Abstract
Background/purpose
Oral squamous cell carcinoma (OSCC) is a highly malignant tumor, and the overall survival (OS) time of patients with OSCC varies considerably. This study aimed to identify reliable biomarkers for OSCC and construct a new prognostic signature, which may guide personalized precision treatment.
Materials and methods
Transcriptome array data of 317 patients with OSCC from The Cancer Genome Atlas Project (TCGA) cohort were retrospectively analyzed. Single-sample gene set enrichment analysis (ssGSEA) and univariate Cox regression were performed to identify the prognostic significance of the hallmarks of each tumor in OSCC. Subsequently, lncRNAs related to glycolysis were identified through co-expression analysis. A glycolysis-related prognostic signature was constructed by combining univariate Cox regression, least absolute shrinkage and selection operator (Lasso) regression, and multivariate Cox regression analyses. Additionally, the infiltration of immune cells in OSCC was evaluated based on data from ssGSEA and TIMER databases.
Results
Glycolysis was identified as the main risk factor for OS in a variety of cancer hallmarks. The 4-lncRNA glycolysis prognostic signature could distinguish high and low-risk patients. This risk signature was found to be an independent prognostic risk factor for OSCC, showing good predictive power compared with other clinicopathological indicators. Immune correlation analysis showed that patients in the low-risk group exhibited higher levels of immune cell infiltration.
Conclusion
The novel 4-lncRNA prognostic signature can predict the clinical outcome of patients with OSCC well, and it is expected to become a promising prognostic biomarker as well as a potential therapeutic target in the future.
Keywords: OSCC, LncRNA, Signature, Prognostic biomarker
Introduction
Oral squamous cell carcinoma (OSCC) is a common malignant tumor worldwide, accounting for 90% of cases of oral cavity cancer.1 Patients are prone to early metastasis as a result of the rapid local invasion of OSCC, and their prognosis is often poor.2 Current reports indicate that more than 350,000 new cases of OSCC were diagnosed in 2018, and more than 170,000 OSCC patients died.3,4 In recent years, with the development of medical technology, treatment methods have also been greatly improved, leading to improved quality of life of patients with OSCC. However, the five-year overall survival (OS) rate of patients has not changed and remains dismal.5 At present, the high mortality rate of patients with OSCC may be attributed to the poor understanding of the molecular genetics and metabolic mechanisms of OSCC, as well as the lack of effective prognostic factors and therapeutic targets.6 At the same time, traditional clinical indicators, including the clinical stage and the tumor size, play a very limited role in assessing the prognosis and survival of patients, which is far from ideal.7 Therefore, there is an urgent need to identify new and reliable prognostic biomarkers to improve the prognosis of patients with OSCC and to better understand the molecular mechanisms of OSCC progression.
Tumor metabolic reprogramming plays an important role in tumor energy metabolism and biosynthetic pathways.8 Glycolysis is the most representative metabolic characterization in the process of tumorigenesis.9 Moreover, glycolysis is also a unique energy metabolism method possessed by tumor cells. Glycolysis reprograms the process of energy acquisition in tumor cells; tumor cells produce a large amount of lactic acid through aerobic glycolysis, which leads to the accumulation of lactic acid, thereby changing the microenvironment in which the tumor cells are located.10 Therefore, glycolysis is more conducive to the proliferation and metastasis of tumor cells. Increasing evidence suggests that inhibiting the tumor glycolysis process may be a promising strategy in tumor therapy.11 So far, the prognostic significance of metabolic characteristics in OSCC has not been fully explored to a large extent. Increasing research shows that long non-coding RNAs (lncRNAs) play an important role in normal physiological processes and disease occurrence. Recent studies have shown that thousands of lncRNAs are abnormally expressed in various cancer types, and some of them are involved in the malignant development of tumors.12 Furthermore, various studies have also shown that abnormally expressed lncRNAs can be used as potential biomarkers for the evaluation of tumor prognosis.13 However, at present, little is known about the function and role of lncRNAs in the glycolytic process of OSCC.
