Abstract
Coimmobilization of the freshwater microalga Chlorella vulgaris and the plant-growth-promoting bacterium Azospirillum brasilense in small alginate beads resulted in a significantly increased growth of the microalga. Dry and fresh weight, total number of cells, size of the microalgal clusters (colonies) within the bead, number of microalgal cells per cluster, and the levels of microalgal pigments significantly increased. Light microscopy revealed that both microorganisms colonized the same cavities inside the beads, though the microalgae tended to concentrate in the more aerated periphery while the bacteria colonized the entire bead. The effect of indole-3-acetic acid addition to microalgal culture prior to immobilization of microorganisms in alginate beads partially imitated the effect of A. brasilense. We propose that coimmobilization of microalgae and plant-growth-promoting bacteria is an effective means of increasing microalgal populations within confined environments.
Microalgae have many uses. They can serve as water bioremediation agents (40), as feed for aquaculture (17), as food for humans and animals (10), in pigment production (25), in bioremoval of heavy metals (49), and in agriculture (33). It is usually desirable to establish large populations of microalgae, especially in aquatic environments where they are often employed. One means of increasing microalgal populations may be to inoculate them with other microorganisms, a strategy that is being tested for its potential to increase yields of agriculturally important plants (5, 23, 32).
One candidate microorganism for coinoculation with microalgae is Azospirillum brasilense, a member of the group of plant rhizosphere bacteria known as plant-growth-promoting bacteria (PGPB) (6, 20, 29). This relatively well-studied diazotrophic bacterium (5) promotes the growth of many terrestrial plants upon seed or root inoculation and increases the yields of numerous crop plants (8, 37). All the known Azospirillum species produce plant hormones, mainly auxins, as do many other PGPB. It is thought that interference with the hormonal metabolism of the host plant is one of the major ways in which PGPB affect plant growth (15, 41).
The aim of this study was to increase the growth of the freshwater microalga Chlorella vulgaris, an important organism in tertiary wastewater treatment (21, 30, 44) and for several industrial research studies (27, 48), by inoculating it with A. brasilense when grown within a confined environment. This study is the first report of the deliberate inoculation of Chlorella sp. with a terrestrial PGPB, perhaps because of the different origins of the two microorganisms. C. vulgaris is not known to harbor any associative beneficial bacteria, and Azospirillum sp. is rarely used for inoculation in aquatic environments (42).
To ensure the close proximity of the two microorganisms in the liquid medium essential for C. vulgaris, they were coimmobilized in alginate beads and were cocultivated under controlled conditions suitable for both, in batch cultures and in continuous flow cultures in a chemostat. Alginate beads of various forms and shapes are convenient inoculant carriers for use in numerous industrial, environmental, and agricultural applications (3, 13, 19, 31, 45). Immobilized A. brasilense has been proposed for semiarid agricultural uses (2, 9), but this work is still in the experimental stage (3, 19). Chlorella sp. coimmobilized with other microorganisms is used in industrial processes where the microalga generates oxygen for the accompanying microorganism involved in compound transformation (14, 28, 39). Continuous culture techniques have been used both for diazotrophic bacteria such as Azospirillum (46, 51) and for microalgae (34).
MATERIALS AND METHODS
Microorganisms and axenic growth conditions.
C. vulgaris Beijerinck (UTEX 2714) was isolated from a secondary effluent of a wastewater treatment stabilization pond near Santafe de Bogota (21) and was purified from associative bacteria using an antibiotic package (L. E. Gonzalez, V. K. Lebsky, J. P. Hernandez, J. J. Bustillos, and Y. Bashan, unpublished data). Prior to immobilization in beads, axenic C. vulgaris was cultivated in a sterile mineral medium (C30) as previously described (21) for 5 days. A. brasilense Cd (DSM 7030) was grown in liquid nutrient broth (Sigma) or N-free OAB medium at 30 ± 2°C for 16 h by standard methods (7) and was used for immobilization after a washing in sterile saline solution (0.85% NaCl).
