Abstract
Objective:
To assess the safety and local recurrence-free survival in patients after cryoablation for treatment of pulmonary metastases.
Methods:
This multicenter, prospective, single-arm, phase 2 study included 128 patients with 224 lung metastases treated with percutaneous cryoablation, with 12 and 24 months of follow-up. The patients were enrolled on the basis of the outlined key inclusion criteria, which include one to six metastases from extrapulmonary cancers with a maximal diameter of 3.5 cm. Time to progression of the index tumor(s), metastatic disease, and overall survival rates were estimated using the Kaplan–Meier method. Complications were captured for 30 days after the procedure, and changes in performance status and quality of life were also evaluated.
Results:
Median size of metastases was 1.0 plus or minus 0.6 cm (0.2–4.5) with a median number of tumors of 1.0 plus or minus 1.2 cm (one to six). Local recurrence-free response (local tumor efficacy) of the treated tumor was 172 of 202 (85.1%) at 12 months and 139 of 180 (77.2%) at 24 months after the initial treatment. After a second cryoablation treatment for recurrent tumor, secondary local recurrence-free response (local tumor efficacy) was 184 of 202 (91.1%) at 12 months and 152 of 180 (84.4%) at 24 months. Kaplan–Meier estimates of 12- and 24-month overall survival rates were 97.6% (95% confidence interval: 92.6–99.2) and 86.6% (95% confidence interval: 78.7–91.7), respectively. Rate of pneumothorax that required pleural catheter placement was 26% (44/169). There were eight grade 3 complication events in 169 procedures (4.7%) and one (0.6%) grade 4 event.
Conclusion:
Percutaneous cryoablation is a safe and effective treatment for pulmonary metastases.
Keywords: Lung metastasis, Cryoablation, Percutaneous ablation, Tumor efficacy, Safety
Introduction
The lung is a common site of metastasis from malignancy, and metastasectomy may be curative.1 Surgical resection of limited pulmonary metastases provides a survival benefit for many histologies in properly selected patients with the survival benefit extending to patients that undergo repeated metastasectomy with recent approaches that emphasize sparing of lung parenchyma.1–3 Extending the use of nonsurgical focal treatments to certain patients with limited pulmonary metastatic disease may result in long-term disease-free survival.4
For patients that are not candidates for pulmonary metastasectomy, stereotactic body radiation therapy (SBRT) and percutaneous ablative therapies may be offered.5–9 Percutaneous ablation of limited metastatic disease is an option for patients with recurrent pulmonary metastatic disease, and who have multiple metastases or metastases located deeper in the lung that would require extensive parenchymal resection, or when comorbidities increase the risk from operation.10
Image-guided percutaneous cryoablation (CA) uniquely allows visualization of the ablation zone with computed tomography (CT), defined by an interstitial or consolidative infiltrate or visible ice, providing complete tumor ablation while also avoiding adjacent normal tissues. In addition, tumors located adjacent to or involving the pleura can be treated with CA without procedural pain or increased risk of bronchopleural fistula.11,12 Several studies have been performed using CA for pulmonary metastases, with promising results.12–17
Previously, we reported the results of a feasibility trial using CA for the treatment of pulmonary metastases with 94.6% local recurrence-free survival at 12 months and 6% grade 3 adverse events in 48 patients with 60 tumors.18 Herein, we present the safety and efficacy at 12- and 24-months posttreatment using percutaneous CA for the treatment of lung metastases less than 3.5 cm in diameter from a prospective, phase 2, multicenter, single-arm study that involves 128 patients with 224 tumors.
Materials and Methods
Design
The Health Insurance Portability and Accountability Act–compliant, single-arm, phase 2, multicenter, prospective study was approved by local institutional review boards (IRBs), Chesapeake IRB, Western IRB, commission scientifique des essais thérapeutiques, or comité consultatif pour la protection des personnes soumis à une recherche biomédicale, and written consent was obtained. The primary objective was to evaluate the efficacy of CA on local recurrence-free response (local tumor efficacy) for each index tumor at 12 months post-CA after a single CA procedure. The secondary end point was overall local tumor efficacy after a single or repeat CA at 12 months. Separate evaluations of primary and secondary efficacy were also made for month 24. Safety data were captured for the incidence and severity of procedural adverse events.
Patients
Patients were enrolled prospectively in 10 centers, including two in Europe and eight in the United States. Inclusion criteria were the following: aged at least 18 years old; pulmonary metastatic disease confirmed by previous biopsy or through imaging as new or growing nodules with histologically proven primary cancer; up to six pulmonary metastases; targeted index tumor(s) defined as intrapulmonary or pleural with a maximum size of 3.5 cm within 4 weeks of the procedure; Karnofsky Performance Scale (KPS) score of at least 60; platelet count greater than 50,000/mm3 within 8 weeks of the procedure; and a life expectancy of more than 3 months. Exclusion criteria included the following: an index tumor that is primary lung cancer; uncontrollable primary or metastatic disease outside the lung; unable to lie flat or with respiratory distress at rest; uncontrolled coagulopathy or bleeding disorder; absolute neutrophil count less than 1000 within 8 weeks of the procedure; evidence of active infection or a medical or psychiatric illness that would preclude informed consent or follow-up; or participation in other clinical trials that could affect the primary end point.
Between April 2014 and March 2016, 130 patients were considered qualified for the trial and treated with CA for a total of 226 metastases (Table 1). Two subjects with one tumor each were enrolled and treated but subsequently identified with primary lung cancer. These patients were included in the safety analysis but were removed from the efficacy data analysis resulting in a final study population of 128 patients with 224 metastases. None of the tumors included in this trial were in previous trials concerning pulmonary metastatic disease treatment. The subjects could have repeat CA of the index tumor(s) with evidence of residual tumor. A total of 114 patients with 202 tumors were evaluable at 12 months and 99 patients and 180 tumors were evaluable at 24 months for local recurrence-free response. A total of 14 subjects with 22 tumors were not evaluated at 12 months owing to missing data at this interval because three patients with three tumors declined further follow-up, two patients with three tumors had operation for other disease with resection of the index tumor or other focal therapy, six patients with 11 tumors had missing data, and three patients with five tumors died resulting in evaluation of 114 patients with 202 tumors. Follow-up at 24 months included 173 tumors in 99 patients for subject-level analysis and an additional seven tumors from two additional patients that were not evaluable for subject-level analysis owing to missing data on other tumors for a total of 180 tumors for tumor level analysis. A total of 29 subjects and 44 tumors were not evaluated at 24 months follow-up, including seven patients with 10 tumors who declined further follow-up, five patients with seven tumors who had missing data, two patients with four tumors who had operation for other disease with resection of the index tumor or other focal therapy, and 15 patients with 23 tumors who died.