The Cancer Genome Atlas (TCGA) is a large-scale cancer genome project that provides a wealth of resources for studying the molecular genetic mechanisms of various tumors and exploring biomarkers. In this study, RNA sequencing (RNA-Seq) data in OSCC samples were obtained from TCGA. A new 4-lncRNA prognostic signature with the excellent performance in the prognostic evaluation of OSCC was identified through combining clinical follow-up data and bioinformatics methods.
Materials and methods
Data acquisition and processing
RNA-seq data of clinical specimens (the alveolar ridge, buccal mucosa, floor of mouth, tongue, lips, oral cavity, and hard palate) were downloaded in the FPKM (Fragments Per Kilobase Million) format from TCGA (The Cancer Genome Atlas, https://portal.gdc.cancer.gov/) database.14 The samples included 32 normal controls and 319 tumor specimens, and the data were converted into the TPM (Transcripts Per Kilobase Million) format. At the same time, the corresponding clinical staging and follow-up data were also downloaded.
Identification of glycolytic metabolic characterization
The hallmark gene set was downloaded from the Molecular Signatures Database (MSigDB, https://www.gsea-msigdb.org/gsea/msigdb).15 To quantify cancer hallmarks in OSCC, single-sample gene set enrichment analysis (ssGSEA) was performed using the “GSVA” package in R. Univariate Cox proportional hazards regression analysis (Cox-PH) was performed using the “survival” package in R to evaluate the impact of cancer hallmarks on the OS of patients with OSCC.
Construction of a glycolysis-related lncRNA prognostic signature
Pearson correlation analysis was performed to construct a glycolysis-related mRNA-lncRNA co-expression network, and the following cut-off values were set: correlation coefficient >0.4 and P < 0.001. Subsequently, the ‘limma’ package in R was used to identify the differentially expressed lncRNAs in OSCC tumor tissues, and the screening criteria were set as follows: |log2FoldChange| >1, false discovery rate (FDR) < 0.05. TCGA cohort was randomly divided into a train set and a test set, and lncRNAs related to OS were identified through univariate Cox regression analysis. Then, the least absolute shrinkage and selection operator (Lasso) Cox algorithm was used to further screen lncRNAs related to OS, and finally, the prognostic signature was constructed by stepwise regression of multivariate Cox analysis. The risk score of the prognostic signature was calculated using the following formula: Risk score = ΣCoefficient (mRNAi) × Expression (mRNAi).
Bioinformatics and statistical analyses of the prognostic signature
R software (version 4.1.1, http://www.r-project.org) was used for data analysis and graphing in this study. The Kaplan–Meier (K-M) method was used to plot survival curves using the ‘survival’ package in R, and the log-rank test was used to evaluate the statistical significance of survival differences between the two groups. Values with P < 0.05 were considered statistically significant. A time-dependent receiver operating characteristic (tROC) graph was plotted using the “survivalROC” package in R to evaluate the predictive performance of the signature. Univariate and multivariate Cox analyses were performed to evaluate the prognostic significance of the glycolytic phenotype and risk signatures in predicting OS. Twenty-nine types of immune-related cells and types in the OSCC queue were estimated based on the ssGSEA method through the “GSVA” package in R. The levels of immune cell infiltration in the high- and low-risk groups were compared through the TIMER (Tumor Immune Estimation Resource, https://cistrome.shinyapps.io/timer/) database, which is a comprehensive website for the analysis of the tumor immune microenvironment.16 Additionally, the “limma” package in R was used to compare the differentially expressed mRNAs in the high- and low-risk groups, and the “clusterProfiler” package in R was used to perform Gene Ontology (GO) and Kyoto Encylopedia of Genes and Genomes (KEGG) enrichment analyses on the differentially expressed genes (DEGs). Furthermore, a protein–protein interaction network was constructed based on the GeneMANIA (http://genemania.org/) database.