Immobilization of microalgae into alginate beads and bead solubilization.
Microorganisms were immobilized using the method described by Bashan (2). Briefly, 20 ml of axenically grown cultures of C. vulgaris containing 6.0 × 106 cells/ml were mixed with 80 ml of a sterile, 6,000-cP 2% alginate solution (a solution made of alginate mixed at 14,000 and 3,500 cP) and stirred for 15 min. The solution was dripped from a sterile syringe into a 2% CaCl2 solution (11) with slow stirring. The beads formed were left for 1 h at 22 ± 2°C for curing and then washed in sterile saline solution. A. brasilense cultures (approximately 109 CFU/ml) were immobilized similarly. Because immobilization normally reduces the number of microorganisms in the beads (2), a second incubation step was necessary, overnight in OAB medium for beads containing A. brasilense and 18 h in 6.5 mM phosphate buffer for beads containing C. vulgaris. The low concentration of phosphate and the short incubation period were insufficient to dissolve the beads. Where cocultures of A. brasilense and the microalga were used, the same concentration of each microorganism used in pure cultures was mixed prior to incorporation with alginate and bead formation, but the volume of each microbial culture was reduced to 10 ml before adding the alginate. Where appropriate, indole-3-acetic acid (IAA) (10−4, 10−5, and 10−6 M; Sigma), dissolved in 100 ml of C30 medium, was added prior to immobilization of C. vulgaris into alginate beads.
Beads were solubilized for cell counts by immersing five beads (one bead per milliliter) in a solution of 0.25 M phosphate-buffered saline (PBS) (pH 7.0 ± 0.2) for 1 h at 30 ± 2°C. A. brasilense was counted by plating a series of dilutions (in PBS) on nutrient agar plates (Difco, Detroit, Mich.), and C. vulgaris was counted using a Neubauer hemocytometer.
Culture conditions for coimmobilized microorganisms or organisms alone.
Coimmobilized microorganisms or C. vulgaris organisms alone were grown in the mineral salts of residual water medium (21) containing the following (in milligrams per liter): NaCl, 7; CaCl2, 4; MgSO4 · 7H2O, 2; K2HPO4, 21.7; KH2PO4, 8.5; Na2HPO4, 33.4; and NH4Cl, 10. These were grown either in batch cultures or in continuous culture in a chemostat. The level of phosphate in the medium was insufficient to dissolve the constructed beads. Batch cultures (500 ml) were incubated in nonbaffled Erlenmeyer flasks at 22 ± 2°C and 150 rpm and with a light intensity of 60 μmol/m2/s for 7 days. Cultures in a chemostat (Virtis, Gardiner, N.Y.) were grown at 28 ± 2°C and 90 rpm with 100% dissolved oxygen, a light intensity of 30 μmol/m2/s, and a medium exchange rate of 1.5 ml/h. Samples for analysis were taken aseptically. Batch cultures were run for 7 to 9 days, and chemostat cultures were run for 5 to 10 days.
Location of C. vulgaris inside the bead.
Random samples of beads from both batch and chemostat cultures were transversely cut and immediately mounted on a glass slide at ambient temperature (23 ± 2°C) for light microscopy (Zeiss) at a magnification of ×400. The location and numbers of C. vulgaris clusters and individual cells within the bead were determined both in the outer periphery (0.5 mm) of the bead and in its interior.
Pigment analysis.
The effect of coculturing with A. brasilense on the quantity of some pigments of C. vulgaris was determined by high-pressure liquid chromatography (series 1100; Hewlett-Packard) (47) after 7 days of incubation. Chlorophylls a and b, β-carotene, lutein, and violoxanthin were analyzed.
Biomass determination.
Ten grams of beads containing coimmobilized microalgae and bacteria was dissolved in 100 ml of PBS as described above. The suspension was then filtered through a 3-μm (pore size) plankton net leaving a pellet of microalgae on the net. This pellet was resuspended in 100 ml of PBS. Aliquots (10 ml) were centrifuged for 3 min at 1,400 × g in tubes containing filter paper (Jecaber 5098) at the bottom. The supernatant containing the bacteria was discarded. The dry weight of the microalgae was measured after extracting and drying the filter paper containing the microalgal pellet at 105°C for 1 h.
