Abstract
Background/purpose
Osteoradionecrosis of the jaw (ORN) often occurs in patients with head and neck cancer undergoing radiotherapy (RT). It has been recommended to extract the tooth before RT that may become source of infection, but in recent years, some investigators have reported that tooth extraction before RT increase the risk of developing ORN and therefore should be avoided. The purpose of the study is to evaluate the risk factors for ORN including tooth extraction before RT.
Materials and methods
This was a retrospective study of 366 patients with oral or oropharyngeal cancer who underwent RT of 50 Gy or more at six university hospitals, with follow-up of at least six months post-RT. The relationship between each factor and ORN incidence was analyzed using the Cox proportional hazard model.
Results
Periapical lesions, more than 50% loss of alveolar bone, and tooth extraction after RT significantly correlated with ORN. Intensity-modulated RT showed a lower incidence than three-dimensional conformal RT, although not statistically different. Tooth extraction before RT significantly reduced ORN incidence, after adjusting the background factors using propensity score matching.
Conclusion
In patients with oral or oropharyngeal cancer who underwent RT, periapical lesions, more than 50% loss of alveolar bone, and tooth extraction after RT significantly increased the risk for ORN. Infected tooth extraction before RT significantly reduced the risk.
Keywords: Radiotherapy, Oral or oropharyngeal cancer, Osteoradionecrosis, Risk factor, Tooth extraction
Introduction
Radiotherapy (RT) is one of the effective treatment methods for head and neck cancer, although various adverse events such as dry mouth, oral mucositis, taste disorders, trismus, and nonhealing ulcers occur during or after RT. Among them, osteoradionecrosis (ORN) is a known serious late adverse event.1 Once ORN develops, it is rarely cured by conservative treatment, and jawbone resection is often required, which can significantly reduce the quality of life. Despite the importance of ORN prevention, there are few reports on effective prevention methods.2
In the analysis of risk factors for developing ORN, some investigators believe that the presence of certain oral conditions before RT, such as poor oral hygiene, dental caries, and periodontal disease, were related to the onset of ORN.3, 4, 5 In contrast, there are reports that post-RT dental caries, periodontal disease, and poor oral hygiene, rather than the pre-RT oral status, were related to the incidence of ORN.6,7
Regarding tooth extraction, it was reported that tooth extraction after RT was related to the onset of ORN,3,5,8 and therefore, the National Comprehensive Cancer Network (NCCN) guidelines recommend pre-RT dental evaluations and extraction of infected teeth at least two weeks before RT.9 However, some authors advocated that tooth extraction before RT did not reduce the risk of developing ORN.10,11 Moreover, some studies showed contradictory results and reported that pre-RT tooth extraction was a major risk factor for developing ORN.12,13
Considering the lack of consensus on the relationship between tooth extraction before RT and ORN onset, this study was undertaken to elucidate this problem. The purpose of this study was to evaluate the risk factors for ORN onset in patients with oral or oropharyngeal cancer undergoing RT and to evaluate whether tooth extraction before RT reduced the risk of developing ORN.
Materials and methods
Patients
We retrospectively investigated 366 patients with head and neck cancer who underwent RT exceeding a dose of 50 Gy at six hospitals between 2008 and 2018. All patients underwent panorama radiographic examination, necessary tooth extractions as much as possible, oral hygiene instructions, removal of dental calculus, and professional mechanical teeth cleaning by a dentist and dental hygienist before RT.
Variables
The data examined were as follows: 1) patient factors: age, sex, primary site, diabetes, minimum leukocyte, and lymphocyte counts during RT, serum creatinine before RT, and serum albumin before RT; 2) treatment factors: total RT dose, RT method (three-dimensional conformal radiotherapy [3D-CRT] or intensity-modulated radiotherapy [IMRT]), combination chemotherapy (RT alone, RT plus anticancer agent, or RT plus cetuximab), and neck dissection before RT; and 3) oral factors: remaining teeth, alveolar bone loss (50% or less, or more than 50% by panoramic X-ray examination), periapical lesion before RT, tooth extraction before RT, and tooth extraction after RT.
Endpoints
Endpoints were the onset and timing of ORN, and background factors described above.