Results
Glycolysis is an important risk factor for OSCC
The prognostic significance of multiple cancer hallmarks in OSCC was evaluated using the ssGSEA algorithm combined with univariate Cox analysis. Oxidative phosphorylation, MYC targets, hypoxia, glycolysis, cholesterol homeostasis, and adipogenesis were identified as risk factors for OSCC, among which glycolysis was defined as the most important risk factor according to hazard ratios and P-values (Fig. 1A). An expression heat map was plotted to show the correlation between the Z score of glycolysis and survival status, and the results showed that the Z score was significantly correlated with survival status (Fig. 1B). At the same time, the Z score of glycolysis was further compared under different survival conditions, and the results showed that the Z score of glycolysis was higher among patients with OSCC who died (Fig. 1C). The effects of glycolysis and multiple clinical factors on the prognosis of OS were analyzed through univariate Cox (Fig. 1D) and multivariate Cox regression analyses (Fig. 1E). The results showed that glycolysis was an important prognostic risk factor for OSCC. Furthermore, the K-M survival curve of glycolysis as a prognostic marker was plotted, and the results showed that patients with high glycolysis scores had a worse OS rate (Fig. 1F). These findings indicated that glycolysis is an unusually important prognostic risk factor in OSCC compared with other cancer hallmarks and clinicopathological factors.
Figure 1.
Glycolysis was identified as the primary risk factor for OSCC. A. Results of univariate Cox regression analysis showed that the glycolytic phenotype affected the prognosis of patients with oral squamous cell carcinoma (OSCC). B. The single-sample gene set enrichment analysis (ssGSEA) score of glycolysis was significantly correlated with the overall survival (OS) status of patients with OSCC. C. Differences in the distribution of glycolytic ssGSEA scores under different living conditions. D. The prognostic significance of glycolysis and other clinicopathological factors in OSCC was assessed through univariate Cox regression analysis. E. Results of multivariate Cox regression analysis showed that glycolysis was an important risk factor for OSCC. F. Results of the Kaplan–Meier analysis showed that patients with high glycolytic ssGSEA scores showed a worse prognosis.
Construction of the lncRNA risk signature related to glycolysis
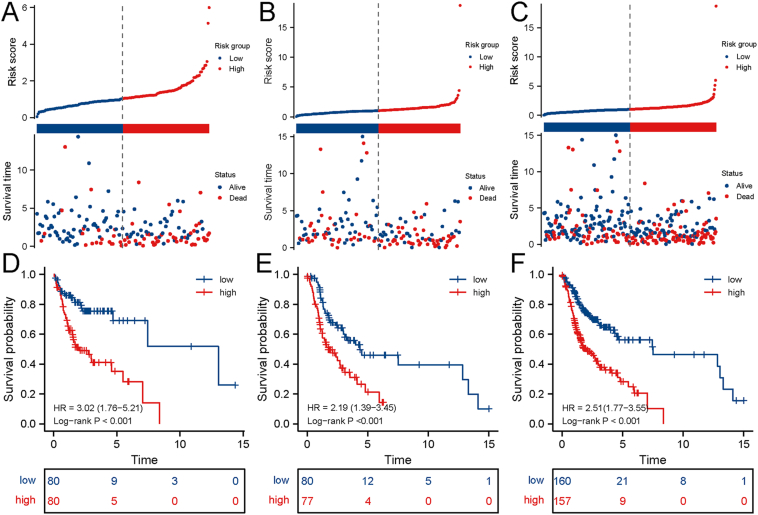
Frist, glycolysis-related genes from MGSigDB were screened out, and then 2520 glycolysis-related lncRNAs were identified through Pearson's correlation analysis (|R2| > 0.4, P < 0.001). Subsequently, the lncRNAs specifically expressed in OSCC tumor tissues were screened out, of which 133 lncRNAs were downregulated and 952 lncRNAs were upregulated in OSCC. Differentially expressed lncRNAs between tumor and normal tissues are shown in the volcano map and heat map (Fig. 2A and B). Subsequently, the TCGA cohort was randomly divided into training and test cohorts, and lncRNAs significantly related to OS (p < 0.05) in the training cohort were identified through univariate Cox regression analysis (Fig. 2C). A total of 10 risk