Experimental design and statistical analysis.
Batch cultures were prepared in triplicate where a single flask served as one replicate, and each experiment was repeated five times. Routine controls were prepared similarly but without microorganisms in the beads. Controls of heat-killed bacteria had no effect on microalgal growth and therefore were not used routinely. Each of the chemostat runs was repeated twice. Five beads were taken randomly (from each culture) for counting of the total number of clusters and the number of cells within each cluster. In each of the five beads, 10 microscopic fields were chosen randomly for counting of microalgae. The dry weight of the microalgae was measured in triplicate, where 10 g of beads dissolved in 100 ml of PBS served as a single replicate. Pigment content was also analyzed in triplicate where the levels of pigments in five dissolved beads served as one replicate. Experiments in which IAA was added to C. vulgaris batch cultures were repeated twice (three flasks per experiment). Results of all repetitions were combined and analyzed by one-way analysis of variance (ANOVA) or by Student's t test (P ≤ 0.05).
RESULTS
Multiplication of C. vulgaris within alginate beads under batch and continuous growth conditions.
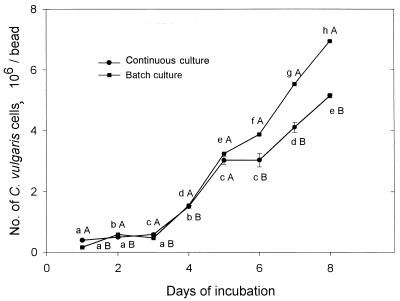
C. vulgaris grew continuously within the alginate beads for 9 days, reaching a population of 7 × 106 cells/bead in batch cultures and only 5 × 106 cells/bead under continuous growth conditions. After 5 days of incubation, significantly more cells developed under batch culture (Fig. 1). Both culture types are useful for the study of C. vulgaris colonization and behavior inside alginate beads.
FIG. 1.
Multiplication of C. vulgaris within alginate beads under batch and continuous growth conditions. Points on each curve denoted by a different lowercase letter differ significantly at P ≤ 0.05 in one-way ANOVA. Points denoted by different capital letters after each day of incubation differ significantly at P ≤ 0.05 in Student's t test. The experiment was repeated five times, and the results presented are from a representative experiment. Bars represent the standard error (SE). When the SE bar is absent, the SE is smaller than the point.
Location and multiplication of C. vulgaris within alginate beads when immobilized alone or when coimmobilized with A. brasilense.
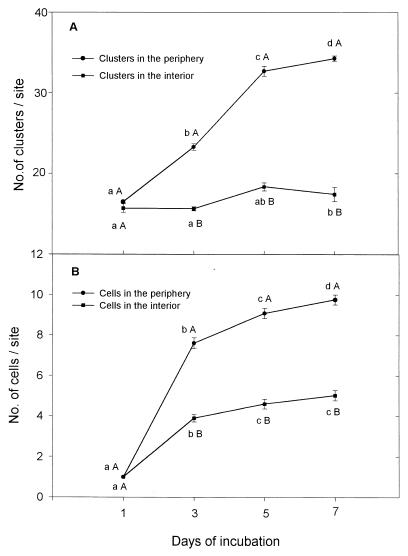
During solidification of the alginate into beads, numerous internal cavities of various sizes are formed randomly. Analysis of these cavities revealed that C. vulgaris preferred to grow in the periphery of the bead. In this area there were significantly more algal clusters (colonies) (Fig. 2A), and each cluster contained more microalga cells (Fig. 2B).
FIG. 2.
Multiplication of C. vulgaris in the periphery and in the interior of alginate beads under continuous culture conditions. Points on each curve denoted by a different lowercase letter differ significantly at P ≤ 0.05 in one-way ANOVA. Points denoted by different capital letters after each day of incubation differ significantly at P ≤ 0.05 in Student's t test. The experiment was repeated five times, and the results presented are from a representative experiment. Bars represent the SE. When the SE bar is absent, the SE is smaller than the point.
