synthesis of aryl metabolites by phospholipid sources in the white-rot fungus Bjerkandera adusta. Arch Microbiol. 1999;171:151–158. [Google Scholar]
- 20.Lapadatescu C, Bonnarme P. Production of aryl metabolites in solid-state fermentations of the white-rot fungus Bjerkandera adusta. Biotechnol Lett. 1999;21:763–769. [Google Scholar]
- 21.Mester T, Swarts H J, Romero Solé S, De Bont J A M, Field J A. Stimulation of aryl metabolite production in the basidiomycete Bjerkandera sp. strain BOS55 with biosynthetic precursors and lignin degradation products. Appl Environ Microbiol. 1997;63:1987–1994. doi: 10.1128/aem.63.5.1987-1994.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muheim A, Waldner R, Leisola M S A, Fiechter A. An extracellular aryl-alcohol oxidase from the white-rot fungus Bjerkandera adusta. Enzyme Microb Technol. 1990;12:204–209. [Google Scholar]
- 23.Muheim A, Waldner R, Sanglard D, Reiser J, Schoemaker H E, Leisola M S A. Purification and properties of an aryl-alcohol dehydrogenase from the white-rot fungus Phanerochaete chrysosporium. Eur J Biochem. 1991;195:369–375. doi: 10.1111/j.1432-1033.1991.tb15715.x. [DOI] [PubMed] [Google Scholar]
- 24.Orndorff S A, Constantino N, Stewart D, Durham D R. Strain improvement of Rhodotorula graminis for production of a novel l-phenylalanine ammonialyase. Appl Environ Microbiol. 1988;54:996–1002. doi: 10.1128/aem.54.4.996-1002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paszczynski A, Crawford R, Huynh V B. Manganese peroxidase of Phanerochaete chrysosporium: purification. Methods Enzymol. 1988;161:264–268. [Google Scholar]
- 26.Peláez F, Martínez M J, Martínez A T. Screening of 68 species of basidiomycetes for enzymes involved in lignin degradation. Mycol Res. 1995;99:37–42. [Google Scholar]
- 27.Tien M, Kirk T K. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium Burds. Science. 1983;221:661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- 28.Welsh W F, Murray W D, Williams R E. Microbiological and enzymatic production of flavour and fragrance chemicals. Crit Rev Biotechnol. 1989;9:105–169. [Google Scholar]
- 29.Yvon M, Thirouin S, Rijnen L, Fromentier D, Gripon J C. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl Environ Microbiol. 1997;63:414–419. doi: 10.1128/aem.63.2.414-419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]