Statistical analyses
All statistical analyses were performed using SPSS software version 24 (Japan IBM Co., Ltd, Tokyo, Japan). Univariate and multivariate analyses were performed using Cox regression to determine the relationship between each factor and the onset of ORN. Further, after the background factors between patients undergoing tooth extraction and those not undergoing extraction before RT were adjusted using the propensity score matching method, the cumulative ORN incidence in those who underwent tooth extraction before RT and those who did not was calculated by the Kaplan–Meier method and the difference between the two groups analyzed by the log-rank test.
Ethics
This study was performed in accordance with the 1964 Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Boards (IRB) of each participating hospital. Because this was a retrospective study, the research plan was published on the homepage of the participating hospitals according to the instructions of the IRB in accordance with the guaranteed opt-out opportunity.
Results
Patient characteristics
The patient characteristics are summarized in Table 1. Of the 366 patients, 272 were men and 94 were women. The average age was 65.8 ± 11.6 years. The primary sites of cancer were the oral cavity in 242 patients and the oropharynx in 123 patients. Regarding the RT method, 277 patients underwent 3D-CRT, whereas 89 underwent IMRT. One hundred and fifteen patients were treated with RT alone, 214 with additional anticancer drugs (platinum in most cases), and 89 with cetuximab. As for the dental findings, 56 patients had periapical lesions at the initiation of RT, and 32 had alveolar bone loss more than 50%. Tooth extraction before RT was performed in 151 patients, while extraction after RT was performed in 34 patients.
Table 1.
Patient characteristics
| Factor | Number of patients / Mean ± standard deviation |
p value | |||
|---|---|---|---|---|---|
| Total | Oral cancer | Oropharyngeal cancer | |||
| Age (years) | 65.8 ± 11.6 | 66.0 ± 12.5 | 65.3 ± 9.67 | 0.582 | |
| Sex | Female | 94 | 80 | 14 | <0.001 |
| Male | 272 | 162 | 110 | ||
| Diabetes | (−) | 301 | 195 | 106 | 0.312 |
| (+) | 65 | 47 | 18 | ||
| Leukocytes | 2969 ± 1234 | 3056 ± 1295 | 2800 ± 1092 | 0.060 | |
| Lymphocytes | 441 ± 310 | 488 ± 325 | 351 ± 258 | <0.001 | |
| Creatinine | 0.877 ± 0.736 | 0.803 ± 0.678 | 1.02 ± 0.822 | 0.007 | |
| Albumin | 3.64 ± 0.537 | 3.67 ± 0.516 | 3.57 ± 0.571 | 0.086 | |
| Total dose | 64.3 ± 5.13 | 62.7 ± 4.95 | 67.6 ± 3.79 | <0.001 | |
| Neck dissection before RT | (−) | 138 | 45 | 93 | <0.001 |
| (+) | 228 | 197 | 31 | ||
| RT method | 3D-CRT | 277 | 211 | 66 | <0.001 |
| IMRT | 89 | 31 | 58 | ||
| Concurrent therapy | RT alone | 115 | 98 | 17 | <0.001 |
| CRT | 214 | 133 | 81 | ||
| BRT | 37 | 11 | 26 | ||
| Remaining teeth | (−) | 26 | 19 | 7 | 0.523 |
| (+) | 340 | 223 | 117 | ||
| Alveolar bone loss | 50% or less | 231 | 163 | 68 | 0.022 |
| more than 50% | 135 | 79 | 56 | ||
| Periapical lesion at the start of RT | (−) | 310 | 206 | 104 | 0.761 |
| (+) | 56 | 36 | 20 | ||
| Severe periodontal disease at the start of RT | (−) | 334 | 218 | 116 | 0.330 |
| (+) | 32 | 24 | 8 | ||
| Tooth extraction before RT | (−) | 215 | 151 | 64 | 0.056 |
| (+) | 151 | 91 | 60 | ||
| Tooth extraction after RT | (−) | 332 | 221 | 111 | 0.573 |
| (+) | 34 | 21 | 13 | ||
| Total | 366 | 242 | 124 | ||
Abbreviations: 3D-CRT: three dimensional conformal radiotherapy; BRT: bio-radiotherapy; CRT: chemo-radiotherapy; IMRT: intensity modulated radiotherapy; RT: radiotherapy.