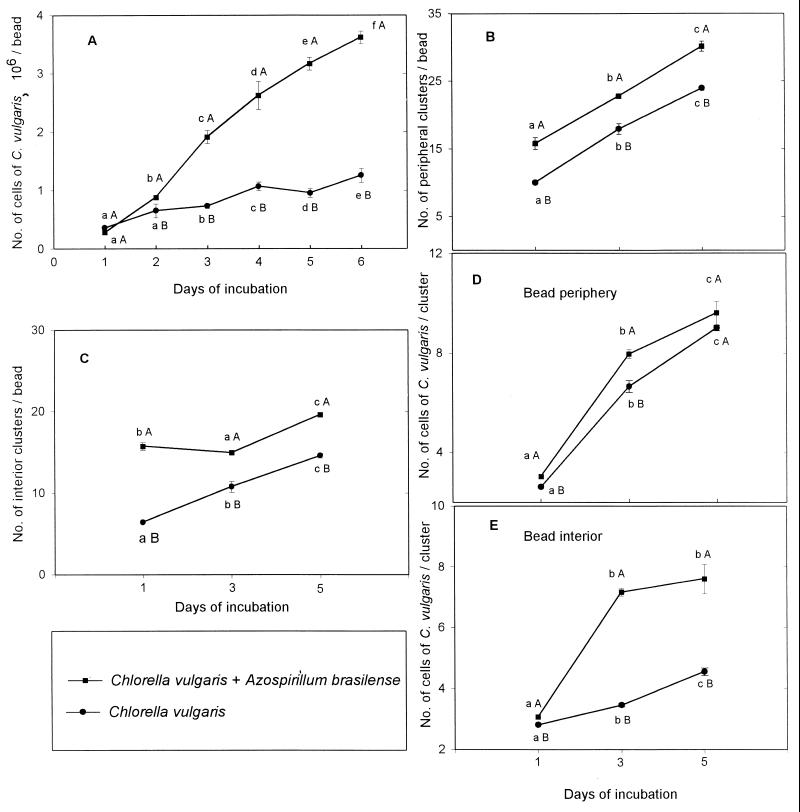
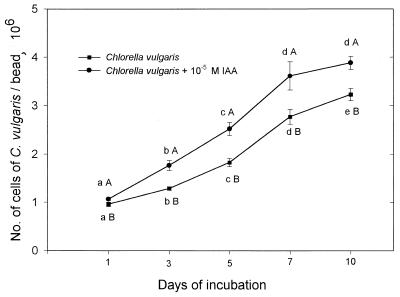
A. brasilense multiplied within the beads over time as was observed previously (2), increasing linearly (y = 3.98x − 2.55; r2 = 98.8) from 1.9 × 107 to 1.35 × 108 CFU/g of beads after 96 h of incubation. When the two microorganisms were coimmobilized in the same bead, significant promotion of C. vulgaris growth occurred within 1 day and continued for 6 days. The total numbers of algal cells (Fig. 3A) and clusters significantly increased, both in the periphery and in the interior of the bead (Fig. 3B and C). Similarly, the number of cells per algal cluster significantly increased both in the periphery and in the interior of the bead (Fig. 3D and E). Fresh weight approximately doubled (from 9 mg of beads per g to 18.9 mg of beads per g), and the dry weight increased even more dramatically (from 0.1 mg of beads per g to 2.4 mg of beads per g). Addition of the auxin significantly increased the multiplication of C. vulgaris within the bead, an effect similar to that observed following coimmobilization with A. brasilense. An IAA concentration of 10−5 M gave the highest growth promotion (Fig. 4).
FIG. 3.