Table 1.
Baseline Patient and Tumor Characteristics (N = 128, tumors = 224)
| Characteristic | Value |
|---|---|
| Median age, SD (range) | 65 ± 12 (32–85) |
| Male sex | 68/128 (53) |
| BMI (SD) | 27.4 ± 5.9 |
| Primary tumor type histology (%) | |
| Colorectal cancer | 63/128 (49) |
| Renal cell carcinoma | 16/128 (12) |
| Sarcoma | 6/128 (5) |
| Breast cancer | 6/128 (5) |
| Endometrial cancer | 6/128 (5) |
| Thyroid cancer | 5/128 (4) |
| Primary lung cancer | 4/128 (3) |
| Pancreatic cancer | 3/128 (2) |
| Melanoma | 2/128 (1) |
| Other | 17/128 (13) |
| Months from primary diagnosis, SD (range) | 64.4 ± 66.7 (1–361) |
| Months from metastatic diagnosis, SD (range) | 40.4 ± 37.2 (1–220) |
| Previous treatments for other lung metastasesa | 73/128 |
| Surgery | 29/73 (40) |
| Radiofrequency ablation | 20/73 (27) |
| Cryoablation | 11/73 (15) |
| Microwave ablation | 6/73 (8) |
| Radiation | 6/73 (8) |
| Systemic therapy | 41/73 (56) |
| Median tumor diameter in cm, SD (range) | 1.0 ± 0.6 (0.2–4.5) |
| Tumor largest diameter (cm) | |
| ≤1.0 | 114/224 (51) |
| 1.1–2.0 | 96/224 (43) |
| 2.1–3.0 | 12/224 (5) |
| 3.1–3.5 | 1/224 (0.5) |
| >3.5b | 1/224 (0.5) |
| Tumor location | |
| Right | 121/224 (54) |
| Left | 103/224 (46) |
| Tumor distribution (per patient) | |
| Unilateral | 103/128 (80) |
| Bilateral | 25/128 (20) |
| Lobe | |
| Upper | 101/224 (45) |
| Middle | 25/224 (11) |
| Lower | 98/224 (44) |
| Lung site | |
| Hilar | 6/224 (3) |
| Intraparenchymal | 83/224 (37) |
| Peripheral | 71/224 (32) |
| Pleural contact | 64/224 (29) |
| Median number of tumors per patient (range) | 1.0 ± 1.2 (1–6) |
| Number of tumors treated per patient | |
| 1 | 79/128 (62) |
| 2 | 23/128 (18) |
| 3 | 15/128 (12) |
| 4 | 5/128 (4) |
| 5 | 2/128 (2) |
| 6 | 4/128 (3) |
Patients may have received more than one previous treatment for history of other lung metastases.
One subject had a tumor that measured 3.5 cm at enrollment and 4.5 cm at treatment.
BMI, body mass index.
CA Procedure
The ablation procedure was performed under general anesthesia, conscious sedation, or regional anesthesia. CA needles, 1.5, 2.1, or 2.4 mm in diameter, were provided by Galil Medical Inc. (Arden Hills, MN) and placed under CT guidance. Cryoprobes were controlled with the Visual-ICE system. Number and configuration of the needles were based on size and location of the tumor while avoiding adjacent anatomical structures. Tumor location(s) within the lung were characterized as pleural when in contact with the pleura, peripheral if within 2 cm of the pleural margin, hilar if within 1 cm of a pulmonary lobar artery, and the remainder were classified as intraparenchymal.
CA was performed with a minimum of three freeze–thaw cycles. The times for each phase were recorded (target times were 3 min freeze, 3 min passive thaw, 7–12 min freeze, 5 min passive thaw, 7–12 min freeze, followed by active thawing). Each procedure was monitored with noncontrast CT imaging typically at 3- to 5-minutes intervals to visualize the evolving ablation zone with the goal of achieving a minimal margin beyond the tumor of 5 mm. After the CA needle(s) were removed, CT images were obtained to assess the overall ablation zone and to identify any potential complications (Table 2).
Table 2.
Cryoablation Treatment Characteristics
| Characteristic | Value |
|---|---|
| Number of tumors | 224 |
| Number of procedures | 167 |
| Number of tumors treated per procedure, median SD (range) | 1.0 ± 0.7 (1–5) |
| 1 | 126/167 (75) |
| 2 | 30/167 (18) |
| 3 | 7/167 (4) |
| 4 | 3/167 (2) |
| 5 | 1/167 (1) |
| Number of tumors treated per patient, median SD (range) | 1.0 ± 1.2 (1–6) |
| 1 | 79/128 (62) |
| 2 | 23/128 (18) |
| 3 | 15/128 (12) |
| 4 | 5/128 (4) |
| 5 | 2/128 (2) |
| 6 | 4/128 (3) |
| Procedure time, min (SD) | 77.7 ± 37.9 |
| Freeze duration per tumor, min (SD) | 20.6 ± 4.3 |
| Mean number of needles per tumor diameter | |
| 0.5–1.0 cm | 1.5 ± 0.5 |
| 1.1–2.0 cm | 1.9 ± 0.6 |
| 2.1–3.0 cm | 2.4 ± 0.5 |
| ≥3.1 cm | 3.0 ± 1.4 |
| Anesthesia | |
| General | 115 (69%) |
| Conscious sedation | 49 (29%) |
| Local | 3 (2%) |
| Median overall hospital stay, days (range) | 1.0 (0–13) |
Follow-Up and Treatment Response
Follow-up was done within the first week and at 1, 3, 6, 12, 18, and 24 months. The patients were clinically evaluated and had a chest CT performed at 1 month which served as the posttreatment baseline study. Technical success was defined as a zone of ground-glass opacity or consolidation encompassing the targeted tumor with at least 5 mm circumferential ablative margin. If the 1-month study was not performed, the 3-month chest CT was used as the posttreatment baseline study. An example of a nodule treatment is found in Figure 1 with the typical appearance on follow-up imaging revealing initial consolidation followed by resolution to a thin scar. Tumor response was calculated comparing the sum of the largest diameter of ablation zones. “Complete” response of index tumor(s) was defined as tumor ablation zone disappearance or reduction of at least 75%, “partial” response as 30% to 75% decrease in size, “stable disease” when there was less than 30% decrease and less than 20% increase in size, and “local failure” when there was an increase of greater than 20% compared with the smallest diameter (nadir) or appearance of nodular enhancement. Local tumor efficacy includes complete, partial, and stable treatment response.