factors and 1 protective factor were identified (Table 1). Subsequently, Lasso regression was used to further screen out the most robust lncRNAs related to OS by applying a 10-fold cross-validation to overcome overfitting, and finally, five lncRNAs were identified (Fig. 2D). Furthermore, a multivariate stepwise Cox regression analysis by backward and forward was performed to screen the independent prognostic factors. Finally, a 4-lncRNA signature was built, and the risk score was calculated using the following formula: Risk score = 0.388∗DDN-AS1 + 0.112∗AL035458.2 + (−1.583∗LINC01281) + 0.022∗AC245041.2. Subsequently, risk scores were calculated in the training, test, and whole set (entire TCGA cohort), and patients were divided into high- and low-risk groups based on the median risk score. Among them, the high-risk group in the training cohort showed a higher risk score, with more deaths in the high-risk group (Fig. 3A). Similar results were also observed in the test cohort and the whole cohort. These findings indicated that the risk signature had a good predictive ability in distinguishing high- and low-risk patients with OSCC (Fig. 3B and C). In addition, results of K-M analysis suggested that in the training, test, and whole cohorts, patients in the high-risk group exhibited a worse OS rate compared to those in the low-risk group (Fig. 3D, E, F). Taken together, these results showed that the 4-lncRNA glycolysis risk signature could distinguish high-risk groups from low-risk groups well.
Figure 2.
Identification of a long non-coding RNA (lncRNA) risk signature related to glycolysis. A. Differential analysis of lncRNAs related to glycolysis. B. A Heat map of differentially expressed lncRNAs related to glycolysis. C. LncRNAs significantly associated with overall survival in the training set were screened out using univariate Cox regression analysis. D. LASSO regression analysis was performed to further screen for robust prognostic markers of oral squamous cell carcinoma.
Table 1.
Identification of overall survival-associated lncRNAs through univariable Cox regression.
| Gene | Description | HR, 95%CI |
|---|---|---|
| LINC02014 | Long Intergenic Non-Protein Coding RNA 2014 | 1.14 (1.05–1.24) |
| AL035458.2 | No data | 1.15 (1.04–1.27) |
| SLC16A1-AS1 | SLC16A1 Antisense RNA 1 | 1.47 (1.13–1.91) |
| AC245041.2 | No data | 1.03 (1.01–1.05) |
| AC122710.2 | No data | 1.14 (1.04–1.26) |
| LINC01281 | Long Intergenic Non-Protein Coding RNA 1281 | 0.14 (0.03–0.59) |
| AL355574.1 | No data | 1.10 (1.03–1.18) |
| AL109615.3 | No data | 1.14 (1.04–1.26) |
| DDN-AS1 | DDN And PRKAG1 Antisense RNA 1 | 1.71 (1.15–2.54) |
| KLHL7-DT | KLHL7 Divergent Transcript | 1.17 (1.04–1.32) |
| ZNF114-AS1 | ZNF114 Antisense RNA 1 | 1.42 (1.09–1.84) |
Hazard ration, HR; Confidence interval, CI.
Figure 3.
The clinical presentation of the prognostic risk signature in the training set, test set, and whole set. The distribution of the risk score and overall survival (OS) status in high and low-risk groups in the training (A), test (B), and whole (C) cohorts. Results of Kaplan–Meier analysis showed that the high-risk group had worse OS rates in the training (A), test (B), and whole (C) cohorts.