Multiplication of C. vulgaris and A. brasilense coimmobilized in the same alginate bead under continuous culture conditions. (A) Total number of cells; (B) number of alga clusters in the periphery; (C) number of alga clusters in the interior of the bead; (D) number of cells per cluster in the periphery; (E) number of cells per cluster in the bead interior. Points on each curve denoted by a different lowercase letter differ significantly at P ≤ 0.05 in one-way ANOVA. Points denoted by different capital letters after each day of incubation differ significantly at P ≤ 0.05 in Student's t test. The experiment was repeated five times, and the results presented are from a representative experiment. Bars represent the SE. When the SE bar is absent, the SE is smaller than the point.
FIG. 4.
The effect of addition of IAA (10−4, 10−5, and 10−6 M) on C. vulgaris growth in batch culture. Points on each curve denoted by a different lowercase letter differ significantly at P ≤ 0.05 in one-way ANOVA. Points denoted by different capital letters after each day of incubation differ significantly at P ≤ 0.05 in Student's t test. Bars represent the SE. When the SE bar is absent, the SE is smaller than the point.
Increase in C. vulgaris pigment production when coimmobilized with A. brasilense.
The microalgal pigment concentration in a mixture of C. vulgaris and A. brasilense coimmobilized in alginate beads was compared to an axenic culture of C. vulgaris immobilized in similar beads in batch cultures. Table 1 shows that the concentration per cell of the five microalgal pigments evaluated significantly increased as a result of coimmobilization of the two microorganisms in the same bead.
TABLE 1.
Pigment production by the microalga C. vulgaris after coimmobilization with A. brasilense in alginate beads and incubated under batch culture for 7 daysa
| Pigment | C. vulgaris (μg/g of cells) | C. vulgaris plus A. brasilense (μg/g of cells) |
|---|---|---|
| Chlorophyll a | 502† | 679.3* |
| Chlorophyll b | 71.9† | 198.6* |
| β-Carotene | 7.1† | 20.3* |
| Lutein | 52† | 131.2* |
| Violoxanthin | 10.4† | 23.9* |
Numbers denoted by a different symbol (* or †) for each pigment differ significantly at P ≤ 0.05 by Student's t test.
DISCUSSION
The use of PGPB to enhance plant growth and crop yield is predicted to become a major activity in contemporary agriculture in the near future (35). These bacteria may also prove useful for increasing the production of microalgae that have important application in aquaculture, environmental cleanup, and the human food and animal feed industries (12, 16). Attempts to increase microalgal biomass have employed various technological, physical, molecular, and environmental methods (16, 40), albeit microalgal growth has limitations under any culture conditions (43).
Our working hypothesis was that some PGPB affect agricultural terrestrial plant performance by interfering with the host plant's hormonal metabolism (41). This hypothesis may be extended to include unicell aquatic plants, such as the microalga C. vulgaris, which, following inoculation with PGPB, may also exhibit enhanced cell proliferation and increased biomass production. Microalga-growth-promoting bacteria have not previously been identified; thus, we chose a nonspecific PGPB, A. brasilense, as a candidate bacterium. In liquid media, both microorganisms disperse: the microalgae through currents (36) and the bacterium by its own motility (4, 50). Effectiveness in industrial and water bioremediation applications would be improved if these free-moving microorganisms were confined to environments where their activities could be better controlled. Furthermore, where mutual effects are expected, it is essential that the microorganisms are in close proximity, as provided by small alginate spheres (26).
We show here that coimmobilization of the two microorganisms in the same bead resulted in enhanced microalgal proliferation, larger quantities of pigment production, and increased culture biomass, effects similar to those found for terrestrial plants following inoculation with Azospirillum sp. Thus, one may speculate that phytohormones such as IAA, produced by A. brasilense (22), may play a role in the stimulation of microalgal growth. This idea is supported by the growth promotion seen following application of exogenous IAA (this study) and the observation that algae are capable of using and being affected by angiosperm hormones (1, 18, 24). Oxygen diffusion is known to be limited inside gel spheres (38), and this may explain why the majority of C. vulgaris proliferation occurred in the periphery of the bead. This indicates that smaller beads may be better for increasing microalgal biomass.