Figure 1.
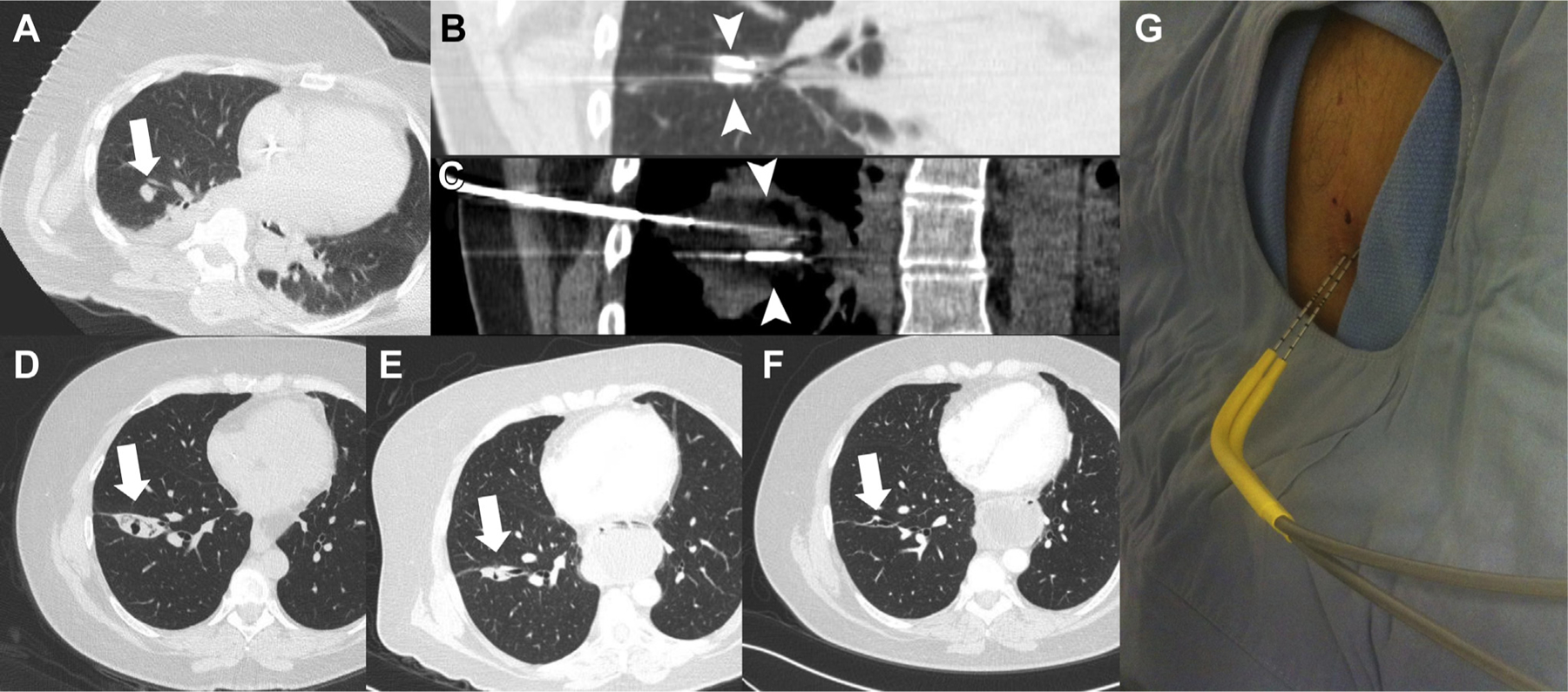
Image-guided cryoablation and follow-up imaging of a metastatic rectal carcinoma contained in the right lung. (A) Noncontrast axial computed tomography (CT) image of the chest with a 1.2 cm nodule (arrow) contained in the right lower lobe positioned for percutaneous cryoablation. (B) Noncontrast coronal CT image of the chest with two cryoprobes (arrow heads) along the superior and inferior margins of the targeted nodule. (C) Noncontrast coronal CT image of the chest with body windows with ice (arrow heads) contained in the cryoablation probes and the targeted nodule. (D) Noncontrast axial CT image of the chest 1 month after the cryoablation treatment revealing an area of consolidation and a small area of central cavitation at the location of the treated nodule (arrow). (E) Noncontrast axial CT image of the chest 6 months after the cryoablation treatment revealing an area of decreasing consolidation and a small area of central cavitation at the location of the treated nodule (arrow). (F) Noncontrast axial CT image of the chest 12 months after the cryoablation treatment revealing a small scar remaining at the location of the treated nodule (arrow).
Data Collection and Review
Data were collected at each institution, anonymized, and sent to a centralized data capture system (Track-It3K, Acumen Healthcare Solutions, LLC, Plymouth, MN). Imaging was stored centrally for review (InteleGRID, Intelemage LLC, Cincinnati, OH), compliant with the Food and Drug Administration’s Title 21 of the Code of Federal Regulations Part 11 guidance. All imaging were reviewed blindly by two interventional radiologists with 9 and 15 years of experience.
Safety and Procedural Tolerance Assessment
Adverse events that occurred within 30 days of the procedure were captured and graded in accordance with the Common Terminology for Adverse Criteria for Adverse Events (CTCAE version 4.03) of the National Cancer Institute.19 Changes in physical function and quality of life were measured using the KPS and the Short Form—12 (SF-12) assessment.20
Statistical Analysis
Continuous variables are expressed as mean, SD, number of patients, median, minimum, and maximum. Categorical variables, including efficacy outcomes and adverse events, are summarized by frequencies and percentages of the patients in each category. The primary end point, rate of local tumor efficacy at month 12, was summarized using a binary proportion with two-sided 95% exact confidence intervals (CIs). The primary null hypothesis was tested by comparing the lower bound of the 95% CI for the estimated rate of local tumor efficacy on the performance goal of 84.0%; if the lower bound was more than 84.0%, the null hypothesis was to be rejected, and the end point was considered met.