Comparing the background factors of oral cancer patients and oropharyngeal cancer patients, oral cancer has more women, has a higher lymphocyte count, lower creatinine level, more postoperative RT, lower RT dose, more 3D-CRT, more RT alone, and less alveolar bone loss than oropharyngeal cancer.
Incidence of osteoradionecrosis
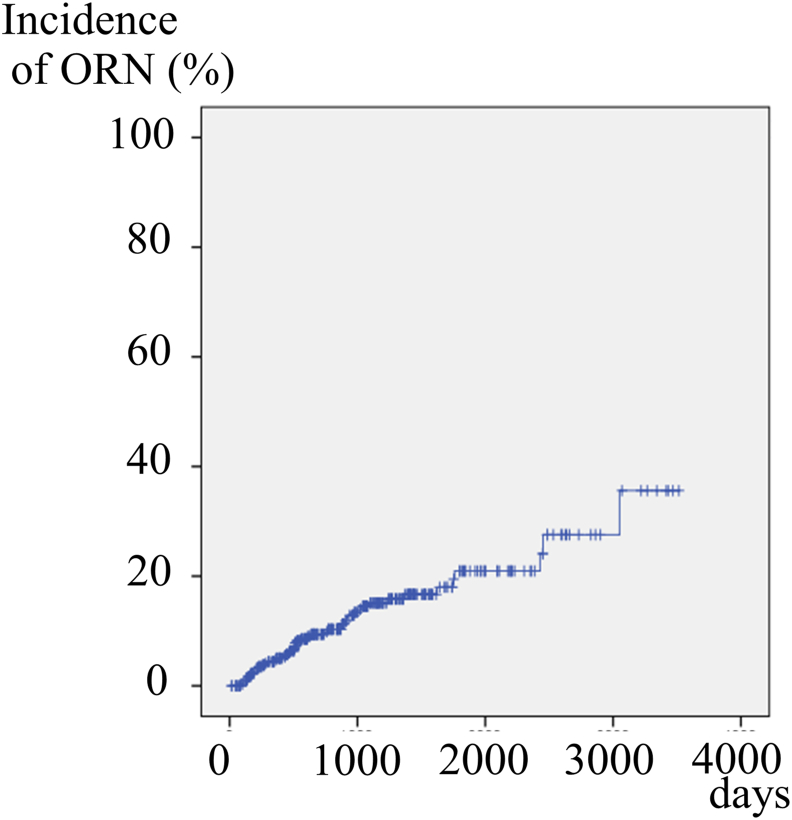
ORN occurred in 49 (13.4%) of the 366 patients. The cumulative incidence increased year by year to 5.0% for 1 year, 9.4% for 2 years, 15.1% for 3 years, 16.7% for 4 years, and 20.9% for 5 years (Fig. 1).
Figure 1.
Cumulative incidence rate of osteoradionecrosis (ORN) in all 366 patients. The cumulative incidence increased year by year and reached 15.1% for 3 years.
Factors related to developing osteoradionecrosis
Results of the univariate analysis showed that three variables, periodontitis at the start of RT, (p-value <0.001, hazard ratio [HR]: 3.714), severe marginal periodontitis at the start of RT, (p-value: 0.007, HR: 2.729), tooth extraction after RT (p-value: 0.006, HR: 2.536), significantly correlated with the occurrence of ORN (Table 2). IMRT had a lower ORN incidence than 3D-CRT (HR: 0.475), although there was no significant difference (p-value: 0.158). In addition, the rate of ORN was low in patients with tooth extraction before RT (HR: 0.588), although the difference was not significant (p-value: 0.095).
Table 2.