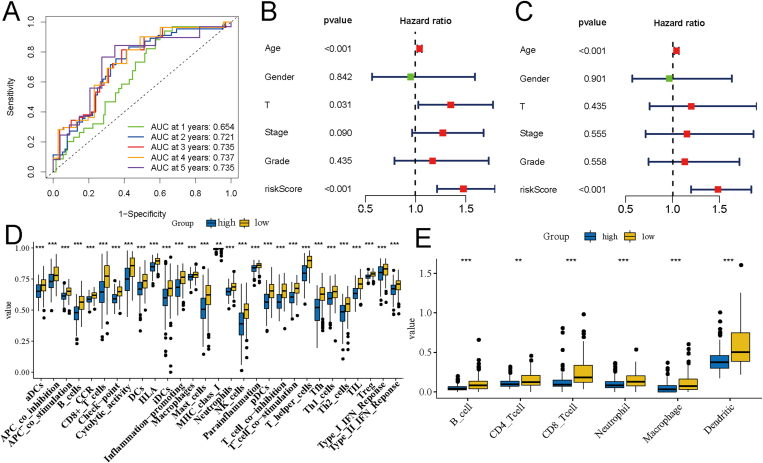
The 4-lncRNA glycolysis signature is an independent prognostic factor for OSCC
The receiver operating characteristic (ROC) curve was plotted to evaluate the effectiveness of the 4-lncRNA glycolysis signature in OSCC prognostic evaluation. The results showed that the signature had a good predictive ability. The area under the ROC curve (AUC) values were all above 0.7 in predicting the 2–5 years OS rate of patients with OSCC (Fig. 4A). Subsequently, univariate Cox regression (Fig. 4B) and multivariate Cox regression (Fig. 4C) analyses were performed to check whether 4-lncRNA was an independent prognostic factor of OSCC. The results showed that the 4-lncRNA signature was the primary prognostic factor for OSCC. In addition, the level of immune cell infiltration was further analyzed in the high- and low-risk groups. A total of 29 types of immune cells and immune function classification were obtained using the ssGSEA algorithm. The results revealed higher infiltration of immune cells, including B cells, CD8+T cells, Th1 cells, Th2 cells, and natural killer cells, in the low-risk group (Fig. 4C). At the same time, the immune cell infiltration was also analyzed in the high- and low-risk groups using the TIMER database, and the results also revealed higher immune cell infiltration in the low-risk group (Fig. 4E). These findings indicated that the level of immune cells in the high-risk group was lower than that in the low-risk group, which further explains the better prognosis of the low-risk group.
Figure 4.
Correlation analysis of clinicopathological factors and immune cell infiltration in the risk signature. A. Results of receiver operating characteristic (ROC) curve analysis showed that the risk signature had a high predictive performance. Univariate Cox (B) and multivariate Cox (C) analyses showed that the risk signature was an independent prognostic risk factor for oral squamous cell carcinoma. D. Differences in the infiltration of immune cells and immune cell functions in the high- and low-risk groups were analyzed using the single-sample gene set enrichment analysis (ssGSEA) algorithm. E. The infiltration of immune cells in the high- and low-risk groups was analyzed using the TIMER database.
Functional enrichment analysis related to glycolysis
The DEGs in the high- and low-risk groups were obtained through differential analysis. The results showed that there were 463 downregulated genes and 159 upregulated genes in the high-risk group (Fig. 5A). Subsequently, GO and KEGG enrichment analyses were performed to explore the potential functions of these DEGs. Results of GO analysis revealed that these DEGs were mainly involved in biological processes such as immunological synapse, immunoglobulin complex, and T-cell receptor complex and cellular components such as T-cell differentiation, T-cell activation, and regulation of lymphocyte activation (Fig. 5B). Moreover, the DEGs were involved in molecular functions such as Th1 and Th2 cell differentiation, Th17 cell differentiation, and cell adhesion molecules, among others. The results of KEGG pathway enrichment analysis revealed that the DEGs were mainly involved in cytokine binding, chemokine binding, cytokine receptor activity, and antigen binding (Fig. 5C). Furthermore, the prognostic significance of these DEGs in OSCC was analyzed through univariate Cox regression analysis, and a total of 38 genes with prognostic significance were obtained, out of which AADACP1 was the most significant (Fig. 5D). Subsequently, the potential interaction network of these prognostic-related genes was constructed using the GeneMANIA database. Results of the functional analysis showed that these molecules were mainly involved in the regulation of cell adhesion, positive regulation of T-cell activation, and positive regulation of cell–cell adhesion (Fig. 6).