Survival rates and time to tumor progression were analyzed using Kaplan–Meier methodology. Distant tumor progression was defined as distant metastatic disease outside of the treatment area, either in the lung or outside the lung. Time to progression of the index tumor was defined as the time from CA procedure to local failure. Patients without progression were censored at the time of death or at the date of their last visit. Overall survival rates were calculated from the day of the first ablation procedure to time of death related to lung cancer and related to any cause. Patients who were alive were censored at the date of their last visit.
KPS and SF-12 analyses used a paired t test to examine differences. SAS version 9.3 (SAS Institute, Cary, NC) statistical software was used for the analyses. p Values less than 0.05 were considered statistically significant.
Results
Patient and Tumors
Patient and tumor characteristics are summarized in Table 1. Among the 128 enrolled patients, colorectal cancer was the most frequent cancer, accounting for 49% of metastases. A total of 73 patients (57%) had previous focal treatment for other lung metastases but without previous treatment to the targeted lung metastases in this study.
CA Treatment
A total of 134 patients were consented for the study. Three enrolled patients were not treated owing to subsequent screen failure, including two patients with tumors larger than 3.5 cm and one patient with a history of primary lung cancer. One patient enrolled and then declined the treatment. A total of 130 patients with 226 tumors were treated with 169 CA procedures and included in the evaluation for complications. After the CA treatment, two patients were excluded from the full analysis owing to screening failure of a patient with primary lung cancer and one patient that was biopsied at the time of the procedure finding primary lung cancer. A total of 128 patients with 224 thoracic metastases treated over 167 procedures were included in the analysis of the efficacy. Of these patients, 80 of 224 tumors (36%) were treated with one CA probe and 144 of the tumors (64%) were treated with two or more cryoprobes. The mean procedure time was 78 minutes (range 30–225 min), including anesthesia management, CA needle placement and ablation, and postprocedural CT evaluation (Table 2). Technical success was achieved in 97.2% (217/224) of evaluable tumors. Repeat treatment of an index tumor was performed on 13 patients with 16 tumors during the 24-month observation at a mean of 8.8 months (range 2–23 months).
Treatment Efficacy
Initial and secondary efficacies are found in Table 3. A total of 114 of 128 patients (89%) with 202 of 224 (90%) tumors were evaluable for the 12-month follow-up analysis. Initial local tumor efficacy was achieved in 172 of 202 (85.1%) (95% CI: 79.5–89.8) after 12 months of follow-up. On a per patient basis, 16 of 114 (14%) had complete, 10 of 114 (9%) partial, and 63 of 114 (55%) stable disease, and 25 of 114 (22%) local treatment failure after the initial treatment. Of the 25 patients that had recurrence with 30 tumors, 11 patients with 12 tumors were retreated with CA and were evaluable 12 months after repeat CA. Overall local tumor efficacy including these patients with retreatment resulted in secondary local recurrence-free response in 184 of 202 (91.1%) (95% CI: 86%–95%). On a per patient basis at 12-month follow-up, overall treatment response was complete for 17 of 114 (15%), partial for 17 of 114 (15%), stable for 65 of 114 (57%), and failure for 15 of 114 (13%) with secondary per patient effectiveness of 99 of 114 (87%).
Table 3.
Cryoblation Tumor Treatment Response at 12 and 24 Months
| Initial treatment |
Repeat treatment |
Secondary treatment |
|||||
|---|---|---|---|---|---|---|---|
| Interval | Characteristic | Response | Efficacy | Response | Efficacy | Response | Efficacy |
| 12 Mo | Number of tumors | 202 | 12 | 202 | |||
| Complete | 33 (16.3%) | 85.1% | 3 (20.0%) | 100% | 36 (17.8%) | 91.1% | |
| Partial | 122 (60.4%) | 8 (66.7%) | 130 (65.3%) | ||||
| Stable | 17 (8.4%) | 1 (13.3%) | 18 (8.9%) | ||||
| Failure | 30 (14.9%) | 0 (0%) | 18 (8.9%) | ||||
| 24 Mo | Number of tumors | 180 | 13 | 180 | |||
| Complete | 49 (37.2%) | 77.2% | 4 (30.8%) | 100% | 53 (29.4%) | 84.4% | |
| Partial | 79 (43.9%) | 7 (53.8%) | 86 (47.8%) | ||||
| Stable | 11 (6.1%) | 2 (15.4%) | 13 (7.2%) | ||||
| Failure | 41 (22.8%) | 0 (0%) | 28 (15.6%) | ||||
A total of 99 of 128 patients (77.3%) with 180 of 224 (80.3%) tumors were evaluable for the 24-month follow-up analysis. Initial local tumor efficacy was achieved in 139 of 180 (77.2%) (95% CI: 70.4–83.1) after 24 months of follow-up. On a per patient basis, 22 of 99 (22.2%) had complete, five of 99 (5.1%) partial, and 42 of 99 (42.4%) stable disease, and 30 of 99 (30.3%) local treatment failure after initial treatment. Of the 30 patients who had recurrence with 41 tumors, 13 patients with 16 tumors were retreated with CA and were followed for 24 months after repeat CA with 11 patients with 13 tumors available for analysis at 24 months. Of this retreatment group that was not evaluable, one patient with one tumor died before 24 months of follow-up and one patient with one tumor had other focal therapy adjacent to the index tumor which obscured further evaluation. A patient with retreatment of three tumors had two evaluable at 24 months with the third tumor treated separately with subsequent patient withdrawal. No treatment failures were observed in the evaluable retreatment group over 24 months of subsequent follow-up. Overall secondary local tumor efficacy was achieved in 152 of 180 (84.4%) at 24 months. On a per patient basis, overall treatment response was complete for 23 of 99 (23.2%), partial for 10 of 99 (10.1%), stable for 44 of 99 (44.4%), and failure for 22 of 99 (22.2%) with secondary per patient effectiveness of 77 of 99 (77.8%).