In summary, this study highlights the potential for using a new agricultural technology, PGPB treatment, to increase microalgal biomass production in aquatic conditions when immobilization is required, as in several bioremediation and industrial processes. This has important implications for industry and the environment. The ability of PGPB to affect unicell plants extends the usefulness of these organisms beyond their original agricultural uses.
ACKNOWLEDGMENTS
We thank Carlos Quitiaquez and Juan Pablo Hernandez for excellent technical assistance, Jesus J. Bustillos for pigment analysis, Ricardo Vazquez-Juarez for assembly of the chemostat, Ellis Glazier for editing the English-language text, and Cheryl Patten for critical reading of the manuscript.
This study was supported by Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología, Francisco José de Caldas (COLCIENCIAS) (Colombia), Consejo Nacional de Ciencia y Tecnología (CONACyT) (Mexico) contracts 26262-B and 28362-B, and the Bashan Foundation.
Footnotes
Y. Bashan participated in this study in memory of the late Avner Bashan of Israel.
REFERENCES
- 1.Bajguz A, Czerpak R. Effect of brassinosteroids on growth and proton extrusion in the alga Chlorella vulgaris Beijerinck (Chlorophyceae) J Plant Growth Regul. 1996;15:153–156. [Google Scholar]
- 2.Bashan Y. Alginate beads as synthetic inoculant carriers for the slow release of bacteria that affect plant growth. Appl Environ Microbiol. 1986;51:1089–1098. doi: 10.1128/aem.51.5.1089-1098.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashan Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol Adv. 1998;16:729–770. [Google Scholar]
- 4.Bashan Y, Holguin G. Root-to-root travel of the beneficial bacterium Azospirillum brasilense. Appl Environ Microbiol. 1994;60:2120–2131. doi: 10.1128/aem.60.6.2120-2131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashan Y, Holguin G. Azospirillum-plant relationships: environmental and physiological advances (1990–1996) Can J Microbiol. 1997;43:103–121. doi: 10.1139/w04-035. [DOI] [PubMed] [Google Scholar]
- 6.Bashan Y, Holguin G. Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol Biochem. 1998;30:1225–1228. [Google Scholar]
- 7.Bashan Y, Holguin G, Lifshitz R. Isolation and characterization of plant growth-promoting rhizobacteria. In: Glick B R, Thompson J E, editors. Methods in plant molecular biology and biotechnology. Boca Raton, Fla: CRC Press; 1993. pp. 331–345. [Google Scholar]
- 8.Bashan Y, Levanony H. Current status of Azospirillum inoculation technology: Azospirillum as a challenge for agriculture. Can J Microbiol. 1990;36:591–608. [Google Scholar]
- 9.Bashan Y, Levanony H, Ziv-Vecht O. The fate of field-inoculated Azospirillum brasilense Cd in wheat rhizosphere during the growing season. Can J Microbiol. 1987;33:1074–1079. [Google Scholar]
- 10.Becker E W. Micro-algae for human and animal consumption. In: Borowitzka M A, Borowitzka L J, editors. Micro-algal biotechnology. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 222–256. [Google Scholar]
- 11.Bettman H, Rehm H J. Degradation of phenol by polymer entrapped microorganisms. Appl Microbiol Biotechnol. 1984;20:285–290. [Google Scholar]
- 12.Borowitzka M A, Borowitzka L J, editors. Micro-algal biotechnology. Cambridge, United Kingdom: Cambridge University Press; 1992. [Google Scholar]
- 13.Cassidy M B, Lee H, Trevors J T. Environmental applications of immobilized microbial cells: a review. J Ind Microbiol. 1996;16:79–101. [Google Scholar]