Relationship between each variable and development of ORN (univariate analysis)
| Variable | p-value | Hazard ratio | 95% confidence interval | |
|---|---|---|---|---|
| Age | 0.253 | 1.014 | 0.990–1.040 | |
| Sex | Female vs. male | 0.443 | 1.303 | 0.663–2.562 |
| Primary site | Oral cavity vs. Oropharyx | 0.750 | 0.901 | 0.473–1.713 |
| Diabetes | (−) vs. (+) | 0.947 | 1.025 | 0.495–2.122 |
| Leukocytes | 0.460 | 1.000 | 1.000–1.000 | |
| Lymphocytes | 0.566 | 1.000 | 0.999–1.001 | |
| Creatinine | 0.116 | 1.219 | 0.952–1.561 | |
| Albumin | 0.935 | 0.978 | 0.564–1.694 | |
| Total dose | 0.271 | 1.032 | 0.976–1.091 | |
| Neck dissection before RT | (−) vs. (+) | 0.297 | 0.737 | 0.415–1.309 |
| RT method | 3D-CRT vs. IMRT | 0.158 | 0.475 | 0.169–1.335 |
| Concurrent therapy | RT alone vs. CRT vs. BRT | 0.455 | 1.215 | 0.729–2.025 |
| Remaining teeth | (−) vs. (+) | 0.258 | 22.170 | 0.104–4745 |
| Alveolar bone loss | (−) vs. (+) | 0.512 | 1.215 | 0.679–2.173 |
| Periapical lesion at the start of RT | (−) vs. (+) | <0.001 | 3.714 | 2.081–6.627 |
| Severe periodontal disease at the start of RT | (−) vs. (+) | 0.007 | 2.729 | 1.317–5.653 |
| Tooth extraction before RT | (−) vs. (+) | 0.095 | 0.588 | 0.315–1.096 |
| Tooth extraction after RT | (−) vs. (+) | 0.006 | 2.536 | 1.307–4.920 |
Abbreviations: 3D-CRT: three-dimensional conformal radiotherapy; BRT: bio-radiotherapy; CRT: chemo-radiotherapy; IMRT: intensity modulated radiotherapy; ORN: osteoradionecrosis; RT: Radiotherapy.
Multivariate Cox regression analysis, in which all factors were considered for and the stepwise selection was performed revealed that the three factors, the periapical lesion (p-value: <0.001, HR: 3.722), severe marginal periodontitis (p-value: 0.006, HR: 2.818), and tooth extraction after RT (p-value: 0.023, HR 2.145), had significantly higher ORN incidence (Table 3).
Table 3.
Relationship between each variable and development of ORN (multivariate analysis)
| Variable | p-value | Hazard ratio | 95% confidence interval | |
|---|---|---|---|---|
| Periapical lesion at the start of RT | (−) vs. (+) | <0.001 | 3.722 | 2.076–6.674 |
| Severe periodontal disease at the start of RT | (−) vs. (+) | 0.006 | 2.818 | 1.351–5.880 |
| Tooth extraction after RT | (−) vs. (+) | 0.023 | 2.145 | 1.110–4.147 |
Abbreviations: ORN: osteoradionecrosis; RT: radiotherapy.
Effect of tooth extraction before radiotherapy
Tooth extraction before RT tended to decrease ORN incidence, although there was no significant difference. However, since there was a large bias between the extracted and non-extracted patients before RT, the background factors were adjusted by the propensity score matching method.