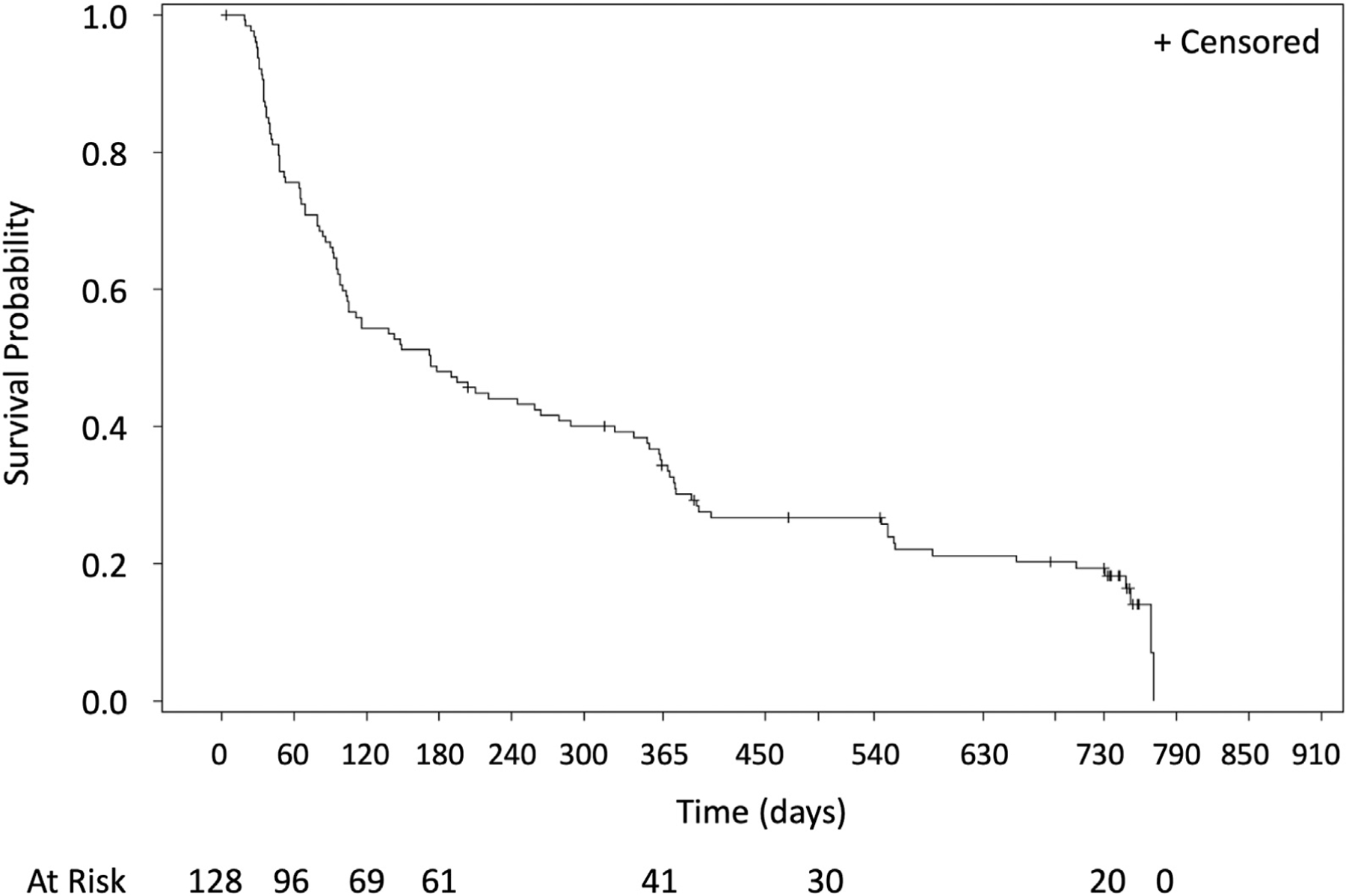
Time to progression, overall survival, time to progression from metastatic lung disease beyond the index tumor, and time to progression from metastatic disease are found in Figures 2 to 5. A total of 10 of the 128 patients treated (7.8%) received systemic therapies during the study. There were three and 12 deaths that occurred within 0 to 12 and 12 to 24 months, respectively, with no procedure-related deaths, 11 deaths related to disease progression, and four un-known. By Kaplan–Meier analysis, the 1-year overall survival was 97.6% and the 2-year overall survival was 86.6% (Fig. 2).
Figure 2.
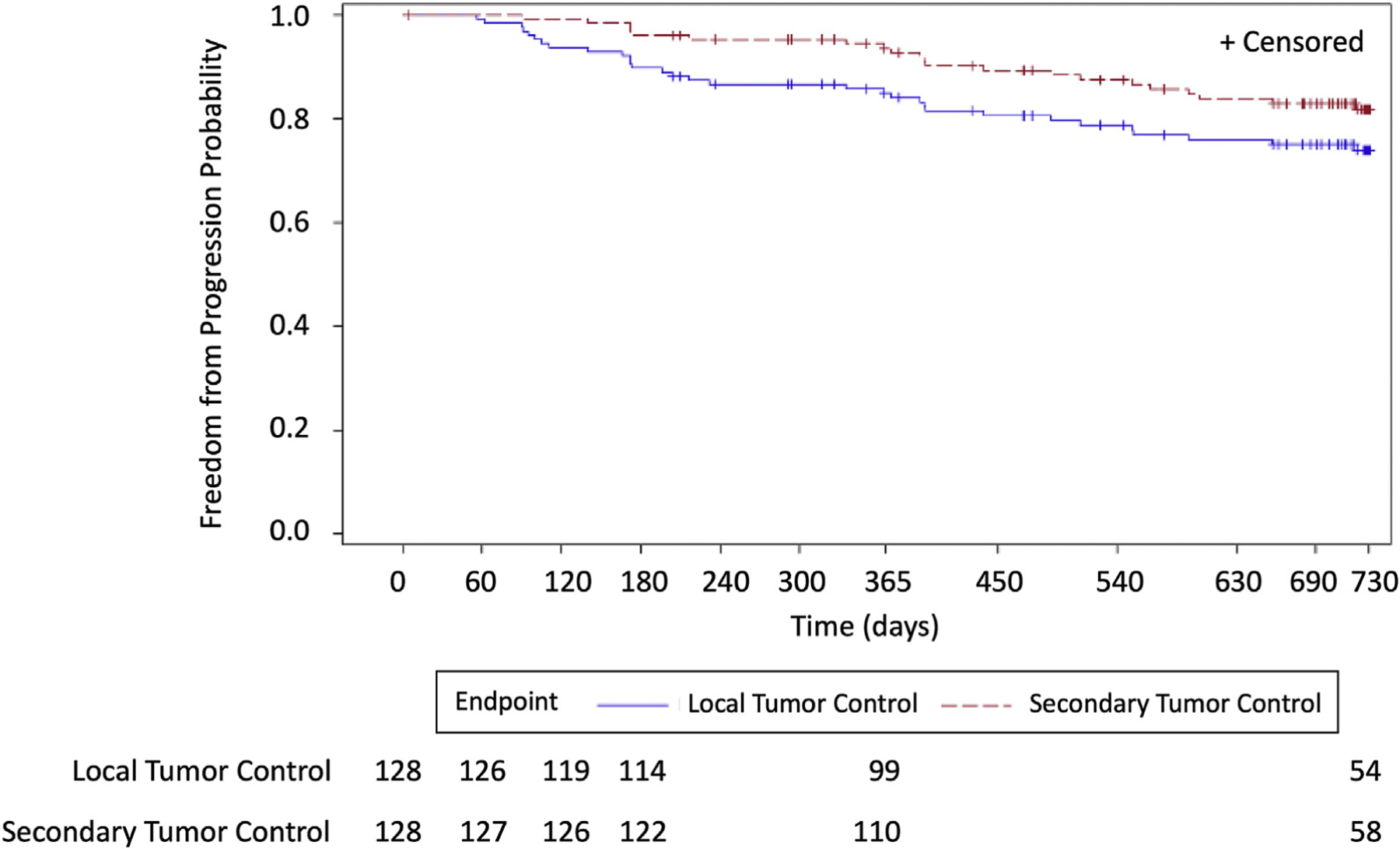
Time to progression of the index tumors for 2 years.