- 14.Chevalier P, De la Noüe J. Behavior of algae and bacteria co-immobilized in carrageenan, in a fluidized bed. Enzyme Microb Technol. 1988;10:19–23. [Google Scholar]
- 15.Costacurta A, Vanderleyden J. Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol. 1995;21:1–18. doi: 10.3109/10408419509113531. [DOI] [PubMed] [Google Scholar]
- 16.De la Noüe J, De Pauw N. The potential of microalgal biotechnology: a review of production and uses of microalgae. Biotechnol Adv. 1988;6:725–770. doi: 10.1016/0734-9750(88)91921-0. [DOI] [PubMed] [Google Scholar]
- 17.De Pauw N, Persoone G. Micro-algae for aquaculture. In: Borowitzka M A, Borowitzka L J, editors. Micro-algal biotechnology. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 197–221. [Google Scholar]
- 18.Dibb-Fuller J E, Morris D A. Studies on the evolution of auxin carriers and phytotropin receptors: transmembrane auxin transport in unicellular and multicellular Chlorophyta. Planta. 1992;186:219–226. doi: 10.1007/BF00196251. [DOI] [PubMed] [Google Scholar]
- 19.Fages J. An industrial view of Azospirillum inoculants: formulation and application technology. Symbiosis. 1992;13:15–26. [Google Scholar]
- 20.Glick B R. The enhancement of plant growth by free-living bacteria. Can J Microbiol. 1995;41:109–117. [Google Scholar]
- 21.Gonzalez L E, Cañizares R O, Baena S. Efficiency of ammonia and phosphorus removal from Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Biores Technol. 1997;60:259–262. [Google Scholar]
- 22.Hartmann A, Zimmer W. Physiology of Azospirillum. In: Okon Y, editor. Azospirillum/plant associations. Boca Raton, Fla: CRC Press; 1994. pp. 15–39. [Google Scholar]
- 23.Holguin G, Bashan Y. Nitrogen-fixation by Azospirillum brasilense Cd is promoted when co-cultured with a mangrove rhizosphere bacterium (Staphylococcus sp.) Soil Biol Biochem. 1996;28:1651–1660. [Google Scholar]
- 24.Jacobs W P. Are angiosperm hormones present in and used by algae? In: Bopp M, editor. Plant growth substances. Berlin, Germany: Springer-Verlag; 1986. pp. 218–226. [Google Scholar]
- 25.Johnson E A, An G H. Astaxanthin from microbial sources. Crit Rev Biotechnol. 1991;11:297–326. [Google Scholar]
- 26.Kaya V M, De la Noüe J, Picard G. A comparative study of four systems for tertiary wastewater treatment by Scenedesmus bicellularis: new technology for immobilization. J Appl Phycol. 1994;7:85–95. [Google Scholar]
- 27.Kayano H, Matsunaga T, Karube I, Suzuki S. Hydrogen evolution by co-immobilized Chlorella vulgaris and Clostridium butyricum cells. Biochim Biophys Acta. 1981;638:80–85. [Google Scholar]
- 28.Khang Y H, Shankar H, Senatore F. Enhanced beta-lactam antibiotic production by coimmobilization of fungus and algae. Biotechnol Lett. 1988;10:867–872. [Google Scholar]
- 29.Kloepper J W, Leong J, Teintze M, Schroth M N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature. 1980;286:885–886. [Google Scholar]
- 30.Lau P S, Tam N F Y, Wong Y S. Wastewater nutrient (N and P) removal by carrageenan and alginate immobilized Chlorella vulgaris. Environ Technol. 1997;18:945–951. [Google Scholar]
- 31.Leenen E J T M, Dos Santos V A P, Grolle K C F, Tramper J, Wijffels R H. Characteristics of and selection criteria for support materials for cell immobilization in wastewater treatment. Water Res. 1996;30:2985–2996. [Google Scholar]
- 32.Lippi D, Cacciari I, Pietrosanti T, Pietrosanti W. Interactions between Azospirillum and Arthrobacter in diazotrophic mixed culture. Symbiosis. 1992;13:107–114. [Google Scholar]
- 33.Metting B. Micro-algae in agriculture. In: Borowitzka M A, Borowitzka L J, editors. Micro-algal biotechnology. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 288–304. [Google Scholar]