Figure 5.
Functional enrichment analysis. A. Differentially expressed genes (DEGs) in the high- and low-risk groups. B, C. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis of DEGs. D. The prognostic significance of DEGs in oral squamous cell carcinoma was analyzed through univariate Cox regression analysis.
Figure 6.
A protein–protein interaction network was constructed using the GeneMANIA database.
Discussion
OSCC is a malignant tumor with a high degree of heterogeneity and high mortality.17 At present, there is still a lack of effective prognostic markers and molecular therapeutic targets for individualized evaluation and treatment.18 Although previous reports have shown the significance of some genes and molecular markers of OSCC for prognostic evaluation, the role of glycolysis in the prognosis of OSCC has not been fully analyzed and explored. Glycolysis is an important metabolic feature of many solid tumors and an important part of tumor cell metabolic reprogramming.19 Abnormal activation of glycolytic pathways in tumor cells has a wide range of effects on tumor cells and the microenvironment, causing changes in the way in which tumor cells obtain energy, producing large amounts of lactic acid, changing the tumor microenvironment, and further leading to malignant proliferation and metastasis of tumor cells.20 At the same time, the abnormal activation of glycolysis also affects other tumor phenotypes, causing the tumor to develop drug resistance and further leading to the malignant process of the tumor and poor clinical outcomes of patients.21 These findings indicate that glycolysis is a promising therapeutic target. At present, some glycolysis-related gene signatures have been constructed for prognostic evaluation of different types of tumors.22,23 However, most signatures are based on coding genes, and there is a lack of glycolysis-related lncRNA risk signatures.
In this study, glycolysis in various cancer phenotypes was identified as the major risk factor affecting the OS rate of OSCC through ssGSEA and univariate Cox regression analyses. When other clinicopathological indicators were combined for multivariate Cox regression analysis, the glycolytic phenotype showed independent prognostic factor characteristics. Subsequently, lncRNAs that were significantly related to glycolysis-encoding genes were identified using Pearson's correlation analysis. Robust prognostic markers were screened through univariate Cox regression and lasso regression analyses. Finally, a multivariate stepwise regression analysis was performed to construct a glycolysis-related 4-lncRNA prognostic signature. Subsequently, the prognostic value of the signature in OSCC was verified in the training, test, and whole datasets. The signature could distinguish high-risk patients from low-risk patients, with significant differences in OS between the two groups. Studies have shown that the abnormal activation of glycolysis in tumor cells is closely related to the immune microenvironment and immune escape. Therefore, the immune cell infiltration in the OSCC cohort was also evaluated through the ssGSEA algorithm and the TIMER database. Interestingly, the low-risk group showed a higher level of immune cell infiltration, thus indirectly suggesting that a certain correlation exists between the abnormal activation of glycolysis and the tumor immune response of OSCC. Meanwhile, the results of enrichment analysis showed that DEGs in the high- and low-risk groups were mainly involved in the activation and regulation of immune cells. These results indicate that glycolysis is closely related to immune regulation in OSCC. The study reported by Tina Cascone et al. demonstrated that inhibiting the expression of glycolysis related molecules could enhance T cell-mediated antitumor immunity in vitro and in vivo.24 Recent reports have also indicated that glycolysis plays a major role in the immune escape of tumor cell by enhancing immune suppression and tumor resistance, which promises to be a novel potential target in immunotherapy.25,26 Our findings provide good theoretical support for further revealing the relationship between immunity and metabolism.