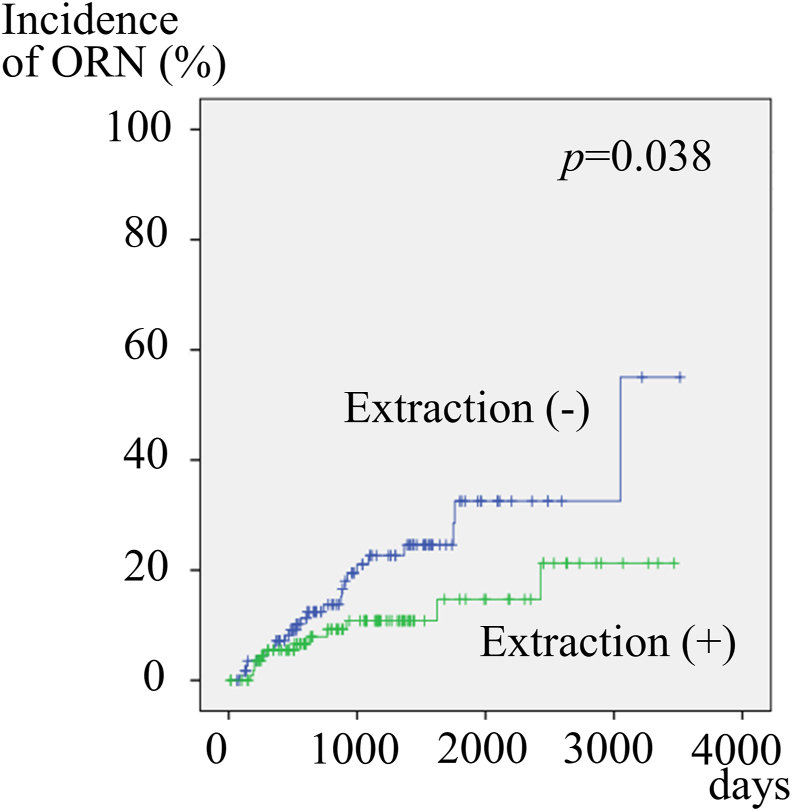
Propensity scores were calculated for 366 participants by logistic regression analysis of all variables with those undergoing or not undergoing tooth extraction before RT. The concordance index (c-index) was 0.740, which indicated a strong ability to differentiate between patients undergoing and not undergoing tooth extraction before RT, and the Hosmer–Lemeshow statistic was non-significant (p = 0.969), indicating good calibration. The propensity scores, which reflected the probability that a patient would undergo tooth extraction before RT or not undergo tooth extraction before RT, ranged from 0.05609 to 0.80144 and from 0.04372 to 0.76656, respectively. Out of 366 patients, 234 were matched. After propensity score matching, 117 patients who underwent tooth extraction before RT showed a significantly lower incidence of developing ORN than the 117 who did not undergo tooth extraction before RT, by a log-rank test (p-value: 0.038) (Fig. 2).
Figure 2.
Cumulative incidence rate of osteoradionecrosis (ORN) based on tooth extraction before radiotherapy (RT) in 234 patients, after propensity score matching. Patients who underwent tooth extraction before RT showed significantly lower incidence of developing ORN.
Discussion
RT is one of the basic treatments for head and neck cancer and is widely used for radical, postoperative, or palliative therapy. In recent years, it is often combined with chemotherapy using agents, such as cisplatin or cetuximab. RT can cause various adverse events and among them, ORN is a serious late adverse event that decreases the patient's quality of life.
According to past reports, the incidence of ORN in head and neck cancer radiotherapy varies from 2% to 50%; the variation may be due to differences in the target patients, RT methods, observation period, etc. In the current study, the ORN incidence increased year by year, with 5.0% for 1 year, 9.4% for 2 years, and 15.1% for 3 years. These findings suggest that even with the advancements of RT methods and supportive care, ORN is still a serious adverse event of RT. However, methods of ORN prevention have not been established yet.
Since ORN is considered to be caused by a dental infection in the damaged jawbone due to RT, various studies have been conducted on the related dental risk factors.