Figure 5.
Time to progression of the patients from the metastatic disease for 2 years.
Baseline characteristics were examined as potential predictive factors of failure, and none were statistically significant other than tumor diameter and primary cancer. Sarcoma, hilar, and local only anesthesia types were suggestive of higher failure rates; however, sample sizes in these subgroups were too small to allow for generalizability. Future work could examine the relative strength of predictive factors along with site experience using a multivariate logistic regression analysis.
Safety and Procedural Tolerance Assessment
The CTCAE grade 3-related adverse procedure-related events within 30 days of the procedure occurred in eight of 169 (4.7%) procedures, including pneumothorax in six patients with pleural catheter placement in five of these patients, one pleural hemorrhage, and one hypoxic event. A grade 4 adverse event was observed in one patient (0.6%) with gas embolism that resolved in less than 24 hours without sequelae. Neither of the two patients that were excluded from the full analysis set had grade 3 or greater adverse events. Pneumothoraces requiring intraprocedural pleural catheter placement occurred in 39 of 169 (23%) of procedures (captured as grade 2 adverse events), and subsequent pleural catheter placement occurred in an additional five of 169 (3%) procedures after discharge from the procedural suite (captured as grade 3 adverse events). Pleural catheters were removed on the next day in nine of 44 patients (20.5%), within 2 days in an additional 23 patients (52.2%), and in 3 or more days in 12 patients (27.3%). No patient required subsequent surgical treatment for bronchopleural fistula or persistent pneumothorax. Hospital length of stay after the procedure varied among centers with the patients treated in the United States having a median of 1.0 day (range 0–13 d) and in Europe with a median of 3.0 days (range 0–7 d) with an overall median length of stay of 1.0 day.
The KPS scores did not change on a clinical basis although analysis found a statistically significant difference from 96.5% at baseline to 95.4%, 94.8%, and 91.3% at the 1-month (p = 0.0658), 12-month (p = 0.0319), and 24-month (p = 0.0073) follow-ups, respectively. The SF-12 quality of life questionnaire revealed no clinically meaningful adverse impact on quality of life after CA, with the general health perception subscale having no statistical difference with 53.4 at baseline and 50.7 at 1 month (p = 0.3155) and 51.3 at 3 months (p = 0.3304).
Discussion
We report data from the largest multicenter prospective study of image-guided percutaneous CA for the treatment of lung metastases. Our results found 77% primary and 84% secondary treatment efficacies at a minimum of 24 months of follow-up in patients achieving the primary end point with secondary treatment. In comparison, SBRT for the treatment of 50 patients with 125 metastatic pulmonary tumors resulted in 83% local efficacy at 18.7 months.21 Sharma et al.6 reported SBRT treatment of 327 metastases in 206 patients with local efficacy of 85% at 2 years.
In this study, the treatment of metastatic pulmonary disease with percutaneous CA was well-tolerated. The CTCAE grade 3 or greater adverse events occurred in 6.2% of patients in this study without sequelae with no grade 5 events. The patients’ functional performance and quality of life did not change on a clinical basis as measured with the KPS score and the SF-12 quality of life questionnaire. In comparison, Chipko et al.22 reported that SBRT for malignant lung tumors resulted in chest wall pain in 33 of 118 patients (28%) 1 year after treatment with eight of 33 reporting grade 3 pain and 36% of patients reporting no resolution of pain. Rib fractures were noted in 29% of patients at a mean time of 22 months with a duration of 25 months.
Other focal image-guided therapies have been utilized for the treatment of metastatic disease to the lung. A prospective study using radiofrequency ablation for metastatic lung disease found 89% local efficacy in 61 patients with a mean follow-up of 15 months23 and de Baere et al.8 reported 4-year local efficacy of 89% in a prospective database of 566 patients.8 Vogl et al.9 reported a single-center study using microwave ablation with a local efficacy of 73.1% in the treatment of 130 tumors with mean follow-up of 9 months.9
Advantages of CA include the use of CT imaging to monitor the zone of ablation and CA can be performed safely adjacent to the pleura with low risk for bronchopleural fistula.12 Although surgical resection is the standard for treatment of patients with limited pulmonary metastatic disease, many patients are not surgical candidates. Unfortunately, many patients develop additional metastatic disease in the lung and repeat surgical treatment may not be an option owing to technical concerns or limited pulmonary reserve. CA treatment can be repeated with recurrence of disease while preserving lung parenchyma. CA treatment of centrally located tumors, when technically feasible, may allow for combination approaches with surgery while sparing lung parenchyma.3
Limitations of our study include having mixed primary histologies, and as a result, the overall cancer-specific survival will require further analyses. Analysis of tumor response using the Response Evaluation Criteria in Solid Tumors utilized the 1-month ablation zone measurement as the baseline for assessment of tumor response to the CA treatment. Over time, the ablation zone decreases in size with residual linear scar formation distinguishable from recurrent tumor. Recurrence was noted as an increase in size of the ablation zone or as an enlarging focal nodule or presence of focal contrast enhancement. As a result of scar formation at the ablation site, complete response to treatment includes measurement of the residual scar rather than disappearance of the treated tumor.
In conclusion, our report of the treatment of limited metastatic disease in the lung represents the largest prospective multicenter trial utilizing image-guided percutaneous CA. The treatment is well-tolerated, and its efficacy is encouraging.