Some of the markers in our signature have been reported in other tumors as well, but a majority of them have not been reported in OSCC, especially in the context of glycolysis. For example, DDN-AS1, a gene with the largest correlation coefficient in our 4-lncRNA signature, is highly expressed in cervical cancer tumor tissues, where it can effectively inhibit the proliferation and metastasis of cervical cancer cells after being knocked down.27 Moreover, LINC01281 has also been reported to be a protective factor in laryngeal and cervical cancers, thus suggesting that it can also be used as a prognostic marker for some types of cancers.28,29 Of note, the two lncRNAs, DDN-AS1 and LINC01281, have been studied in squamous cell carcinomas, such as cervical cancer, laryngeal cancer, and OSCC (mentioned in this study), thus indicating that these two lncRNAs may play an important role in the occurrence of squamous cell carcinomas. AC245041.2 is related to the KRAS-mutant pathogenesis of pancreatic cancer, and its high expression may be an important marker of the poor prognosis of pancreatic cancer.30 Furthermore, AC245041.2 is significantly related to the poor prognosis of renal clear cell carcinoma, and it also constitutes a prognostic signature of renal clear cell carcinoma with other lncRNAs.31 However, AL035458.2 has not been reported as a prognostic marker in cancers. Therefore, in this study, all four lncRNA-encoding genes have been reported to be related to the abnormal activation of glycolysis in OSCC cells for the first time.
There are still some limitations to our study. First, because this study is based on retrospective data analysis, further verification of this risk signature in large-scale prospective cohort studies is necessary. Second, further in vivo and in vitro experiments will help in the understanding of the specific mechanisms by which these molecules are involved in OSCC glycolysis.
Summary, in this study, a new glycolysis-related lncRNA risk signature was constructed to distinguish high-risk patients with OSCC from the low-risk group. The lncRNA signature, which is an independent prognostic factor of OSCC, could assess the prognosis of patients with OSCC with strong reliability. Furthermore, the glycolysis gene signature also has good recognition ability in immune cell infiltration, which can provide personalized treatment management for future immunotherapy.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We acknowledge TCGA database for providing their platforms and contributors for uploading their meaningful datasets.
References
- 1.Rivera C., Venegas B. Histological and molecular aspects of oral squamous cell carcinoma. Onco Lett. 2014;8:7–11. doi: 10.3892/ol.2014.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascitti M., Orsini G., Tosco V., et al. An overview on current non-invasive diagnostic devices in oral oncology. Front Physiol. 2018;9:1510. doi: 10.3389/fphys.2018.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. Ca - Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: glogocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Kain J.J., Birkeland A.C., Udayakumar N., et al. Surgical margins in oral cavity squamous cell carcinoma: current practices and future directions. Laryngoscope. 2020;130:128–138. doi: 10.1002/lary.27943. [DOI] [PubMed] [Google Scholar]
- 6.Li C.-C., Shen Z., Bavarian R., Yang F., Bhattacharya A. Oral cancer: genetics and the role of precision medicine. Surg Oncol Clin. 2020;29:127–144. doi: 10.1016/j.soc.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Yap T., Celentano A., Seers C., McCullough M.J., Farah C.S. Molecular diagnostics in oral cancer and oral potentially malignant disorders-a clinician's guide. J Oral Pathol Med. 2020;49:1–8. doi: 10.1111/jop.12920. [DOI] [PubMed] [Google Scholar]
- 8.Vitório J.G., Duarte-Andrade F.F., Dos Santos Fontes Pereira T., et al. Metabolic landscape of oral squamous cell carcinoma. Metabolomics. 2020;16:105. doi: 10.1007/s11306-020-01727-6. [DOI] [PubMed] [Google Scholar]
- 9.Abbaszadeh Z., Çeşmeli S., Biray Avcı Ç Crucial players in glycolysis: cancer progress. Gene. 2020;726:144158. doi: 10.1016/j.gene.2019.144158. [DOI] [PubMed] [Google Scholar]