Some investigators have stated that oral conditions before RT, such as poor oral hygiene, dental caries, and periodontal disease, were related to the onset of ORN.3, 4, 5 In contrast, others have reported that post-RT oral status, including dental caries, periodontal disease, and poor oral hygiene, correlated more with the incidence of ORN, rather than the pre-RT oral conditions.6,7 ORN often occurs after tooth extraction. Some authors reported a significant correlation between tooth extraction after RT and development of ORN,3,5,8,14 and therefore, NCCN guidelines recommend pre-RT extraction of infected teeth at least 2 weeks before RT.9 In contrast, Chang reported that the risk of ORN was not reduced by tooth extraction before RT,10 and Koga reported that tooth extraction before and after RT was not related to the development of ORN.11 Furthermore, there are reports that hyperbaric oxygen therapy may reduce the incidence of ORN when tooth extraction and implants are performed,15 and ORN may develop from pressure ulcers on dentures.16 Regarding IMRT, some investigators reported that the risk of ORN was reduced in comparison with conventional 3D-CRT, although there was no significant difference.12, 13, 14 There are various reports on the risk factors of ORN, but there are currently few established preventive methods of ORN, as described in the Cochrane systematic review.2
In a previous study,17 the risk factors mentioned for developing ORN were threefold: oral or oropharyngeal cancer, a tooth with the periapical lesion, and tooth extraction after RT. Therefore, only oral cancer and oropharyngeal cancer were registered in this study. There are some differences between RT for oral cancer and nasopharyngeal cancer. In the former, postoperative irradiation in patients at high risk of recurrence is more frequent and 3D-CRT is relatively more common, whereas in the latter, curative irradiation is more frequent and IMRT is more common. However, since the jawbone was included in the RT field in both cases and there was no difference in the incidence of ORN in this study, oral cancer and oropharyngeal cancer were analyzed together. In addition, there are many reports in which ORN was caused by dental caries that progressed rapidly after RT, and most of the ORN occurred in the mandibular molars. Therefore, extraction of mandibular molars that might become a potential infection source or of those in which long-term prognosis was not expected, and strict follow-up after RT by a dentist to prevent ORN were advocated.17 However, in recent reports, Christensen et al. and Moon et al. describe that pre-RT tooth extraction was a major risk factor for developing ORN.12,13 Therefore, it is questionable whether current pre-RT tooth extraction protocols are appropriate. It is speculated that patients who need tooth extraction before RT have a poorer oral condition than those who do not. In the reports discussed above, oral conditions, such as periapical lesions and periodontal disease, were not included in the independent variables; therefore, we think that a large selection bias might have existed between the pre-RT tooth extraction group and the non-extraction group. This study was conducted to clarify whether pre-RT tooth extraction increases or decreases ORN risk.
In this study, univariate and multivariate analyses showed that three factors: apical lesion, alveolar bone loss of more than 50%, and post-RT tooth extraction significantly increased the ORN incidence. Although it is presumed that patients who underwent pre-RT tooth extraction often had poor oral status, the incidence of ORN was decreased in those undergoing pre-RT tooth extraction in univariate analysis, but this was not statistically significant. Therefore, to minimize the bias between pre-RT extracted cases and non-extracted cases, the background factors, including oral conditions, were adjusted for by the propensity score matching method, and analysis was then performed. Therefore, it was found that tooth extraction before RT significantly decreased the incidence of ORN. These results reveal that during RT for oral or oropharyngeal cancer, apical lesions, alveolar bone loss of more than 50%, and post-RT tooth extraction significantly increase the risk of ORN development, and extraction of the infected tooth or the tooth with a poor prognosis significantly reduces the risk.
The current study had some limitations. First, although the results were analyzed by multivariate and propensity score matching analyses, it is a retrospective observational study, and the possibility of unknown confounding factors and bias cannot be denied. Second, since it was a retrospective study, it was not possible to directly observe the condition of soft tissues such as the gingiva, and only the observation of hard tissues by panoramic radiographic photography was performed. Furthermore, since this study is targeted at patients who underwent dental evaluation and treatment before RT, it is unclear whether the obtained results can be generalized to all oral and oropharyngeal cancer patients. However, to the best of our knowledge, this study is the first to make a clear conclusion regarding the effectiveness of tooth extraction before RT by taking into consideration the oral conditions, in a large number of cases. It is necessary to verify these results with a prospective study.
The risk factors for developing ORN in RT patients with oral or oropharyngeal cancer were periapical lesions, alveolar bone loss of more than 50%, and tooth extraction after RT. Furthermore, by adjusting for the background factors using the propensity score matching method, it was shown that tooth extraction before RT reduced the risk of developing ORN.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing. This study was supported by Grants-in-Aid for Scientific Research Japan; Japan Society for the Promotion of Science, Japan (No. 16K11878).