Figure 3.
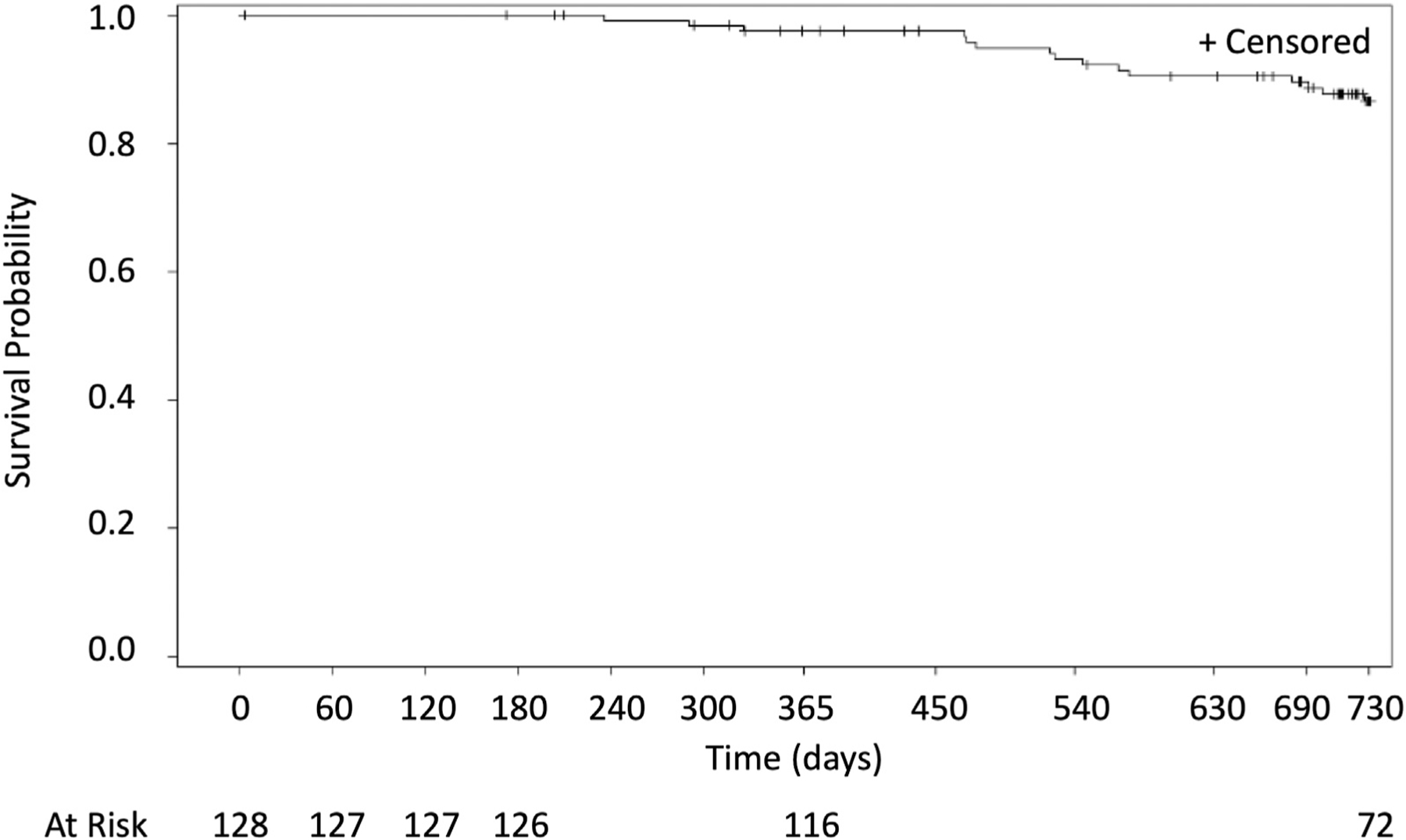
Overall survival of the patients after the image-guided percutaneous cryoablation of pulmonary metastatic disease for 2 years.
Figure 4.
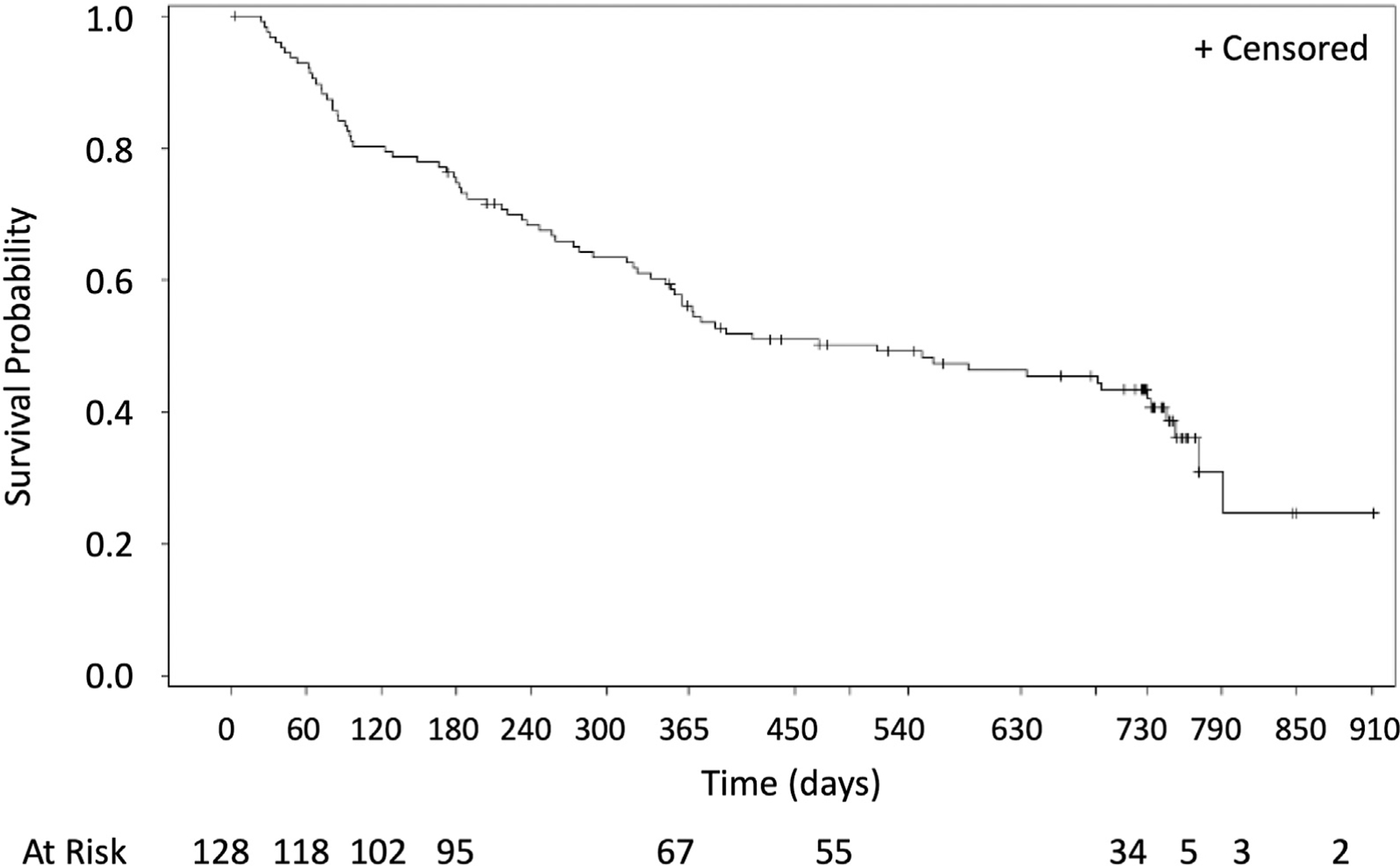
Time to progression of the patients from the metastatic disease in the lung beyond the targeted tumor for 2 years.