- 10.Marcucci F., Rumio C. Glycolysis-induced drug resistance in tumors-a response to danger signals? Neoplasia. 2021;23:234–245. doi: 10.1016/j.neo.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J., DeBerardinis R.J. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metabol. 2019;30:434–446. doi: 10.1016/j.cmet.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng W.X., Koirala P., Mo Y.Y. Lncrna-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhan A., Soleimani M., Mandal S.S. Long noncoding rna and cancer: a new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein J.N., Collisson E.A., Mills G.B., et al. Cancer Genome Atlas Research Network The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramanian A., Tamayo P., Mootha V.K., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T., Fu J., Zeng Z., et al. Timer2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:509–514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborty D., Natarajan C., Mukherjee A. Advances in oral cancer detection. Adv Clin Chem. 2019;91:181–200. doi: 10.1016/bs.acc.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Madhura M.G., Rao R.S., Patil S., Fageeh H.N., Alhazmi A., Awan K.H. Advanced diagnostic aids for oral cancer. Dis Mon. 2020;66:101034. doi: 10.1016/j.disamonth.2020.101034. [DOI] [PubMed] [Google Scholar]
- 19.Feng J., Li J., Wu L., et al. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39:126. doi: 10.1186/s13046-020-01629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves A.P., Mamede A.C., Alves M.G., et al. Glycolysis inhibition as a strategy for hepatocellular carcinoma treatment? Curr Cancer Drug Targets. 2019;19:26–40. doi: 10.2174/1568009618666180430144441. [DOI] [PubMed] [Google Scholar]
- 21.Han W., Shi J., Cao J., Dong B., Guan W. Emerging roles and therapeutic interventions of aerobic glycolysis in glioma. OncoTargets Ther. 2020;13:6937–6955. doi: 10.2147/OTT.S260376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Z., Liu Z., Li M., Chen C., Wang X. Increased glycolysis correlates with elevated immune activity in tumor immune microenvironment. EBioMedicine. 2019;42:431–442. doi: 10.1016/j.ebiom.2019.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang J., Luo Y., Wu G. A glycolysis-related gene expression signature in predicting recurrence of breast cancer. Aging. 2020;12:24983–24994. doi: 10.18632/aging.103806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cascone T., McKenzie J.A., Mbofung R.M., et al. Increased tumor glycolysis characterizes immune resistance to adoptive t cell therapy. Cell Metabol. 2018;27:977–987. doi: 10.1016/j.cmet.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganapathy-Kanniappan S. Linking tumor glycolysis and immune evasion in cancer: emerging concepts and therapeutic opportunities. Biochim Biophys Acta Rev Cancer. 2017;1868:212–220. doi: 10.1016/j.bbcan.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Guo C., Chen S., Liu W., et al. Immunometabolism: a new target for improving cancer immunotherapy. Adv Cancer Res. 2019;143:195–253. doi: 10.1016/bs.acr.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z., Wu M., Shi H., Huang C., Luo S., Song X. Ddn-as1-mir-15a/16-tcf3 feedback loop regulates tumor progression in cervical cancer. J Cell Biochem. 2019;120:10228–10238. doi: 10.1002/jcb.28307. [DOI] [PubMed] [Google Scholar]
- 28.Zhang G., Fan E., Zhong Q., et al. Identification and potential mechanisms of a 4-lncrna signature that predicts prognosis in patients with laryngeal cancer. Hum Genom. 2019;13:36. doi: 10.1186/s40246-019-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye J., Chen X., Lu W. Identification and experimental validation of immune-associate lncrnas for predicting prognosis in cervical cancer. OncoTargets Ther. 2021;14:4721–4734. doi: 10.2147/OTT.S322998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian C., Li X., Ge C. High expression of lama3/ac245041.2 gene pair associated with kras mutation and poor survival in pancreatic adenocarcinoma: a comprehensive tcga analysis. Mol Med. 2021;27:62. doi: 10.1186/s10020-021-00322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S., Chai K., Chen J. A novel prognostic nomogram based on 5 long non-coding rnas in clear cell renal cell carcinoma. Oncol Lett. 2019;18:6605–6613. doi: 10.3892/ol.2019.11009. [DOI] [PMC free article] [PubMed] [Google Scholar]