References
- 1.Jereczek-Fossa B.A., Orecchia R. Radiation-induced mandibular bone complications. Cancer Treat Rev. 2002;28:65–74. doi: 10.1053/ctrv.2002.0254. [DOI] [PubMed] [Google Scholar]
- 2.El-Rabbany M., Duchnay M., Raziee H.R., et al. Interventions for preventing osteoradionecrosis of the jaws in adults receiving head and neck radiotherapy. Cochrane Database Syst Rev. 2019;2019(11) doi: 10.1002/14651858.CD011559.pub2. CD011559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raguse J.D., Hossamo J., Tinhofer I., et al. Patient and treatment-related risk factors for osteoradionecrosis of the jaw in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:215221. doi: 10.1016/j.oooo.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Niewald M., Mang K., Barbie O., et al. Dental status, dental treatment procedures and radiotherapy as risk factors for infected osteoradionecrosis (IORN) in patients with oral cancer -a comparison of two 10 years' observation periods. SpringerPlus. 2014;3:263. doi: 10.1186/2193-1801-3-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuther T., Schuster T., Mende U., Kübler A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients -a report of a thirty-year retrospective review. Int J Oral Maxillofac Surg. 2003;32:289–295. doi: 10.1054/ijom.2002.0332. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi W., Teh B.G., Kimura H., Kakehata S., Kawaguchi H., Takai Y. Comparison of osteoradilnecrosis of the jaw after superselective intra-arterial chemotherapy versus conventional concurrent chemoradiotherapy of oral cancer. J Oral Maxillofac Surg. 2015;73:994–1002. doi: 10.1016/j.joms.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Katsura K., Sasai K., Sato K., Saito M., Hoshina H., Hayashi T. Relationship between oral health status and development of osteoradilnecrosis of the mandible: a retrospective longitudinal study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:731–738. doi: 10.1016/j.tripleo.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Thorn J.J., Hansen H.S., Specht L., Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg. 2000;58:1088–1093. doi: 10.1053/joms.2000.9562. [DOI] [PubMed] [Google Scholar]
- 9.NCCN clinical practice guidelines in oncology [NCCN guidelines] Head and Neck Cancers version 1. 2020. https://www.nccn.org/professionals/physician_gls/default.aspx#site Date accessed. [DOI] [PubMed]
- 10.Chang D.T., Sandow P.R., Morris C.G., et al. Do pre-irradiation dental extractions reduce the risk of osteoradionecrosis of the mandible? Head Neck. 2007;29:528–536. doi: 10.1002/hed.20538. [DOI] [PubMed] [Google Scholar]
- 11.Koga D.H., Salvajoli J.V., Kowalski L.P., Nishimoto I.N., Alves F.A. Dental extractions related to head and neck radiotherapy: ten-year experience of a single institution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e1–e6. doi: 10.1016/j.tripleo.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Aarup-Kristensen S., Hansen C.R., Forner L., Brink C., Eriksen J.G., Johansen J. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: risk factor and dose-volume correlations. Acta Oncol. 2019;58:1373–1377. doi: 10.1080/0284186X.2019.1643037. [DOI] [PubMed] [Google Scholar]
- 13.Moon D.H., Moon S.H., Wang K., et al. Incidence of, and risk factor for, mandibular osteoradionecrosis in patients with oral cavity and oropharynx cancers. Oral Oncol. 2017;72:98–103. doi: 10.1016/j.oraloncology.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Willaert R., Nevens D., Laenen A., Batstone M., Politis C., Nuyts S. Does intensity-modulated radiation therapy lower the risk of osteoradionecrosis of the jaw? A long-term comparative analysis. Int J Oral Maxillofac Surg. 2019;48:1387–1393. doi: 10.1016/j.ijom.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Shaw R.J., Butterworth C.J., Silcocks P., et al. HOPON (hyprebaric oxygen for the prevention of osteonecrosis): a randomized controlled trial of hyperbaric oxygen to prevent osteonecrosis of the irradiated mandible after dentoalveolar surgery. Int J Radiat Oncol Biol Phys. 2019;104:530–539. doi: 10.1016/j.ijrobp.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 16.Abed H., Burke M., Scambler S., Scott S.E. Denture use and osteoradianecrosis following radiotherapy for head and neck cancer: a systematic review. Gerontology. 2020;37:102–109. doi: 10.1111/ger.12456. [DOI] [PubMed] [Google Scholar]
- 17.Kojima Y., Yanamoto S., Umeda M., et al. Relationship between dental status and development of osteoradionecrosis of the jaw: a multicenter retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124:39–145. doi: 10.1016/j.oooo.2017.04.012. [DOI] [PubMed] [Google Scholar]