Acknowledgments
This study was sponsored by Galil Medical Inc. (a BTG International group company). The authors thank the study medical monitors, Hiran Fernando, MD (Inova Alexander Health Center, Cardiovascular and Interventional Radiology), Antoine Hakime, MD (Gustave Roussy Hospital), Hussein D. Aoun, MD (Karmanos Cancer Center), and Hollins Clark, MD (Wake Forest University Baptist Medical Center) for centralized review of the study; Ms. Maria Plentl (BTG) for her coordination with data management; and Ms. Claire Daugherty (BTG) for assistance with the statistical analysis.
Footnotes
Disclosure: The authors declare no conflict of interest.
Presented at the European Conference on Interventional Oncology Congress 2017 Clinical Focus Session: CF 801–Lung Metastases, April 24, 2017; Preliminary Safety Outcomes.
References
- 1.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37–49. [DOI] [PubMed] [Google Scholar]
- 2.Headrick JR, Miller DL, Nagorney DM, et al. Surgical treatment of hepatic and pulmonary metastases from colon cancer. Ann Thorac Surg 2001;71:975–979 [discussion: 979–980]. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson KJ, Blackmon SH. Results of pulmonary resection: colorectal carcinoma. Thorac Surg Clin 2016;26: 41–47. [DOI] [PubMed] [Google Scholar]
- 4.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8–10. [DOI] [PubMed] [Google Scholar]
- 5.Mouli SK, Kurilova I, Sofocleous CT, Lewandowski RJ. The role of percutaneous image-guided thermal ablation for the treatment of pulmonary malignancies. AJR Am J Roentgenol 2017;209:740–751. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A, Duijm M, Oomen-de Hoop E, et al. Survival and prognostic factors of pulmonary oligometastases treated with stereotactic body radiotherapy. Acta Oncol 2019;58:74–80. [DOI] [PubMed] [Google Scholar]
- 7.Hess A, Palussiere J, Goyers JF, Guth A, Auperin A, de Baere T. Pulmonary radiofrequency ablation in patients with a single lung: feasibility, efficacy, and tolerance. Radiology 2011;258:635–642. [DOI] [PubMed] [Google Scholar]
- 8.de Baere T, Auperin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 2015;26:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogl TJ, Naguib NN, Gruber-Rouh T, Koitka K, Lehnert T, Nour-Eldin NE. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 2011;261:643–651. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621–628. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed A, Littrup P. Percutaneous cryotherapy of the thorax: safety considerations for complex cases. AJR Am J Roentgenol 2006;186:1703–1706. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Nakatsuka S, Yashiro H, et al. Percutaneous cryoablation of lung tumors: feasibility and safety. J Vasc Interv Radiol 2012;23:295–302 [quiz 305]. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi Y, Izumi Y, Kawamura M, et al. Percutaneous cryoablation of pulmonary metastases from colorectal cancer. PLoS One 2011;6:e27086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asimakopoulos G, Beeson J, Evans J, Maiwand MO. Cryosurgery for malignant endobronchial tumors: analysis of outcome. Chest 2005;127:2007–2014. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura M, Izumi Y, Tsukada N, et al. Percutaneous cryoablation of small pulmonary malignant tumors under computed tomographic guidance with local anesthesia for nonsurgical candidates. J Thorac Cardiovasc Surg 2006;131:1007–1013. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology 2005;235:289–298. [DOI] [PubMed] [Google Scholar]
- 17.Pusceddu C, Sotgia B, Fele RM, Melis L. CT-guided thin needles percutaneous cryoablation (PCA) in patients with primary and secondary lung tumors: a preliminary experience. Eur J Radiol 2013;82:e246–e253. [DOI] [PubMed] [Google Scholar]
- 18.de Baere T, Tselikas L, Woodrum D, et al. Evaluating cryoablation of metastatic lung tumors in patients—safety and efficacy: the ECLIPSE trial—interim analysis at 1 year. J Thorac Oncol 2015;10:1468–1474. [DOI] [PubMed] [Google Scholar]
- 19.Common terminology criteria for adverse events v 3. 0 (CTCAE) http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Published August 09, 2006. Accessed September 30, 2013.
- 20.Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 21.Okunieff P, Petersen AL, Philip A, et al. Stereotactic body radiation therapy (SBRT) for lung metastases. Acta Oncol 2006;45:808–817. [DOI] [PubMed] [Google Scholar]
- 22.Chipko C, Ojwang J, Gharai LR, Deng X, Mukhopadhyay N, Weiss E. Characterization of chest wall toxicity during long-term follow up after thoracic stereotactic body radiation therapy. Pract Radiat Oncol 2019;9:e338–e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chua TC, Sarkar A, Saxena A, Glenn D, Zhao J, Morris DL. Long-term outcome of image-guided percutaneous radiofrequency ablation of lung metastases: an open-labeled prospective trial of 148 patients. Ann Oncol 2010;21:2017–2022. [DOI] [PubMed] [Google Scholar]