- 34.Moo-Young M, editor. Bioreactor immobilized enzymes and cells: fundamentals and applications. New York, N.Y: Elsevier Applied Science; 1988. [Google Scholar]
- 35.Ogoshi A, Kobayashi K, Homma Y, Kodama F, Kondo N, Akino S, editors. Plant growth-promoting bacteria—present status and future prospects. Sapporo, Japan: Hokkaido University Press; 1997. [Google Scholar]
- 36.Oh-Hama T, Miyachi S. Chlorella. In: Borowitzka M A, Borowitzka L J, editors. Micro-algal biotechnology. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 3–26. [Google Scholar]
- 37.Okon Y, Labandera-Gonzalez C A. Agronomic applications of Azospirillum: an evaluation of 20 years of worldwide field inoculation. Soil Biol Biochem. 1994;26:1591–1601. [Google Scholar]
- 38.Omar S H. Oxygen diffusion through gels employed for immobilization. 2. In the presence of microorganisms. Appl Microbiol Biotechnol. 1993;40:173–181. [Google Scholar]
- 39.O'Reilly A M, Scott J A. Defined coimmobilization of mixed microorganism cultures. Enzyme Microb Technol. 1995;17:636–646. [Google Scholar]
- 40.Oswald W J. Micro-algae and waste-water treatment. In: Borowitzka M A, Borowitzka L J, editors. Micro-algal biotechnology. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 305–328. [Google Scholar]
- 41.Patten C L, Glick B R. Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol. 1996;42:207–220. doi: 10.1139/m96-032. [DOI] [PubMed] [Google Scholar]
- 42.Puente M E, Holguin G, Glick B R, Bashan Y. Root surface colonization of black mangrove seedlings by Azospirillum halopraeferans and Azospirillum brasilense in seawater. FEMS Microbiol Ecol. 1999;29:283–292. [Google Scholar]
- 43.Raven J A. Limits to growth. In: Borowitzka M A, Borowitzka L J, editors. Micro-algal biotechnology. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 331–356. [Google Scholar]
- 44.Tam N F Y, Lau P S, Wong Y S. Wastewater inorganic N and P removal by immobilized Chlorella vulgaris. Water Sci Technol. 1994;30:369–374. [Google Scholar]
- 45.Tanaka A, Nakajima H. Applications of immobilized growing cells. Adv Biochem Eng Biotechnol. 1990;42:97–131. doi: 10.1007/BFb0000732. [DOI] [PubMed] [Google Scholar]
- 46.Ueckert J, Fendrik I. Continuous culture application in physiological investigations on diazotrophic bacteria. In: Fendrick I, Del Gallo M, Vanderleyden J, de Zamaroczy M, editors. Azospirillum VI and related microorganisms. Genetics-physiology-ecology. NATO Series. G37. Berlin, Germany: Springer-Verlag; 1995. pp. 291–298. [Google Scholar]
- 47.Vidussi F, Claustre H, Bustillos-Guzman J, Cailliau C, Marty J C. Determination of chlorophylls and carotenoids of marine phytoplankton: separation of chlorophyll a from divinyl-chlorophyll a and zeaxanthin from lutein. J Plankton Res. 1996;18:2377–2382. [Google Scholar]
- 48.Wikstrom P, Szwajcer E, Brodelius P, Nilsson K, Mosbach K. Formation of keto acids from amino acids using immobilized bacteria and algae. Biotechnol Lett. 1982;4:153–158. [Google Scholar]
- 49.Wilde E W, Benemann J R. Bioremoval of heavy metals by the use of microalgae. Biotechnol Adv. 1993;11:781–812. doi: 10.1016/0734-9750(93)90003-6. [DOI] [PubMed] [Google Scholar]
- 50.Zhulin I B, Armitage J P. The role of taxis in the ecology of Azospirillum. Symbiosis. 1992;13:199–206. [Google Scholar]
- 51.Zimmer W, Stephan M P, Bothe H. Denitrification by Azospirillum brasilense Sp7. I. Growth with nitrate as respiratory electron acceptor. Arch Microbiol. 1984;138:206–211. [Google Scholar]